Abstract
The effects of intranasal oxytocin, a neuropeptide involved in prosocial behavior and modulation of neural networks underlying social cognition and emotion regulation, have been studied in schizophrenia. We tested the hypothesis that twice-daily intranasal oxytocin administered for 12-weeks would improve tertiary and exploratory outcomes of self-reported social symptoms, empathy and introspective accuracy from the Jarskog et al. (2017) randomized controlled trial. Sixty-eight stable outpatients with schizophrenia or schizoaffective disorder were randomized to receive oxytocin (24 IU twice daily) or placebo. Introspective accuracy was assessed with the Specific Level of Functioning Scale and the Interpersonal Perception Task. Empathy was assessed with the Interpersonal Reactivity Index (IRI), and social symptoms were assessed with the Liebowitz Social Anxiety Scale and the Green et al. Paranoid Thoughts Scales. Outcomes were assessed at baseline, six, and twelve weeks. Results demonstrated limited effect of oxytocin with some improvement on the IRI Perspective-Taking Subscale. No additional between-group differences emerged on self-reported symptoms, empathy, or introspective accuracy.
Keywords: Oxytocin, Social behavior, Psychosis, Schizophrenia, Schizoaffective disorder
1. Introduction
A promising psychopharmacological intervention for schizophrenia is the neuropeptide oxytocin (Rosenfeld, Lieberman, & Jarskog, 2011). Oxytocin modulates networks involved in social cognition and emotion regulation and is shown to play a key role in social behaviors (Lee, Macbeth, Pagani, & Young, 2009; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011; Rosenfeld et al., 2011). Several studies have investigated the effects of adjunctive intranasal oxytocin in schizophrenia with mixed findings (Bradley & Woolley, 2017; Burkner, Williams, Simmons, & Woolley, 2017; Cacciotti-Saija et al., 2015; Feifel, Shilling, & MacDonald, 2016; Mercedes Perez-Rodriguez, Mahon, Russo, Ungar, & Burdick, 2015; Oya, Matsuda, Matsunaga, Kishi, & Iwata, 2016).
To address heterogeneity in findings and the file drawer effect (Rosenthal, 1979), especially as documented in oxytocin treatment research (Lane, Luminet, Nave, & Mikolajczak, 2016), the present study presents tertiary (i.e., self-report measures) and exploratory outcomes (i.e., introspective accuracy or IA) from Jarskog et al. (2017). Previously, Jarskog et al. (2017) found limited effects of oxytocin on the secondary outcomes of social functioning (i.e., improvements in social skills) and negative symptoms but not on the primary outcomes of social cognition (i.e., emotion perception, theory of mind, attributional style). Given mixed findings and no previous research of oxytocin effects on IA, investigation of self-report and IA outcomes in the present study are exploratory.
2. Methods
2.1. Study design, participants, and randomization
Individuals with a diagnosis of schizophrenia or schizoaffective disorder participated in a double blind, randomized treatment study between June 2011 and September 2014. Participants completed screening, baseline, and assessment visits at six and 12 weeks. Participants were randomized to twice-daily intranasal oxytocin or placebo stratified by sex and total PANSS score. The Institutional Review Board at the University of North Carolina at Chapel Hill approved all procedures. Participants were compensated for participation. See Jarskog et al. (2017) for a comprehensive description of study methods, adverse events, and tolerability.
2.2. Intervention
Participants continued established medication regimens and administered intranasal spray twice daily before morning and evening meals for 12 weeks (see Jarskog et al. (2017) for drug and dosage details). To assess adherence, study drugs were weighed prior to dispensing and upon return and participants completed a daily medication diary.
2.3. Introspective accuracy measures
IA is the ability to accurately judge one’s own performance or impairment in terms of symptoms, cognition, functioning, and potential for achievement, and is a better predictor of functional outcomes compared with cognitive or functional performance in schizophrenia (Gould et al., 2015; Harvey & Pinkham, 2015; Harvey, Pinkham, & Penn, 2017). Measures with two informant sources (e.g., self and an informant report or objective performance on a task) were considered sources of IA. Differences between self and informant assessment or task performance were calculated with lower difference scores reflecting better IA. The present study included two IA outcomes.
Individuals and an informant source completed the Specific Level of Functioning Scale (SLOF; Schneider, 1983) with higher scores reflecting better functioning. Between and within-group differences on SLOF scores are presented in Jarskog et al. (2017).The difference between self-reported SLOF scores and informant SLOF scores measured IA.
The Interpersonal Perception Task (IPT; Costanzo & Archer, 1989; Dane & Mark, 1993) is a measure of social perception processes. Videos of common social interactions were shown followed by multiple-choice questions. IPT task performance outcomes are presented in Supplementary Table 1. Participants were asked to indicate how many items they answered correctly. The difference between number of perceived correct responses and number of correct responses measured IA.
2.4. Self-report measures
Self-report measures included a measure of empathy, Interpersonal Reactivity Index (IRI; Davis, 1980; Davis, 1983), as well as two measures assessing symptoms, Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987) and the Green et al. Paranoid Thought Scales (GPTS; Green et al., 2008). All self-report measures demonstrate good validity and have been used extensively with schizophrenia populations (Davis, 1983; Green et al., 2008; Liebowitz, 1987; Pallanti, Quercioli, & Hollander, 2004). The reliability of self-report measures in the present sample ranged from acceptable to excellent (Cronbach’s αs .74 - .96).
2.5. Statistical Analysis
Individuals receiving at least one dose of study medication and a post-baseline assessment were included in the present analysis. The effect of oxytocin on outcomes was analyzed using mixed models with random intercepts for each participant and fixed effects of visit, treatment, and treatment-by-visit interactions. An unstructured covariance pattern modeled correlations within participants over time. Least squares means for change from baseline were estimated using full information maximum likelihood. Unadjusted means are presented in supplementary materials (S2 and S3).
3. Results
3.1. Baseline characteristics, randomization, and treatment adherence
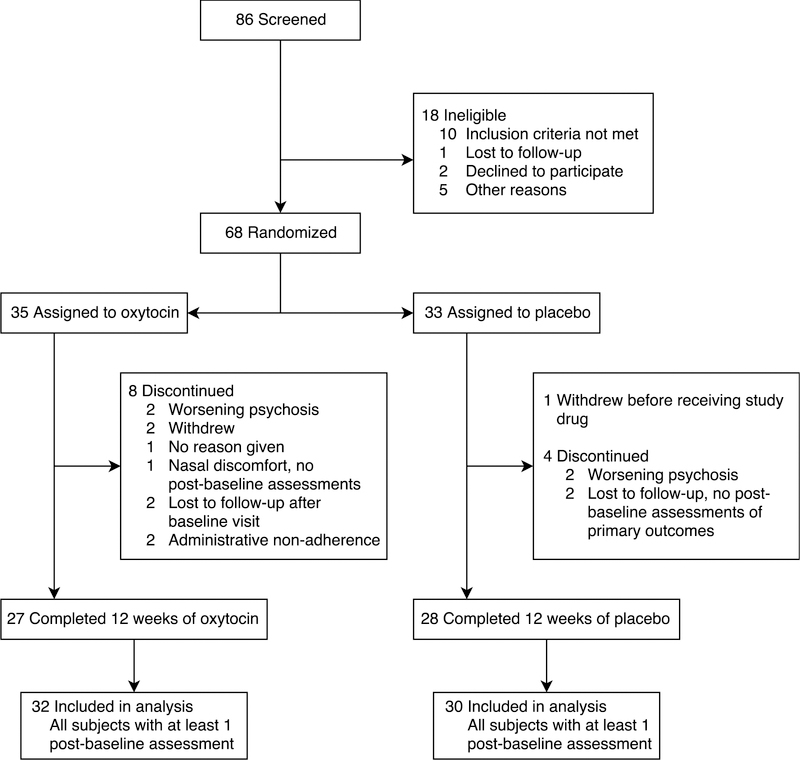
Figure 1 provides a CONSORT flowchart of study screening and inclusion. Thirty individuals in the placebo group and 32 in the oxytocin group were included in the study.
Figure 1.
Flowchart of study inclusion and data analysis adapted from Jarskog et al. (2017).
Baseline demographic and clinical information are presented in Table 1. The placebo group had significantly higher levels of paranoia indicated by PANSS items assessing social functioning (i.e., suspiciousness/persecution, hostility, passive/apathetic social withdrawal, uncooperativeness, and active social avoidance) compared with the oxytocin group, t(60) = 2.29, p = .03. Age (t(60) = 1.75, p = .09) and taking mood stabilizers (X2(1) = 3.34, p = .07) differed at a trend level. Subsequent analyses controlled for baseline PANSS social items and age given statistical and trend-level baseline differences. Mood stabilizers were not included in analyses given the relatively small proportion of the sample taking mood stabilizers. No additional significant between-group differences were observed at baseline.
Table 1.
Baseline Demographic and Clinical Characteristics
| Oxytocin N = 32 |
Placebo N = 30 |
p Values | |
|---|---|---|---|
| Age, years | 41.4± 12.3 | 35.9± 12.5 | .09 |
| Male % (N) | 75 (24) | 76.7(23) | .88 |
| Education, years | 12.6± 1.8 | 13 ± 2 | .41 |
| Race % (N) | |||
| White | 46.9(15) | 53.3(16) | .61 |
| Black | 43.8(14) | 40 (12) | .76 |
| Other | 9.3 (3) | 6.7 (2) | .70 |
| Schizophrenia % (N) | 59.4(19) | 65.5(20) | .55 |
| Schizoaffective % (N) | 40.6(13) | 34.5(10) | .55 |
| WRAT standard score | 93.63 ± 14.7 | 98.38 ± 14 | .20 |
| PANSS | |||
| Total | 65.75 ± 13 | 68.5± 10.3 | .36 |
| Negative | 17.4± 4.6 | 18.67 ± 4.2 | .26 |
| Positive | 16.7± 4.7 | 17.2± 4.9 | .68 |
| General psychopathology | 31.7± 6.9 | 32.7± 5.8 | .54 |
| Sociala | 10.5± 3.3 | 12.5± 3.6 | .03* |
| Medications % (N) | |||
| 1st generation antipsychotics | 12.5(4) | 13.3 (4) | .92 |
| 2nd generation antipsychotics | 84.3(27) | 86.7 (26) | .80 |
| Antidepressants | 46.8(15) | 33.3 (10) | .28 |
| Mood stabilizers | 37.5(12) | 16.6 (5) | .07 |
| Benzodiazepines | 18.7(6) | 30 (9) | .30 |
Note: Data presented as mean ± standard deviation unless otherwise indicated. WRAT – Wide Range Achievement Test. PANSS – Positive and Negative Symptom Scale.
Significant differences between treatment groups on baseline demographics or clinical characteristics, p < .05.
PANSS items assessing social functioning: suspiciousness/persecution, hostility, passive/apathetic social withdrawal, uncooperativeness, and active social avoidance.
Seventy five percent of individuals in the oxytocin group and 81.2% in the placebo group demonstrated excellent (80%−100%) or good (60% - 80%) adherence to study drug based on bottle weights, adherence diaries, and any other available information. No significant between-group differences on adherence emerged.
3.2. Introspective accuracy
No significant differences on IA abilities measured by the IPT and the SLOF were observed between treatment groups (Table 2). However, improved IA, measured by the IPT task, was observed within the placebo group at 12 weeks (MLS = −1.7, 95% CI [−3.1, −0.3], p = .02).
Table 2.
Change from Baseline for Introspective Accuracy Outcomes
| Introspective Accuracy Measure | Time Point (wks) |
LS Mean Change [95% CI] | Trt Diffb | |||
|---|---|---|---|---|---|---|
| Oxytocin N = 32 |
Placebo N = 30 |
|||||
| Interpersonal Perception Task | 6 | 0.3 | [−1.1, 1.7] | −0.5 | [−1.9, 1.0] | 0.8 |
| 12 | −0.5 | [−1.9, 1.0] | −1.7a | [−3.1, −0.3] | 1.2 | |
| Specific Levels of Functioning | ||||||
| Interpersonal Relationships |
12 | 2.7 | [−0.6, 6.0] | 1.7 | [−2.2, 5.6] | 1 |
| Social Acceptability | 12 | −0.9 | [−3.0, 1.1] | −0.5 | [−2.8, 1.9] | −0.4 |
| Activities | 12 | 2.4 | [−1.1, 6.0] | −2.0 | [−6.4, 2.3] | 4.4 |
| Work Skills | 12 | −0.01 | [−2.2, 2.2] | −0.1 | [−2.6, 2.4] | 0.09 |
| Total | 12 | 4.6 | [−1.6, 0.8] | −1.2 | [−9.1, 6.6] | 5.8 |
Note: Bold values indicate significant between group differences, p <.05.
Indicates significant within group differences, p <.05.
Values reflect differences between treatment groups, i.e., oxytocin group compared with the placebo group, at each time point. Models adjusted for baseline value, age, and baseline PANSS social items.
3.3. Self-report measures
The oxytocin group (MLS = 0.4, 95% CI [−1.1, 1.9]) exhibited improved IRI Perspective Taking at week 12 compared with the placebo group (MLS = −1.8, 95% CI [−3.3, −0.4], F(1, 109) = 4.77, p = .031) (Table 3). No other significant between-group differences were observed on the empathy subscales.
Table 3.
Change from Baseline for Self-Report Outcomes
| Self-Report Measure | Time Point (wks) |
LS Mean Change [95% CI] | Trt Diffb | |||
|---|---|---|---|---|---|---|
| Oxytocin N = 32 |
Placebo N = 30 |
|||||
| Interpersonal Reactivity Index | ||||||
| Fantasy | 6 | −0.8 | [−2.4, 0.8] | −1.5 | [−3.1, 0.1] | 0.7 |
| 12 | −1.3 | [−2.9, 0.4] | −1.1 | [−2.7, 0.5] | −0.2 | |
| Emotional Concern | 6 | −1.2 | [−2.5, 0.1] | −1.4a | [−2.7, −0.1] | 0.2 |
| 12 | −1.2 | [−2.5, 0.1] | −1.1 | [−2.3, 0.2] | −0.1 | |
| Perspective Taking | 6 | −0.4 | [−1.9, 1.0] | −1.4 | [−2.8, 0.1] | 1 |
| 12 | 0.4 | [−1.1, 1.9] | −1.8a | [−3.3, −0.4] | 2.2 | |
| Personal Distress | 6 | 0.6 | [−0.9, 2.1] | −0.4 | [−1.8, 1.1] | 1 |
| 12 | 0.2 | [−1.3, 1.7] | −0.3 | [−1.8, 1.1] | 0.5 | |
| Lieberman Anxiety Scale | ||||||
| Total | 6 | −0.3 | [−8.3, 7.7] | −1.6 | [−9.7, 6.4] | 1.3 |
| 12 | −1.3 | [−9.6, 6.9] | −8.0 | [−16.1, 0.2] | 6.7 | |
| Fear | 6 | −0.2 | [−4.6, 4.2] | 1.7 | [−2.7, 6.0] | −1.9 |
| 12 | −0.7 | [−5.2, 3.8] | −1.6 | [−5.9, 2.8] | 0.9 | |
| Avoidance | 6 | −0.1 | [−4.3, 4.0] | −3.7 | [−7.8, 0.5] | 3.6 |
| 12 | −0.6 | [−4.9, 3.7] | −5.4 | [−9.5, −1.3] | 4.8 | |
| Social Fear | 6 | −0.1 | [−2.4, 2.2] | −0.1 | [−2.4, 2.2] | 0 |
| 12 | −0.6 | [−2.9, 1.8] | −1.4 | [−3.7, 0.9] | 0.8 | |
| Social Avoidance | 6 | 0.4 | [−1.6, 2.3] | −1.2 | [−3.1, 0.8] | 1.6 |
| 12 | −0.1 | [−2.0, 1.9] | −2.7a | [−4.6, −0.7] | 2.6 | |
| Performance Fear | 6 | −0.1 | [−2.6, 2.3] | 1.7 | [−0.7, 4.2] | −1.8 |
| 12 | −0.2 | [−2.7, 2.4] | −0.2 | [−2.6, 2.2] | 0 | |
| Performance Av | 6 | −0.5 | [−2.9, 1.8] | −1.1 | [−3.4, 1.3] | 0.6 |
| 12 | −0.6 | [−3.0, 1.8] | −1.9 | [−4.2, 0.5] | 1.3 | |
| Green Paranoid Thought Scale | ||||||
| Social Reference | 6 | −0.3 | [−3.8, 3.2] | −4.7a | [−8.2, −1.2] | 4.4 |
| 12 | −0.3 | [−3.8, 3.3] | −3.5 | [−6.9, 0.1] | 3.2 | |
| Persecution | 6 | −0.2 | [−4.1, 3.9] | −3.4 | [−7.4, 0.6] | 3.2 |
| 12 | −0.9 | [−5.0, 3.3] | −2.1 | [−6.1, 1.8] | 1.2 | |
| Total | 6 | −0.4 | [−6.9, 6.2] | −8.1a | [−14.6, −1.7] | 7.7 |
| 12 | −1.0 | [−7.7, 5.7] | −5.6 | [−12.1, 0.8] | 4.6 | |
Note: Bold values indicate significant between group differences, p <.05.
Indicates significant within group differences.
Values reflect differences between treatment groups, i.e., oxytocin group compared with the placebo group, at each time point. Models adjusted for baseline value, age, and baseline PANSS social items.
Significant within-group changes in empathy were only observed in the placebo group. The placebo group exhibited worse empathy abilities over time on the Emotional Concern Subscale of the IRI (MLS = −1.4, 95% CI [−2.7, −0.1], t(109) = 2.2, p = .03) at six weeks as well as on the Perspective Taking Subscale of the IRI (MLS = −1.8, 95% CI [−3.3, −0.4], t(110) = 2.5, p =.02) at 12 weeks.
No significant between-group differences were observed on self-reported symptom outcomes. Significant within-group changes in symptoms were only observed in the placebo group. Significantly better social avoidance measured by the LSAS Social Avoidance Subscale was observed in the placebo group at 12 weeks (MLS = −2.7, 95% CI [−4.6, −0.7], t(106) = 2.7, p =.007). The placebo group also demonstrated significantly better paranoia at six weeks measured by the GPTS Social Reference Subscale (MLS = −4.7, 95% CI [−8.2, −1.2], t(108) = 2.7, p =.008) and total score (MLS = −8.1, 95% CI [−14.6, −1.7], t(108) = 2.5, p =. 01).
4. Discussion
Twice-daily administration of intranasal oxytocin showed limited evidence for improving self-reported symptoms, empathy, and IA in patients with schizophrenia. Individuals in the oxytocin group showed improvement on the Perspective Taking subscale of the IRI only but not on other self-reported outcomes and IA. Improvements in empathy in the oxytocin group extend findings from an oxytocin treatment study of a shorter duration (i.e., 6 weeks) where improvements on empathic perspective taking as measured by the IRI were also observed (Gibson et al., 2014).
No improvements in IA or self-reported symptoms of anxiety and paranoia were observed with oxytocin compared to placebo. These findings contrast previous support for oxytocin as a potential anxiolytic in schizophrenia (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Pedersen et al., 2011); no other previous studies have examined the effects of oxytocin on IA.
The present study had a number of limitations. First, although Jarskog et al (2017) is one of the largest treatment studies of oxytocin in schizophrenia to date, limited power precluded investigation of potential moderators such as age and psychotropic medication (Bradley & Woolley, 2017). Second, investigation of IA outcomes may be underestimated due to heterogeneity in informant source (e.g., roommate, friend, family member). Finally, adherence to study drug was carefully monitored with over 75% of participants demonstrating good or excellent adherence. However, intranasal delivery likely introduced individual variability in drug absorption dose (Guastella et al., 2013).
In summary, we found little support for the effect of twice-daily intranasal oxytocin on self-reported social symptoms, empathy and IA. Presentation of these tertiary and exploratory outcomes should contribute to any potential file drawer effect in oxytocin treatment research.
Supplementary Material
Acknowledgements:
Odum Institute (University of North Carolina at Chapel Hill)
Role of funding source: This work was supported by a grant from NIMH (grant number R01MH093529 – PIs CAP, DLP)
Footnotes
Clinicaltrials.gov identifier: NCT01394471
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflicts of interest.
References
- Bradley ER, & Woolley JD (2017). Oxytocin effects in schizophrenia: reconciling mixed findings and moving forward. Neuroscience & Biobehavioral Reviews 10.1016/j.neubiorev.2017.05.007 [DOI] [PMC free article] [PubMed]
- Burkner PC, Williams DR, Simmons TC, & Woolley JD (2017). Intranasal oxytocin may improve high-level social cognition in schizophrenia, but not social cognition or neurocognition in general: A multilevel bayesian meta-analysis. Schizophrenia Bulletin 10.1093/schbul/sbx053 [DOI] [PMC free article] [PubMed]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, … Guastella AJ (2015). A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophrenia Bulletin, 41(2), 483–493. 10.1093/schbul/sbu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, & Archer D (1989). Interpreting the expressive behavior of others - the Interpersonal Perception Task. Journal of Nonverbal Behavior, 13(4), 225–245. 10.1007/bf00990295 [DOI] [Google Scholar]
- Dane A, & Mark C (1993). The Interpersonal perception task (IPT-15): Berkeley, CA: : University of California, Extension Center for Media and Independent Learning, 1993. [Google Scholar]
- Davis M (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10(85). [Google Scholar]
- Davis M (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126. [Google Scholar]
- Feifel D, Shilling PD, & MacDonald K (2016). A review of oxytocin’s effects on the positive, negative, and cognitive domains of schizophrenia. Biological Psychiatry, 79(3), 222–233. 10.1016/j.biopsych.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, & Pedersen CA (2014). A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophrenia Research, 156(2–3), 261–265. 10.1016/j.schres.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O’Connor M, Torres I, & Carter CS (2008). Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Research, 98(1–3), 247–255. 10.1016/j.schres.2007.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F, McGuire LS, Durand D, Sabbag S, Larrauri C, Patterson TL, … Harvey PD (2015). Self-assessment in schizophrenia: Accuracy of evaluation of cognition and everyday functioning. Neuropsychology, 29(5), 675–682. 10.1037/neu0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Freeman D, Kuipers E, Bebbington P, Fowler D, Dunn G, & Garety PA (2008). Measuring ideas of persecution and social reference: the Green et al. Paranoid Thought Scales (GPTS). Psychological Medicine, 38(1), 101–111. 10.1017/S0033291707001638 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38(5), 612–625. 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Harvey P, & Pinkham A (2015). Impaired self-assessment in schizophrenia: why patients misjudge their cognition and functioning: observations from caregivers and clinicians seem to have the most validity. Current Psychiatry, 14(4), 53. [Google Scholar]
- Harvey P, Pinkham A, & Penn D (2017). Introspective accuracy as a predictor of real-world outcomes. Schizophrenia Bulletin, 43(suppl_1), S35–S36. 10.1093/schbul/sbx021.093 [DOI] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, & Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. 10.1016/s0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Pedersen CA, Johnson JL, Hamer RM, Rau SW, Elliott T, & Penn DL (2017). A 12-week randomized controlled trial of twice-daily intranasal oxytocin for social cognitive deficits in people with schizophrenia. Schizophrenia Research 10.1016/j.schres.2017.01.008 [DOI] [PMC free article] [PubMed]
- Lane A, Luminet O, Nave G, & Mikolajczak M (2016). Is there a publication bias in behavioural intranasal oxytocin research on humans? Opening the file drawer of one laboratory. Journal of Neuroendocrinology, 28(4). 10.1111/jne.12384 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, & Young WS 3rd. (2009). Oxytocin: the great facilitator of life. Progress in Neurobiology, 88(2), 127–151. 10.1016/j.pneurobio.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Mercedes Perez-Rodriguez M, Mahon K, Russo M, Ungar AK, & Burdick KE (2015). Oxytocin and social cognition in affective and psychotic disorders. European Neuropsychopharmacology, 25(2), 265–282. 10.1016/j.euroneuro.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews: Neuroscience, 12(9), 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Oya K, Matsuda Y, Matsunaga S, Kishi T, & Iwata N (2016). Efficacy and safety of oxytocin augmentation therapy for schizophrenia: an updated systematic review and meta-analysis of randomized, placebo-controlled trials. European Archives of Psychiatry and Clinical Neuroscience, 266(5), 439–450. 10.1007/s00406-015-0634-9 [DOI] [PubMed] [Google Scholar]
- Pallanti S, Quercioli L, & Hollander E (2004). Social anxiety in outpatients with schizophrenia: a relevant cause of disability. American Journal of Psychiatry, 161(1), 53–58. 10.1176/appi.ajp.161.1.53 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, … Penn DL (2011). Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia Research, 132(1), 50–53. 10.1016/j.schres.2011.07.027 [DOI] [PubMed] [Google Scholar]
- Rosenfeld AJ, Lieberman JA, & Jarskog LF (2011). Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophrenia Bulletin, 37(5), 1077–1087. 10.1093/schbul/sbq015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638–641. 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- Schneider LC (1983). SLOF: a behavioral rating scale for assessing the mentally ill. Social Work Research and Abstracts, 19(3), 9–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.