Abstract
Omega-3 treatment studies for multi-episode schizophrenia or clinical high risk for conversion to psychosis states have had variable, and often negative, results. To examine adjunctive omega-3 treatment for recent onset psychosis, participants aged 15 - 40 years with recent onset schizophrenia-spectrum (n=46) or bipolar (n=4) disorders and current psychotic symptoms were treated for 16 weeks with risperidone and randomly-assigned omega-3 (EPA 740 mg and DHA 400 mg daily) or matching placebo. The primary outcome measure was the Brief Psychiatric Rating Scale (BPRS) total score. Mean lifetime antipsychotic exposure was 18.1 days. Length of time in treatment, risperidone dose and number of omega-3/placebo capsules taken did not differ between conditions. Longitudinal analysis of the total BPRS score revealed a trend level (p=0.0826) treatment effect favoring omega-3 treatment. Lorazepam was an allowed concomitant medication. Among the subgroup (N=23) who did not receive lorazepam, the treatment effect on BPRS total scores favoring omega-3 was significant (p=0.0406) and factor scores analyses revealed a substantial decrease in depression-anxiety with omega-3 but no change with placebo (treatment-by-time interaction, p=0.0184). Motor side effects did not differ between conditions. Analysis of Systematic Assessment for Treatment Emergent Events assessments revealed fewer adverse events overall with omega-3 compared with placebo with the largest differences between conditions (all favoring omega-3) on confusion, anxiety, depression, irritability, and tiredness/fatigue. These results suggest that omega-3 adjuvant treatment is a potential option for depression and anxiety symptoms of people with recent onset psychosis. Further research is needed to confirm this potential.
Keywords: omega-3, recent onset psychosis, risperidone, adjuvant treatment, depression, anxiety
Introduction
The limitations of current antipsychotic medications supports efforts to develop additional psychosis treatment strategies. The rationale for examining omega-3s as psychosis treatments includes the evidence that omega-3 polyunsaturated fatty acid levels are lower in patients with schizophrenia compared to healthy volunteers (Assies et al., 2001; Yao et al., 2002; Khan et al., 2002; Arvindakshan et al., 2003; Evans et al., 2003; McNamara et al., 2007; Sethom et al., 2010; van der Kemp et al., 2012), including patients who are antipsychotic drug-naïve (Reddy et al., 2004), and evidence from rodent studies that developmental omega-3 insufficiency leads to enduring abnormalities in neurochemical systems associated with the pathophysiology of schizophrenia, including glutamate (McNamara et al., 2017) and dopamine (Zimmer et al., 2002).
Results with multi-episode patients have been variable with studies finding positive omega-3 effects (Peet et al., 2001; Emsley et al., 2002; Jamilian et al., 2014), no effect (Fenton et al., 2001; Peet et al., 2002; Emsley et al., 2006) or less improvement (Bentsen et al., 2013) compared with placebo treatment. At the opposite end of the illness continuum, an initial study (Amminger et al., 2010) raised the possibility of preventing psychosis onset among high risk individuals with omega-3 treatment but the subsequent larger NEURAPRO (McGorry et al., 2017) and NAPLS studies (Cadenhead et al., 2017) found no effect.
Results with first-episode populations have been more positive (Chen et al., 2015). Peet and colleagues (Peet et al., 2001) found better outcomes with first-episode patients (N=30) treated with omega-3 monotherapy versus placebo. Berger and colleagues (Berger et al., 2007) found no advantage for omega-3 supplementation of antipsychotics with their broadly defined psychosis sample (N=69), but among participants with non-affective psychoses found a trend for faster response time and higher response rates with omega-3 treatment. Pawelczky and colleagues (Pawełczyk et al., 2016) randomized 71 patients with first-episode schizophrenia to 24 weeks of treatment with antipsychotics and either 1.32 g/day of EPA plus 0.88g/day of DHA or placebo. Significantly more improvement with omega-3 was found on the PANSS (Kay et al., 1987) total score and the general symptoms subscale but not on the positive or negative subscales. Better scores on the Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., 1992) were also found.
One possibility for the variable omega-3 psychosis trial results is that omega-3s may be helpful for only subgroups of psychosis patients. We present the clinical outcomes of an initial, exploratory study to further understanding of whether there are subgroups of patients with psychosis who respond to omega-3 treatment. Mediator and moderator analyses with omega-3 levels and imaging measures will be presented separately. The primary clinical hypothesis tested was that patients with recent onset psychosis treated with risperidone and omega-3s would have greater reductions in Brief Psychiatric Rating Scale (BPRS) (Woerner et al., 1988) total scores than patients treated with risperidone and placebo. Total scale scores were chosen as a broad symptom measure to detect effects with an adjuvant to active antipsychotic study design. Risperidone was chosen as the antipsychotic as it has been widely studied with recent onset patients (e.g. (Schooler et al., 2005; Robinson et al., 2006; McEvoy et al., 2007; Robinson et al., 2015a)), is frequently used in clinical practice for this patient group (Robinson et al., 2015b, 2018) and may augment omega-3 biosynthesis and membrane composition (McNamara et al., 2009).
Experimental Materials and Methods
Setting:
Participants were recruited from a large acute care not-for-profit psychiatric facility serving a diverse patient population from the New York City area. The study was conducted under the guidance of the Northwell Health Institutional Review Board and the Data Safety and Monitoring Board of The Zucker Hillside Hospital. Data were collected from June 2013 to September 2015.
Inclusion Criteria were: (1) current DSM-IV-defined diagnosis of schizophrenia, schizophreniform, schizoaffective disorder, psychosis NOS or bipolar 1 disorder; (2) does not meet DSM-IV criteria for a current substance-induced psychotic disorder, a psychotic disorder due to a general medical condition, delusional disorder, brief psychotic disorder, shared psychotic disorder, or major depressive disorder with psychotic features; (3) current positive symptoms rated ≥4 (moderate) on one or more of these BPRS items: conceptual disorganization, grandiosity, hallucinatory behavior, unusual thought content; (4) early phase illness as defined by having taken antipsychotic medications for a cumulative lifetime period of 2 years or less, (5) age 15 to 40 years; (6) competent and willing to sign informed consent; and (7) for women, a negative pregnancy test and agreement to use a medically accepted birth control method.
Exclusion Criteria were: (1) serious neurological or endocrine disorder or any medical condition or treatment known to affect the brain; (2) requiring treatment with a medication with psychotropic effects; (3) significant risk of suicidal or homicidal behavior; (4) cognitive, language or other limitations that would preclude participants providing informed consent; (5) medical contraindications to risperidone treatment (e.g. neuroleptic malignant syndrome with prior risperidone exposure), omega-3 supplements (e.g. bleeding disorders) or placebo capsules (e.g. allergies to capsule components); (6) lack of response to a prior adequate risperidone trial; and 7) taking omega-3 supplements.
Consent:
After a complete study description, participants aged 18 years or older provided written informed consent before initiation of study procedures. Participants younger than 18 provided written assent and their parents/guardians written consent.
Randomization to omega-3 or placebo treatment:
Prior to study initiation, the study statistician (M.J.) developed a computer-generated randomization list. Randomization to omega-3 and placebo was on a 1:1 basis stratified by sex and length of antipsychotic exposure (< versus ≥ 16 weeks). Participants and staff obtaining consent, providing treatment or performing assessment were masked to the randomization.
Treatment:
Treatment lasted 16 weeks based upon data that this is the optimal trial length with risperidone treatment of recent onset patients (Gallego et al., 2011). All participants received open-label flexibly-dosed risperidone. The initial dosing schedule was: 1 mg qhs days 1-3; 2 mg qhs on days 4-6 and 3 mg on day 7. The target dose was 3 mg daily but doses up to 6 mg daily were allowed if needed. Dose reductions were permitted for participants with side effects that did not improve with the allowed adjuvant medications. With participants taking antipsychotics at study entry, the prior antipsychotic was terminated and risperidone initiated as above. Allowed concomitant medications were: benztropine mesylate; lorazepam, propranolol, and if lorazepam was contraindicated for insomnia treatment, zolpidem or rozerem. These were used sparingly and discontinued as soon as possible.
Omega-3 or placebo treatment:
Omega-3 capsules and matching placebo (a soybean/corn blend) was provided by Ocean Nutrition Canada. Each omega-3 capsule contained 370 mg EPA and 200 mg DHA as well as 2 mg/g tocopherol. Both capsule types were identically colored and flavored with natural lemon-lime, to mask them. Participants took one randomized capsule in the morning and one capsule in the evening (i.e. the total daily dose for omega-3 participants was 740 mg of EPA and 400 mg of DHA) beginning on study day 1 and continuing until study completion. The dose was chosen to be comparable to that used in the NAPLS clinical high risk study. Participants were asked to not take omega-3 supplements from other sources or change their normal dietary patterns during the trial.
Clinical Assessments relevant to this report:
Research visits occurred at baseline, week 1, 2, 3, 4, 6, 8, 10, 12, 14 and 16. Diagnosis (baseline): the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-I/P) (First et al., 1994); Psychopathology (all visits): the Brief Psychiatric Rating Scale – Anchored version (BPRS-A) (Woerner et al., 1988), Schedule for Assessment of Negative Symptoms (SANS) Hillside Clinical Trials Version (Robinson et al., 2000) and Clinical Global Impressions Scale (CGI) (Guy and Bonato, 1976); General Adverse Events (all visits): Modified Systematic Assessment for Treatment Emergent Events (Specific Inquiry (SAFTEE-SI) (Levine and Schooler, 1986); Motor Side Effects: Simpson-Angus Scale for EPS (Simpson and Angus, 1970) Hillside version and Barnes Akathisia Scale (Barnes, 1989) at all visits; Simpson Dyskinesia (Simpson et al., 1979) at baseline and study end; Vital Signs (all visits); Metabolic Indices (baseline and study end): hemoglobin A1C, cholesterol (HDL, LDL, total), triglycerides; Pregnancy testing at baseline and study end for female participants of child bearing potential; Erythrocyte fatty acid composition (baseline, week 8 and week 16).
Statistical Analysis:
Distributions of all variables were inspected using histograms, q-q plots and Shapiro-Wilks tests before conducting statistical analysis. Differences in patient characteristics between groups were examined using chi-square analysis for categorical variables, and independent samples t-test or Wilcoxon rank sum test for continuous variables. The primary outcome for the study was the total score on the BPRS measured at baseline and at each of the follow-up time-points in the study. We assumed that the missing data mechanism was Missing At Random (MAR). Intent to treat analysis with a mixed models approach with a random intercept was the analysis strategy for primary and secondary outcomes.
Secondary analyses:
For hypothesis generation, we examined several outcomes relevant to evaluating omega-3 treatment: BPRS factor and SANS global scores to characterize more specific treatment effects than those assessed with the BPRS total score; potential metabolic effects of omega-3 treatment and adverse events with treatment. As these were exploratory analyses, no adjustments for multiple comparisons were conducted (Savitz and Olshan, 1995).
Results
Participants:
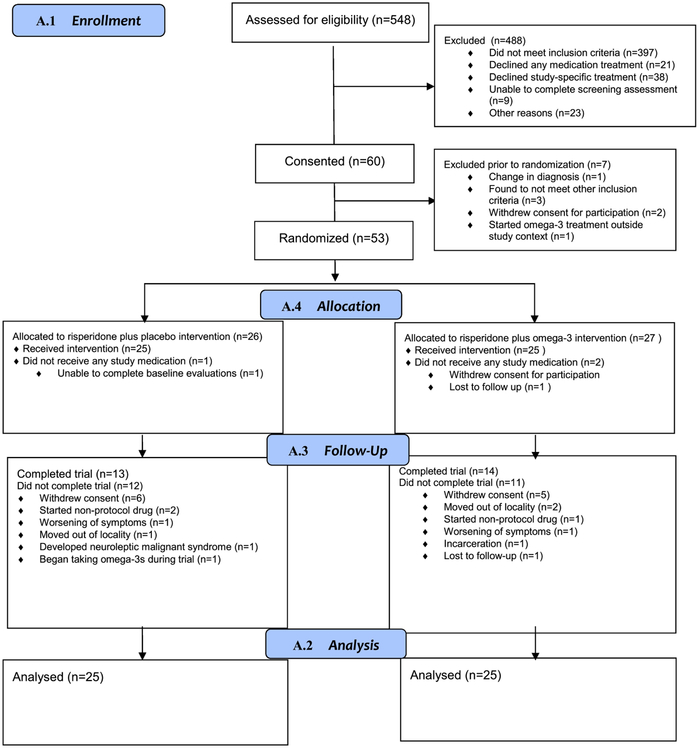
Figure 1 presents participant flow. The sample included 50 participants, equally assigned to omega-3 or placebo. Participants across conditions did not differ on any of the demographic and clinical characteristics presented in Supplemental Table 1. Participants were mostly male with a mean age in the early twenties. Forty-six participants had schizophrenia-spectrum disorders and 4 had bipolar 1 disorder with psychotic features. Six placebo participants had a lifetime history of an alcohol use disorder and 11 had a lifetime history of a cannabis use disorder; the corresponding numbers for omega-3 participants was 3 and 9. One placebo participant had comorbid panic disorder and one omega-3 participant had an anxiety disorder not otherwise specified; the remainder no anxiety diagnoses. The mean lifetime days of antipsychotic treatment prior to study entry was 18.1 (SE=6.326) and the median number of days was 3.0. 84% of participants had taken antipsychotics for 14 days or less in their lifetime.
Figure 1.
Study Progression
Treatment received:
Mean time in controlled treatment was 11.04 (SD=6.67) weeks with placebo and 11.16 (SD=6.72) weeks with omega-3 (t = −0.0634, df = 47.996, p-value = 0.9497). Supplemental Table 2 presents the number of participants at each study week. Longitudinal analyses of risperidone daily dose (averaged separately for each treatment week) revealed an effect of time (F=2.34, df=16, 464, p=0.0025) but no treatment-by-time interaction (F=0.88, df=16,464, p=0.5925) or effect of treatment condition (F=0.10, df=48.9, p=0.7588). Least squares means estimates of risperidone average mg daily dose across the trial was 3.4634 (SE=0.2481) for placebo and 3.3533 (SE=0.2559) for omega-3 treatment. There were no significant treatment-by-time, time or treatment effects in analysis of the average number of masked omega-3/placebo capsules taken based upon patient report during each study week. Least squares means estimates of the daily masked omega-3/placebo capsules taken over the trial course was 1.6973 (SE=0.07688) for placebo and 1.7705 (SE=0.07890) for omega-3 treatment (p=0.5107). Consistent with the actual ingestion of the omega-3 supplements, longitudinal analyses of the EPA + DHA percentage of total erythrocyte fatty acids (Supplemental Figure 1) revealed a significant treatment-by-time interaction (F=26.62, df=2, 42.3, p<0.0001) with greater percentages at week 8 (p=0.0001) and week 16 (p<0.0001) but not at baseline (p=0.4858) with omega-3 compared with placebo assigned participants. Estimated mean percentages at baseline and week 16 were: 4.3840 (SE=0.229) and 4.6946 (SE=0.2870) with placebo and 4.0994 (SE=0.3090) and 6.7441 (SE=0.3923) with omega-3.
Rates of concomitant medications for motor side effects did not differ between conditions but analyses of lorazepam daily dose (averaged over each treatment week) revealed a treatment-by-time interaction (F=2.98, df=16, 396, p=0.0001). Lorazepam average dose was significantly lower in the placebo condition at weeks 1 (p<0.0007) and 2 (p=0.0012) and at a trend level at baseline (p=0.0984) and week 3 (p=0.0588).
BPRS Total Score Outcomes
Table 1 presents outcomes for the entire sample. Because lorazepam may affect some symptoms (e.g. anxiety) and the potential that differential lorazepam use early in the study might have carryover effects on later outcomes, separate analyses are also presented for participants who received (N=27) or did not receive (N=23) any lorazepam during the trial. As expected with risperidone treatment, scores for all 3 groups decreased significantly. With the entire sample, there was a trend level (p=0.0826) treatment effect favoring omega-3 (Supplemental Figure 2). Among participants who did not receive lorazepam, a significant treatment effect (p=0.0406) and a trend level treatment-by-time interaction (p=0.08676) was revealed favoring omega-3. BPRS scores were significantly lower among omega-3 compared to placebo-treated participants at weeks 14 and 16 and at a trend level at weeks 3 and 12 (Supplemental Table 3). Mean scores decreased by approximately 8.5 points with placebo and 20 points with omega-3. Among participants who received lorazepam, mean score change was very similar between treatment conditions.
Table 1.
Least Squares Means Estimates of Brief Psychiatric Rating Scale (BPRS) Total Scores Over Time
| Placebo | Omega 3 | Treatment-by-time interaction | Effect of Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F | df | df | P | F | df | df | p | |
| Entire sample (N=50)1 | ||||||||||||
| Baseline | 42.3200 | 1.5717 | 41.8000 | 1.4485 | 0.65 | 10 | 312 | 0.7728 | 3.03 | 1 | 312 | 0.0826 |
| week 16 | 28.8419 | 2.0808 | 24.0912 | 1.8734 | ||||||||
| Restricted to participants who did not receive lorazepam (N=23)2 | ||||||||||||
| Baseline | 42.0833 | 2.2528 | 43.6364 | 2.0395 | 1.71 | 10 | 117 | 0.0867 | 4.29 | 1 | 117 | 0.0406 |
| week 16 | 33.5130 | 3.6285 | 23.8372 | 2.5976 | ||||||||
| Restricted to participants who received lorazepam at any point (N=27)3 | ||||||||||||
| Baseline | 42.5385 | 2.1782 | 40.3571 | 2.0063 | 0.33 | 10 | 175 | 0.9721 | 0.81 | 1 | 175 | 0.3691 |
| week 16 | 26.5460 | 2.5505 | 23.7965 | 2.6232 | ||||||||
effect of time, F=24.36, df=10, 312; p<0.0001
effect of time, F=9.85, df=10, 117; p<0.0001
effect of time, F=17.14, df=10, 175; p<0.0001
General Adverse Events:
Supplemental Table 4 presents data from the entire sample on the presence of each adverse event assessed by the SAFTEE. Rates were greater among placebo-treated compared with omega-3 treated participants for 56 (61.1%) of the 86 adverse event types elicited, the same for 3 (3.5%) and less for 27 (31.4%). The adverse events with the five largest differences in rates between conditions were confusion, anxiety, depression, irritability and tiredness/fatigue; all were less among omega-3 compared with placebo treated participants.
Metabolic Outcomes:
Analyses with the entire sample are presented in Table 2. There were no significant treatment-by-time interactions or significant treatment effects for weight, BMI, LDL cholesterol, triglycerides, hemoglobin A1c or fasting glucose. There was a significant (p=0.0446) treatment effect for total cholesterol. Total cholesterol was significantly lower (F=7.23, df=1,57, p=0.0094) at baseline among omega-3 compared with placebo participants but levels did not differ at week 16 (F=1.22, df=1,71, p=0.2728). There was a trend level (p=0.0738) treatment effect for HDL cholesterol but the difference between baseline and week 16 for both groups was very small and not significant (effect of time, F=0.18, df=1,29.4, p= 0.6711) and there were no significant differences between treatment groups at either baseline or week 16.
Table 2:
Metabolic Outcomes, Entire Sample (N=50 at baseline)
| Placebo | Omega 3 | Treatment-by-time interaction1 |
Effect of treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F | df | df | p | F | df | df | p | ||
| Weight (kg)1 | 1.27 | 10 | 305 | 0.2442 | 0.81 | 1 | 48 | 0.3725 | |||||
| Baseline | 70.3884 | 3.3990 | 73.7813 | 3.3883 | |||||||||
| week 16 | 75.7992 | 3.4317 | 82.0922 | 3.4063 | |||||||||
| Body Mass Index2 | 1.47 | 10 | 311 | 0.1493 | 1.86 | 1 | 47.8 | 0.1795 | |||||
| Baseline | 23.1327 | 0.9576 | 24.6226 | 0.9546 | |||||||||
| week 16 | 24.8618 | 0.9685 | 27.3169 | 0.9612 | |||||||||
| Total Cholesterol (mg/dL)3 | 1.74 | 1 | 29.7 | 0.1976 | 4.24 | 1 | 51.5 | 0.0446 | |||||
| Baseline | 160.48 | 5.9037 | 136.44 | 6.7137 | |||||||||
| week 16 | 175.75 | 6.8789 | 163.91 | 8.2170 | |||||||||
| LDL Cholesterol (mg/dL)4 | 2.59 | 1 | 26.6 | 0.1191 | 2.24 | 1 | 50.6 | 0.1405 | |||||
| Baseline | 88.8400 | 5.5127 | 71.3600 | 5.9703 | |||||||||
| week 16 | 97.7130 | 6.1093 | 91.1811 | 6.9516 | |||||||||
| HDL Cholesterol (mg/dL) | 0.24 | 1 | 29.4 | 0.6277 | 3.33 | 1 | 51.5 | 0.0738 | |||||
| Baseline | 57.1200 | 2.7542 | 50.8000 | 2.8654 | |||||||||
| week 16 | 58.6912 | 3.1409 | 50.6954 | 3.3087 | |||||||||
| Triglycerides (mg/dL)5 |
0.21 | 1 | 27.7 | 0.6489 | 0.04 | 1 | 48.5 | 0.8383 | |||||
| Baseline | 73.1200 | 8.9376 | 71.1200 | 10.3982 | |||||||||
| week 16 | 97.4367 | 11.9673 | 104.62 | 13.6578 | |||||||||
| Hemoglobin A1c | 0.80 | 1 | 37.6 | 0.3780 | 0.30 | 1 | 38.8 | 0.5896 | |||||
| Baseline | 5.3560 | 0.04553 | 5.7800 | 0.3512 | |||||||||
| week 16 | 5.4639 | 0.06126 | 5.3627 | 0.4693 | |||||||||
| Fasting Glucose (mg/dL) | 1.02 | 1 | 41.7 | 0.3188 | 0.05 | 1 | 52.8 | 0.8230 | |||||
| Baseline | 82.8000 | 2.5621 | 85.8000 | 2.0099 | |||||||||
| week 16 | 87.2541 | 3.4723 | 85.6240 | 2.5355 | |||||||||
effect of time, F=37.52, df=10,305, p<0.0001
effect of time, F=39.46, df=10, 311, p<0.0001
effect of time, F=21.32, df=1, 29.7, p<0.0001
effect of time, F=17.82, df=1, 26.6, p=0.0003
effect of time, F=8.40, df=1, 27.7, p=0.0072
Motor Outcomes With the Entire Sample:
Simpson-Angus total scores increased over time (F=3.69, df=10,357, p=0.0001) but there was no significant treatment-by-time interaction (F=0.65, df=10,357, p=0.7666) or treatment effect (F=0.16, df=1,50.9, p=0.6866). There was no difference between conditions in the rate of Parkinsonism (log-rank Chi-square = 0.3002, p=0.5838). Analyses of Barnes Akathisia Scale global scores revealed no significant time (F= 1.48, df=16,335, p=0.1061), treatment condition (F=0.76, df=1, 82, p=0.3869) or treatment-by-time interaction (F=0.81, df=15,338, p=0.6624) effects.
BPRS Factors and SANS Global Scores:
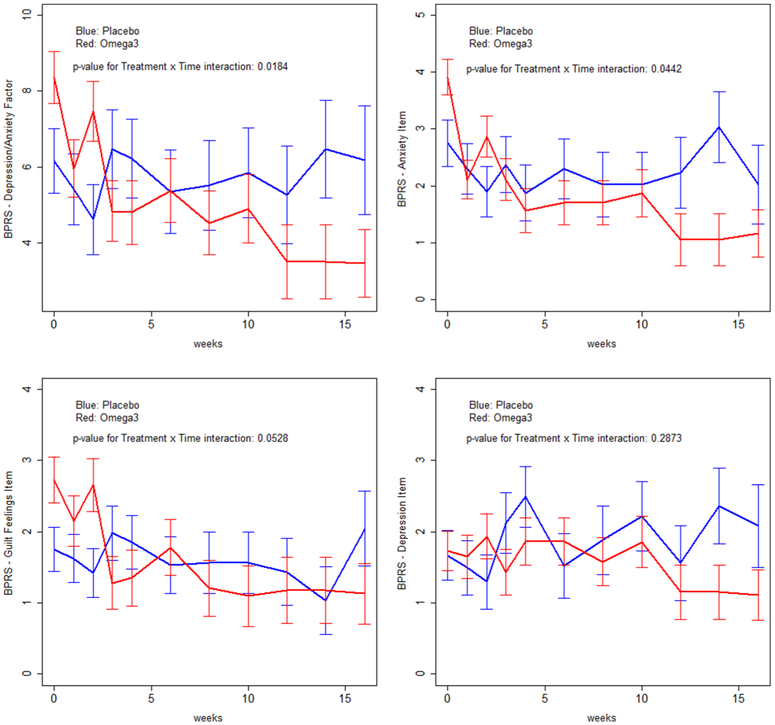
BPRS total scores are a broad symptom measure. To explore whether omega-3 treatment had specific effects, we analyzed BPRS factor scores (Ventura et al., 2000) and SANS global scores. These analyses were performed with data from participants who did not receive lorazepam as omega-3 effects on BPRS total scores were most prominent among this group. These (Table 3) revealed a significant treatment-by time interaction (P=0.0184) favoring omega-3 on the BPRS depression-anxiety factor; mean scores were very similar at baseline (6.1667 (SE=0.85100) and week 16 (6.1801 (SE=1.4345)) with placebo but decreased substantially (from 8.3636 (SE=0.6849) to 3.4542 (SE=0.8983)) with omega-3. Analysis revealed a trend level treatment effect (P=0.0945) on the SANS asociality/anhedonia global score favoring omega-3. Figure 2 presents in graph format analyses of the depression-anxiety factor and its BPRS item components. A significant treatment-by-time interaction was found for anxiety (F=1.96, df=10,117, p=0.0442) and at a trend level (F=1.89, df=10,117, p=0.0528) for guilt favoring omega-3 treatment. Graph inspection suggests a signal for omega-3 effects on depressed mood but there were no significant treatment-by-time or treatment effects in analyses of depressed mood.
Table 3.
Participants Who Did Not Receive Lorazepam (N=23): Least Squares Means Estimates of Brief Psychiatric Rating Scale (BPRS) Factor Scores and Scale for the Assessment of Negative Symptoms (SANS) scores over time
| Placebo | Omega 3 | Treatment-by-time interaction |
Effect of Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | F | Df | df | p | F | df | df | p | ||
| BPRS Factor Scores1 | |||||||||||||
| Negative symptoms | 0.71 | 10 | 117 | 0.7133 | 0.30 | 1 | 117 | 0.5872 | |||||
| baseline | 4.5000 | 0.5733 | 5.3636 | 0.6414 | |||||||||
| week 16 | 5.9250 | 0.9014 | 4.8257 | 0.8326 | |||||||||
| Depression-anxiety | 2.27 | 10 | 117 | 0.0184 | 0.84 | 1 | 117 | 0.3605 | |||||
| baseline | 6.1667 | 0.8510 | 8.3636 | 0.6849 | |||||||||
| week 16 | 6.1801 | 1.4345 | 3.4542 | 0.8983 | |||||||||
| Hostile-uncooperativeness2 | 1.41 | 10 | 117 | 0.1844 | 2.67 | 1 | 117 | 0.1051 | |||||
| baseline | 6.5833 | 0.4304 | 5.9091 | 0.3299 | |||||||||
| week 16 | 5.8269 | 0.7040 | 4.7586 | 0.4120 | |||||||||
| Positive symptoms3 | 0.45 | 10 | 117 | 0.9181 | 0.72 | 1 | 117 | 0.3977 | |||||
| baseline | 12.2500 | 0.8454 | 12.3636 | 0.7569 | |||||||||
| week 16 | 7.2597 | 1.2787 | 5.8353 | 0.9049 | |||||||||
| SANS Global Scores | |||||||||||||
| Affective flattening | 0.76 | 10 | 114 | 0.6690 | 0.70 | 1 | 114 | 0.4050 | |||||
| baseline | 1.5000 | 0.2199 | 1.8182 | 0.2427 | |||||||||
| week 16 | 1.9015 | 0.3202 | 1.9148 | 0.3172 | |||||||||
| Alogia | 1.15 | 10 | 114 | 0.3326 | 0.82 | 1 | 114 | 0.3668 | |||||
| baseline | 1.2500 | 0.1818 | 1.5455 | 0.1374 | |||||||||
| week 16 | 1.5748 | 0.2723 | 1.0313 | 0.1745 | |||||||||
| Avolition/apathy4 | 1.61 | 10 | 114 | 0.1129 | 2.20 | 1 | 114 | 0.1405 | |||||
| baseline | 1.7500 | 0.2729 | 2.0000 | 0.2521 | |||||||||
| week 16 | 2.8167 | 0.4126 | 1.8015 | 0.3332 | |||||||||
| Asociality/anhedonia5 | 1.57 | 10 | 113 | 0.1260 | 2.84 | 1 | 113 | 0.0945 | |||||
| baseline | 1.6667 | 0.2393 | 1.6364 | 0.2469 | |||||||||
| week 16 | 2.3600 | 0.3524 | 1.4822 | 0.3227 | |||||||||
defined in (Ventura et al., 2000)
effect of time, F=2.93, df=10,117; p=0.0026
effect of time, F=13.89, df=10,117; p<0.0001
effect of time, F=2.83, df=10,144; p<=0.0035
effect of time, F=2.20, df=10,113; p=0.0226
Figure 2.
Brief Psychiatric Rating Scale Depression/Anxiety Factor and Component Items Among Participants Who Did Not Receive Lorazepam (N=23)
DISCUSSION
The favorable side effect profile of omega-3s and the high patient acceptability of treatment with a “natural” product suggested studying omega-3s with a broad range of patients with psychosis. Given the usual focus in schizophrenia trials on positive symptoms, it was also natural that these should be the focus of omega-3 trials. However, the variable results from studies with patients with established illness coupled with the inability to replicate initial promising findings with clinical high risk groups suggest that a broad application may not be optimal. With our recent onset population, we found trend level differential improvement with omega-3s in total BPRS scores with the entire sample and significant improvement among the subgroup of participants who did not receive lorazepam. Differences among participants who did not receive lorazepam were substantial (mean baseline versus week 16 scores were 43.6364 vs. 23.8372 with omega-3 and only 42.0833 vs. 33.5130 with placebo). We found several indications that omega-3 effects were most robust on the depression-anxiety domain. Among participants who did not receive lorazepam there was a significant differential improvement in the depression-anxiety factor on the BPRS. Depression-anxiety scores did not change with placebo treatment but decreased from 8.3636 to 3.4542 with omega-3. The SAFTEE assessments, done by different masked raters than the raters for the BPRS, also found less anxiety, depression and related symptoms among omega-3 treated participants. Our results are in broad agreement with those from the study of Pawelczky and colleagues (Pawełczyk et al., 2016). The two studies suggest that recent onset patients maybe a responsive population and that depression-anxiety a prime treatment target.
Adjuvant treatment with benzodiazepines is common with acutely psychotic recent onset patients. Rates of lorazepam use were very similar between our study (64% with omega-3 and 52% with placebo treatment) and that of Pawelczyk and colleagues (69.9% and 51.4%, respectively). Non-random lorazepam assignment raises two possibilities for interpreting our finding of substantial effects with participants who did not receive lorazepam but not with those who did receive lorazepam. First, lorazepam may have improved lorazepam-responsive symptoms among the placebo group to the extent that additional omega-3 beneficial effects were not detectable. This is consistent with the lower 16 week scores for placebo-treated patients who received, compared to those who did not receive, lorazepam. Second, omega-3 treatment may not be beneficial for patients who have the symptom patterns requiring adjuvant lorazepam in our study. Additional research is needed to distinguish between these possibilities.
Expanding treatment options for depression and anxiety among people with psychosis can have public health impact given the frequent occurrence of these symptoms and their deleterious effects on outcome. Prevalence rates of depression in schizophrenia are estimated to be approximately 25% across all illness stages (Buckley et al., 2009) with higher rates (up to 75%) among recent onset populations (Koreen et al., 1993; Häfner et al., 2005). Depression is associated with poor social functioning and quality of life (Sim et al., 2004; Conley et al., 2007), higher rates of relapse and rehospitalization (e.g. (Johnson, 1988); for review see (Olivares et al., 2013)) and is a risk factor for suicidal thoughts and actions (e.g. (Barnes et al., 1989; Fenton, 2000)). Anxiety is also frequent among people experiencing schizophrenia with reported rates of around 40% (Achim et al., 2011; Karpov et al., 2016). Some patients have co-occurring specific anxiety disorders (e.g. obsessive-compulsive disorder) but others have anxiety due to schizophrenia alone and are the type of anxiety symptoms among our study patients with a few exceptions. Higher levels of general anxiety in schizophrenia are associated with poor outcomes including more severe hallucinations, social withdrawal, depression and hopelessness and poorer functioning (Lysaker and Salyers, 2007; Karpov et al., 2016).
We have treatments for co-occurring depression and anxiety in schizophrenia but additional options would be beneficial given the limitations of current therapies. A recent meta-analysis (Helfer et al., 2016) suggested only a small beneficial effect (effect size 0.25) for antidepressants for depression in schizophrenia. Benzodiazepines are often effective for anxiety but chronic benzodiazepine use by people with schizophrenia is associated with up to a 70% higher risk of death compared with no use (Tiihonen et al., 2016). This increased mortality and the reluctance of many clinicians to prescribe benzodiazepines to a patient group with vulnerability to substance abuse suggests the need for more treatment options.
Cardiovascular disease is a primary cause of the excess mortality of people with schizophrenia (Olfson et al., 2015) and dyslipidemias are frequent even early in the illness (Correll et al., 2014). Although our data did not provide compelling evidence for preventative omega-3 effects, our results should be considered in the context of a recent meta-analysis (Nam Gina E. et al., 2017) of omega-3 studies with medical populations demonstrating more triglyceride lowering effects among individuals with high baseline triglyceride levels (as opposed to our population with mean levels in the normal range) and dose dependent effects up to 3.9 gram per day (as opposed to our total 1.1 gram dose). We found a significant treatment effect for total cholesterol. There were significant differences between conditions at baseline (cholesterol lower with omega-3 assigned participants) but not at week 16. Caution is need in interpreting this finding as the treatment effect may simply be due to the baseline differences. Prior omega-3 metabolic studies with antipsychotic-treated participants have been limited. Two investigations (Fetter et al., 2013; Freeman et al., 2015) reported positive effects but other studies have found no omega-3 effects (Emsley et al., 2008; Faghihi et al., 2012) or positive effects on triglycerides but increases in total and LDL cholesterol (Caniato et al., 2006). More research is needed on omega-3 metabolic effects and, if present, their effect on the risk-to-benefit assessment for omega-3s treatment.
Our study has limitations. The sample size precludes making definitive conclusions. We had very few participants with bipolar disorder so the efficacy of omega-3 treatment for this group is unclear. A study strength is that participants had a mean duration of lifetime antipsychotic use of only 18.1 days. Whether omega-3s benefit patients who have had more prolonged treatment is unknown. Our participants were not selected for the presence of depression-anxiety at baseline. The outcome with omega-3 treatment for patients selected for the presence of these symptoms warrants exploration. Finally, further research on dosing of omega-3s for psychosis is needed. Our results are broadly similar to those of Pawelczyk and colleagues who used higher doses than ours. However, more effects might have been obtained with different dosing.
In summary, our data suggest that omega-3 adjuvant treatment has the potential to be effective at improving depression and anxiety symptoms of people with recent onset psychosis and that it is premature to discontinue study of omega-3s for people with psychotic disorders. Further studies are needed to replicate our findings and address the unanswered questions posed above.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of our participants without whom the study would not have been possible. We also acknowledge the contributions of the support staff of our research department for their assistance with all aspects of the study. We also thank our clinical colleagues who facilitated participant referral.
Funding
This work was supported by grants R21MH101746 (Dr. Robinson and Dr. Szeszko) and R01DK097599 (Dr. McNamara) from the National Institutes of Health, Bethesda, Maryland USA and the Empire Clinical Research Investigator Program award Targeting Omega-3 Treatment in First-episode Psychosis from the New York State Department of Health to Dr. Malhotra. The funding sources had no input in the development of this manuscript.
Clinical trial registration: NCT01786239
Footnotes
Conflicts of interest
Dr. Robinson has been a consultant to Costello Medical Consulting, Innovative Science Solutions, Janssen, Lundbeck, Otsuka and US WorldMeds and has received research support from Otsuka. Dr. Zhang has received research support from Genomind Inc and Janssen. Dr. McNamara has received research support from Royal DSM Nutritional Products, LLC, and Kyowa Hakko Bio Co., LTD, and served as a consultant for VAYA Pharma Inc., and Vifor Pharma Inc. Dr. Malhotra has been a consultant to Genomind, Concert Pharma and Biogen.
Dr. Gallego, Dr. John, Dr. Hanna, Dr. Birnbaum, Ms. Greenberg, Ms. Naraine, Dr. Peters and Dr. Szeszko have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy M-A, 2011. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull 37, 811–821. 10.1093/schbul/sbp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E, Joyce J, 1992. Reliability and validity of a depression rating scale for schizophrenics. Schizophr. Res 6, 201–208. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE, 2010. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry 67, 146–154. 10.1001/archgenpsychiatry.2009.192 [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP, 2003. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr. Res 62, 195–204. [DOI] [PubMed] [Google Scholar]
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH, 2001. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol. Psychiatry 49, 510–522. [DOI] [PubMed] [Google Scholar]
- Barnes TR, 1989. A rating scale for drug-induced akathisia. Br J Psychiatry 154, 672–676. [DOI] [PubMed] [Google Scholar]
- Barnes TR, Curson DA, Liddle PF, Patel M, 1989. The nature and prevalence of depression in chronic schizophrenic in-patients. Br J Psychiatry 154, 486–491. [DOI] [PubMed] [Google Scholar]
- Bentsen H, Osnes K, Refsum H, Solberg DK, Bøhmer T, 2013. A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E+C in schizophrenia. Transl Psychiatry 3, e335 10.1038/tp.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger GE, Proffitt T-M, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD, 2007. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry 68, 1867–1875. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ, 2009. Psychiatric comorbidities and schizophrenia. Schizophr Bull 35, 383–402. 10.1093/schbul/sbn135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead K, Addington J, Cannon T, Cornblatt B, Mathalon D, McGlashan T, Perkins D, Seidman LJ, Tsuang M, Walker E, Woods S, 2017. 23. Omega-3 Fatty Acid Versus Placebo in a Clinical High-Risk Sample From the North American Prodrome Longitudinal Studies (NAPLS) Consortium. Schizophr Bull 43, S16–S16. 10.1093/schbul/sbx021.042 [DOI] [Google Scholar]
- Caniato RN, Alvarenga ME, Garcia-Alcaraz MA, 2006. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry 40, 691–697. 10.1080/j.1440-1614.2006.01869.x [DOI] [PubMed] [Google Scholar]
- Chen AT, Chibnall JT, Nasrallah HA, 2015. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann Clin Psychiatry 27, 289–296. [PubMed] [Google Scholar]
- Conley RR, Ascher-Svanum H, Zhu B, Faries DE, Kinon BJ, 2007. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr. Res 90, 186–197. 10.1016/j.schres.2006.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM, 2014. Cardiometabolic Risk in Patients With First-Episode Schizophrenia Spectrum Disorders: Baseline Results From the RAISE-ETP Study. JAMA Psychiatry 71, 1350–1363. 10.1001/jamapsychiatry.2014.1314 [DOI] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ, 2002. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry 159, 1596–1598. 10.1176/appi.ajp.159.9.1596 [DOI] [PubMed] [Google Scholar]
- Emsley R, Niehaus DJH, Koen L, Oosthuizen PP, Turner HJ, Carey P, van Rensburg SJ, Maritz JS, Murck H, 2006. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr. Res 84, 112–120. 10.1016/j.schres.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Emsley R, Niehaus DJH, Oosthuizen PP, Koen L, Ascott-Evans B, Chiliza B, van Rensburg SJ, Smit RM, 2008. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry Res 161, 284–291. 10.1016/j.psychres.2007.06.029 [DOI] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP, 2003. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot. Essent. Fatty Acids 69, 393–399. [DOI] [PubMed] [Google Scholar]
- Faghihi T, Jahed A, Mahmoudi-Gharaei J, Sharifi V, Akhondzadeh S, Ghaeli P, 2012. Role of Omega-3 fatty acids in preventing metabolic disturbances in patients on olanzapine plus either sodium valproate or lithium: a randomized double-blind placebo-controlled trial. Daru 20, 43 10.1186/2008-2231-20-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WS, 2000. Depression, suicide, and suicide prevention in schizophrenia. Suicide Life Threat Behav 30, 34–49. [PubMed] [Google Scholar]
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M, 2001. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 158, 2071–2074. 10.1176/appi.ajp.158.12.2071 [DOI] [PubMed] [Google Scholar]
- Fetter JC, Brunette M, Green AI, 2013. N-3 fatty acids for hypertriglyceridemia in patients taking second-generation antipsychotics. Clin Schizophr Relat Psychoses 7, 73–77A. 10.3371/CSRP.FEBR.012513 [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 1994. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-I/P). [Google Scholar]
- Freeman MP, Mclnerney K, Sosinsky AZ, Kwiatkowski MA, Cohen LS, 2015. Omega-3 fatty acids for atypical antipsychotic-associated hypertriglyceridemia. Ann Clin Psychiatry 27, 197–202. [PubMed] [Google Scholar]
- Gallego JA, Robinson DG, Sevy SM, Napolitano B, McCormack J, Lesser ML, Kane JM, 2011. Time to Treatment Response in First-Episode Schizophrenia: Should Acute Treatment Trials Last Several Months? J Clin Psychiatry. 10.4088/JCP.10m06349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W, Bonato RR, 1976. CGI: Clinical global impressions. ECDEU Assessment Manual for Psychopharmacology, Revised 217–222. [Google Scholar]
- Häfner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Könnecke R, 2005. Schizophrenia and depression: challenging the paradigm of two separate diseases--a controlled study of schizophrenia, depression and healthy controls. Schizophr. Res 77, 11–24. 10.1016/j.schres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Helfer B, Samara MT, Huhn M, Klupp E, Leucht C, Zhu Y, Engel RR, Leucht S, 2016. Efficacy and Safety of Antidepressants Added to Antipsychotics for Schizophrenia: A Systematic Review and Meta-Analysis. Am J Psychiatry 173, 876–886. 10.1176/appi.ajp.2016.15081035 [DOI] [PubMed] [Google Scholar]
- Jamilian H, Solhi H, Jamilian M, 2014. Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Glob J Health Sci 6, 103–108. 10.5539/gjhs.v6n7p103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, 1988. The significance of depression in the prediction of relapse in chronic schizophrenia. Br J Psychiatry 152, 320–323. [DOI] [PubMed] [Google Scholar]
- Karpov B, Joffe G, Aaltonen K, Suvisaari J, Baryshnikov I, Näätänen P, Koivisto M, Melartin T, Oksanen J, Suominen K, Heikkinen M, Paunio T, Isometsä E, 2016. Anxiety symptoms in a major mood and schizophrenia spectrum disorders. European Psychiatry 37, 1–7. 10.1016/j.eurpsy.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13, 261–276. [DOI] [PubMed] [Google Scholar]
- Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP, 2002. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res 58, 1–10. [DOI] [PubMed] [Google Scholar]
- Koreen AR, Siris SG, Chakos M, Alvir J, others, 1993. Depression in first-episode schizophrenia. The American journal of psychiatry 150, 1643. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR, 1986. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull 22, 343–381. [PubMed] [Google Scholar]
- Lysaker PH, Salyers MP, 2007. Anxiety symptoms in schizophrenia spectrum disorders: associations with social function, positive and negative symptoms, hope and trauma history. Acta Psychiatr Scand 116, 290–298. 10.1111/j.1600-0447.2007.01067.x [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD, 2007. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164, 1050–1060. 10.1176/ajp.2007.164.7.1050 [DOI] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Mossaheb N, Schlögelhofer M, Smesny S, Hickie IB, Berger GE, Chen EYH, de Haan L, Nieman DH, Nordentoft M, Riecher-Rössler A, Verma S, Thompson A, Yung AR, Amminger GP, 2017. Effect of ω-3 Polyunsaturated Fatty Acids in Young People at Ultrahigh Risk for Psychotic Disorders: The NEURAPRO Randomized Clinical Trial. JAMA Psychiatry 74, 19–27. 10.1001/jamapsychiatry.2016.2902 [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek R, Rider T, Tso P, 2009. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: evidence for augmented biosynthesis. Schizophr. Res 107, 150–157. 10.1016/j.schres.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Asch RH, Schurdak JD, Lindquist DM, 2017. Glutamate homeostasis in the adult rat prefrontal cortex is altered by cortical docosahexaenoic acid accrual during adolescence: An in vivo 1H MRS study. Psychiatry Research: Neuroimaging 270, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn C-G, Richtand NM, Stanford KE, 2007. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr. Res 91, 37–50. 10.1016/j.schres.2006.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Gina E, Myung Seung-Kwon, Choi Yoon-Jung, 2017. Use of Omega-3 Fatty Acid Supplements Has Insufficient Clinical Evidence for Treatment of Hypertriglyceridemia: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. European Journal of Lipid Science and Technology 119, 1700111 10.1002/ejlt.201700111 [DOI] [Google Scholar]
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS, 2015. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry 72, 1172–1181. 10.1001/jamapsychiatry.2015.1737 [DOI] [PubMed] [Google Scholar]
- Olivares JM, Sermon J, Hemels M, Schreiner A, 2013. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry 12, 32 10.1186/1744-859X-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawełczyk T, Grancow-Grabka M, Kotlicka-Antczak M, Trafalska E, Pawełczyk A, 2016. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J Psychiatr Res 73, 34–44. 10.1016/j.jpsychires.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK, 2001. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr. Res 49, 243–251. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF, E-E Multicentre Study Group, 2002. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 36, 7–18. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK, 2004. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull 30, 901–911. [DOI] [PubMed] [Google Scholar]
- Robinson D, Woerner M, Schooler N, 2000. Intervention research in psychosis: issues related to clinical assessment. Schizophr Bull 26, 551–556. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang J-P, Lopez L, Braga RJ, Sevy SM, Addington J, Kellner CH, Tohen M, Naraine M, Bennett N, Greenberg J, Lencz T, Cornell CU, Kane JM, Malhotra AK, 2015a. A Randomized Comparison of Aripiprazole and Risperidone for the Acute Treatment of First-Episode Schizophrenia and Related Disorders: 3-Month Outcomes. Schizophr Bull 41, 1227–1236. 10.1093/schbul/sbv125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Schooler NR, Correll CU, John M, Kurian BT, Marcy P, Miller AL, Pipes R, Trivedi MH, Kane JM, 2018. Psychopharmacological Treatment in the RAISE-ETP Study: Outcomes of a Manual and Computer Decision Support System Based Intervention. Am J Psychiatry 175, 169–179. 10.1176/appi.ajp.2017.16080919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Schooler NR, John M, Correll CU, Marcy P, Addington J, Brunette MF, Estroff SE, Mueser KT, Penn D, Robinson J, Rosenheck RA, Severe J, Goldstein A, Azrin S, Heinssen R, Kane JM, 2015b. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry 172, 237–248. 10.1176/appi.ajp.2014.13101355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Woerner MG, Napolitano B, Patel RC, Sevy SM, Gunduz-Bruce H, Soto-Perello JM, Mendelowitz A, Khadivi A, Miller R, McCormack J, Lorell BS, Lesser ML, Schooler NR, Kane JM, 2006. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry 163, 2096–2102. 10.1176/ajp.2006.163.12.2096 [DOI] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF, 1995. Multiple comparisons and related issues in the interpretation of epidemiologic data. American journal of epidemiology 142, 904. [DOI] [PubMed] [Google Scholar]
- Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, McGorry PD, Van Hove I, Eerdekens M, Swyzen W, De Smedt G, Early Psychosis Global Working Group, 2005. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry 162, 947–953. 10.1176/appi.ajp.162.5.947 [DOI] [PubMed] [Google Scholar]
- Sethom MM, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, Hechmi Z, Kaabachi N, 2010. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids 83, 131–136. 10.1016/j.plefa.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Sim K, Mahendran R, Siris SG, Heckers S, Chong SA, 2004. Subjective quality of life in first episode schizophrenia spectrum disorders with comorbid depression. Psychiatry Res 129, 141–147. 10.1016/j.psychres.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW, 1970. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212, 11–19. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Lee JH, Zoubok B, Gardos G, 1979. A rating scale for tardive dyskinesia. Psychopharmacology 64, 171–179. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A, 2016. Mortality and Cumulative Exposure to Antipsychotics, Antidepressants, and Benzodiazepines in Patients With Schizophrenia: An Observational Follow-Up Study. Am J Psychiatry 173, 600–606. 10.1176/appi.ajp.2015.15050618 [DOI] [PubMed] [Google Scholar]
- van der Kemp WJM, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE, 2012. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr. Res 141, 153–161. 10.1016/j.schres.2012.08.014 [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA, 2000. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res 97, 129–135. [DOI] [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM, 1988. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 24, 112–117. [PubMed] [Google Scholar]
- Yao J. k, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW, 2002. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol. Psychiatry 52, 823–830. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S, 2002. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr 75, 662–667. 10.1093/ajcn/75.4.662 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.