Lay Summary
Attention to faces is an important means for infants to learn about the social world. The complexity of the social scene and an infant’s early social environment both affect the amount of time infants at high- and low-risk for ASD look at faces during the first postnatal year. For infants at high-risk for ASD, greater attention to faces was associated with better social skills. Understanding an infant’s social environment may have a positive impact on social communicative development.
Keywords: infancy, visual social attention, broad autism phenotype, social development, autism risk
Abstract
Diminished attention to socially relevant information appears to be an early emerging risk factor associated with autism spectrum disorders (ASD). However, inconsistencies across studies suggest that atypicalities in visual social attention in infants at high-risk for ASD during the first postnatal year may be subtle and more apparent under certain contexts. Here we explore factors that may moderate developmental trajectories in attention to faces, including the social complexity of the dynamic visual stimuli used to measure visual social attention and the early social environment of the infant as indexed by parental affectedness of ASD-related traits. Across infants at both high (HR) and low risk (LR) for ASD, attention to faces increased during the first postnatal year, with overall greater attention being allocated to schematic faces in the simpler video stimulus. Moreover, greater parental affectedness of ASD-related traits was associated with reduced developmental gains in attention to faces. For HR infants, greater attention to faces was positively associated with social communicative competence, including better joint attention skills and lower social impairments. Altogether, our findings highlight the importance of considering developmental level when selecting stimuli to longitudinally examine visual social attention, and the clinical relevance of including measures of infant’s social environment in understanding early markers of ASD risk.
Introduction
Faces are a rich source of social communicative cues and constitute a particularly salient class of stimulus in an infant’s environment (Gliga, Elsabbagh, & Andravizou, 2009; Grossmann & Johnson, 2007). Infants preferentially orient to face-like stimuli shortly after birth (Morton & Johnson, 1991) and, in typical development, they increasingly look at faces during the first postnatal year (Frank, Vul, & Johnson, 2009; Frank, Vul, & Saxe, 2011). In contrast, infants who develop autism spectrum disorder (ASD) may deviate from normative patterns of attention to faces during the first postnatal year (Jones & Klin, 2013). Visual attention is an important mechanism for learning across social and cognitive developmental domains during infancy (Amso, Fitzgerald, Davidow, Gilhooly, & Tottenham, 2010; Johnson, Amso, & Slemmer, 2003), and attention to faces, in particular, provides quantifiable indices of social and communicative functioning in both typical and atypical development (Campbell, Shic, Macari, & Chawarska, 2014; Tsang, Atagi, & Johnson, 2018; Young, Merin, Rogers, & Ozonoff, 2009). Early disruptions in attention to socially relevant stimuli likely impose cascading effects on social communicative development and is theorized to be a contributing factor to the emergence of the social impairments diagnostic to ASD (Elsabbagh & Johnson, 2016; Klin, Jones, Schultz, & Volkmar, 2003). An examination of factors that moderate developmental trajectories of attention to faces during infancy provide greater specificity on how visual social attention may be impacted in infants at risk for ASD.
Infants with an older sibling with ASD have approximately a 20% risk of developing ASD themselves (Ozonoff et al., 2011), and are also more likely to exhibit suboptimal developmental outcomes than the general population. The prospective study of high-risk (HR) infant siblings who subsequently receive an ASD diagnosis has begun to elucidate the degree to which attenuated visual attention to faces constitutes a prodromal symptom of the disorder. There is evidence that infants who develop ASD show a reduced preference for social information within the first postnatal year (Chawarska, Macari, & Shic, 2013; Jones & Klin, 2013; Shic, Macari, & Chawarska, 2014); however, diminished visual attention to faces does not appear to be an unequivocal early marker of ASD in infancy (for review, see Jones, Gliga, Bedford, Charman, & Johnson, 2014). Similar to young children and adolescents with ASD (Chawarska, Macari, & Shic, 2012; Speer, Cook, McMahon, & Clark, 2007), deviations from normative patterns of attention to faces during infancy may be superficially masked under simple social contexts where distractors from social targets are minimized, and accentuated under more complex social contexts such as dynamic social interactions.
To date, only a few eye-tracking studies have examined context-dependent variability in attention to faces in HR infants using dynamic social stimuli (Elsabbagh et al., 2013; Shic et al., 2014). Elsabbagh and colleagues (2013) examined face scanning patterns while 7- to 14-month old infants at high versus low risk (LR) for ASD viewed a woman either performing a social communicative gesture (i.e., peek-a-boo) or its constituent features (i.e., separately moving the eyes, hands, and mouth). Regardless of developmental outcomes, the researchers found no categorical differences in face scanning patterns between HR and LR infants; however, relative attention to a moving mouth during simple, non-communicative displays was associated with worse language scores and symptom severity in HR infants, suggesting that potential mechanisms underlying attention to faces and/or face processing may be impacted by familial risk status. Shic and colleagues (2014) examined visual attention to a woman’s face in social scenes in 6-month-old HR and LR infants and found that differences in face looking became apparent under more socially ostensive contexts; HR infants later diagnosed with ASD showed similar visual attention to smiling and neutral faces as their unaffected peers, but looked less to speaking faces. Altogether there is preliminary evidence to suggest that familial risk for ASD confers vulnerabilities in face processing, which can be made more apparent under certain experimental contexts. Varying the social complexity of the stimuli used to evaluate face looking may provide a more sensitive metric of the atypicalities in visual social attention in infants at high risk for ASD.
Moreover, examining developmental trajectories, rather than individual time points, may more appropriately capture deviations in attention to faces and their association with social communicative development. It has been theorized that infants who develop ASD increasingly diverge from normative patterns of face looking as a cumulative effect of atypical interactions with their social environment (Elsabbagh et al., 2013). Indeed, a longitudinal eye-tracking study that sampled multiple time points within the first postnatal year found that deviations in normative trajectories in face scanning are predictive of both individual diagnostic classification and level of social disability at 24 months in HR infants who developed ASD (Jones & Klin, 2013). In light of these findings, the current study examined attention to faces in HR and LR infants while viewing social scenes of varying social complexity (i.e., schematic/cartoon-like versus naturalistic, live-action social scenes) across the first postnatal year. Comparing viewing patterns between schematic versus naturalistic social scenes will further inform whether context affects developmental differences in face looking between HR and LR infants.
In addition to stimulus-specific qualities that may emphasize developmental differences in attention to faces, understanding the impact of potential social environmental influences on visual attention to faces may further inform how familial risk for ASD confers social vulnerabilities. There is some evidence that the landscape of HR infants’ social environments, such as the overture and quality of reciprocal social interactions that shape early social learning, may differ from those of LR infants, possibly by affecting the availability of social learning opportunities afforded to them from both parent and sibling interactions (e.g., Bontinck et al., 2018). Subclinical characteristics associated with the autism phenotype, such as difficulties with communication, aloofness, and rigidity, are continuously distributed in the general population and are particularly common among first degree relatives of individuals with ASD (Constantino & Todd, 2003; Lundström et al., 2012; Pickles, Starr, Kazak et al., 2000; Sucksmith, et al., 2011; Constantino & Lajonchere, 2006). Collectively, these traits are referred to as the “broader autism phenotype” (BAP). Although the methods used to measure BAP features in the general population vary across studies, including questionnaires that ascertain behaviors associated with ASD symptoms (e.g., Constantino & Todd, 2003; Dawson et al., 2007; Hurley, Losh, Parlier, Reznick, & Piven, 2007) and interview/direct observation of personality characteristics and social functioning (e.g., Losh, Childress, Lam, & Piven, 2008; Pickles et al., 2013), there is converging evidence for elevated social and communicative difficulties and rigidity in parents of children with ASD and developmental concerns.
Prior work evaluating BAP features in parents of children with ASD have primarily focused on intergenerational transmission of autism-related traits (Bernier, Gerdts, Munson, Dawson, & Estes, 2012; Dawson et al., 2007; Gerdts, Bernier, Dawson, & Estes, 2013; Lyall et al., 2014); however, little work has been done to examine associations between parental affectedness of autism-related traits and their children’s social communicative functioning. One study using the Social Responsiveness Scale (SRS, Constantino, 2002), a questionnaire-based measure that quantifies affectedness of autism-related traits, found that elevated BAP features in parents was associated with greater level of social impairments in their children (Lyall et al., 2014; cf Schwichtenberg et al., 2010). Elevated BAP features in parents with children with ASD not only indicate heritability of core autism traits, but also suggest potential differences in the early social environment of HR families relative to families without a history of ASD (Gerdts et al., 2013). Thus, familial risk for ASD may not only impose increased genetic susceptibility for atypical social communicative development but also manifest through the reciprocal social interactions between parent and child. Clinical observation of parents with multiple children with autism noted reduced mutual eye-gaze, social smiling, and elevated rigid behaviors (Gerdts et al., 2013), which may inadvertently impact the quality of parent-child interactions in HR families (Wan et al., 2013). Moreover, the effectiveness of parent-mediated interventions for HR infants on improving infant attentiveness and parental responsiveness to infant’s communicative bids, as well as reducing prodromal ASD symptoms (Green et al., 2015, 2017), further highlight the likelihood that parent-child interactions shape social functioning. Whether parental affectedness of ASD-related traits also influences developmental trajectories of visual social attention to faces in infancy is a point of investigation here.
The current study examined developmental trajectories in attention to faces in infants from 3- to 12-months of age. Specifically, we evaluated the extent to which complexity of dynamic social stimuli and parents’ ASD-related traits moderate developmental trajectories in face looking in HR and LR infants. We hypothesized that the magnitude of attenuated attention to faces between HR and LR infants may be more apparent while viewing complex realistic social scenes than while viewing simpler, schematic/cartoon scenes. Moreover, we hypothesized that the developmental trajectories of attention during viewing of complex, realistic versus simpler, schematic social scenes to faces would increasingly diverge between HR and LR infants. In addition, more ASD-related traits in parents may be associated with lower social attention in infants.
Furthermore, we examined the relation between developmental trajectories in attention to faces and behavioral measures of social communicative competence. We used two metrics of social communicative competence. The first was engagement in joint attention, or the coordination of attention between social partners to share interest in an object or event, as measured by the Early Social Communication Scales (ESCS; Mundy, Delgado, & Block, 2003). Impairments in joint attention behaviors have been documented as an early risk marker of ASD (Zwaigenbaum, Bryson, & Garon, 2013); such impairments differentiate toddlers with ASD from individuals with more general developmental delays (Dawson et al., 2004) and are associated with symptom severity in toddlers with ASD (Charman, 2003). Our second measure of social communicative competence was the degree of social impairment specific to ASD, as operationalized by the Autism Diagnostic Observation Schedule-Toddler Module (ADOS-T) (Luyster et al., 2009). We predicted that greater developmental increases in attention to faces would be associated with more spontaneous joint attention behaviors—a sign of better social communicative skills—and lower levels of social ASD symptoms.
Methods
Participants
Infant participants were enrolled in this study as part of a larger, longitudinal project aimed to examine early biomarkers of ASD risk (ACE; NICHD P50 HD055784). Informed consent from participating families was obtained prior to any experimental procedures under protocols approved by the University of California, Los Angeles Institutional Review Board. Infant participants were grouped in accordance to family risk status. Low-risk infants had no family history of ASD or related neurodevelopmental disorders. High-risk infants had at least one older sibling with an ASD diagnosis. Proband diagnosis was confirmed in writing by a licensed clinician prior to the infant sibling’s enrollment in the study. When available, external validation of the proband’s ASD diagnosis was confirmed by the Social Communication Questionnaire (Rutter et al., 2003).
Infants participated in 4 eye-tracking sessions at approximately 3, 6, 9, and 12 months of age. The final sample comprised 83 infants (52 HR: 59.6% male; 31 LR: 58.06% male). The number of trials (e.g., quantity of data) provided by each infant did not differ by risk status; from 3- to 12-months, HR and LR infants contributed an average of 4.67± 2.22 and 5.45 ± 1.88 trials, respectively and participated an average of 2.60 ± 1.11 and 2.94 ± 0.93 sessions (trial: t(81)=1.61, p = .11; session t(81)=1.77, p = .08). An additional 26 (13 HR, 23 LR) 3-month-old, 28 (11 HR, 17 LR) 6-month-old, 20 (9 HR 11 LR) 9-month-old, and 8 (2 HR, 6 LR) 12 month-old infants were observed but excluded due to excessive fussiness (e.g., crying and/or excessive movement) which compromised data quality by impacting the visibility of the infant’s eye. This was operationalized as point-of-gaze recorded for less than 30% of viewing time. Infants were also excluded due to a failure to complete the eye-tracking calibration procedure described below. Pearson 2-sided Chi-square tests confirmed that the number of infants included/excluded based on this data quality criteria did not differ by risk status (3-month: X2(1)=0.480, p = .488; 6-month: X2(1)=0.001, p = .980; 9-month: X2(1)=0.284, p = .594; 12-month: X2(1)=0.020, p = .888). Altogether, data quality and quantity were comparable between HR and LR infants across time points.
As part of an ongoing longitudinal study, diagnostic outcomes are conferred at 36 months by clinician’s best estimate upon reviewing all available information including performance on social communicative and language measures. At present, diagnostic outcomes are available for 23 HR infants; the remaining infants in the study have not yet aged to 36 months. Of the 23 HR infants, 7 were diagnosed with ASD, 4 with other concerns (e.g., BAP and/or Global Developmental Delays), and 2 with Speech and Language Delays. The remaining 10 HR infants were considered to be typically developing infants. Thus, the HR group contains a diverse range of developmental outcomes, with the majority demonstrating developmental vulnerabilities.
Behavioral Measures
The Social Responsiveness Scale (SRS, Constantino, 2002) was used to quantify the degree of autistic-like traits for each infant’s parents. The SRS is a questionnaire that provides a quantitative measure of reciprocal social communicative behaviors. The SRS yields a single score that captures both social communicative and restricted and repetitive behaviors, as determined in a factor analysis, and is continuously distributed in the general population (Frazier et al., 2014). The SRS has high test-retest reliability; correlation between scores for test-retest intervals spanning 3 to 6 months ranges between 0.88–0.95 in adults, indicating that it is a stable measure of autism-related traits (Bruni, 2014). Parents completed the SRS for each other upon study enrollment. The average of the parents’ scores was used as a quantitative metric of parental social responsiveness (Lyall et al., 2014), reflecting both genetic and environmental influences on their infant’s social communicative (Virkud et al., 2008; Schwichtenberg, Young, Sigman et al., 2010). The average parental SRS score is referred to as parental affectedness of ASD-related traits.
At 12-months, infants were administered the Early Social Communication Scales (ESCS; Mundy, Delgado, & Block, 2003) and the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The ESCS is a play-based standardized assessment of nonverbal social communication skills, including frequency of initiating (IJA) and responding to joint attention (RJA) cues. The MSEL is a standardized, normed developmental assessment and provides an overall metric of cognitive functioning (i.e., the Early Learning Composite [ELC]).
At 18 months, infants were administered the Autism Diagnostic Observation Schedule-Toddler Module (ADOS-T; Luyster et al., 2009) to measure core ASD symptomatology. Social symptoms are reflected in the Social Affect (SA) Score. Higher values indicate greater level of impairment.
Eye-tracking Stimuli
Infants viewed two 2-minute full-color video excerpts of a cartoon (A Charlie Brown Christmas) and a children’s television program (Sesame Street, see Frank et al., 2009 and Frank et al., 2014 for example stills). Videos were a) matched for duration, b) featured background music, and c) contained comparable content, described as follows. While the Charlie Brown excerpt was themed around Christmas and the Sesame Street excerpt was themed around a neighborhood gathering, both clips included scenes depicting 2–3 characters engaged in dialogue, one character speaking in a monologue to a group, and action sequences featuring a group of characters. The Charlie Brown video featured animated characters engaged in conversation (~80 s), speaking to a crowd (~20 s), and gathered in a group cheering and dancing (~20 s); background music was present for approximately 80% of the clip. One animated character was an anthropomorphized dog, which was present in approximately 20 cumulative, but nonconsecutive, seconds of the clip; all other animated characters were depictions of human children. The Sesame Street video featured children playing in a group setting (~50 s), an adult, male actor walking in an urban neighborhood greeting neighborhood children (~35 s), and the adult actor conversing with two human puppets (~40 s); background music was present for approximately 60% of the clip and urban sounds (e.g., cars honking, foot traffic) were present for the remaining 40% of the clip. Both stimuli included multisensory redundancy, such as moving mouths synchronized with speech. Relative to Charlie Brown, Sesame Street is a more complex stimulus insofar that it contained more visual information to process such as fast-paced camera movements (e.g., panning, zooming) and detailed background scenery (e.g., signs with text, building façades, moving vehicles).
Video frames were 8-bit color images and 720 by 480 pixels in resolution. Each frame was hand-traced for areas of interest (AOIs) encompassing each character’s face, including the animated anthropomorphized dog and human puppets, as in prior research using identical stimuli (Frank, Amso, & Johnson, 2014; cf. Frank et al., 2009) using software written in MATLAB (MathWorks, Inc; Natick, MA). The pixels that included faces (e.g., the area of face AOIs) on average comprised 38.21% of the visual display across both stimuli−−37.42 ± 16.49% of the screen in Charlie Brown and 39.01 ± 13.33% of the screen in Sesame Street. The area of face AOIs did not differ between the two stimuli; the 95% bootstrapped confidence interval of p-values was .144-.165 as calculated from 1000 independent t-tests between 100 randomly sampled frames from Charlie Brown and 100 randomly sampled frames from Sesame Street, both with replacement.
The eye tracker classified points of gaze that fell within a 30-pixel radius for a minimum of 100 ms as visual fixations. Valid on-screen viewing time was operationalized as the percent of total fixations directed to any part of the display relative to the total duration of the video stimuli. This measure of valid looking time was also used as a measure of overall task engagement. The primary dependent eye-tracking measure was percent looking at faces, computed as fixations directed to face AOIs relative to any part of the display (e.g., on-screen viewing time).
Eye-tracking Procedure
Eye-tracking protocols were completed at 3, 6, 9, and 12 months of age using a Tobii T60XL, a remote corneal-reflection binocular eye-tracker, which records eye movements at high spatial and temporal resolution (spatially < .5 degree of a visual angle; temporally samples at 60Hz). Infants sat on a caregiver’s lap approximately 60 cm from the 65-cm video display monitor. Caregivers were explicitly instructed not to distract their infant’s attention from the screen during stimuli presentation. Each infant’s point-of-gaze (POG) was calibrated using a 9-point calibration scheme prior to data collection. The calibration scheme was repeated until infant’s POG was within 1o of the center of the target and repeated between the two trials (i.e., between presentation of Charlie Brown and Sesame Street). The video stimuli were presented only after the calibration criterion had been reached to ensure accuracy in POG data that were included in the analyses.
Statistical Analyses
Percent looking at faces was longitudinally analyzed with a 3-level hierarchical linear model (individual time points nested within clip type within infant; risk status and parental affectedness were time-invariant subject-level predictors) using the nlme package in R (Pinheiro, Bates, DebRoy, Sarkar et al., 2018). On-screen valid viewing time was similarly longitudinally analyzed. Age at session, risk status (HR vs LR), parental affectedness (averaged parental SRS score), and clip type (Charlie Brown vs. Sesame Street) were included as predictors in the model. The age variable was centered at 3 months such that the intercept represents mean attention to faces at 3 months of age. Similarly, parental SRS scores were mean centered for ease of interpretation.
As noted previously, we were interested in evaluating whether developmental trajectories are moderated by a) familial risk status, b) parental affectedness and c) clip type. This was statistically modeled as the interaction between age and each of the moderating variables. Additional interactions of interest concerned effects of risk status and parental SRS on developmental trends in attention to faces by clip type (i.e., three-way interaction between age, risk status, and clip type, as well as between age, parental SRS, and clip type). We were also interested in evaluating a potential interaction effect between risk status and parental SRS on the developmental trajectories in attention to faces (i.e., a three-way interaction between age, risk status, and parental SRS). This would reveal whether there is a dosage effect of parental SRS on developmental trajectories in attention to faces by risk status (e.g., greater parental SRS scores in HR infants may have more of an impact on developmental trends in face-looking than greater affectedness in low-risk infants).
To examine how developmental trends in attention to faces may be associated with measures of social competence, we ran Pearson correlations between rate of change in attention to faces and ADOS-T SA scores and ESCS IJA and RJA scores. We hypothesized that these measures of social development would be inter-correlated with one another, and that greater developmental change in face-looking would be associated with better social functioning. Estimates of individual trajectories in attention to faces from 3- to 12-months were derived from the model using the coef function in R. This metric is a sum of the fixed effect and random effect for the age term for each infant and provides the estimated slope for face-looking from 3–12 months. Because prior work has found that cognitive level is associated with ASD symptom severity in young children with ASD (Ben-itzchak & Zachor, 2007) and cognitive profiles moderate trajectories of visual social attention to faces in youth with ASD (Rice, Moriuchi, Jones, & Klin, 2012), we also ran correlations partialling out the effect of cognitive skill level (Mullen ELC) when evaluating associations between ADOS-T scores and developmental trajectories in visual attention to faces (e.g., Bedford et al., 2012). This specifically examines the relation between face-looking and social impairments over and above the effects of cognitive functioning. Zero-order and partial correlations are both reported.
Results
Behavioral Profiles
Parental SRS scores did not differ between HR and LR infants (t(81)=1.712, p = .09). At 12 months, HR and LR infants had comparable cognitive scores (t(77)=1.67, p=.099) and similar rates of spontaneous initiation of, and response to, joint attention bids (ESCS IJA: t(70)=1.499, p =.138; ESCS RJA: t(70)=1.208, p = .231). At 18 months, HR infants had higher ADOS-T SA scores (i.e., showed greater social impairments) than LR infants (t(66)=2.007, p = .04, see Table 1).
Table 1.
Behavioral Profiles of Infants at High vs. Low-Risk for ASD
| HR | LR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Range | N | Mean | SD | Range | ||
| Age at Eye- tracking Session, months | 3 month | 24 | 3.11 | 0.30 | 2.57–3.87 | 19 | 3.15 | 0.24 | 2.77–3.83 |
| 6 month | 40 | 6.12 | 0.39 | 5.57–7.7 | 25 | 6.11 | 0.30 | 5.53–6.87 | |
| 9 month | 34 | 9.10 | 0.46 | 8.53–10.07 | 21 | 9.18 | 0.41 | 8.5–10.17 | |
| 12 month | 37 | 12.15 | 0.37 | 11.43–13.1 | 26 | 12.12 | 0.36 | 11.57–12.83 | |
| % Valid Looking Time | 3 month | 65.56 | 21.71 | 30.14–98.96 | 75.24 | 19.05 | 33.60–99.45 | ||
| 6 month | 68.63 | 19.21 | 30.00–99.42 | 68.54 | 20.63 | 30.04–98.07 | |||
| 9 month | 67.26 | 19.47 | 31.21–99.35 | 74.69 | 20.87 | 30.38–98.45 | |||
| 12 month | 68.81 | 18.66 | 31.86–99.75 | 72.36 | 23.02 | 30.72–99.82 | |||
| % Face | 3 month | 41.20 | 17.18 | 9.61–76.50 | 39.86 | 18.55 | 9.92–68.71 | ||
| 6 month | 49.79 | 18.58 | 13.68–82.32 | 49.75 | 17.41 | 18.69–80.07 | |||
| 9 month | 54.83 | 19.17 | 11.51–84.99 | 54.23 | 17.08 | 22.14–83.24 | |||
| 12 month | 54.69 | 19.47 | 17.47–90.24 | 57.71 | 20.40 | 19.81–91.16 | |||
| Age at Assessment | Mullen/ESCS | 49 | 12.15 | 0.40 | 11.43–13.43 | 31 | 12.14 | 0.36 | 11.57–12.83 |
| ADOS-T | 43 | 18.27 | 0.74 | 17.33–22.03 | 26 | 18.24 | 0.34 | 17.7–19.4 | |
| Parent SRS | 50 | 36.62 | 3.93 | 28.51–43.84 | 31 | 35.37 | 4.36 | 25.75–44.98 | |
| Assessment Scores | Mullen ELC | 104.89 | 14.37 | 68–137 | 109.91 | 14.37 | 79–133 | ||
| ESCS IJA | 0.87 | 0.53 | 0–2.12 | 0.69 | 0.44 | 0–1.6 | |||
| ESCS RJA | 0.26 | 0.27 | 0–1 | 0.33 | 0.22 | 0–0.75 | |||
| ADOS T SA | 6.37 | 4.71 | 0–18 | 4.15 | 3.60 | 0–15 | |||
| Parent SRS | 28.25 | 15.07 | 7–79.5 | 22.33 | 15.71 | 3–68 | |||
Notes. ELC, Early Learning Composite; ESCS, Early Social Communication Scales; IJA, Initiation of joint attention; RJA, Response to joint attention; ADOS T SA, social affect score. Ages at the Mullen, ESCS, and ADOS-T are in months; Parent SRS are in years (i.e., parent’s average age). The Mullen and ESCS were administered on the same day.
Across all infants, parental affectedness correlated with Mullen ELC (r=−.30, p = .007) and ADOS-T SA (r=.30, p =.012), but not ESCS IJA and RJA scores. Mullen ELC was positively correlated with ESCS RJA scores (r=.283, p = .016) and negatively correlated with ADOS-T SA (r=−.370, p = .028).
On-screen Viewing Time
A preliminary, unconditional growth model was first run to evaluate the proportion of variance explained by each level and, in particular, to evaluate whether clip type was an appropriate level to model. Intraclass correlation coefficients (ICC) were calculated to examine the correlation among observations within the same level. The ICC for clip type nested within subject was .07, suggesting that clip type was not an informative level to evaluate trends in on-screen viewing time; that is, the observations for Charlie Brown were no more similar than the observations for Sesame Street and vice versa. The ICC for between-subject variability was .29 and within-subject variability was .63. Thus, the hierarchical linear model reported below excludes clip type as a separate level and only includes infant age at evaluation, parental affectedness, risk status, and their interactions as predictors and percent on-screen viewing time as the criterion (see Table 2).
Table 2:
Results from Linear Mixed Effects Model Predicting On-Screen Viewing
| Fixed Effects | Estimate | Standard Error | df | t | Pr (>|t|) |
|---|---|---|---|---|---|
| Intercept | 67.501 | 3.445 | 326 | 19.592 | <.001 |
| Age | 0.433 | 0.484 | 326 | 0.895 | .371 |
| Parental Affectedness | −0.396 | 0.223 | 78 | −1.772 | .080 |
| Risk | .390 | 4.376 | 78 | 0.089 | .929 |
| Age X Parental Affectedness | 0.049 | 0.031 | 326 | 1.588 | .113 |
| Age X Risk | −.561 | .633 | 326 | −0.888 | .375 |
| Parental Affectedness X Risk | .602 | .259 | 78 | 2.322 | .023 |
| Age X Parental Affectedness X Risk | −0.058 | 0.036 | 326 | −1.610 | .108 |
Note. T-tests are calculated using Satterthwaite approximations of degrees of freedom
On-screen viewing time did not vary with age (β=0.43, t=0.89, p=.37) and developmental trajectories in on-screen viewing time did not vary by parental affectedness (β=.05, t=1.58, p=.11) or infant risk status (β=−0.56, t=−0.89, p=.38). The dynamic stimuli were thus equally engaging for all infants across all time points.
Developmental Trends in Attention to Faces
Similar to above, we first ran a preliminary unconditional growth model to calculate the ICC for clip type, between-, and within-subject variance. ICC for clip type nested within subject was .55, justifying its inclusion as a level for the hierarchical linear model (e.g., 55% of the variance in face-looking was accounted for by clip type). ICC for between-subject variance was .14 and for within-subject variance was .31. The hierarchical linear model reported below includes clip type as a separate level and evaluates the effect of infant age at eye-tracking, parental affectedness, risk status, and their interactions on percent looking to faces. We initially explored the effect of sex, but did not observe a significant effect (β=0.726 t=0.377 p=.71) and thus excluded it from the aforementioned model.
Attention to faces increased from 3- to 12-months of age across all infants (β=2.020, t=4.860, p<.001, f2= 1.239). There was also a main effect of clip type; infants looked less to faces in Sesame Street than in Charlie Brown (β=−26.589, t=−8.734, p<.001, f2= 0.801). There was no main effect of either parental SRS (β=0.162, t=1.627, p= .11, f2=0.033) or risk status (β=0-.214, t=−0.060, p=.952, f2= 0.024) on overall attention to faces (see Figure 1).
Figure 1.
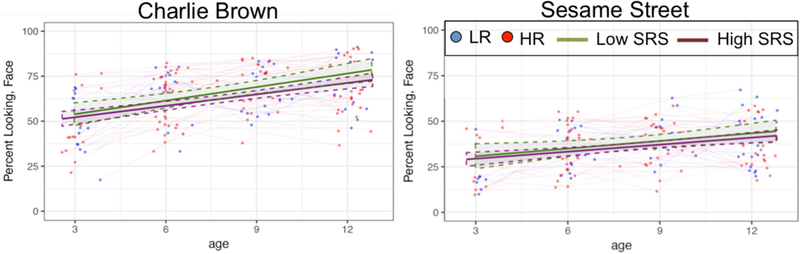
For visualization purposes, infants were grouped based on their parent’s average SRS scores. Low SRS includes infants whose parents’ SRS scores were below the group average; High SRS includes infants whose parents’ SRS scores were above the group average.
There was a significant three-way interaction between age, parental SRS, and clip type of a small-to-medium effect size (Cohen, 1988); β=0.447, t=2.687, p = .008, f2=0.08, see Table 3. To decompose this interaction, separate hierarchical linear models were run for eye-tracking data corresponding to Charlie Brown and Sesame Street with age, parental SRS, and their interaction as predictors (see Table 4). Given that these two additional models were post-hoc tests, we evaluated significance against a Bonferroni corrected alpha threshold of p<.025 =.05/2. There was a significant interaction of a medium effect size between age and parental SRS for Charlie Brown (β=−0.39, t=−2.819, p = .006, f2=0.153) but not for Sesame Street (β=0.002, t=0.147, p = .883, f2=0.042); higher values in parental SRS (e.g., greater level of ASD-related traits) were associated with lower developmental change in face-looking in Charlie Brown from 3- to 12-months.
Table 3:
Results from Linear Mixed Effects Model Predicting Looking at Faces
| Fixed Effects | Estimate | Standard Error | df | t | Pr (>|t|) |
|---|---|---|---|---|---|
| Intercept | 54.026 | 2.732 | 246 | 19.778 | <.001 |
| Age | 2.019 | 0.415 | 246 | 4.859 | <.001 |
| Parental SRS | 0.162 | 0.099 | 79 | 1.629 | .108 |
| Clip Type | −26.588 | 3.044 | 74 | −8.734 | <.001 |
| Risk | −.214 | 3.488 | 79 | −0.059 | .953 |
| Age X Parental SRS | −0.038 | 0.016 | 246 | −2.338 | .020 |
| Age X Clip Type | −0.348 | 0.489 | 246 | −0.711 | .477 |
| Age X Risk | 0.149 | 0.554 | 246 | 0.269 | .788 |
| Clip Type X Parental SRS | −0.217 | 0.104 | 74 | −2.077 | .042 |
| Clip Type X Risk | 4.748 | 4.050 | 74 | 1.172 | .245 |
| Age X Parental SRS X Clip Type | 0.045 | 0.017 | 246 | 2.687 | .008 |
| Age X Risk X Clip Type | −0.899 | 0.658 | 246 | −1.368 | .173 |
| Age X Parental SRS X Risk | −0.0004 | 0.011 | 246 | −0.034 | .973 |
Note. T-tests are calculated using Satterthwaite approximations of degrees of freedom
Table 4:
Results from Post-Hoc Breakdown of 3-Way Interaction
| Fixed Effects | Estimate | Standard Error | df | t | Pr (>|t|) |
|---|---|---|---|---|---|
| Attention to Faces in Charlie Brown | |||||
| Intercept | 53.790 | 1.77 | 135 | 30.342 | <.001 |
| Age | 2.144 | 0.268 | 135 | 8.010 | <.001 |
| Parental SRS | 0.176 | 0.096 | 79 | 1.827 | .072 |
| Age X Parental SRS | −.039 | 0.014 | 135 | −2.819 | .006 |
| Attention to Faces in Sesame Street | |||||
| Intercept | 30.115 | 1.729 | 114 | 17.411 | <.001 |
| Age | 1.252 | 0.260 | 114 | 4.823 | <.001 |
| Parental SRS | −0.036 | 0.089 | 76 | −0.403 | .688 |
| Age X Parental SRS | 0.002 | 0.013 | 114 | 0.147 | .883 |
Note. T-tests are calculated using Satterthwaite approximations of degrees of freedom. Significance is evaluated at Bonferonni corrected alpha threshold of p<.025 = .05/2.
Associations between Developmental Trends in Attention to Faces and Social Communicative Competence
The null interaction between age and clip type suggests that developmental trends in attention to faces for Charlie Brown and Sesame Street were comparable (e.g., the magnitude of linear change in attention to faces was not different between clip types). Indeed, estimates of individual slopes in attention to face while viewing Charlie Brown and Sesame Street were correlated with one another, which implies measurement consistency across clips (r=.425, p<.001). Thus, estimates of overall attention to faces across clip types were used to examine associations between developmental trends in face looking and social communicative competence. Across HR and LR participants, greater attention to faces was associated with lower social symptom severity on the ADOS-T (r=−.245, p=.044, see Figure 2), even when partialling out the effects of cognitive level (r=−.267, p=.034). The correlation between ADOS-T SA and face looking was significant for HR infants, over and above the effects of cognitive level (r=−.319, p = .048), but not for LR infants. Greater developmental increases in attention to faces were associated with lower levels of social symptom severity at 18 months.
Figure 2.
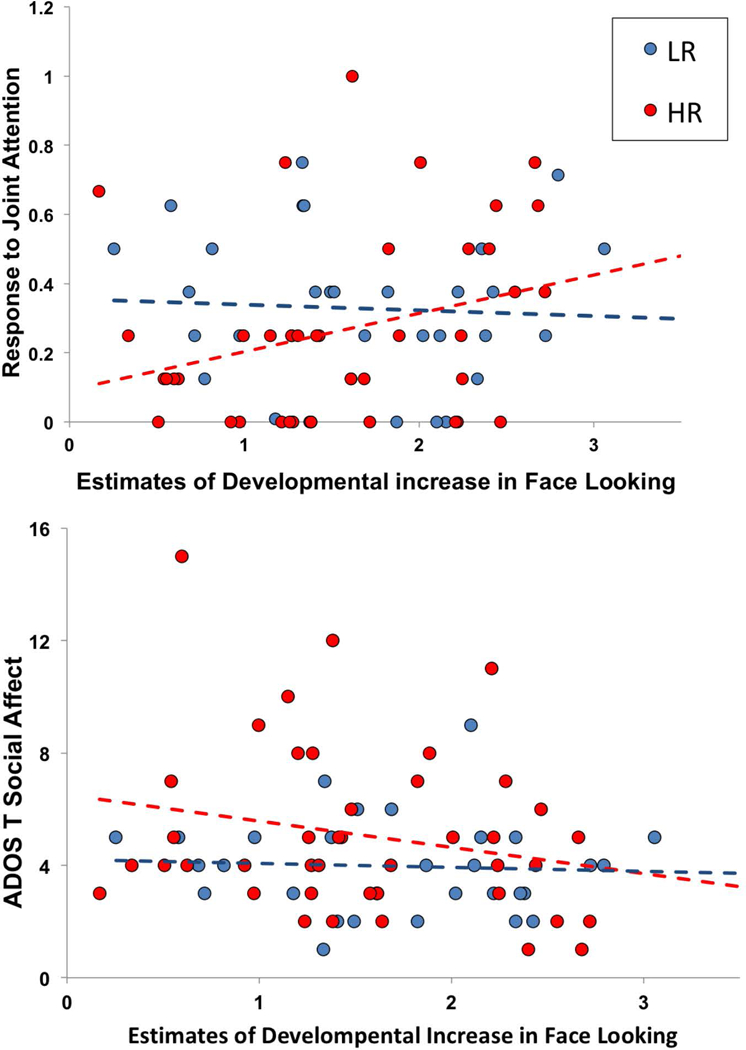
Zero-order scatterplots in estimates of developmental increases in face looking and ESCS RJA scores, and ADOS-T SA scores. For HR infants, increased developmental rates in face looking were associated with better response to joint attention skills. Increased developmental rates in face looking were associated with lower ASD social symptom severity across all infants, and in HR infants when controlling for cognitive level.
Attention to faces from 3- to 12-months in HR infants was positively correlated with 12 month ESCS RJA scores (r=.316, p = .04) but not IJA scores (r = −.045, p = .780). The relation was not significant across HR and LR participants (ESCS IJA: r=−.016, p =.895; ESCS RJA: r=.165, p=.166, see Figure 2).
Discussion
Attenuated engagement with the social world is a hallmark feature of ASD that also reflects the degree of social impairments in children and adults with ASD (Jones, Carr, & Klin, 2008; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Frazier et al., 2016). There is some evidence that diminished attention to socially relevant stimuli is an early emerging risk marker for ASD (Chawarska et al., 2013; Shic et al., 2014). Here, we explored factors that may influence observed developmental trajectories in attention to faces in infants at high and low risk for ASD, and the extent to which individual differences in attention to faces constitute a performance-based measure of social communicative competence. We did not find overall between-group differences in attention to faces from 3- to 12-months in our HR and LR infants. Rather, stimulus type and parental SRS scores influenced attention to faces during the first postnatal year. These findings highlight that experimental stimuli may impact observed trends in attention to faces, as well as inform potential modifiable aspects of early visual social attention.
Stimulus-specific Features Influence Visual Social Attention
We found that stimulus complexity influenced infants’ social attention, regardless of risk status. While developmental trends in attention to faces were comparable between Charlie Brown and Sesame Street (i.e., simple versus complex stimuli), infants overall looked more to faces in the simple schematic social stimulus. This is consistent with prior eye-tracking studies demonstrating that visual behaviors are context-dependent and vary as a function of the social demands of the task in both typical development (Tenenbaum, Shah, Sobel, Malle, & Morgan, 2013) and ASD (Chawarska et al., 2012; Elsabbagh et al., 2013).
The ability to detect faces in complex, dynamic displays appears contingent upon an infant’s attentional capacities (Frank et al., 2014). Although possible, we do not attribute the presence of puppets in Sesame Street as a likely source for the differences in face looking between the types of stimuli. Rather, the fast-paced camera and people motions that were predominantly featured in Sesame Street may have made face detection more challenging by necessitating rapid deployment of attention (e.g., Frank, Amso, & Johnson, 2014). The exaggerated features of the schematic faces in Charlie Brown may represent a supernormal stimulus that facilitated the attentional capture of faces for both high- and low-risk infants. Moreover, the simpler presentation of the social scenes in Charlie Brown may have distilled the complexity of the social interactions such that irrelevant objects and events were not depicted and accentuated relevant social actions. For instance, the background in Sesame Street contained details, such as building facades and street signs, which may have detracted attention from faces. The departure from the busy complexity of naturalistic social situations, in conjunction with the exaggerated facial shapes and features in Charlie Brown, may have accounted for the elevated overall attention to faces in our schematic versus live-action stimuli.
Attention to faces in the two kinds of social stimulus did not differ across the HR and LR infants in this study. Prior research has found that diminished attention to faces is specific to HR infants who develop ASD and not necessarily an endophenotype common to all HR infants (e.g., Chawarska et al., 2013). Given the heterogeneity in developmental outcomes among the HR infants in our sample, it is not surprising that familial risk for ASD does not uniformly confer social vulnerabilities. Regardless, the stimuli used here appear to be valid in quantifying behavioral correlates of social competence in HR infants. Notably, greater increases in attention to faces from 3- to 12-months of age was associated with greater responses to joint attention cues at 12 months, and lower levels of social symptom severity at 18 months, independent of cognitive levels. This corroborates prior work demonstrating that reduced attention to the eyes between 2–6 months in infants who develop ASD is associated with greater social impairment at 24 months (Jones & Klin, 2013).
Although significant, the correlations between face-looking and metrics of social competence were of small-to-moderate size (i.e., r’s ranging between .245 and .319), suggesting that attention to faces may be one of many means for infants to learn about the social world. Alternatively, overall of attention to faces may be a less sensitive metric of social competence than moment-to-moment attention to faces (e.g., looking to the most socially relevant face at the right time; Klin et al., 2003, 2002), which would account for our observed null effect between developmental increases in attention to faces and response to joint attention skills across all infants, as well as the lack of a between-group difference in attention to faces in HR and LR infants. Nonetheless, our findings provide converging evidence that performance-based measures of spontaneous attention to socially relevant information during infancy may capture several aspects of social communicative development, further highlighting the clinical significance of these early behaviors. More normative patterns of visual social attention in infancy may be a potential protective factor for HR infants with regards to their social functioning.
Parental Affectedness of ASD-Related Traits Influence Visual Social Attention
One aim of the study was to examine the effects of parental affectedness of ASD traits on their infant’s visual social attention to faces. Our findings provide preliminary evidence of an association between ASD-related traits in parents and developmental trends in attention to faces in their infants. Similar to prior work (Bernier et al., 2012), we did not observe differences in BAP features between parents of HR and LR infants. The comparable distribution of parental SRS scores across risk cohorts suggests that parents of HR infants in our study were not qualitatively different than parents of LR infants. Notably however, we found that higher parental SRS scores predicted slower developmental increases in attention to faces across all infants, and increased rates of face-looking was associated with greater social competence in HR infants. Inter-generational transmission of autism-related traits, which likely entails the conjoined influence of parental behaviors and shared genetic risk, may manifest as altered developmental trajectories of attention to faces in infancy. Future work focusing on intervention strategies may aim to capitalize on this behavioral influence; improving social responsiveness in parents may have positive effects in promoting social communicative development in infants at risk for ASD and other developmental disabilities that affect social function.
The observed association between parental SRS scores and developmental trajectories in attention to faces may not be necessarily attributed to the availability or overture of social communicative bids from the caregiver. If this were the case, we would have observed a significant association between degree of parent’s SRS scores and infants’ joint attention and language skills (e.g., Siller & Sigman, 2002). Shared genetic variation between parent and infant may account for the effect of social communication function in parents on infant’s trajectories of visual social attention (e.g., Constantino et al., 2017). It is also important to consider how early parent-child interactions shape the landscape of an infant’s social environment and thus their social communicative development (e.g., Granat et al., 2017). Elevated BAP features in parents may impact the quality of parent-child interactions, such that they are more directive and less synchronous with the communicative needs of the infant (Parr, Gray, Wigham, & Mcconachie, 2015). Infants, including those at high risk for ASD, are sensitive to reciprocity in dyadic interactions and divert their gaze from their mother’s face when there are breaks in contingency (Rozga et al., 2011). Reduced affect and synchrony in infant-initiated interactions in 4-month-old HR infants, however, suggest that HR infants may be less adept at engaging their caregivers in a reciprocal social interactions (Yirmiya et al., 2006). Following a transactional model of parent-infant interaction on social development, these two factors may iteratively compromise the social learning opportunities afforded by face-to-face interactions in HR infants (whose parents themselves may have elevated BAP features). This may manifest as reduced attention to faces, again presenting implications for developing interventions geared at ameliorating prodromal social symptoms in HR infants.
There is general support for the possibility that parent-mediated interventions can promote social communicative skills in children with ASD (Mcconachie & Diggle, 2007), and earlier age of enrollment in parent-mediated intervention is associated with greater degree of improved social functioning (Rogers et al., 2012). Preliminary evidence from a randomized trial of parent-mediated intervention in 7- to 10-month-old HR infants suggests that optimizing parent-child interactive behaviors (e.g., parental responsiveness and emotional attunement to their infant’s communication needs) can ameliorate behavioral and attentional prodromal symptoms of ASD (Green et al., 2017). For parents who may exhibit more social or language difficulties associated with BAP, such training may be particularly beneficial in promoting positive and sensitive parent-child interactions. Although prior work has found that parents with ASD are equally attuned to their child’s communicative needs as parents of children with developmental delays and typical development, children with ASD may require higher levels of parent-child synchrony than their peers to attain benefits in joint attention and language skills (Siller & Sigman, 2002). Further research will be necessary to pinpoint how parent-mediated interventions affect autism-related traits in parents, and whether developmental changes in attention to faces can serve as an intermediary, and objective, outcome measure associated with social learning and social communicative competence in such interventions.
Conclusions
Taken together, the findings from the present study contribute to our understanding of developmental differences in visual social attention among infants at high vs. low risk for ASD in three important ways. First, our results highlight the importance of considering developmental level when selecting dynamic social stimuli in longitudinal designs of visual social attention to faces. During the first postnatal year, infants transition from primarily engaging in dyadic social interactions to participating in triadic interactions. Stimuli that require monitoring triadic interactions may not be the most sensitive in identifying early deviations in visual social attention in high-risk infants, unlike the case for older individuals with ASD (Klin et al., 2002; Rice et al., 2012). Second, the comparable developmental trends in HR and LR infants’ looking to faces in our study suggest that familial risk for ASD does not confer gross deficits in visual social attention, though this may in part reflect the genetic heterogeneity in the HR group. Rather, it may be possible that more subtle difficulties in modulating visual attention initially impede the capacity to attend to the most socially relevant information in complex dynamic stimuli (Elsabbagh & Volein, 2009). Research examining whether lower-level deficits in attention affects higher-order visual social attention in HR infants could clarify this issue. Finally, we found that ASD-related traits in parents moderated developmental trajectories in face looking during the first postnatal year, and that increased rates in HR infants’ attention to faces were associated with greater social competency. This is a point of optimism because it identifies a modifiable aspect of an early symptom-based marker of ASD and presents clinical implications for developing interventions aimed at promoting social communicative skills in HR infants.
Supplementary Material
Acknowledgements
This work was supported by grants from National Institute of Child Health and Human Development (NICHD P50 HD055784). Additional support was provided by the National Institute of Health (F31HD090937). We wish to thank the Child and Adult Neurodevelopmental Clinic for their contribution in behavioral characterization of the sample. The authors do not have any conflicts of interest to declare. We also wish to thank the infants and their families for their time and participation in the study.
References
- Amso D, Fitzgerald M, Davidow J, Gilhooly T, & Tottenham N (2010). Visual exploration strategies and the development of infants’ facial emotion discrimination. Frontiers in Psychology, 1(November), 180 10.3389/fpsyg.2010.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, & Johnson MH (2012). Precursors to social and communication difficulties in infants at-risk for autism: gaze following and attentional engagement. Journal of Autism and Developmental Disorders, 42(10), 2208–18. 10.1007/s10803-012-1450-y [DOI] [PubMed] [Google Scholar]
- Ben-itzchak E, & Zachor DA (2007). The effects of intellectual functioning and autism severity on outcome of early behavioral intervention for children with autism, 28, 287–303. 10.1016/j.ridd.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Bernier R, Gerdts J, Munson J, Dawson G, & Estes A (2012). Evidence for broader autism phenotype characteristics in parents from multiple-incidence autism families. Autism Research : Official Journal of the International Society for Autism Research, 5(1), 13–20. 10.1002/aur.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontinck C, Warreyn P, Demurie E, Bruyneel E, Boterberg S, & Roeyers H (2018). Social Interactions Between 24-Month-Old Children and Their Older Sibling with Autism Spectrum Disorder : Characteristics and Association with Social-Communicative Development. Journal of Autism and Developmental Disorders, 0(0), 0 10.1007/s10803-018-3660-4 [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Shic F, Macari S, & Chawarska K (2014). Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: a subtyping analysis. Journal of Autism and Developmental Disorders, 44(2), 431–42. 10.1007/s10803-013-1885-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, & Nagell K (1998). Social Cognition, Joint Attention, and Communicative Competence from 9 to 15 Months of Age. Monographs of the …, 63(4). Retrieved from http://www.jstor.org/stable/10.2307/1166214 [PubMed] [Google Scholar]
- Charman T (2003). Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 315–24. 10.1098/rstb.2002.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari SL, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203. 10.1016/j.biopsych.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22428993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. 2nd. [Google Scholar]
- Constantino JN, Kennon-McGill S, Weichselbaum C, Marrus N, Haider A, Glowinski AL, Gillespie S, Klaiman C, Klin A, & Jones W (2017). Infant viewing of social scenes is under genetic control and indexes risk for autism. Nature. 10.1038/nature22999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, & Lajonchere C (2006). Autistic social impairment in the siblings of children with pervasive developmental disorders. American Journal of …, (February), 294–296. Retrieved from http://journals.psychiatryonline.org/article.aspx?articleid=178051 [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2003). Autistic traits in the general population: a twin study. Archives of general psychiatry, 60(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Csibra G, & Gergely G (2006). Social learning and social cognition: The case for pedagogy. Processes of change in brain and cognitive development. Attention and performance XXI, 21, 249–274. [Google Scholar]
- Dawson G, Estes A, Munson J, Schellenberg G, Bernier R, & Abbott R (2007). Quantitative assessment of autism symptom-related traits in probands and parents: Broader Phenotype Autism Symptom Scale. Journal of Autism and Developmental Disorders, 37(3), 523–36. 10.1007/s10803-006-0182-2 [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, & Liaw J (2004). Early Social Attention Impairments in Autism : Social Orienting , Joint Attention , and Attention to Distress. Developmental Psychology, 40(2), 271–283. 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, & Johnson MH (2013). What you see is what you get: contextual modulation of face scanning in typical and atypical development. Social Cognitive and Affective Neuroscience. 10.1093/scan/nst012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, & Johnson MH (2016). Autism and the Social Brain: The First-Year Puzzle Biological Psychiatry. Elsevier; 10.1016/j.biopsych.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, & Volein A (2009). Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child …, 5, 637–642. 10.1111/j.1469-7610.2008.02051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Amso D, & Johnson SP (2014). Visual search and attention to faces during early infancy. Journal of Experimental Child Psychology, 118(1), 13–26. 10.1016/j.jecp.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Vul E, & Johnson SP (2009). Development of infants’ attention to faces during the first year. Cognition, 110(2), 160–70. 10.1016/j.cognition.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Vul E, & Saxe R (2011). Measuring the Development of Social Attention Using Free-Viewing. Infancy, 1(21). [DOI] [PubMed] [Google Scholar]
- Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, & Constantino JN (2014). Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism, 18(1), 31–44. 10.1177/1362361313500382 [DOI] [PubMed] [Google Scholar]
- Gerdts JA, Bernier R, Dawson G, & Estes A (2013). The broader autism phenotype in simplex and multiplex families. Journal of Autism and Developmental Disorders, 43(7), 1597–605. 10.1007/s10803-012-1706-6 [DOI] [PubMed] [Google Scholar]
- Gliga T, Elsabbagh M, & Andravizou A (2009). Faces Attract Infants ‘ Attention in Complex Displays, 14(5), 550–562. 10.1080/1525oooO9O3 [DOI] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, … Booth R (2015). Parent-mediated intervention versus no intervention for infants at high risk of autism : a parallel , single-blind , randomised trial, 133–140. 10.1016/S2215-0366(14)00091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Pickles A, Pasco G, Bedford R, Wan MW, Elsabbagh M, … Cheung C (2017). Randomised trial of a parent-mediated intervention for infants at high risk for autism : longitudinal outcomes to age 3 years, 12, 1330–1340. 10.1111/jcpp.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, & Johnson MH (2007). The development of the social brain in human infancy. The European Journal of Neuroscience, 25(4), 909–19. 10.1111/j.1460-9568.2007.05379.x [DOI] [PubMed] [Google Scholar]
- Hurley RSE, Losh M, Parlier M, Reznick JS, & Piven J (2007). The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders, 37(9), 1679–90. 10.1007/s10803-006-0299-3 [DOI] [PubMed] [Google Scholar]
- Johnson SP, Amso D, & Slemmer JA (2003). Development of object concepts in infancy: Evidence for early learning in an eye-tracking paradigm. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10568–73. 10.1073/pnas.1630655100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews, 39C, 1–33. 10.1016/j.neubiorev.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Carr K, & Klin A (2008). Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry, 65(8), 946–54. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18678799 [DOI] [PubMed] [Google Scholar]
- Jones W, & Klin A (2013). Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature, 504(7480), 427–431. 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, & Volkmar F (2003). The enactive mind, or from actions to cognition: lessons from autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 345–60. 10.1098/rstb.2002.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, & Cohen D (2002). Visual Fixation Patterns During Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals With Autism. JAMA Psychiatry, 59 Retrieved from https://jamanetwork.com/journals/jamapsychiatry/fullarticle/206705 [DOI] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, & Piven J (2008). Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics : The Official Publication of the International Society of Psychiatric Genetics, 147B(4), 424–33. 10.1002/ajmg.b.30612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, … Catherine Lord. (2009). The Autism Diagnostic Observation Schedule — Toddler Module : A New Module of a Standardized Diagnostic Measure for Autism Spectrum Disorders. J Autism Dev Disord, 39, 1305–1320. 10.1007/s10803-009-0746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, & Santangelo SL (2014). Parental Social Responsiveness and Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry, 71(8), 936–942. 10.1001/jamapsychiatry.2014.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconachie H, & Diggle T (2007). Parent implemented early intervention for young children with autism spectrum disorder : a systematic review. Journal of Evaluation in Clinical Practice, 13, 120–129. 10.1111/j.1365-2753.2006.00674.x [DOI] [PubMed] [Google Scholar]
- Mundy P, Delgado C, & Block J (2003). Early social communication scales (ESCS). Coral Gables, FL: …, (305). Retrieved from http://www.ucdmc.ucdavis.edu/mindinstitute/ourteam/faculty_staff/escs.pdf [Google Scholar]
- Mundy P, & Gomes A (1998). Individual differences in joint attention skill development in the second year. Infant Behavior and Development, 21(3), 469–482. 10.1016/S0163-6383(98)90020-0 [DOI] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–95. 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr JR, Gray L, Wigham S, & Mcconachie H (2015). Research in Autism Spectrum Disorders Measuring the relationship between the parental Broader Autism Phenotype , parent – child interaction , and children ‘ s progress following parent mediated intervention. ., 20, 24–30. 10.1016/j.rasd.2015.07.006 [DOI] [Google Scholar]
- Pickles A, Parr JR, Rutter ML, De Jonge MV, Wallace S, Le Couteur AS, … Bailey a J. (2013). New interview and observation measures of the broader autism phenotype: impressions of interviewee measure. Journal of Autism and Developmental Disorders, 43(9), 2082–9. 10.1007/s10803-013-1810-2 [DOI] [PubMed] [Google Scholar]
- Rice K, Moriuchi JM, Jones W, & Klin A (2012). Parsing heterogeneity in autism spectrum disorders: visual scanning of dynamic social scenes in school-aged children. Journal of the American Academy of Child and Adolescent Psychiatry, 51(3), 238–248. 10.1016/j.jaac.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Ph D, Estes A, Ph D, Lord C, Ph D, … Ph D (2012). Effects of a Brief Early Start Denver Model ( ESDM )- Based Parent Intervention on Toddlers at Risk for Autism Spectrum Disorders : A Randomized Controlled Trial. JAAC, 51(10), 1052–1065. 10.1016/j.jaac.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, & Sigman M (2011). Behavioral profiles of affected and unaffected siblings of children with autism: contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders, 41(3), 287–301. 10.1007/s10803-010-1051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Young GS, Sigman M, Hutman T, & Ozonoff S (2010). Can family affectedness inform infant sibling outcomes of autism spectrum disorders? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(9), 1021–30. 10.1111/j.1469-7610.2010.02267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shic F, Macari S, & Chawarska K (2014). Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry, 75(3), 231–7. 10.1016/j.biopsych.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller M, & Sigman M (2002). The Behaviors of Parents of Children with Autism Predict the Subsequent Development of Their Children ‘ s Communication, 32(2). [DOI] [PubMed] [Google Scholar]
- Speer LL, Cook AE, McMahon WM, & Clark E (2007). Face processing in children with autism: Effects of stimulus contents and type. Autism, 11(3), 265–277. 10.1177/1362361307076925 [DOI] [PubMed] [Google Scholar]
- Striano T, & Stahl D (2005). Sensitivity to triadic attention in early infancy. Developmental Science, 4, 333–343. [DOI] [PubMed] [Google Scholar]
- Tenenbaum EJ, Shah RJ, Sobel DM, Malle BF, & Morgan JL (2013). Increased focus on the mouth among infants in the first year of life: A longitudinal eye-tracking study. Infancy : The Official Journal of the International Society on Infant Studies, 18(4), 534–553. 10.1111/j.1532-7078.2012.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T, Atagi N, & Johnson SP (2018). Selective attention to the mouth is associated with expressive language skills in monolingual and bilingual infants. Journal of Experimental Child Psychology, 169, 93–109. 10.1016/j.jecp.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, & Plummer F (2013). Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(7), 763–71. 10.1111/jcpp.12032 [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-cohen S, & Sigman M (2006). The development of siblings of children with autism at 4 and 14 months : social engagement , communication , and cognition, 5, 511–523. 10.1111/j.1469-7610.2005.01528.x [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, & Ozonoff S (2009). Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science, 12(5), 798–814. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2732664&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, & Garon N (2013). Early identification of autism spectrum disorders Behavioural Brain Research. Elsevier B.V. 10.1016/j.bbr.2013.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


