Abstract
Purpose:
To determine the feasibility of employing telemedicine consultations in the evaluation of recovered donor corneas for transplant suitability.
Methods:
This study aims to establish and test the minimum imaging requirements for telemedical consultations of corneal tissue by remote eye bank medical directors. Digital images from the slit lamp, optical coherence tomography, and/or specular microscope were assembled into telemedical consults and emailed to 4 eye bank medical directors (MAT, JW, CSS, NKR). Surgeon feedback on the minimum image requirements for each corneal finding was collected. After establishing a standardized imaging and presentation protocol, test cases were presented to the medical directors to examine the validity of these remote consults. To establish a benchmark for the study’s parameters, one medical director (JW) examined each case in-person after his initial remote review. Examiners were masked to each other’s responses.
Results:
Minimum image requirements for determination of corneal findings were defined and were specific to each anatomic layer of the cornea (epithelial, stromal, or endothelial). Using a defined set of digital images for a set of common corneal findings, the rate of agreement between remote evaluators, eye bank staff, and the in-person evaluator was 100% (11 out of 11 examples). For ambiguous test cases, all remote evaluators agreed on 80% of the cases (4 out of 5).
Conclusions:
Results from this pilot study suggest that telemedical review of corneal tissue using high quality digital images may be adequate for accurate identification of specific corneal findings commonly encountered by eye banks.
Keywords: cornea, eye banking, telemedicine, tissue evaluation, remote consultation, slit lamp, specular microscopy
INTRODUCTION
Applications of ophthalmic telemedicine have become increasingly diverse. Studies have established the use of telemedicine in the evaluation of a range of ocular diseases.1 Most notably, numerous studies carried out in the United States and abroad have demonstrated the utility of telemedicine approaches in screening for diabetic retinopathy.2–11 Additional work has established an increasing role for a telemedical approach in the monitoring of retinopathy of prematurity,12–14 and other recent studies have assessed the potential utility of telemedicine in remote evaluation of neovascular age-related macular degeneration15,16 and glaucoma.17–19 Relatively fewer studies have focused on applications of telemedicine in detection of anterior segment disease. Some investigations have validated the use of the video slit lamp or digital slit lamp images in remote evaluation of anterior segment eye diseases,20,21 and others have relied on images obtained from portable cameras or smartphone camera attachments for remote evaluation of corneal disease.22–24 To our knowledge, no prior study has described the use of telemedicine in eye bank tissue evaluation.
The use of eye bank technology in telemedical evaluation of corneas provide several exciting advantages. The use of telemedicine in eye banking has the potential, for example, to increase the efficiency of consultations between eye bank staff and medical directors. The logistics of physical transfer of corneas from the eye bank to the medical director consultant’s office is often inconvenient for the eye banker and medical director alike, and the busy and sometimes unpredictable schedules of medical directors can compromise the timely completion of consults. These factors result in incomplete consults and unnecessarily discarded corneal tissue. In addition, the need to travel to present tissue to the medical director results in additional costs incurred by eye banks. Finally, for telemedical consults eye banks and consulting medical directors are not confined by geographical distance, thus the successful application of telemedicine in eye banking has the potential to not only improve consult turnaround time but can also expand access to include feedback from multiple, remote medical directors.
In the present study, we aim to establish a standardized approach for the application of telemedicine in the evaluation of corneal tissue for transplantation at eye banks. We delineate the minimum image requirements (including the types and quality) of images required for successful remote detection of common findings in donor corneas.
METHODS
Image acquisition and equipment
Digital slit lamp images (including slit beam, diffuse lighting, and back lighting) were collected using a Haig-Streit BX900 slit lamp equipped with a Canon EOS 30D digital SLR camera (Haig-Streit Diagnostics, Mason, OH. USA). Optical coherence tomography (OCT) images were captured on an Optovue RTVue100 (Optovue Inc., Fremont, CA. USA). Specular images were acquired using either a Konan KSS-EB10 or CellChekD+ specular microscope (Konan USA, Irvine, CA. USA). Some back lit images were acquired using the ‘Finder View’ mode on the CellChekD+ specular microscope.
Defining an imaging protocol
Eye bank technicians initially established imaging requirements for different layers of the cornea (Online Table 1). Sample images for common corneal findings were obtained using this initial protocol, assembled using Microsoft PowerPoint (Microsoft Corporation, Redmond, WA. USA), and emailed to 4 medical directors of Lions VisionGift (LVG) (CSS, MAT, JW, NKR) for feedback. Each image set included any combination of three slit lamp views (diffuse, slit beam, or retroillumination), OCT, and/or specular microscopy images as appropriate for the anatomic location (corneal layer) of the finding. Technicians were intentionally selective about which modalities were used to provide medical directors only the most relevant information for identifying the finding. For example, a specular image of the endothelium would not be included in a consult for an epithelial or stromal feature (Table 1). Medical director feedback was sought to establish the minimum imaging requirements for each finding (Table 1 and Supplemental Table 1).
Table 1.
Refined Image Requirements for Remote Evaluation of Specific Corneal Features*
| Corneal Finding | Slit Lamp (Diffuse View) |
Slit Lamp (Slit Beam) |
Slit Lamp (Backlit View) |
OCT | Specular Microscopy |
|---|---|---|---|---|---|
| Epithelium | |||||
| Exposure | Y | Y | N | Y | N |
| Stroma | |||||
| IOL scars | Y | Y | N | Y | N |
| LASIK scar | Y | Y | N | Y | N |
| Folds | Y | Y | N | Y | Y |
| Arcus | Y | Y | N | N | N |
| Opacity | Y | Y | Y | Y | N |
| Infiltrate | Y | Y | Y | Y | N |
| Pterygium# | Y | Y | N | Y | N |
| Endothelium | |||||
| Guttae | Y | N | N | Y+ | Y |
| Endothelial stress | Y | Y | Y | N | Y |
| Other | |||||
| Sutures | Y | Y | Y | Y | N |
The minimum image requirements required for remote evaluation of tissues as established in early study stages with feedback from medical directors. Y: yes, image included for telemedical assessment; N: no, image not included for telemedical assessment
Pterygium was group with stromal findings because it is possible for pterygium to extend into this layer. Therefore, the imaging requirements for pterygium is similar to other stromal findings.
Although OCT images are included for guttae, they may not always review the presence of guttae and should not be used as a substitution for any of the other two modalities.
Format of telemedical consults
Appropriate images were assembled into telemedical consults using Microsoft PowerPoint and emailed to the same medical directors above. Consults were put together by one of four trained eye bank technicians who routinely evaluate tissue for surgical suitability and can identify when a medical director consult is needed. The format of the PowerPoint file is as follows: several slides containing images of the finding captured by an eye bank technician followed by a blank buffer slide so that the medical director knows the image presentation is over and they can have the opportunity to make their own assessment of the presented finding. On the final slide, evaluators were asked a) if they agreed with the finding determined by eye bank staff, b) whether images provided were sufficient for making the remote determination, and c) if the images were not adequate for making the determination, what additional images each evaluator would require. No other information (i.e. donor medical history and tissue specifications) was provided. To establish a benchmark for the study’s parameters, one medical director (JW) assessed each sample in-person four to five days after his initial remote review. All medical directors were masked to each other’s findings.
Telemedical consult pilot studies
Digital images (of findings routinely encountered and identified by eye bank technicians) were collected and distributed to evaluators as delineated above. Medical directors were asked if, based on the images provided, they agreed with the eye bank technician’s assessment. The corneas used in these remote consults were then examined in-person by one of the medical directors (JW) 4 to 5 days later. We purposely waited this amount of time so that it would be more difficult for the medical director to connect the in-person consult to a specific set of digital images to minimize bias. Corneas were re-examined at the eye bank on the day of the in-person consult to ensure that the feature in question has not changed during the elapse 4–5 days in storage. It is our eye bank’s standard operating procedure to take the medical director’s in-person consult findings as the final determination used for tissue suitability.
To test the effectiveness of telemedical consults for eye bank tissue evaluation, 5 ambiguous cases were consulted. In these cases, eye bank technicians were unable to determine if the stromal finding was an infiltrate (a rule-out for transplantation) or focal edema (not a rule-out for transplantation). Remote and in-person consults were performed as described above.
RESULTS
Evaluators’ feedback included requests for higher resolution images, improved focus in slit lamp images, and highlighting the finding with an arrow or circle for each consult image. Based on this feedback and with accumulated experience throughout the course of the study, eye bank technicians were able to improve their image acquisition techniques to clearly represent the features they aim to capture in the image. Minimum image requirements were defined based on the anatomic location of each corneal finding (Table 1).
A total of eleven donor corneal findings were assessed remotely by four medical directors. Evaluators were consulted on common corneal findings—including epithelial exposure and incision scars (N=2), stromal features (N=7), and endothelium morphology (N=2). Remote evaluators agreed with eye bank evaluators’ assessments on all 11 examples (Table 1). There was 100% agreement between the remote consults and in-person consults.
In ambiguous cases of infiltrate vs. edema, there was agreement between all remote and in-person evaluations for 4 out of 5 cases. In the fifth case, 2 out of 3 remote consultants determined that the features shown represented an infiltrate, while the third determined that the feature was ‘mucous plaque with focal edema’. The in-person consult confirmed the observation of focal edema.
When assessing whether a finding is an infiltrate versus local edema, a focused image is critical (Fig. 1). One distinct feature that is taken into consideration during the examination of an infiltrate is the sharp transition border between the infiltrate and the native stroma (Fig. 1 C-D). A ‘fuzzy’ border would suggest that the feature is local edema, which is not a rule out for transplantation. Likewise, for consults surrounding endothelial pathology, a high-quality specular image can help the medical director determine whether dark areas on the endothelium are areas within folds of an edematous cornea or if they are indicative of a more serious condition such as Fuchs’ corneal dystrophy (Fig. 2). In the case of Fuchs’ dystrophy described in this study (likely advanced Fuchs’) bright ‘specs’ can also be seen on the Descemet’s membrane (Fig. 2).
Figure 1.

Sample image set for a stromal infiltrate. The dashed outline, used during telemedical consults, indicates the area of the feature that is being consulted.
A) Slit lamp image using diffuse illumination.
B) Slit lamp image using retro-illumination (back lighting).
C) Slit lamp image using narrow slit beam.
D) OCT image showing stromal ingression of the infiltrate.
Figure 2.
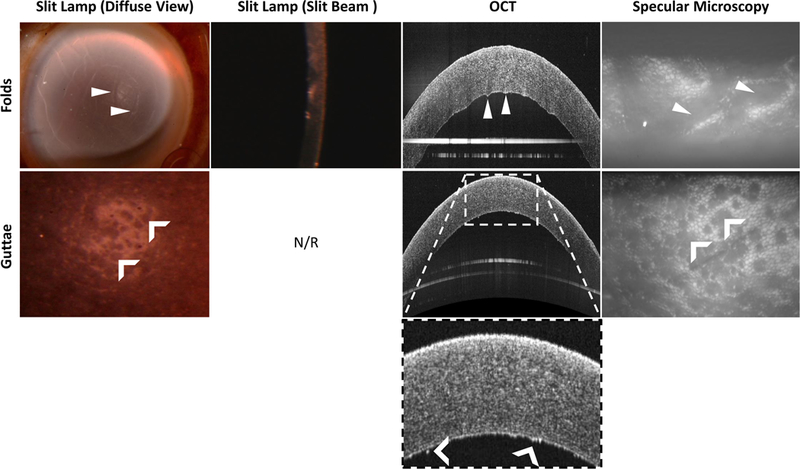
Example of how different imaging modalities can be used to distinguish between stromal folds (top row) and guttae (bottom).
Top, Arrowheads indicate features representing folds in the diffuse view, OCT, and corresponding specular image.
Bottom, Dark areas of cell death (or drop out) can be seen using both diffuse illumination, OCT, and specular microscopy (chevrons). The bright ‘specs’ in the OCT image may represent guttae. N/R = not required. Slit beam images does not have the appropriate resolution to visualize guttae in this modality.
DISCUSSION
The idea of applying a telemedical consult to aid in the identification of ambiguous corneal findings is not novel,1,5,8,21 as clinicians routinely consult their colleagues when faced with a difficult diagnosis or when they simply need a second opinion. For example, users of Keranet (an online listserv for Ophthalmologists) routinely share interesting findings for educational and consultation purposes among peers and to draw upon the knowledge and experience of a collective that possess a wide range of experience and background. Further, the use of telemedicine in ophthalmology continues to be developed and is becoming increasingly widespread. Applications of telemedicine in the emergency setting and in the screening of more prevalent ocular diseases such diabetic retinopathy25 and glaucoma26 have established the potential of teleophthalmology to provide cost-effective ways to achieve wider patient access to ophthalmic care than previously possible. Prior studies on telemedicine in the diagnosis of corneal disease have focused on validating the use of portable cameras in remote evaluation of corneal disease.20,23,24 It will be particularly exciting to explore the utility of telemedicine consultation of eye bank medical directors in determining the transplant suitability of corneal tissue—a use of telemedicine that has the potential to universally enhance tissue transplant-suitability determination by making medical director consults more accessible and more efficient.
A key factor to a successful telemedical consult for donor corneas is high quality images. It is important to have clear images that show the features in question in a focused manner to minimize any ambiguity that may be introduced by image acquisition. In the present study, we have focused on the optimization of image acquisition using standard ophthalmologic equipment available at eye banks. Using a combination of digital images acquired from slit lamp microscopy, OCT, and specular microscopy at an eye bank, four surgeons were able to remotely identify common findings in donor corneal tissue at high rates of agreement with an in-person surgeon-evaluator and eye bank evaluators. While we describe the minimum image requirements necessary for a medical director to identify common donor cornea findings (Table 1), we note that it will be imperative to employ all imaging modalities outlined in Supplemental Table 1 when consulting truly unknown features. This study provides a protocol that can serve as a framework for other eye banks interested in providing a similar telemedicine consultation option to its medical directors and may allow for the potential involvement by multiple medical directors in assessing an ambiguous corneal finding.
A recent resource has become available that may provide additional references for future studies of telemedical consults for donor corneal evaluation. The journal Cornea recently published a Supplemental Article Series describing the use of current and new eye bank imaging technologies to document various features that can be found on donor corneas.27 Indeed, some of the features associated with folds and guttae described in this manuscript is similar to those recently described in the Comparative Image Atlas section of these supplement articles.28 Thus, there is even more technology currently available to aid in the future development of telemedicine in eye banking.
One limitation of this study is the small number of samples examined. However, despite this limited sample size, the samples used represent a diverse set of findings commonly encountered by eye banks and serve as a good starting point for establishing whether image acquisition was adequate for medical directors to accurately ascertain tissue findings. Ongoing work at our eye bank aim to further validate this method using a larger consult series that will include additional cornea findings that eye banks may encounter and that would be more appropriately powered to test accuracy.
Another potential limitation of this study is the potential bias introduced by the remote evaluators being asked if they agreed with the eye bank’s preliminary diagnosis (rather than being asked, without a prompt, to provide their own diagnosis), or whether they believe it is one feature or another. This may have resulted in an excessively high rate of agreement among evaluators. However, even during in-person consults, eye bank evaluators typically ask medical directors very targeted questions that reflect their impression of the finding (e.g. ‘Is this an infiltrate?’, or ‘Is this an infiltrate or is it edema?’). Altogether, bias limitations in our experimental consult design are undoubtedly something to consider in future studies that involve more complex consult findings and determinations of transplant suitability. As this is a pilot study focused on the feasibility of telemedicine consults in eye banking, we have not tested our telemedical consult platform for unknown findings, but this is an ongoing area of research in our laboratory.
Despite these potential limitations, the high-level of agreement between all study participants (remote evaluators, in-person evaluator, and eye bank technicians) for findings examined in the present study suggests that our approach to telemedical consultation with eye bank medical directors may be a feasible alternative to in-person consultations. In addition to its potential application at other eye banks, we hope that this eye bank telemedicine approach can be applied to more complex consultations in the future.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by a NIH T32GM007739 to the Tri-Institutional MD-PhD program (ROA).
Footnotes
Conflict of interest statement: No conflicts to declare.
REFERENCES:
- 1.Rathi S, Tsui E, Mehta N, et al. The Current State of Teleophthalmology in the United States. Ophthalmology 2017;124:1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tozer K, Woodward MA, Newman-Casey PA. Telemedicine and Diabetic Retinopathy: Review of Published Screening Programs. J Endocrinol Diabetes 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol 2008;2:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology 2013;120:2604–2610. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez CR, Silva PS, Cavallerano JD, et al. Ocular telemedicine for diabetic retinopathy and the Joslin Vision Network. Semin Ophthalmol 2010;25:218–224. [DOI] [PubMed] [Google Scholar]
- 6.Silva PS, Horton MB, Clary D, et al. Identification of Diabetic Retinopathy and Ungradable Image Rate with Ultrawide Field Imaging in a National Teleophthalmology Program. Ophthalmology 2016;123:1360–1367. [DOI] [PubMed] [Google Scholar]
- 7.Walton OBt, Garoon RB, Weng CY, et al. Evaluation of Automated Teleretinal Screening Program for Diabetic Retinopathy. JAMA Ophthalmol 2016;134:204–209. [DOI] [PubMed] [Google Scholar]
- 8.Ng M, Nathoo N, Rudnisky CJ, et al. Improving access to eye care: teleophthalmology in Alberta, Canada. J Diabetes Sci Technol 2009;3:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramoff MD, Suttorp-Schulten MS. Web-based screening for diabetic retinopathy in a primary care population: the EyeCheck project. Telemed J E Health 2005;11:668–674. [DOI] [PubMed] [Google Scholar]
- 10.Olson JA, Strachan FM, Hipwell JH, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med 2003;20:528–534. [DOI] [PubMed] [Google Scholar]
- 11.Scanlon PH, Malhotra R, Thomas G, et al. The effectiveness of screening for diabetic retinopathy by digital imaging photography and technician ophthalmoscopy. Diabet Med 2003;20:467–474. [DOI] [PubMed] [Google Scholar]
- 12.Chiang MF, Melia M, Buffenn AN, et al. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology. Ophthalmology 2012;119:1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn GE, Ying GS, Daniel E, et al. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol 2014;132:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel E, Quinn GE, Hildebrand PL, et al. Validated System for Centralized Grading of Retinopathy of Prematurity: Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity (e-ROP) Study. JAMA Ophthalmol 2015;133:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Powell AM, Hooper PL, et al. Prospective evaluation of teleophthalmology in screening and recurrence monitoring of neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 2015;133:276–282. [DOI] [PubMed] [Google Scholar]
- 16.Thomas M, Wolfson Y, Zayit-Soudry S, et al. Qualifying to Use a Home Monitoring Device for Detection of Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol 2015;133:1425–1430. [DOI] [PubMed] [Google Scholar]
- 17.Bergua A, Mardin CY, Horn FK. Tele-transmission of stereoscopic images of the optic nerve head in glaucoma via Internet. Telemed J E Health 2009;15:439–444. [DOI] [PubMed] [Google Scholar]
- 18.Kiage D, Kherani IN, Gichuhi S, et al. The Muranga Teleophthalmology Study: Comparison of Virtual (Teleglaucoma) with in-Person Clinical Assessment to Diagnose Glaucoma. Middle East Afr J Ophthalmol 2013;20:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jampel HD, Friedman D, Quigley H, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol 2009;147:39–44 e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Yogesan K, Constable IJ. Telemedical diagnosis of anterior segment eye diseases: validation of digital slit-lamp still images. Eye (Lond) 2009;23:652–660. [DOI] [PubMed] [Google Scholar]
- 21.Shimmura S, Shinozaki N, Fukagawa K, et al. Real-time telemedicine in the clinical assessment of the ocular surface. Am J Ophthalmol 1998;125:388–390. [DOI] [PubMed] [Google Scholar]
- 22.Maamari RN, Ausayakhun S, Margolis TP, et al. Novel telemedicine device for diagnosis of corneal abrasions and ulcers in resource-poor settings. JAMA Ophthalmol 2014;132:894–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward MA, Musch DC, Hood CT, et al. Teleophthalmic Approach for Detection of Corneal Diseases: Accuracy and Reliability. Cornea 2017;36:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward MA, Bavinger JC, Amin S, et al. Telemedicine for ophthalmic consultation services: use of a portable device and layering information for graders. J Telemed Telecare 2017;23:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kortuem K, Fasler K, Charnley A, et al. Implementation of medical retina virtual clinics in a tertiary eye care referral centre. Br J Ophthalmol 2018. [DOI] [PubMed]
- 26.Gunn PJG, Marks JR, Au L, et al. Acceptability and use of glaucoma virtual clinics in the UK: a national survey of clinical leads. BMJ Open Ophthalmol 2018;3:e000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray KE. Wide-Field Ex Vivo Dual Imaging Microscopy. Cornea 2018;37:S11–S13. [DOI] [PubMed] [Google Scholar]
- 28.Comparative Image Atlas of Current and New Technologies in Corneal Donor Tissue Evaluation. Cornea 2018; S14–S35. Available at: https://journals.lww.com/corneajrnl/Fulltext/2018/06001/Comparative_Image_Atlas_of_Current_and_New.6.aspx, 37. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


