Abstract
Chronic inflammation of the gastric mucosa, often caused by autoimmune gastritis and/or infection with Helicobacter pylori, can lead to atrophy of acid-secreting parietal cells with metaplasia of remaining cells. The histological pattern marks a critical step in the progression from chronic gastritis to gastric cancer, yet underlying mechanism(s) of inflammation-induced cell death of gastric epithelial cells are poorly understood. We investigated direct effects of a type 1 cytokine associated with autoimmunity and infection, interferon-γ (IFN-γ), on gastric epithelial cells. IFN-γ was applied to three dimensional organoid cultures of gastric epithelial cells derived from gastric corpus gland (gastroids) of control and IFN-γ receptor-deficient mice. Gastroids were also treated with supernatants from activated immune cells isolated from a mouse model of autoimmune-mediated atrophic gastritis (TxA23) with and without IFN-γ expression. Finally, histopathological analysis of atrophy and metaplasia severity was performed in TxA23 mice and compared to TxA23xIfng−/− mice. Gastric epithelial cells in gastroid cultures expressed IFN-γ receptor in the basolateral membrane, and gastroids died when treated with IFN-γ in an IFN-γ receptor-dependent manner. Supernatants from immune cells containing high levels of IFN-γ were highly toxic to gastroids, and toxicity was tempered when IFN-γ was either neutralized using a monoclonal antibody or when supernatants from Ifng−/− mouse immune cells were used. Finally, TxA23xIfng−/− mice showed near complete abrogation of pre-cancerous histopathological atrophy and metaplasia versus IFN-γ-sufficient controls. We identify IFN-γ as a critical promoter of parietal cell atrophy with metaplasia during the progression of gastritis to gastric atrophy and metaplasia.
Keywords: Atrophic gastritis, Spasmolytic Polypeptide Expressing Metaplasia (SPEM), inflammation, paligenosis
Introduction
Gastric cancer is the fifth most common and the third most deadly cancer in the world, leading to more than 700,000 deaths/year [1]. 95% of gastric cancers are adenocarcinomas derived from glandular epithelium of the gastric mucosa [2]. The cancerous transformation of normal gastric epithelium has been strongly correlated with chronic inflammation, most commonly caused by Helicobacter pylori infection and autoimmune gastritis in humans [3, 4]. The progression from gastritis to gastric cancer has been proposed to involve a stepwise progression of lesions (the Correa pathway) in which chronic gastritis leads to the loss of acid-secreting parietal cells and the nearly concomitant development of Spasmolytic Polypeptide-Expressing Metaplasia (SPEM)[5]. These are critical precursor lesions for the downstream development of dysplasia and, eventually, frank adenocarcinoma [6, 7]. The cause(s) of parietal cell atrophy have not been fully described and may include autoantibodies, inflammatory cytokines, Sonic hedgehog signaling, and/or other inducers of cell death (e.g. FAS-FASL) [8–11]. A better understanding of how chronic immune responses regulate mucosal lesion progression is needed to better identify those at risk for developing gastric cancer in the context of gastritis.
In both Helicobacter pylori infection and autoimmune gastritis, type 1 immune responses characterized by Th1 CD4+ T cells and the production of interferon-γ (IFN-γ) are critical drivers of disease pathology [12–15]. However, the mechanistic link between this immune phenotype and the development of gastric cancer has not been established. IFN-γ is a critical component of type 1 immune responses, including the activation of macrophages, the differentiation of Th1 CD4+ T cells, and the induction of MHC molecules on the surface of target cells [16]. However, the direct effects of IFN-γ on epithelial cells have not been examined in detail. Several studies have shown a significant association of single nucleotide polymorphisms in genes that encode cytokines and the risk of developing gastric cancer [17–19]. A limited number of studies have implicated a role for cytokines, including in inducing atrophy and/or metaplasia [8, 20–22]. Here, we tested, for the first time, the hypothesis that the direct action of IFN-γ on epithelial cells is critical for the development of atrophy and metaplasia.
To determine the role IFN-γ plays in promoting parietal cell atrophy, we used a TCR transgenic mouse model of chronic atrophic gastritis induced by CD4+ T cells that are autoreactive against the H+/K+-ATPase expressed by parietal cells. This model, referred to as TxA23, mimics many aspects of atrophic gastritis and gastric metaplasia in human patients including: chronic inflammation and anti-parietal cell autoantibodies; infiltration of numerous IFN-γ producing cells into the gastric mucosa; and the development of parietal cell atrophy, mucous neck cells hyperplasia, SPEM, and eventually gastric intraepithelial neoplasms [8, 14, 23]. In the present study, we observed IFN-γ receptor expression on gastric epithelial cells and used three-dimensional gastric corpus organoid cultures to show that IFN-γ directly induces organoid degeneration in a receptor-dependent manner. We also demonstrated that supernatants from stimulated TxA23 immune cells were highly toxic to organoid cultures, but this toxicity was reduced in supernatants from IFN-γ-deficient TxA23 immune cells. Finally, histopathologic analysis of IFN-γ deficient mice revealed minimal atrophy and metaplasia versus wild-type TxA23 mice, demonstrating that IFN-γ expression is critical for disease progression in vivo. Together, these findings show that IFN-γ, a cytokine produced in large quantities during chronic gastritis, directly promotes the death of gastric epithelial cells and might, therefore, contribute to the development of chronic atrophic gastritis and SPEM, which are critical determinants of gastric cancer risk.
Materials and Methods
Animals.
Wild-type Balb/c and C57BL/6, and Rosa26/loxP-membrane tdTomato-loxP-membrane GFP (mTmG) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). TxA23 TCR transgenic mice have been previously described [8, 14, 23, 24]. Ifngr1−/− mice were a gift from Dr. Robert Schreiber (Washington University in St. Louis, USA). TxA23xIfng−/− mice were generated by breeding TxA23 mice onto an IFN-γ deficient background purchased from Jackson Laboratories. Mice were maintained in a specified pathogen-free barrier facility under a 12 h light cycle and all procedures were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee or Saint Louis University Institutional Animal Care and Use Committee.
Histopathology
Stomachs were fixed in 10% neutral-buffered formalin, and embedded in paraffin. After deparaffinization, tissue sections were stained with H&E and examined by two independent pathologists without knowledge of the specimen’s group. Pathology scores were determined according to methods adapted from Rogers et al [25]. Sections were assigned scores from 0 (absent) to 4 (severe) to indicate the severity of inflammation, oxyntic atrophy, and mucous neck cell hyperplasia.
Immunofluorescence
Tissue sections (5 μm) were deparaffinized, submitted to antigen retrieval with 10 mM sodium citrate (pH 6.0), washed with PBS and blocked in 1% BSA and 0.3% Triton X-100 in PBS followed by overnight incubation with primary antibodies: rabbit anti-IFNγR1 (1:200, sc-700, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-Ezrin (1:100, Santa Cruz Biotechnology), goat anti-VEGFB (1:100, sc-1876, Santa Cruz Biotechnology), and rat anti-CD44v9 (1:10,000, Cosmo Bio USA, Carlsbad, CA, USA; note that CD44v9 is the human splice designation, so we refer henceforth to this protein in our murine studies as “CD44v”). After washing, sections were incubated with secondary antibodies and Griffonia simplicifolia II lectin (GSII lectin, a marker for mucous neck cells), washed, stained with bisbenzimide and mounted in PBS:glycerol (1:1).
Corpus gastroid culture.
Gastric glands from the corpus region of the stomach were isolated and cultured into gastroids as described previously [26, 27]. In brief, the corpus gastric mucosa was separated from muscle layers, cut into small pieces and incubated in chelation buffer containing 10 mM EDTA (3 h, shaking, 4 °C). EDTA removal was followed by mechanical dissociation by carefully pipetting the tissue to release glands. Isolated glands were mixed with Matrigel (BD Biosciences, Franklin Lanes, NJ, USA), distributed in culture plates and grown in Advanced DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA), 50% Wnt3a conditioned medium, 10% R-Spondin1 and Noggin conditioned medium supplemented with 10 mM HEPES, 1X N-2, 1X B27, 1X GlutaMAX (Invitrogen), 2.5 mM N-Acetylcysteine (Sigma-Aldrich, St. Louis, MO, USA), 50 ng/ml EGF, 100 ng/ml FGF10 (Peprotech, Rocky Hill, NJ, USA) and 10 nM gastrin (Sigma-Aldrich). For the first 3 days, 10 μM ROCK inhibitor (Y-27632, Sigma-Aldrich) was added. Three days after the first passage, gastroids were treated with IFN-γ (Peprotech) and all wells were scanned microscopically every 24 h using a Cytation 3 Cell Imaging Multi-Mode Reader (Biotek, Winooski, VT, USA). Montages formed of stitched images were used to count the number of dead gastroids. Several figure panels show stitching junctions and for clarity these are not marked.
IFN-γ-coated beads
Agarose beads (Affi-gel Blue Media, Bio-Rad, Hercules, CA, USA) were washed 5x with Dulbecco’s modified PBS (DPBS). Under a stereoscope, beads were selected by size and incubated overnight at 4 °C with IFN-γ or albumin (control). After washing with DPBS, beads were placed next to individual gastroids. Images of the gastroids were acquired daily, and ImageJ software was used to measure the diameter of the gastroid and the distance of the furthest dead cell from the bead.
Supernatant generation and treatment of gastroids
Isolation of cells from gastric lymph nodes of TxA23 and TxA23xIfng−/− mice was performed as described previously [8, 14]. In brief, gastric lymph nodes were removed, homogenized, passed through a 40-μm-pore nylon filter and the isolated cells were counted. One million cells were cultured in supplemented RPMI and stimulated with plate bound anti-CD3 and anti-CD28 antibodies (BD Biosciences). After 72 h, cytokines in the supernatant were measured using the Th1/Th2/Th17 BDTM Cytometric Bead Array kit (BD Biosciences) according to the manufacturer.
Three days after the first passage, supernatants were added to gastroid medium. Supernatant from TxA23 cells was diluted to expose gastroids to 3 ng/ml of IFN-γ in the culture medium, and the same dilution was used for the TxA23xIfng−/− supernatant. Additionally, groups of gastroids were pre-incubated with anti-IFN-γ (50 μg/ml, 10 min) and maintained in the medium containing TxA23 supernatant. In another group, IFN-γ (3 ng/ml) was added to TxA23xIfng−/− supernatant. Plates were microscopically scanned every 24 h, and the number of live/dead gastroids was counted.
Statistical analysis
Results were analyzed using the Mann-Whitney U test, unpaired Student’s t-test, or two-way ANOVA followed by Tukey’s post hoc test (*P<.05; **P<.01; ***P<.001) using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA).
Results
Gastric epithelial cells express IFNγR1 and are killed by IFN-γ
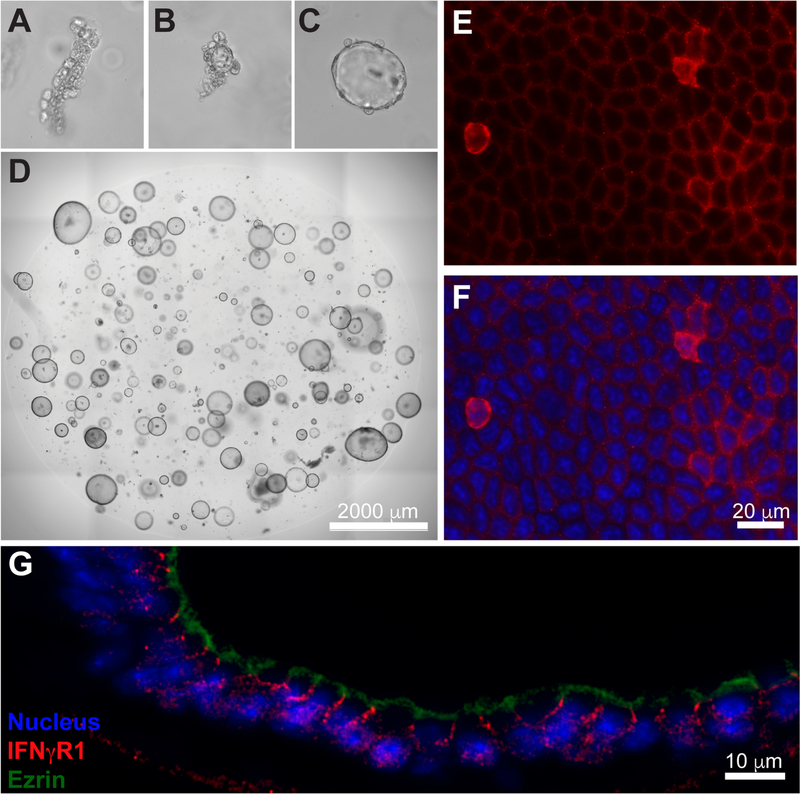
To determine if IFN-γ is capable of acting directly on gastric epithelial cells, we first examined organoids from isolated corpus glands of the gastric mucosa (“gastroids”) [28]. Gastroids are essentially indefinite ex vivo cultures of exclusively epithelial cells (Figure 1A-D). Whole mount histological imaging showed IFNγR1 expression at varying levels on the cell surface with almost all cells expressing detectable levels (Figure 1E,F). Cross-sections showed that IFNγR1 was clearly expressed on the basolateral surface of gastroids epithelial cells opposite the apical marker ezrin (Figure 1G).
Figure 1. Gastric epithelial cells express the IFN-γ receptor.
(A-D) Representative photomicrographs of murine corpus gastroid culture at 0, 1, 2 and 6 days, respectively. (E-F) Representative immunofluorescence images of whole mount corpus gastroids: anti-IFNγR1 = red; nuclei = blue. (G) Immunofluorescence imaging of a cross-sectioned organoid indicating basolateral localization of IFN-γ receptor (red) relative to apical marker ezrin (green).
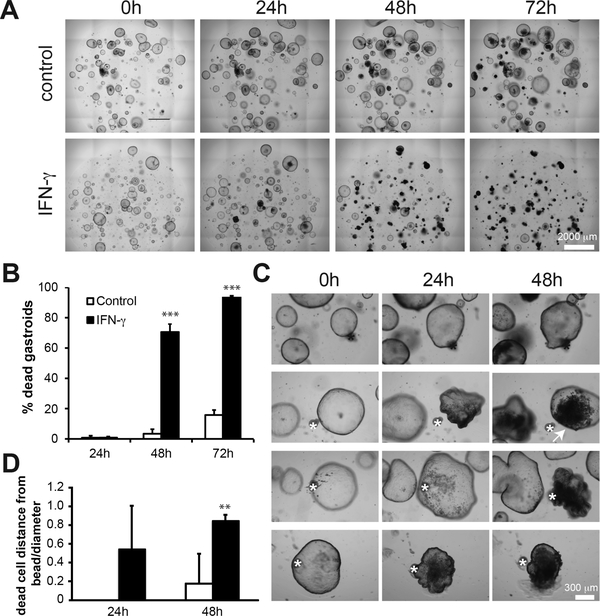
To determine the effect(s) of IFN-γ on gastroids, we performed a dose-response assay (0.15–3 ng/ml) over a 3 day time course, scanning the plate every 24 h and tracking each gastroid individually and longitudinally over time (supplementary material, Figure S1). In subsequent experiments, we used a dose of 3 ng/ml. We found that IFN-γ induced cell death in the majority of gastroids, as confirmed by propidium iodide staining (supplementary material, Figure S2). Death rate was significantly increased by 48 h and was even more pronounced at 72 h, when nearly all gastroids in IFN-γ-treated cultures stopped growing and collapsed, while most gastroids without the cytokine continued to grow (Figure 2A,B). To test if this was a direct effect of IFN-γ, we used IFN-γ-coated agarose beads to induce local concentration gradients of the cytokine. BSA-coated control beads (black asterisks) had no effect on gastroids, which continued growing throughout the experiment (Figure 2C). IFN-γ-coated beads (white asterisks), however, induced cell death in the epithelial cells in gastroids beginning with those cells closest to the beads with death radiating out from the bead focal point (Figure 2C,D, arrow). These results show that gastric epithelial cells express the IFN-γ receptor and that IFN-γ causes them to die.
Figure 2. Gastric epithelial organoids are killed by IFN-γ.
(A) Photomicrographs of control and IFN-γ treated corpus organoids 0, 24, 48, and 72 h following addition of IFN-γ to cultures. (B) The mean ± SD of the percentage of dead organoids in each culture condition at 24, 48, and 72 h following the addition of IFN-γ. N=6 cultures per group from 2 separate experiments. Results were analyzed by ANOVA followed by Tukey’s post hoc test. For this and all other plots: *=p<0.05, **=p<0.01, ***=p<0.001. (C) Photomicrographs of organoid cultures in which BSA-coated agarose beads (marked with a black asterisk) and IFN-γ coated beads have been added to cultures (marked with a white asterisk). (D) The mean ± SD of the distance of the furthest dead cell from the bead as a fraction of the diameter of the whole gastroid at 24 and 48 h after placing IFN-γ- or BSA-coated beads. N=3–8 gastroids per group from 2 separate experiments. Statistical significance of IFN-γ versus control at each timepoint by Mann-Whitney U test.
IFN-γ directly induces cell death of gastric epithelial cells through IFNγR1
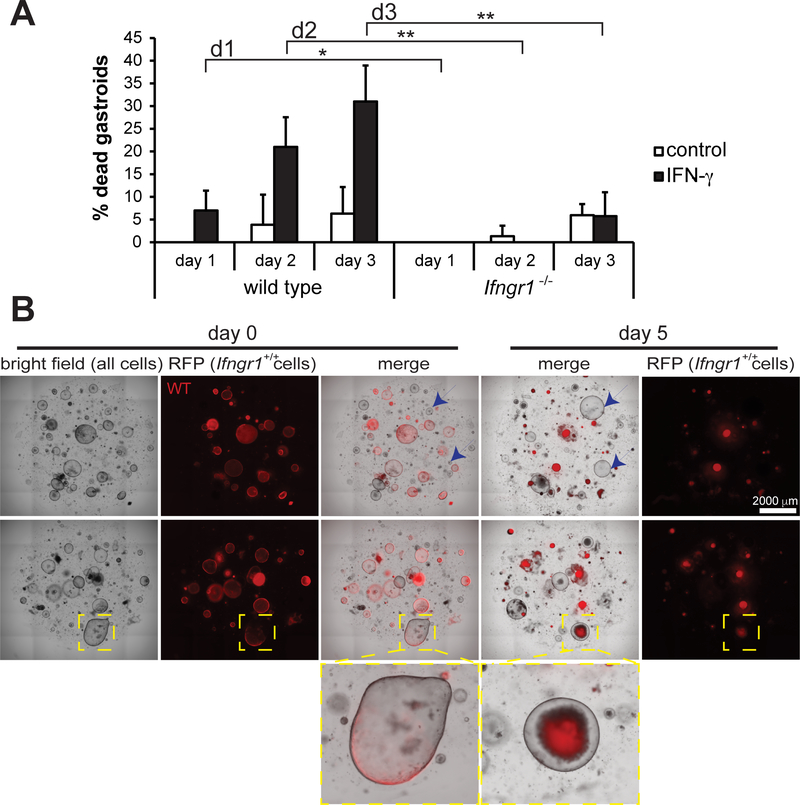
To determine if IFN-γ-induced death required the IFN-γ receptor, we treated gastroids from Ifngr1−/− mice and wild-type controls with IFN-γ. The Ifngr1−/− gastroids exhibited few deaths whether they were exposed to IFN-γ or not, and when treated with IFN-γ gastroid deaths were significantly fewer than in control gastroids (Figure 3A). From these results we concluded that IFNγR1 is required for IFN-γ-induced gastric epithelial cell death. Next, we addressed whether this was a direct effect of IFN-γ or whether death was caused indirectly by the release of other factors that induce apoptotic signals. Gastroids from wild-type mice expressing a red fluorescent reporter (RFP) were cultured with gastroids generated from Ifngr1−/− mice (no reporter), and treated with IFN-γ. In the same tissue culture wells, WT gastroids (RFP+) died following exposure to IFN-γ, whereas Ifngr1−/− gastroids (non-fluorescent) were still alive and growing after 5 days (blue arrows) (Figure 3B). Interestingly, chimeric gastroids composed of both wild-type and Ifngr1−/− cells (yellow box) could be observed. Upon IFN-γ treatment, the wild-type RFP+ cells in such chimeras died and were shed into the gastroid lumen, such that only Ifngr1−/− cells remained lining the gastroid (Figure 3B). These results further support the interpretation that IFN-γ induces a programmed cell death by directly triggering signals that lead to cell death only in cells expressing IFNγR1 and not indirectly through secondary and/or non-cell autonomous factors.
Figure 3. IFN-γ receptor is required for IFN-γ-induced death of corpus gastroids.
(A) The mean ± SD of the percentage of dead organoids in wild-type and Ifngr1−/− organoid cultures on days 1, 2, and 3 following the addition of IFN-γ. N=6 cultures per group from 2 separate experiments. Results were analyzed by ANOVA followed by Tukey’s post hoc test. (B) Light- and fluorescence-microscopy images of gastroid cultures in which only wild-type organoids express RFP while Ifngr−/− do not (blue arrows) demonstrating that only cells expressing the IFN-γ receptor die as a result of culture with IFN-γ (yellow box highlights a chimeric organoid in which all RFP+ cells are expelled into the organoid lumen).
IFN-γ is a major inducer of gastric epithelial cell death as a component of the inflammatory milieu
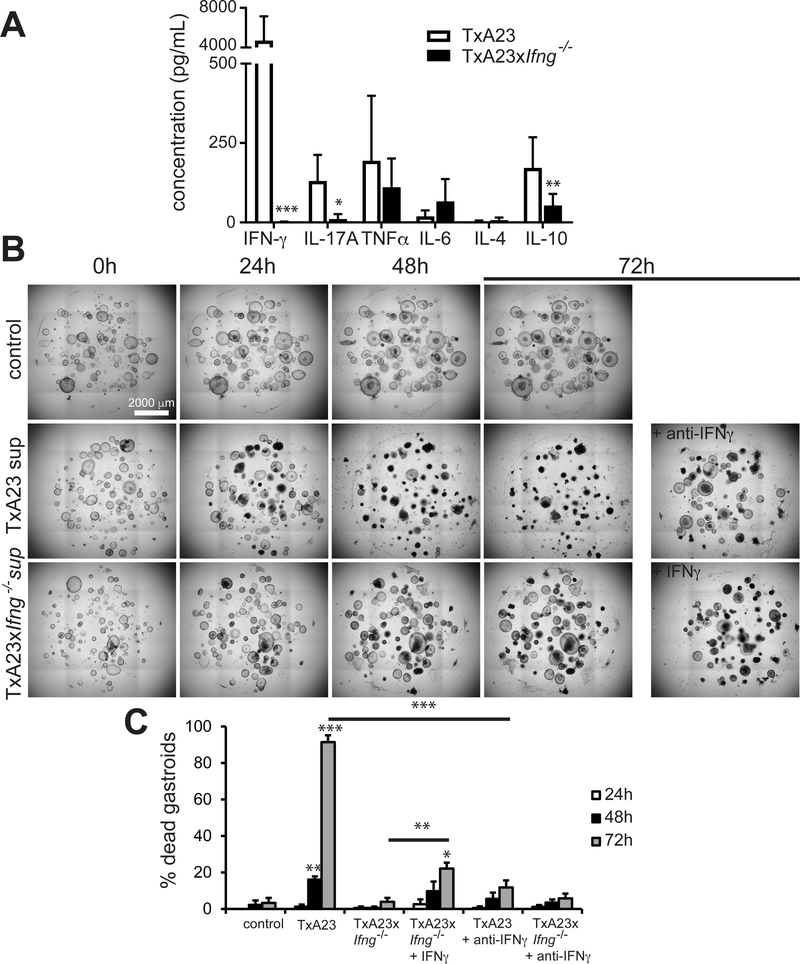
We demonstrated that recombinant IFN-γ directly induces death in gastric epithelial cells. However, the inflammatory milieu present during autoimmune gastritis and Helicobacter infection contains a multitude of secreted factors that could influence the effects of IFN-γ. To explore the role of IFN-γ as a component of a more representative environment of inflammatory cytokines, we isolated and cultured cells from the gastric lymph nodes of TxA23 mice, the mouse model of autoimmune gastritis [14]. Lymphoid cells from TxA23 and TxA23xIfng−/− mice were re-stimulated in vitro, and supernatants were collected 72 h later. We confirmed that IFN-γ was produced at markedly high levels (>4000 pg/ml) by immune cells from TxA23 mice and was undetectable in the TxA23xIfng−/− supernatants. Concentrations of other common immune cytokines that we measured were either much lower (e.g. IL-17A and IL-10) or not statistically dependent on mouse IFN-γ expression (e.g. TNFα, IL-4, and IL-6) (Figure 4A). Thus, the key difference in the inflammatory cytokine milieu of TxA23 and TxA23xIfng−/− is the absence of IFN-γ.
Figure 4. IFN-γ present in the inflammatory milieu is a major inducer of gastroid death.
(A) The mean ± SEM of the concentration of several cytokines secreted by immune cells from a mouse model of inflammatory atrophic gastritis, TxA23, and an IFN-γ deficient model, TxA23xIfng-/−. N=7–10 mice per group from 2 or 3 separate experiments. (B) Photomicrographs of gastroid cultures 0, 24, 48, and 72 h following the addition of TxA23 supernatants diluted to a final IFN-γ concentration of 3 ng/ml compared to control and IFN-γ deficient supernatant conditions. (C) Mean ± SD of the percentage of dead gastroids 24, 48, and 72 h after treatment with control, TxA23, or TxA23xIfng−/− supernatants ± IFN-γ or anti- IFN-γ (72 h cultures under those conditions shown at right in panel B). N=6 organoid cultures from 2 separate experiments. Results were analyzed by ANOVA followed by Tukey’s post hoc test.
We treated wild-type gastroids with both supernatants at a dilution calculated to reproduce the 3 ng/ml concentration of IFN-γ used in previous experiments. TxA23 supernatant induced gastroid death significantly when compared to the control group (~90% death by 72 h), whereas the fraction of dead gastroids in the TxA23xIfng−/− supernatant group was similar to the control (<10% death by 72 h) (Figure 4B,C). Furthermore, when the TxA23 supernatant was pre-incubated with anti-IFN-γ to neutralize the cytokine, gastroid death was significantly reduced (~15% gastroid death by 72 h) (Figure 4B,C). Also, addition of recombinant IFN-γ to TxA23xIfng−/− supernatant stimulated more gastroid death relative to the supernatant from TxA23xIfng−/− lymphocytes (~25% gastroid death by 72 h) (Figure 4B,C). Overall, these results show that IFN-γ is the most abundant cytokine detected in a well-characterized model of inflammation-induced atrophic gastritis, and it is critical for inducing death of gastric epithelial cells even in a complex milieu of several other cytokines.
IFN-γ is required for atrophy and metaplasia in a model of chronic atrophic gastritis
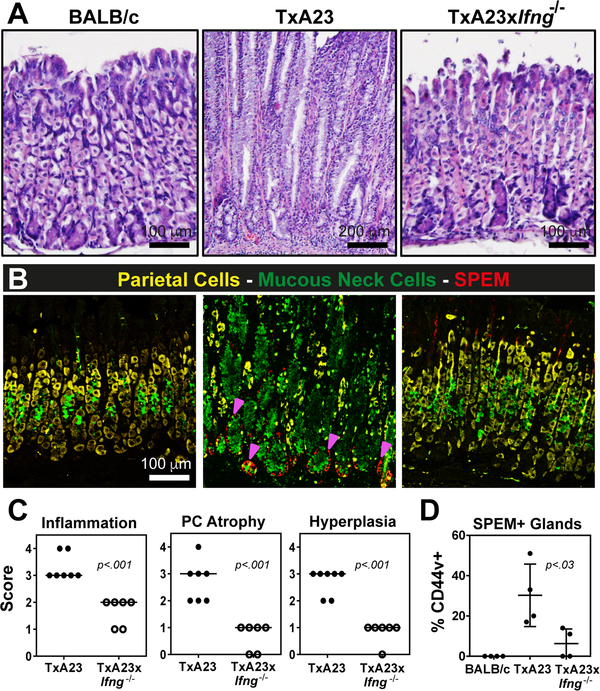
We used various markers of gastric cell lineages to determine the composition of our gastroids, and we found all principal gastric epithelial cell types represented (supplementary material, Figure S3). Therefore, our results indicated that essentially all gastric epithelial cells express IFNγR1, and that death caused by IFN-γ depended on expression of IFNγR1. Furthermore, in a model of inflammatory gastric atrophy and metaplasia, the TxA23 mouse, IFN-γ is the most abundant cytokine in the cytokine milieu [14]. Thus, we reasoned that IFN-γ plays a role in parietal cell loss and the onset of metaplasia, and might be a critical driver of disease progression during chronic atrophic gastritis in vivo. To test this hypothesis, we analyzed the development of critical preneoplastic epithelial cell changes (e.g. parietal cell atrophy, mucous neck cell hyperplasia, and SPEM) in the TxA23 mouse. As mentioned above, TxA23 mice develop chronic progressive atrophic gastritis and the replacement of the digestive-enzyme-secreting chief cell lineage by metaplastic cells that co-express markers of chief cells and progenitor cells [14], a metaplasia known as SPEM [6]. Changes in TxA23 mice that are similar to the progression to human cancer include: atrophy, metaplasia, and in some mice, lesions that resemble dysplasia in humans [14, 23]. These histological changes are accompanied by increased abundance of important cytokines, chiefly IFN-γ and, to a lesser degree, IL-17A [8]. As previously reported, by 4 months TxA23 mice exhibited diffuse chronic inflammation and oxyntic atrophy, accompanied by mucous neck cell hyperplasia, SPEM, and occasional dysplasia-like lesions. Here we quantified disease at an even later stage, 5–7 months, and compared TxA23 control and TxA23xIfng─/─ mice. At 7 months, TxA23 mice had severe inflammation, atrophy, and hyperplasia as well as diffuse SPEM development. In contrast, TxA23 mice lacking IFN-γ showed markedly decreased inflammation and little or no atrophy or hyperplasia (Figure 5A). Immunofluorescence staining confirmed few parietal cells (yellow, anti-VEGFB) in TxA23 stomachs, extensive mucous cell hyperplasia (green, GSII), and evidence of metaplasia throughout the base of the gastric units, as indicated by induction of CD44v (red) [29, 30] and overlap of GSII and the chief cell marker gastric intrinsic factor GIF (not shown) [31, 32]. In striking contrast, the vast majority of glands in the stomachs of TxA23xIfng−/− mice contained numerous healthy parietal cells, scant mucous neck cell hyperplasia, and little/no evidence of metaplasia (Figure 5B). These data corroborate our in vitro findings on gastroids and demonstrate that disease was significantly reduced in TxA23xIfng−/− compared to TxA23 mice, quantified using both a pathological scoring system and an analysis of the percentage of glands containing CD44v+ SPEM (Figure 5C,D). Thus, IFN-γ is a critical component in the progression from gastritis caused by anti-parietal T-cells to the pre-cancerous stage of atrophic gastritis and SPEM and is thus required also for progression to subsequent stages of the classical progression to gastric cancer [33, 34].
Figure 5. IFN-γ is a critical driver of parietal cell atrophy and SPEM development in vivo.
(A) Representative hematoxylin and eosin stained sections from the gastric corpus of BALB/c, TxA23, and TxA23xIfng−/− mice at 5–7 months of age. (B) Representative fluorescence images showing parietal cells (anti-VEGFB, yellow), mucous neck cells (GS-II, green), and SPEM (anti-CD44v, red). Magenta arrows indicate CD44v+ cells. (C) Scoring of individual stomachs for the degree of inflammatory infiltrate, parietal cell atrophy, and mucosal hyperplasia in TxA23 and TxA23xIfng−/− mice. Each dot represents one mouse, 6–7 mice per group from 2 or 3 separate experiments. (D) Quantification of the fraction of corpus glands containing CD44v+ cells at the base. N=4 mice per group from 2 separate experiments.
Discussion
Gastric adenocarcinomas are thought to arise via sequential pathological lesions starting with chronic inflammation and progressing through atrophy/metaplasia and eventually to dysplasia and neoplasia. Helicobacter pylori infection and autoimmune-mediated gastritis, principal risk factors for the development of gastric cancer, upregulate various pro-inflammatory cytokines including IFN-γ [8, 14]. Previous studies have implicated cytokines as mediators of parietal cell atrophy [12, 13, 21], but a role for IFN-γ in directly inducing parietal cell death and thereby promoting the progression from superficial gastritis to atrophic gastritis has previously not been elucidated.
Several studies have touched upon the role of IFN-γ in Helicobacter-mediated gastric diseases and gastric cancer in general, but its specific role in disease progression remains controversial and poorly understood. In one study, overexpression of IFN-γ in the stomach was shown to induce metaplasia and dysplasia [35]. Another study, however, indicated a blockade of those processes, suggesting that IFN-γ had protective effects against gastric carcinogenesis [36]. In both of these studies IFN-γ was overexpressed transgenically using an epithelial promoter without additional inflammatory signals associated with infection and autoimmunity. It seems likely that additional signals are important and may account for differences between those studies and with the results reported here. Furthermore, it has not been determined which cells might be responsive to IFN-γ in the stomach. Other previous studies have shown that infusion of IFN-γ triggered hyperplasia of mucous neck cells (which is also a feature of TxA23 mice) caused in chronic inflammation induced by the loss of acid seen during gastrin-deficiency and was required for the mucous neck cell hyperplasia caused by parietal cell atrophy secondary to a genetic abnormality [37, 38].
Here we provide additional clarity to the cellular targets and potential direct effects of IFN-γ by showing that in gastroid cultures and in a mouse model of autoimmune gastritis, IFN-γ acts directly on gastric epithelium and is critical for the progression from gastritis to atrophic gastritis and metaplasia. The atrophy and metaplasia stage is the first in the gastric cancer progression sequence that carries heightened cancer risk. Moreover, other mouse models, like the TxA23 autoimmune gastritis model used here, phenocopy the progression seen in humans [39, 40]. Thus, if IFN-γ is the key factor mediating the transition to atrophy/metaplasia, it may be critically important for determining which patients with chronic gastritis are at risk for progression to gastric cancer.
There are several new aspects to the present study. For one, we used both in vivo and ex vivo (i.e. organoids cultured directly from gastric epithelium) models to investigate the effect of IFN-γ on gastric epithelial cell biology. We established that gastric epithelial cells express the receptor for IFN-γ and, in vitro, respond to IFN-γ by undergoing cell death in a direct, receptor-dependent manner. This is a critical observation, as IFN-γ regulates many cellular behaviors and it was, prior to these studies, entirely plausible that the effects of IFN-γ on gastric epithelium could be an indirect effect caused by downstream products of IFN-γ signaling. Another feature of our study is that we show that atrophy and metaplasia in TxA23 mice, which eventually progress to marked cancer-like dysplasia [8, 14], depend nearly entirely on IFN-γ, as TxA23xIfng−/− mice have inflammation but minimal other pathology, even at advanced age (5–7 months) for this model. We specifically determine that it is IFN-γ within the supernatants from draining-lymphocytes of wild-type mice that is elevated in TxA23 mice and lost in Ifng−/− mice, with other cytokines not being significantly affected. Moreover, antibody depletion of wild-type supernatant IFN-γ blocked supernatant killing, so other cytokines were not sufficient in the absence of IFN-γ to cause cell death. Thus, even as a part of a highly complex cytokine milieu, IFN-γ is a critical driver of epithelial cell death. This is important to note as cytokines are capable of either antagonistic or synergistic effects during an immune response, and the results are a strong indicator of the relative importance of IFN-γ as a driver of disease progression.
The finding that the predominant cytokine produced during both Helicobacter infection and autoimmune gastritis is capable of directly inducing epithelial cell death should advance our understanding of the pathogenesis of inflammation-induced gastric carcinogenesis. We have previously shown that another major component of the inflammatory milieu, IL-17A, can directly induce parietal cell atrophy via apoptosis [8]. It should be noted that data from both that study and this current one indicate that IFN-γ is roughly 100x more concentrated and 10x more potent at inducing cell death compared to IL-17A alone, and it is reasonable to hypothesize that there is a strong potential for synergy between these two arms of the immune response given that they have little overlap in their signaling pathways. Recently, Miska et al. hypothesized that gastric epithelial destruction caused by type 1 and type 17 immune responses was a major initiating factor for the development of gastric metaplasia and eventually neoplasia [41]. The current study, in combination with our previous work, provides mechanistic support for that hypothesis by showing that both IFN-γ and IL-17A, the canonical cytokines produced during type 1 and type 17 responses, are capable of directly inducing gastric epithelial death and are critical for the downstream development of parietal cell atrophy and preneoplastic metaplasia.
Much work still needs to be done with regard to determining the mediators of the entire constellation of changes seen during atrophy and metaplasia. Our current studies do not allow us to determine if specific epithelial cell populations are targeted by IFN-γ in vivo. For example, the assumption is that it is parietal cell death that triggers the SPEM-type metaplasia response (though we recently showed that this assumption may be simplistic, because parietal cell apoptosis alone does not cause metaplasia [29]). However, IFNγR1 staining in gastroids seems to be in all the epithelial cells (and the first-generation gastroids we use here have all corpus cell types represented). In addition, the organoid cultures chimeric for wild-type and Ifngr1−/− cells indicate that all wild-type epithelial cells in culture die (at the concentrations we used) when treated with IFN-γ, while Ifngr1−/− epithelial cells survive – even if the Ifngr1−/− cells are in the same gastroid with dying wild-type cells.
Overall, our results indicate, in a way not possible before the advent of organoid culture, that multiple gastric epithelial cells express IFNγR1 and can respond by dying when treated with IFN-γ. So why, in tissue, do only some cells die, while others become metaplastic? Our bead-organoid assay showed that IFN-γ-mediated death can be localized to a cluster of nearby cells. One possibility is therefore that in vivo, immune cells provide specificity by homing only to specific cell lineages and releasing IFN-γ locally to kill those cells specifically. Additionally, as mentioned above, there is a complex inflammatory milieu in vivo during disease, comprising numerous other factors that might enhance or temper the death-inducing properties of IFN-γ on each cell lineage. Moreover, given that parietal cell apoptosis alone is insufficient to induce metaplasia [29], it is possible that death of other cell lineages may also be important. For example, it has been proposed that SPEM cells arise from chief cells via a stereotyped cellular retooling known as paligenosis, whereas another proposal is that gastric epithelial stem cells may be an important cell-of-origin for SPEM [40, 42, 43); in either case, IFN-γ-induced death of some chief cells, as well as parietal cells, might be required.
Multiple lines of evidence support a critical, epithelial-specific role for IFN-γ in the transition to the first stage in tumorigenesis. Understanding how IFN-γ increases cancer risk may lead us to new opportunities for therapeutic intervention, either by blocking the production or signaling of IFN-γ, or by inhibiting the downstream cellular death induced by IFN-γ signaling. Further elucidation of the roles and epithelial targets of immune cytokines will inform the development of more targeted small molecule and antibody-based therapies. The ability to effectively intervene at early stages of disease upon the detection of precursor lesions in an at-risk individual could have wide-spread implications for patient care by preventing disease progression before gastric cancer develops.
Supplementary Material
Figure S1. Dose titration of IFN-γ on corpus gastroids
Figure S2. Two representative fields of IFN-γ-treated corpus gastroids stained with the propidium iodide that was used to verify bright-field assessment of organoid death
Figure S3. Gastric epithelial cell types present in gastroids. Fluorescence images of gastroids positive for different cell markers: gastric intrinsic factor (GIF, zymogenic cells), Griffonia simplicifolia II lectin (GSII lectin, mucous neck cells), Anguilla anguilla agglutinin (AAA lectin, pit cells), Doublecortin-like kinase 1 (DCLK1, tuft cells), H+/K+-ATPase (parietal cells), Ki-67 (cell proliferation).
Acknowledgements
The authors thank Dr. Grant Kolar, Barbara Nagel, and Caroline Murphy from the Saint Louis University Research Microscopy and Histology Core for generation of tissue sections and the Saint Louis University and Washington University Comparative Medicine Departments for assistance in maintaining mouse colonies. The authors also thank the Advanced Imaging and Tissue Analysis Core of the Washington University Digestive Disease Research Cores Center. JCM is supported by grants NIDDK R01s (DK094989, DK105129) and the DeNardo Education & Research Foundation. RJD and JCM have research support through the NIH NIDDK (R01 DK110406), and RJD, BBM, and JCM from the Digestive Diseases Research Core Center of the Washington University School of Medicine NIDDK P30DK52574. RJD was also funded by the AGA Funderburg Research Award. BBM and JCM are supported by the Alvin J. Siteman Cancer Center/Barnes Jewish Hospital Foundation Cancer Frontier Fund and the Cancer Research Foundation (Young Investigator Award). BBM is additionally supported by NIH NIDDK (K01-DK093885, R03-DK108764). LHO was supported by CNPq – Brazil (fellowship 249477/2013–0). KAB is supported by an NIH/NIDDK NRSA Predoctoral fellowship (F30 DK118873). Animal work was performed in a facility supported by NCRR grant C06 RR012466.
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz GK. Invasion and metastases in gastric cancer: in vitro and in vivo models with clinical correlations. Semin Oncol 1996; 23: 316–324. [PubMed] [Google Scholar]
- 3.Correa P Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995; 19: S37–S43. [PubMed] [Google Scholar]
- 4.Landgren AM, Landgren O, Gridley G, et al. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer 2011; 117: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P A human model of gastric carcinogenesis. Cancer Res 1988; 48: 3554–3560. [PubMed] [Google Scholar]
- 6.Yamaguchi H, Goldenring JR, Kaminishi M, et al. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci 2002; 47: 573–578. [DOI] [PubMed] [Google Scholar]
- 7.Lennerz JK, Kim SH, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 2010; 177: 1514–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bockerstett KA, Osaki LH, Petersen CP, et al. Interleukin–17A promotes parietal cell atrophy by inducing apoptosis. Cell Mol Gastroenterol Hepatol 2018; 5: 678–690 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusak E, Chobot A, Krzywicka A, et al. Anti-parietal cell antibodies - diagnostic significance. Adv Med Sci 2016; 61: 175–179. [DOI] [PubMed] [Google Scholar]
- 10.Marshall AC, Alderuccio F, Toh BH. Fas/CD95 is required for gastric mucosal damage in autoimmune gastritis. Gastroenterology 2002; 123: 780–789. [DOI] [PubMed] [Google Scholar]
- 11.Merchant JL, Ding L. Hedgehog Signaling Links Chronic Inflammation to Gastric Cancer Precursor Lesions. Cell Mol Gastroenterol Hepatol 2017; 3: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest 2007; 117: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peek RM Jr., Crabtree JE Helicobacter infection and gastric neoplasia. J Pathol 2006; 208: 233–248. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TL, Khurana SS, Bellone CJ, et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res 2013; 73: 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawai N, Kita M, Kodama T, et al. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun 1999; 67: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96: 41–101. [DOI] [PubMed] [Google Scholar]
- 17.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000; 404: 398–402. [DOI] [PubMed] [Google Scholar]
- 18.Lee WP, Tai DI, Lan KH, et al. The −251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res 2005; 11: 6431–6441. [DOI] [PubMed] [Google Scholar]
- 19.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003; 124: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 20.Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018; 67: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 2008; 14: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzzelli JN, Chalinor HV, Pavlic DI, et al. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell Mol Gastroenterol Hepatol 2015; 1: 203–221 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TL, Dipaolo RJ. A new mouse model of inflammation and gastric cancer. Oncoimmunology 2013; 2: e25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHugh RS, Shevach EM, Margulies DH, et al. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol 2001; 31: 2094–2103. [DOI] [PubMed] [Google Scholar]
- 25.Rogers AB, Taylor NS, Whary MT, et al. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res 2005; 65: 10709–10715. [DOI] [PubMed] [Google Scholar]
- 26.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6: 25–36. [DOI] [PubMed] [Google Scholar]
- 27.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013; 155: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher MA, Aihara E, Feng R, et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol 2015; 593: 1809–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burclaff J, Osaki LH, Liu D, et al. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology 2017; 152: 762–766 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khurana SS, Riehl TE, Moore BD, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem 2013; 288: 16085–16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013; 104: 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavros Y Initiation and maintenance of gastric cancer: a focus on CD44 variant isoforms and cancer stem cells. Cell Mol Gastroenterol Hepatol 2017; 4: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–6740. [PubMed] [Google Scholar]
- 34.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012; 13: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syu LJ, El-Zaatari M, Eaton KA, et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol 2012; 181: 2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu SP, Quante M, Bhagat G, et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res 2011; 71: 4247–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang W, Rathinavelu S, Samuelson LC, et al. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest 2005; 85: 702–715. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Demitrack ES, Keeley TM, et al. IFNgamma contributes to the development of gastric epithelial cell metaplasia in Huntingtin interacting protein 1 related (Hip1r)-deficient mice. Lab Invest 2012; 92: 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen CP, Mills JC, Goldenring JR. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol 2017; 3: 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 2018; 15: 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miska J, Lui JB, Toomer KH, et al. Initiation of inflammatory tumorigenesis by CTLA4 insufficiency due to type 2 cytokines. J Exp Med 2018; 215: 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burclaff J, Mills JC. Plasticity of differentiated cells in wound repair and tumorigenesis, part I: stomach and pancreas. Dis Model Mech 2018; 11: dmm033373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 2018; 37: e98311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dose titration of IFN-γ on corpus gastroids
Figure S2. Two representative fields of IFN-γ-treated corpus gastroids stained with the propidium iodide that was used to verify bright-field assessment of organoid death
Figure S3. Gastric epithelial cell types present in gastroids. Fluorescence images of gastroids positive for different cell markers: gastric intrinsic factor (GIF, zymogenic cells), Griffonia simplicifolia II lectin (GSII lectin, mucous neck cells), Anguilla anguilla agglutinin (AAA lectin, pit cells), Doublecortin-like kinase 1 (DCLK1, tuft cells), H+/K+-ATPase (parietal cells), Ki-67 (cell proliferation).