Abstract
Introduction
Patients with schizophrenia have an elevated mortality risk compared to the general population, with cardiovascular-related deaths being the leading cause. The role of clozapine use in the long-term mortality risk is unclear. While clozapine treatment may increase the risk for cardiovascular mortality, it may have protective effects regarding suicidal behavior.
Methods
We systematically searched EMBASE, MEDLINE, and PsycINFO and reviewed studies that used a long-term follow-up (ie, >52 weeks) and reported on mortality in adults diagnosed with schizophrenia-spectrum disorders who had received clozapine treatment.
Results
Altogether, 24 studies reported on 1327 deaths from any causes during 217691 patient years in patients treated with clozapine. The unadjusted mortality rate in 22 unique samples during a follow-up of 1.1–12.5 (median = 5.4) years was 6.7 (95% confidence interval [CI] = 5.4–7.9) per 1000 patient years. Long-term, crude mortality rate ratios were not significantly lower in patients ever treated with clozapine during follow-up, but significantly lower in patients continuously treated with clozapine compared to patients with other antipsychotics (mortality rate ratio = 0.56, 95% CI = 0.36–0.85, P-value = .007). Few studies reported on rates of long-term cause-specific mortality (suicide and ischemic heart disease), which showed no significant difference in patients using clozapine compared to patients using other antipsychotics. Statistical heterogeneity was high in all analyses.
Discussion
Continuous clozapine treatment in schizophrenia patients was associated with a significantly lower long-term all-cause mortality rate compared to other antipsychotic use. These findings, combined with the known efficacy of clozapine, give reason to re-evaluate the hesitancy to prescribe clozapine in regular care settings.
Trial registration
PROSPERO CRD42017069390.
Keywords: mortality, clozapine, antipsychotics, schizophrenia
Introduction
Patients with schizophrenia-spectrum disorders have an estimated 2.5 times elevated mortality risk compared to the general population1,2 and live 15–25 years shorter.3,4 The main cause of death in these patients has been related to cardiovascular diseases.5 Considering the efficacy of antipsychotics, while also acknowledging their potential role in elevating the risk of developing cardiovascular diseases, the benefit-risk ratio considering mortality risk has been equivocal.6 Clozapine is a unique antipsychotic agent with superior efficacy in patients with schizophrenia who are treatment-resistant7–9 or have suicidal ideations and behaviour.10
The question of long-term mortality risk is of special clinical interest for patients treated with clozapine. In 1975, clozapine was immediately withdrawn from the international markets after reports of agranulocytosis leading to death, but was reintroduced around 1990 due to its efficacy with strict blood monitoring requirements.11 Consequently, the use of clozapine is in many countries restricted to patients with schizophrenia who have not adequately responded to at least 2 other antipsychotics.12 Apart from agranulocytosis, there are several reasons why clozapine might be associated with higher long-term mortality compared to other antipsychotics. For instance, clozapine is—compared to other antipsychotics—associated with the highest risk of metabolic adverse effects, such as weight gain, dyslipidemia, and hyperglycemia.13 All of these cardiometabolic adverse effects are part of the metabolic syndrome that has been associated with cardiovascular morbidity and mortality risk, which is the main cause of premature death in patients with schizophrenia.13 Nevertheless, several large cohort studies have shown a decreased risk of death for clozapine compared to other antipsychotics, but this risk reduction was not always statistically significant.3,14,15
On the other hand, clozapine has proven to be effective in the prevention of suicide, as shown by a meta-analysis of long-term studies focusing on suicide (n = 240564), which demonstrated a 2.9-fold (95% CI = 1.5–5.7) overall risk reduction of completed suicide during long-term exposure to clozapine.16Despite a meta-analysis6 and a systematic review17 each having investigated the association between the use of antipsychotics and long-term mortality there are, to the best of our knowledge, no systematic reviews or meta-analyses that focus on clozapine and its association with long-term mortality risk from all causes. Consequently, it is currently uncertain to what degree clozapine might play a role in the all-cause mortality excess of patients diagnosed with schizophrenia in the long-term.
In summary, whether the benefits of enhanced clinical efficacy outweigh the potential long-term harmful side-effects of clozapine remains an open question. Performing a systematic review and meta-analysis that investigates the long-term mortality rates of clozapine can help provide answers on this clinically relevant topic. Therefore, we aimed to study (1) the long-term mortality rates and (2) specific causes of death in patients with schizophrenia-spectrum disorders treated with clozapine compared to patients treated with other antipsychotics or no antipsychotics.
Methods
This review was performed according to the guidelines of the PRISMA statement.18 The protocol was registered in the PROSPERO database under registration number CRD42017069390. The search strategy was developed and conducted with the help of a clinical librarian (supplement 1). Relevant studies were identified through searching MEDLINE, EMBASE, and PsycINFO from database inception through 27th of June 2017. The reference lists of retrieved articles were hand searched (forward and backward tracking of the literature up to March 2018) to identify additional eligible studies.
Selection of Studies
Two reviewers (M.v.d.K. and G.v.R.) independently screened titles and abstracts to identify eligible studies. The following inclusion criteria were used: The study (1) included patients ≥18 years old diagnosed with schizophrenia-spectrum disorders (including schizophrenia, schizoaffective disorder, schizophreniform disorder, and psychotic disorder not otherwise specified); (2) patients ever or currently used clozapine (at any dose); (3) had mortality as an outcome; (4) was an original research article that used a follow-up design longer than 52 weeks). The first 30 conflicts in study inclusion were resolved in consensus meetings and since overlap was high, other conflicts were reviewed by one author (M.v.d.K.). The full article was obtained for further inspection, in case a clear decision concerning inclusion criteria could not be made during abstract screening. Subsequently, studies were excluded by several authors (G.v.R., M.v.d.K., A.S., and C.C.) during full-text reading if: the study (1) had a follow-up duration of ≤52 weeks, and/or (2) only cause-specific mortality was available even after contacting the authors (eg, several studies reported the number of patients who died due to myocarditis during treatment with clozapine, but did not provide the total number of deaths). To obtain homogenous samples, authors from articles that included >10% patients diagnosed with other diagnoses (eg, neurological diseases, cognitive disorders) were contacted to request number of death within the patient group diagnosed with a schizophrenia-spectrum disorder (in case these numbers were not available articles were excluded).
Data Extraction
Data were extracted by 2 independent researchers (JV and MvdK) and accuracy was discussed in regular meetings. Corresponding authors were contacted to provide additional data when studies lacked sufficient information (which was the case for 6 studies). We extracted the following data: country of study, years of data collection, follow-up in years or patient years, sample source, characteristics of population (eg, elderly, high risk of suicide or treatment-resistant), diagnoses and diagnostic assessment, primary and secondary outcome(s), comparison group(s), sample size (clozapine and comparison group(s)), number of death (all-cause and cause-specific), death assessment, statistical method for adjusting for group differences, medication details (dosage, length of exposure, and concomitant medications), age at inclusion, sex, and if possible information regarding confounders (eg, duration of illness, medical history, smoking status). In the case of overlapping samples, the study with the smallest sample size was excluded. Risk of bias of the studies was assessed on outcome level with the Cochrane Risk of bias tool for randomized studies or the Newcastle Ottawa scale (NOS) for observational studies (range = 0–9).19,20 For observational studies with an ineligible comparison group or convenience samples, we used the NOS (range = 0–6)19 without the items regarding comparison groups. A NOS score of ≤5 was deemed as indicating high risk of bias.
Statistical Analyses
Mortality Rates in Patients Who Used Clozapine
All statistical analyses were performed using Comprehensive Meta-Analysis, version 3.0. To determine all-cause mortality rates, we calculated crude rates for patients exposed to clozapine per 1000 patient years. The following formula was used to estimate patient years if not provided by the authors: [(Number of people at risk at the beginning of the time interval + number of people at risk at the end of the time interval) / 2] × (number of years in the time interval). The number of patients at risk at the end of the time interval was the total sample size minus the number of deaths and the number of patients lost to follow-up. All studies that reported data on number of deaths for patients treated with clozapine were used to pool a one armed summary mortality rate per 1000 patient years and presented in a forest plot using a DerSimonian–Laird random-effects model.21 Between-study heterogeneity was assessed using Cochran’s Q and the I2-statistic. According to convention, a chi-squared test <0.05 or I2 ≥50% indicates significant heterogeneity.22 Publication bias was visually inspected by a funnel plot and statistically assessed using an Egger’s test if applicable. A comprehensive series of sensitivity analyses and subgroup analyses were performed to examine possible explanations for the observed heterogeneity. Additionally, meta-regression analysis was applied to examine the potential effect of continuous moderators (mean sample age, % males, % patients diagnosed with schizophrenia, risk of bias based on the NOS score of cohort studies) if reported by at least 10 studies, as suggested by Borenstein et al.21
Mortality in Patients Who Ever or Continuously Used Clozapine During Follow-up Compared to Those Treated With Other or No Antipsychotics
Furthermore, we calculated for a subset of all included cohort studies that had a control group, all-cause mortality numbers and patient years not only for clozapine-exposed patients, but also exposure to other antipsychotics and exposure to no antipsychotics. Analyses were further divided into studies that included patients who continuously or ever used clozapine during follow-up. This decision was based on previous literature23 and the assumption that patients who were ever exposed to clozapine during follow-up but discontinued treatment (eg, due to a lack of clinical response or intolerability) would likely have a higher mortality risk than those who continued clozapine during the entire follow-up period. Group comparisons were conducted for studies that included: (1) clozapine vs other antipsychotics, or (2) clozapine vs no antipsychotics. If possible, results were pooled and presented in a forest plot using a DerSimonian–Laird random-effects model when 3 or more studies were available.21
Cause-Specific Mortality for Clozapine Users
The studies that also reported on cause-specific mortality were included to perform one-armed meta-analyses of pooled cause-specific mortality rates (ie, suicide and death due to ischemic heart disease). These 2 causes were selected in line with the largest previously published study so far.3 Again, whenever possible, analyses were further divided into studies that included patients who continuously or ever used clozapine during complete follow-up.
Results
Study Selection
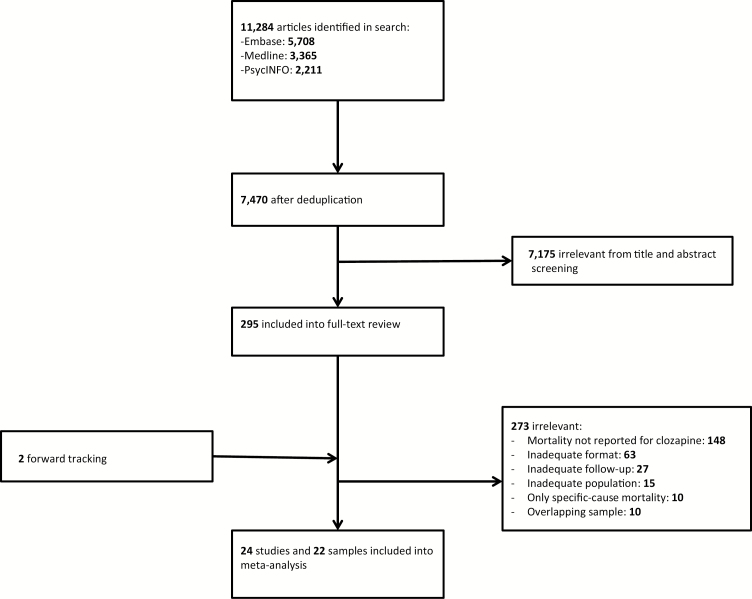
The initial search yielded 11284 articles, of which 295 remained after title and abstract screening. After full-text reading 273 studies were excluded and 2 articles were added by forward tracking. Most of the excluded articles had no assessment of mortality or an inadequate format (ie, conference papers). Ten overlapping samples were removed, resulting in 24 studies and 22 unique samples eligible for meta-analysis.3,10,14,23–43 Details of the selection process are shown in figure 1.
Fig. 1.
Flowchart of study selection.
Study Characteristics
Table 1 summarizes the 24 study characteristics by type of study design.3,10,14,23–43 One study had an overlapping sample and was excluded from the all-cause mortality analysis, but could be included in the analyses with a comparison group.27 Two studies had an overlapping sample: one of these studies provided data regarding all-cause mortality,32 while the other study31 provided cause-specific mortality data and was therefore only used in the analyses regarding cause-specific mortality. Considerable differences existed between studies regarding methodological and clinical characteristics (eg, study designs and patient subgroups). We included one randomized controlled trial and 23 observational studies. Most of the studies (n = 19) originated from Western countries. The follow-up duration of all included studies ranged from 1.1 to 12.5 (median = 5.4) years (table 1). The selected studies primarily included patients with a diagnosis of schizophrenia (n = 7) or schizophrenia spectrum disorders (n = 15) and 3 studies included <10% patients with bipolar disorder or unspecified diagnosis.33,35,38 One corresponding author provided additional data for patients with schizophrenia and schizoaffective disorder, which made it possible to exclude patients with other diagnoses from the results.26 The reported data regarding length of exposure to clozapine varied extensively between studies. For example, some studies provided mortality for continuous users during the entire length of follow-up,3,14,23,27,32 whereas the remaining studies reported “ever” use during follow-up with discontinuation rates. The mean age across all studies was 49.1 (SD = 3.2) (range: 28.7–67.4) years, and some studies only presented the age of the participants in strata. All-cause mortality was the primary outcome in 6 studies3,14,23,26,30,32 and several specific causes of death were the primary outcome in 4 studies.26–29 The risk of bias of the studies was assessed as 45.8% being low (NOS > 5) and 13 studies (54.2%) being high (supplements 2 and 3).
Table 1.
Study Characteristics of All 24 Studies Included in the Current Study
| Authors (Year) | Country | Total Sample Size (n), Source | Length of Follow-up in Years (Years of Data Collection) | DSM/ ICD | Diagnoses (n), Subgroup Details | Comparator Group | Mean Age | Male (%) |
|---|---|---|---|---|---|---|---|---|
| Randomized trial, open label | ||||||||
| Meltzer et al10 | Multinational | 980, n.s. | 2 (1998–2001) | DSM | Schizophrenia (609), schizoaffective (371); high risk suicide | Olanzapine | 37.1 | 602 (61.4) |
| Cohort studies | ||||||||
| Girgis et al24 | China | 160, medical records | 9 (1995–2007) | DSM | Schizophrenia (122), schizophreniform (38); in- and outpatients, first episode, treatment- naive patients | Chlorpromazine | 28.7 | 84 (52.2) |
| Dickson et al25 | Canada | 26, n.s. | 3 (n.s.) | DSM | Schizophrenia (26); in- and outpatients, treatment-resistant | Continuers, discontinued, and interrupted clozapine usersa | 31.8 | 24 (92.3) |
| Hayes et al26 | UK | 14754,b medical records | 3 (2007–2011) | ICD | Schizophrenia (9437), bipolar disorder (4512),b schizoaffective (805); in- and outpatients, newly prescribed clozapine | First- and second- generation antipsychotics or no antipsychotics | 43.2b | 7985 (54.1)b |
| Hennessy et al27 | USA | 124718c, database | 1.1c (1993–1996) | n.s. | Schizophrenia (95632); outpatients, individuals with limited resources | Other antipsychotics or patients without schizophreniac | n.s. (median age group 35–44) | 47812 (50.0) |
| Kelly et al28 | USA | 1686, database | 6–10 (1994–2000) | DSM | Schizophrenia (964), schizoaffective (561), psychosis NOS (161) | Risperidone | 39.0d | 1059 (62.8) |
| Modai et al29 | Israel | 5479, medical records | 6.7 (1991–1997) | ICD | Schizophrenia (n.s.); inpatients | Other antipsychotics | n.s. | n.s. |
| Pridan et al30 | Israel | 527, medical records | 5 (2007–2012) | DSM | Schizophrenia (527); elderly inpatients, treatment-resistant | Other antipsychotics (first and second generation) | 67.4 | 245 (46.5) |
| Ringbäck et al31 | Sweden | 26046, database | 6 (2006–2011) | ICD | Schizophrenia (n.s.), schizoaffective (n.s.); in- and outpatients | First- and second- generation antipsychotics or no antipsychotics | 48.4 | 14397 (55.3) |
| Taipale et al32 | Sweden | 29823, database | 5.7 (2006–2013) | ICD | Schizophrenia (n.s.), schizoaffective (n.s.); in- and outpatients | Other antipsychotics (first and second generation) and no antipsychotics | 44.9 | 16999 (57) |
| Tiihonen et al3 | Finland | 66881, database | 8.6e (1996–2006) | ICD | Schizophrenia (66881); outpatients, inpatients deaths were excludedf | Other antipsychotics and no antipsychoticsf | 51.0f | 30803f (46.0) |
| Walker et al14 | USA | 67072,g database | 1.47 (1991–1993) | n.s. | n.s.; in- and outpatients | Current, recent, or past clozapine usersh | n.s. (median age group 35–39) | n.s. |
| Wimberley et al23 | Denmark | 2370, database | 6.8i (1996–2013) | ICD | Schizophrenia and related disorders (2370); in- and outpatients, treatment-resistant | Other antipsychotics or no antipsychotics | n.s.(median age 30.1) | 1284 (54.2) |
| Case–control studies | ||||||||
| Mela and Depiang33 | Canada | 98, medical records | 2 (1984–2012) | DSM | Psychotic disorder and related disorders (98); offenders | Other antipsychotics (first and second generation) | n.s. | 94 (95.9) |
| Schulte et al34 | Netherlands | 94, medical records | 12.3 (1989–2010) | n.s. | Schizophrenia (81), schizoaffective (13); in- and outpatients, patients without diabetes mellitus at start of follow-up | Other antipsychotics | 39.0 | 74 (78.7) |
| Taylor et al35 | UK | 779, database | 4.67 (2002–2006) | n.s. | Schizophrenia (n.s.), schizoaffective (n.s.), bipolar disorder (n.s.),j other (n.s.)j | Risperidone long- acting injection | 36.4j | n.s. |
| Convenience sample studies | ||||||||
| Gaertner et al36 | Germany | 23, n.s. | 3.8 (1993–n.s.) | ICD | Schizophrenia (23); outpatients, patients with complete remission of positive symptoms | No comparison | 40.0 | 18 (78.2) |
| Khan et al37 | Australia | 503, n.s. | 9 (2009–2015)k | n.s. | Schizophrenia (503); outpatients, treatment-resistant | No comparison | 44.0 | 397 (78.9) |
| Lee et al38 | Canada | 94, database | 1.4 (2009–2010) | n.s. | Schizophrenia (75), schizoaffective (17), bipolar disorder (1), delusional disorder (1); patients without anemia before clozapine initiation | No comparison | 35.9 | 68 (72.3) |
| Munro et al39 | UK/Ireland | 12720, database | 8 (1990–1997) | n.s. | Schizophrenia (12760); Treatment-resistant | No comparison | n.s. (modal age 25–35) | 8533 (67.1) |
| Srivastava et al40 | India | 25, n.s. | 3 (1994–1997) | ICD | Schizophrenia (25); treatment-resistant | No comparison | 28.8 | 18 (72.0) |
| Davis et al41 | USA | 320, database | n.s.l (1993–2007) | DSM | Schizophrenia (n.s.), schizoaffective (n.s.); in- and outpatients, veterans with treatment intolerance or significant risk of suicidal behavior | No comparison | 48.0 | 292 (91.3) |
| Lindström42 | Sweden | 96, recruited by clinicians | 12.5 (1974–1986) | ICD | Schizophrenia (89), schizoaffective (7); in- and outpatients, treatment-resistant or with distressing side effects | No comparison | 36.1 | 64 (66.7) |
| Rimón et al43 | Finland | 103, medical records | 3.4 (n.s.) | DSM | Schizophrenia (96), schizoaffective (7); in- and outpatients, treatment-resistant | No comparison | 36.9 | 59 (57.3) |
Note: n.s., not specified; DSM, Diagnostic System of Mental disorders; ICD, International Classification of Diseases.
aComparison group not eligible for our analyses.
bPatients with bipolar disorder were excluded from our analyses.
cTotal sample size of the complete study included patients diagnosed with glaucoma and psoriasis. Only patients with schizophrenia were used in our analyses. As length of follow-up was not provided, we calculated the length of follow-up for patients using clozapine by dividing the patient years by number of patients using clozapine.
dMean age of clozapine group.
eMean follow-up for patients with antipsychotics.
fInpatients were excluded from the analyses.
gTotal sample size, patients with ages 55–94 years were excluded.
hThis study was used in the first analyses about mortality rates in clozapine users.
iMedian follow-up.
jDiagnoses and mean age only specified for discontinuers.
kMean follow-up reported in study does not correspond to the years of data collection.
lNo mean follow-up reported, the mean duration of clozapine exposure was 5.7 years for the patients who were deceased.
Mortality Rates in Patients Who Used Clozapine
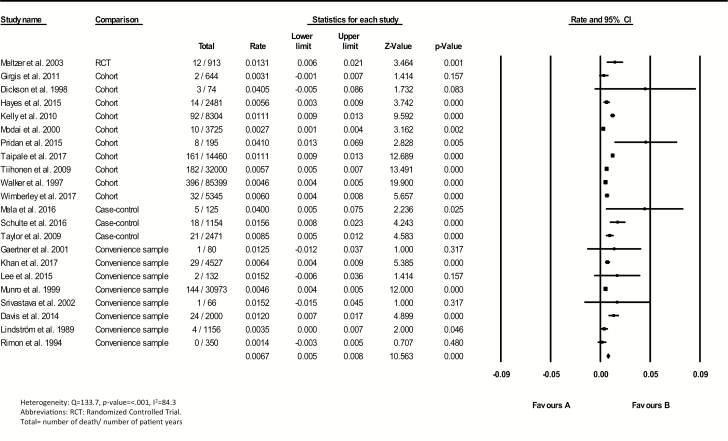
A total of 1327 deaths from any causes during 217691 patient years were reported for patients with schizophrenia spectrum disorders across 24 samples. Mortality rates differed widely, ranging from 0 to 41.0 deaths per 1000 patient years. A forest plot with the pooled summary rate 6.7 (95% CI = 5.4–7.9) per 1000 patient years is shown in figure 2. The Egger’s test suggested some evidence of publication bias (β = 1.64, SE = 0.61, P-value = .015). Seven subgroup analyses were performed (table 2). Large difference in the certainty of continuous exposure to clozapine during the whole period of follow-up was found between studies (eg, some studies included patients who filled ≥1 prescription of clozapine or used clozapine ever during follow-up). The subgroup of studies that included patients who continuously used clozapine during follow-up showed a pooled all-cause mortality rate of 6.7 (95% CI = 4.6–8.9) per 1000 patient years. Besides, the subgroup of patients ever exposed to clozapine showed a pooled mortality rate of 7.1 (95% CI = 5.1–9.0) per 1000 patients years (P = .838). The subgroup meta-analyses of studies with a minimum sample size and patient years or a minimum of 100 patients and a minimum of 5 years follow-up also revealed high heterogeneity levels. After visual inspection of the funnel plot of all studies, we progressively excluded studies with highest and lowest values, but the resulting pooled rate estimates did not yield low heterogeneity levels (I2 < 50%).
Fig. 2.
Crude mortality rates for clozapine users.
Table 2.
Meta-analysis of Mortality Rate in Patients Treated With Clozapine, Including Subgroup and Sensitivity Analyses
| Number of Samples | Total Number of Deaths | Total Number of Patient Years | Number of Deaths per Patient Year | Lower 95% CI | Upper 95% CI | I 2 (%) | |
|---|---|---|---|---|---|---|---|
| Mortality rate in patients treated with clozapine continuously or ever during follow-up (main analysis) | 22 | 1161 | 196574 | 0.0067 | 0.0054 | 0.0079 | 84.3 |
| Subgroup analysis | |||||||
| On clozapine ever during follow-up | 18 | 390 | 59370 | 0.0071 | 0.0051 | 0.0090 | 78.8 |
| On clozapine continuously during follow-up | 4 | 771 | 137204 | 0.0067 | 0.0046 | 0.0089 | 94.4 |
| All studies with a comparator group using other antipsychotics | 8 | 523 | 75339 | 0.0066 | 0.0042 | 0.0090 | 93.4 |
| On clozapine ever during follow-up and other antipsychotic comparator group | 4 | 124 | 14705 | 0.0077 | 0.0020 | 0.0133 | 92.5 |
| No antipsychotic comparator group | 3 | 207 | 22286 | 0.0077 | 0.0039 | 0.0115 | 89.0 |
| >100 patients, >500 patient years | 12 | 1117 | 192598 | 0.0069 | 0.0056 | 0.0082 | 89.7 |
| >100 patients, >5 years follow-up | 5 | 477 | 63834 | 0.0073 | 0.0044 | 0.0102 | 94.1 |
| Categorical sensitivity analysis (between group P-value) | |||||||
| On clozapine ever or continuously during follow-up (P = .838) | |||||||
| Ever | 18 | 390 | 59370 | 0.0071 | 0.0051 | 0.0090 | 78.8 |
| Continuously | 4 | 771 | 137204 | 0.0067 | 0.0046 | 0.0089 | 94.4 |
| Continent (P = .227) | |||||||
| Asia | 4 | 21 | 4630 | 0.0044 | −0.0008 | 0.0096 | 60.7 |
| Australia | 1 | 29 | 4527 | 0.0064 | NA | NA | NA |
| Europe | 10 | 577 | 90470 | 0.0064 | 0.0047 | 0.0081 | 85.4 |
| Multicontinental | 1 | 12 | 913 | 0.0131 | NA | NA | NA |
| North-America | 6 | 522 | 96034 | 0.0104 | 0.0051 | 0.0158 | 89.0 |
| Treatment-resistant (P = .103) | |||||||
| Treatment-resistant patients only | 7 | 217 | 41530 | 0.0052 | 0.0032 | 0.0072 | 60.0 |
| Other | 15 | 944 | 155044 | 0.0074 | 0.0057 | 0.0091 | 88.1 |
| Treatment setting (P = .621) | |||||||
| Inpatient only | 2 | 18 | 3920 | 0.0191 | −0.0181 | 0.0563 | 85.6 |
| Outpatient only | 3 | 212 | 36607 | 0.0058 | 0.0050 | 0.0066 | 0.0 |
| In- and outpatients | 10 | 654 | 113036 | 0.0066 | 0.0043 | 0.0090 | 88.1 |
| Risk of biasa (P = .749) | |||||||
| Low | 9 | 900 | 149171 | 0.0078 | 0.0058 | 0.0099 | 91.2 |
| High | 13 | 249 | 43034 | 0.0058 | 0.0039 | 0.0076 | 69.2 |
| Diagnosis (P = .087) | |||||||
| Schizophrenia only | 7 | 196 | 39640 | 0.0049 | 0.0027 | 0.0071 | 64.5 |
| Schizophrenia and related disorders | 15 | 965 | 156934 | 0.0073 | 0.0057 | 0.0089 | 87.4 |
| Start of study conduct (P = .193) | |||||||
| ≤1980 | 1 | 4 | 1156 | 0.0035 | NA | NA | NA |
| 1980–1990 | 4 | 167 | 32602 | 0.0070 | 0.0015 | 0.0126 | 80.4 |
| 1991–2000 | 11 | 755 | 138550 | 0.0064 | 000048 | 0.0080 | 82.7 |
| 2001–2010 | 6 | 235 | 24266 | 0.0054 | 0.0054 | 0.0116 | 76.0 |
| Sample size patient years (P = .463) | |||||||
| 0–100 | 3 | 5 | 220 | 0.0175 | 0.0000 | 0.0350 | 0 |
| >100 | 4 | 15 | 802 | 0.0202 | −0.0005 | 0.0410 | 77.0 |
| >500 | 2 | 14 | 1557 | 0.0076 | −0.0021 | 0.0174 | 80.9 |
| >1000 | 9 | 244 | 31163 | 0.0073 | 0.0049 | 0.0097 | 84.4 |
| >10000 | 4 | 883 | 162832 | 0.0063 | 0.0046 | 0.0080 | 94.5 |
| Years of follow-up (P = .905) | |||||||
| 0–5 years | 11 | 463 | 92286 | 0.0069 | 0.0041 | 0.0098 | 62.3 |
| >5 years | 4 | 227 | 25530 | 0.0077 | 0.0032 | 0.0123 | 94.3 |
| >7.5 years | 5 | 449 | 76448 | 0.0063 | 0.0045 | 0.0081 | 86.7 |
| >10 years | 2 | 22 | 2310 | 0.0091 | −0.0028 | 0.0210 | 88.8 |
aRisk of bias of the studies was assessed on outcome level with the Cochrane Risk of bias tool for randomized studies or the Newcastle Ottawa scale (NOS, range = 0–9) for observational studies. For observational studies with an ineligible comparison group or convenience samples, we used the NOS (range = 0–6) without the items regarding comparison groups, with a score of ≤5 indicating high risk of bias.
Categorical sensitivity analyses showed no significant results. The following variables did not significantly moderate mortality rates for patients treated with clozapine: on clozapine ever or continuously during follow-up (P = .838), continent (P = .227), treatment-resistant (P = .103), treatment setting (P = .621), risk of bias (P = .749); diagnosis (P = .087), year of start of the data collection (P = .193), sample size in patient years (P = .463), and years of follow-up (P = .905) (table 2). Meta-regression with the variable % of males, mean age of the total sample, and the NOS score of cohort studies also revealed no significant differences in mortality of clozapine users. A significant result was found for the variable % of patients diagnosed with schizophrenia, indicating a lower mortality rate of clozapine users in studies with a higher percentage of patients diagnosed with schizophrenia only and less patients with schizophrenia related disorders (P = .032, table 3).
Table 3.
Mixed-Effects Meta-Regression of Moderators of Mortality Rates of Patients Treated With Clozapine
| Moderator Variable | Number of Comparisons | β | 95% CI Lower Limit | 95% CI Upper Limit | P Value |
|---|---|---|---|---|---|
| % Males | 19 | 0.0001 | −0.0000 | 0.0003 | .072 |
| % Patients with schizophrenia | 14 | −0.0002 | −0.0004 | −0.0000 | .032 |
| Mean age total sample | 17 | 0.0002 | −0.0001 | 0.0006 | .192 |
| NOS score of cohort studies | 10 | 0.0010 | −0.0002 | 0.0021 | .099 |
Note: NOS, Newcastle Ottawa scale.
Mortality in Patients Who Ever or Who Continuously Used Clozapine During Follow-up Compared to Those Treated With Other or With No Antipsychotics
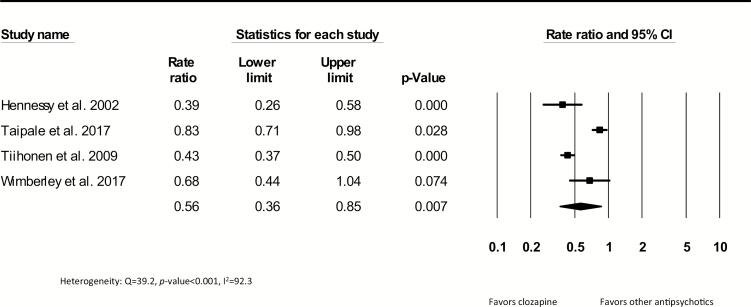
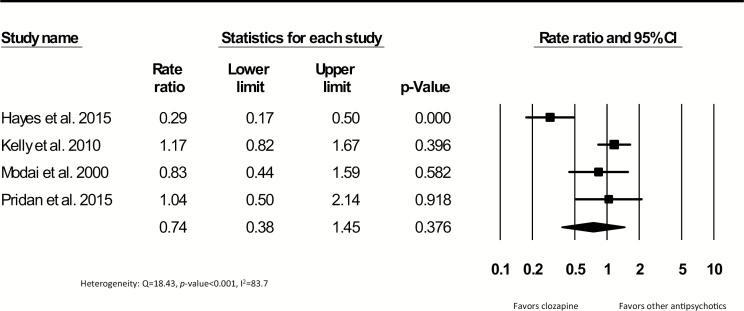
A total of 7304 deaths in 630368 patient years were reported in 8 cohort studies comparing patients exposed to clozapine to patients exposed to other antipsychotics. Studies including continuous users had a median follow-up of 7.2 years and the studies including patients who ever used clozapine during follow-up had a median length of 5.9 years. Crude mortality rate ratios are shown in figures 3 and 4. The pooled mortality rate ratio was 0.56 (95% CI = 0.36–0.85, P = .007, k = 4), indicating a lower mortality rate for patients continuously exposed to clozapine compared to patients continuously exposed to other antipsychotics. Statistical heterogeneity was high (Q = 39.2, P < .001, I2 = 92.3), but the Egger’s test showed no evidence of publication bias (β = −0.44, SE = 5.28, P-value = .940). The pooled rate ratio of studies including patients who ever used clozapine during follow-up compared to other antipsychotics was not significant (0.74; 95% CI = 0.38–1.45, P = .376, k = 4) (figure 4). In the studies that compared patients on continuous or ever use of clozapine treatment during follow-up to patients without the use of antipsychotics, a significant pooled rate ratio of 0.34 was yielded in favor of patients using clozapine (95% CI = 0.19–0.62, P ≤ 0.001, k = 3) (supplement 4). Due to the limited data, meta-regression and sensitivity analyses in continuous and ever users during follow-up could not be conducted. To illustrate this, we summarized the adjusted all-cause mortality ratios of clozapine users compared to other antipsychotics from the 4 largest samples included (supplement 5). Only one of these 4 studies3,23,27,31 showed a significantly lower adjusted mortality rate in continuous clozapine users compared to perphenazine users (adjusted hazard ratio [aHR] = 0.74, 95% CI = 0.60–0.91).3
Fig. 3.
Crude all-cause mortality rate ratios comparing patients who continuously used clozapine during follow-up with patients continuously using other antipsychotics.
Fig. 4.
Crude all-cause mortality rate ratios comparing patients who ever used other antipsychotics.
Cause-Specific Mortality
Twenty studies reported data on specific causes of death concerning 58.0% (n = 5019) of all patients who died. Classification of causes of death was heterogeneous and often incomplete across studies (eg, cardiovascular-related mortality was defined using different criteria). Subcategorizing data by natural vs unnatural causes was discarded since 2 large cohort studies presented incomplete data by only addressing suicide and/or ischemic heart disease mortality numbers.3,31 Therefore, we decided to further explore death from suicide and death from ischemic heart disease. Thirteen studies reported data on mortality from suicide. Crude pooled suicide rates are presented in supplements 6–8. To summarize, throughout the 13 analyzed studies suicide rates ranged widely from 0.00 to 27.03 suicides per 1000 patient years. A numerical, but nonsignificantly lower pooled crude suicide rate (P = .455) was found in patients exposed to clozapine compared to other antipsychotics (supplement 9).3,29,31
Death from ischemic heart disease was reported in 9 studies.3,10,14,25,29,35,36,40,43 We found few studies that reported on rates of long-term cause-specific mortality (suicide and ischemic heart disease) (supplements 10 and 11). Data about rate ratios for death from ischemic heart disease were reported in only 2 studies and therefore a meta-analyses could not be performed.3,10 Tiihonen et al3 used the largest sample size and found for continuously use of clozapine a nonsignificant adjusted hazard ratio of 0.78 (95% CI = 0.54–1.12) with perphenazine as a reference group.
Discussion
To our knowledge, this is the first systematic review and meta-analysis investigating the long-term risk of death from all causes for patients diagnosed with schizophrenia-spectrum disorders that were continuously or ever treated with clozapine during follow-up. The major finding of our study is that although clozapine is known to induce severe side effects, the unadjusted long-term mortality rate during a median of 7 years follow-up in continuous clozapine users was significantly lower compared to those who were continuously treated with other antipsychotics. This finding, combined with the known superior efficacy of clozapine for treatment-resistant schizophrenia,7–10 is clinically highly relevant and may lead to alleviation of earlier concerns about the mortality risk when switching patients to clozapine.
Mortality Rates for Patients Continuously or Ever Treated With Clozapine
We found a wide range of mortality rates for schizophrenia patients who were continuously or ever treated with clozapine. By pooling mortality rates, we found an unadjusted mortality rate of 6.7 per 1000 patient years. This pooled rate is slightly higher than the unadjusted rates that were found in the 2 largest cohort studies that we included.3,14 Tiihonen et al3 and Walker et al14 reported unadjusted mortality rate of 5.7 respectively 4.6 per 1000 patient years, respectively. Given the large and international samples that could be included in the current meta-analyses, the provided mortality rate is likely generalizable and a more precise indication of the worldwide 5-year mortality rate in schizophrenia patients treated with clozapine.
Comparison of Mortality in Patients Continuously or Ever Treated With Clozapine Compared to Patients Treated With Other Antipsychotics or No Antipsychotics
The significantly lower long-term all-cause mortality rate ratio of continuous clozapine users compared to patients treated with other antipsychotics is a new finding. Additionally, the all-cause mortality rate ratio was not statistically significantly different when comparing patients ever treated with clozapine during follow-up compared to patients ever treated with other antipsychotics. Different factors might explain these findings when both results are combined (ie, significantly lower mortality rates in patients continuously treated with clozapine but nonsignificant findings in ever treated patients). These findings suggest an exposure–response relationship, meaning that continuous effects of clozapine are most beneficial for prolonging life expectancy and that this effect seems to be diminished or lost when clozapine is discontinued. Another potential explanation could be that patients who were ever treated with clozapine, discontinued treatment due to being unresponsive to clozapine. Consequently, the nonsignificant findings in the ever-treated subgroup might be a reflection of more severe psychopathology which may be associated with increased risk for premature mortality. Nevertheless, there are also findings indicating that patients stopping clozapine are at an increased risk of mortality compared to patients never treated with clozapine,23 supporting the notion that clozapine is used in severely ill patients at high risk for mortality and that this protective effect is lost when clozapine is stopped.
A lower mortality risk for schizophrenia patients who are continuously treated with clozapine probably reflects a multifactorial etiology. First of all, clozapine has been found to be highly effective in lowering psychopathological symptoms, which likely increases the level of functioning.16 Higher levels of functioning could go hand in hand with improvement of several risk factors, such as improved healthy lifestyle and health care seeking behaviors and a higher socioeconomic status, which have been clearly associated with a lower risk of mortality.44–46 Additionally, as mentioned earlier, another possible explanation of a lower mortality risk in clozapine users might be that patients who are prescribed clozapine undergo frequent clinical monitoring (known as performance bias). Performance bias has been mentioned in the light of the improved effectiveness of clozapine (although this was not confirmed in a randomized controlled clinical trial on this topic).10 Nevertheless, it could be hypothesized that increased monitoring and medical surveillance of cardiometabolic risk factors (eg, hypertension or hyperglycaemia) or adverse lifestyle behaviors (eg, smoking) may be one of the mechanisms by which mortality is reduced in patients treated with clozapine, even though they tend to be generally sicker and more severely ill than patients not started on clozapine.
Similarly, the lower mortality observed in clozapine users might also be, at least in part, due to confounding by contraindication, in that a subgroup of patients who already suffer from severe somatic comorbidities (eg, cardiac comorbidity) and who may therefore be at particularly high risk for mortality may preferentially not be prescribed clozapine. While this potential selection bias excluding a subgroup of patients with severely medical illness may artificially lower the mortality rate in clozapine users, it is unclear how large this group really is. Moreover, it is even more uncertain whether the exclusion of this relatively small group would compensate for the overall greater psychiatric8 and medical illness severity44 in treatment-resistant patients who are the subgroup in whom clozapine is used, whereas the majority of patients on nonclozapine antipsychotics are not as severely ill or treatment refractory.
On the other hand, one could expect a higher risk of mortality in patients who use clozapine compared to other antipsychotics due to confounding by indication (ie, clozapine is indicated for treatment-resistant patients who are arguably among the most severely ill patients).
This potential confounding could be diminished by survival treatment bias since patients must survive other treatments before clozapine is indicated (ie, clozapine is not a first-choice treatment and international guidelines advice prescription of clozapine after nonresponse to 2 other antipsychotics).47 Additionally, clozapine-treated patients are a subgroup of patients who agree to take this medication requiring special monitoring. Altogether, our findings of a substantially lower long-term all-cause mortality risk in patients continuously treated with clozapine compared to those treated with no or other antipsychotics point toward the fact that the long-term beneficial effects of clozapine outweigh its well-documented risks.13,48 An additional point for consideration is that all of the individual studies using adjusted ratios for patients treated with clozapine also showed a lower, but mostly nonsignificant difference in mortality risk favoring patients with clozapine treatment.3,23,27,31 Although, our findings require additional validation with adjustment for important confounders, the current unadjusted findings do not support the hypotheses that the lives saved via clozapine’s reduction in suicide may be offset by the deaths due to an increase of cardiovascular risk factors.49,50
As recently mentioned by Kane,51 clinicians seem to be too cautious in prescribing clozapine. Nielsen et al48 investigated prescription habits of clinicians and showed that discontinuation of clozapine is often not warranted, as adverse effects are treatable in most cases. Therefore, the findings of the overall lower mortality favoring clozapine should encourage clinicians to investigate treatment response to clozapine in every patient in case of unresponsiveness to 2 other antipsychotics (taken at an adequate dose and for a sufficient length of time).
Mortality Rates From Specific Causes
We were unable to draw firm conclusions regarding the long-term causes of death of patients with schizophrenia due to the incomplete and inconsistent reporting of data. For example, cardiovascular-related mortality was reported according to various definitions (eg, Tiihonen et al3 merely reported deaths due to ischemic heart disease). Therefore, 2 subgroups were explored in more detail: death from suicide and from ischemic-heart disease. Regarding the first outcome, we did not find a significant difference in patients that were treated with clozapine compared to other antipsychotics. A previous meta-analysis into long-term risk of suicide did find a significant difference favoring clozapine.16 The findings regarding the association between clozapine use and a lower or higher cardiovascular mortality were contradictory in individual studies.3,28 Future studies, using uniform definitions of cause-specific mortality (eg, following the ICD-11 index as provided by the World Health Organization) and having a substantial length of follow-up for cardiovascular mortality to occur, are recommended to study this relationship. Studying causes of death is crucial, as by reviewing the causes of death, prevention and interventions to improve the health of patients with schizophrenia can be prioritized.
Methodological Limitations
These findings should be interpreted in the light of the following limitations. First, despite the systematic search, the number of included studies in the main analyses was still relatively small. Second, a high level of heterogeneity was present for most outcomes of interest, despite the numerous additional subgroup and sensitivity analyses that were performed. The sensitivity analyses yielded no significant results for categorical variables. We found no difference between studies including treatment-resistant patients only and other studies. However, although in the other studies, the diagnosis was often solely given as schizophrenia and treatment-resistance was neither defined nor described, it is highly likely that a large proportion of patients in fact was clinically treatment-resistant, as clozapine is rather underutilized in the severely ill and refractory patients than overutilized in the nonrefractory patient group.52,53 Additionally, due to mixed antipsychotic comparison groups in most studies, we were unable to perform a subgroup analysis of clozapine’s mortality reducing effect vs first- or vs second-generation antipsychotics. Future research should examine this further. In general, high heterogeneity indicates that variation in clinical and statistical characteristics within and between the individual studies was important, which poses a limitation to a reliable interpretation of the results. Nevertheless, all individual studies pointed toward a lower mortality in patients with continuous clozapine use vs continuous nonclozapine antipsychotic use, indicating that the observed heterogeneity does not challenge the main finding of our meta-analysis. In other words, the heterogeneity was not about whether or not continuous clozapine use is associated with lower all-cause mortality, but rather around the magnitude of this benefit. Therefore, moderators and mediators of the mortality reducing effects of clozapine should be investigated in further studies. Third, we presented and pooled unadjusted mortality rates and performed a meta-regression with 3 potential confounders, yielding no significant results. However, performing a meta-regression with other important confounders was limited by the fact that uniform reporting of relevant sociodemographic and clinical confounders was often lacking. Consequently, our findings need further validation by adjusted rates using more potential confounders.
Fourth, we used rates per patient years to account for sample size and length of follow-up. We encountered several studies from which we had to estimate the length of follow-up based on data collection years, and this could have resulted in an underestimation of mortality rates. Due to the scarcity of high quality studies, this approach represented the most pragmatic way of handling the data.
Fifth, we established broad criteria regarding study design, which lead to the inclusion of low quality studies with methodologically weaker results. However, we performed subgroup analyses based on the risk of bias, but this did not significantly affect the findings. Additionally, to investigate long-term outcomes such as mortality, large sample sizes (including thousands of patients) are required, which is impossible to include in randomized controlled trials (RCTs). As a consequence, the evidence is currently merely based on observational studies.51
Sixth, only a few studies provided antipsychotic exposure estimates to investigate the association between cumulative exposure to antipsychotics and mortality rates. For example, dissimilar measures were used (eg, defined daily dosages or chlorpromazine equivalents) and information regarding dosages or concomitant medication was frequently missing. Consequently, it was impossible to add cumulative exposure as a covariate in the meta-analysis. Nevertheless, it would be interesting to include this in future studies since earlier research indicated that there is an “U-shaped” relation between mortality rate and antipsychotic exposure (higher mortality risk for no and high exposure to antipsychotics and lowest mortality risk for modest exposure to antipsychotics).54 Nevertheless, it is unclear if higher mortality rates in higher antipsychotic dose strata are related to antipsychotic dose per se, or whether there is confounding by indication: more severely ill patients with related variables that are associated with greater mortality risk receive higher antipsychotic doses.
Seventh, we subdivided the analyses into continuous users vs ever clozapine users, based on theoretical grounds and in line with previous findings.23 The assumption that the mortality risk would differ between those 2 groups was not reflected in a subgroup analysis, in which we tested the differences in mortality rates between these 2 groups yielding nonsignificant results. However, in contrast, when comparing each of these clozapine subgroups to nonclozapine users, as hypothesized the continuous clozapine users, but not the ever clozapine users were at significantly reduced risk for all-cause mortality.
Finally, and most significantly, although we intended to include long-term follow-up studies, the median follow-up period of 5 years across all included studies may not have been long enough to expose the overall mortality risk. This may specifically influence mortality due to natural causes (eg, cardiovascular) since mortality by suicide tends to occur early during the disorder,55 while mortality by natural causes increases over time.
Besides these (methodological) limitations of the current study, certain forms of bias that apply to the included studies should also be considered. First, survival treatment bias could be in place (eg, patients had already survived treatment with—often—2 other types of antipsychotics before clozapine was prescribed which may impose a lower mortality risk).47 Ideally, this potential bias should be accounted for, especially when comparing patients treated with clozapine to patients with other or no antipsychotics in observational designs. Unfortunately, most studies were not able to account for this type of bias and only corrected for the available clinical and sociodemographic confounders.23 This omission is often due to the retrospective character of database studies, which frequently lack data about important confounders. Therefore, future long-term cohort studies that collect decisive clinical confounders are strongly suggested. Second, as earlier mentioned, performance bias could be in place. Since patients who use clozapine are requested to have more clinical contacts, due to frequent blood monitoring, they could have better access to care and may be more adequately treated. Performance bias can be adjusted for, most easily in randomized controlled trials, as shown by Meltzer et al,10 but also in observational studies when accounting for the number of clinical contacts.26 However, this confounder was only measured and controlled for in one observational study.26
Conclusion
This meta-analysis showed that continuous clozapine treatment in schizophrenia patients is associated with a significantly lower long-term all-cause mortality rate compared to treatment with other antipsychotics. Future studies with substantial length of follow-up, uniform reporting of causes of death and inclusion of crucial confounders are needed to validate these findings. Nevertheless, these findings are important, given the known effectiveness of clozapine in treating patients with treatment-resistant schizophrenia. Our findings highlight the need of re-evaluation of the role of clozapine in clinical practice. The concern of clinicians that prescribing clozapine will increase the long-term mortality risk of schizophrenia patients by inducing cardiovascular-related adverse effects is not empirically supported in the current systematic review and meta-analysis.
Supplementary Material
Acknowledgments
The authors would like to thank Joost Daams for his help during the search, as well as Richard Garcia for his helpful comments on the first drafts of this article. Conflicts of interest: Dr Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Angelini, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ROVI, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Pfizer, Roche, and ROVI. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a shareholder of LB Pharma. All other authors have nothing to declare.
References
- 1. Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-Year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 4. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6:e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–1181. [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen J, van Rooijen G, Doedens P, Numminen E, van Tricht M, de Haan L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis. Psychol Med. 2017;47:2217–2228. [DOI] [PubMed] [Google Scholar]
- 7. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine V. first-and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:387–394. [DOI] [PubMed] [Google Scholar]
- 8. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73:199–210. [DOI] [PubMed] [Google Scholar]
- 10. Meltzer HY, Alphs L, Green AI, et al. ; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82–91. [DOI] [PubMed] [Google Scholar]
- 11. Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs. 2016;30:149–161. [DOI] [PubMed] [Google Scholar]
- 13. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8:114–126. [DOI] [PubMed] [Google Scholar]
- 14. Walker AM, Lanza LL, Arellano F, Rothman KJ. Mortality in current and former users of clozapine. Epidemiology. 1997;8:671–677. [DOI] [PubMed] [Google Scholar]
- 15. Kiviniemi M, Suvisaari J, Koivumaa-Honkanen H, Häkkinen U, Isohanni M, Hakko H. Antipsychotics and mortality in first-onset schizophrenia: prospective Finnish register study with 5-year follow-up. Schizophr Res. 2013;150:274–280. [DOI] [PubMed] [Google Scholar]
- 16. Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73:139–145. [DOI] [PubMed] [Google Scholar]
- 17. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113:1–11. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 4, 2016.
- 21. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wimberley T, MacCabe JH, Laursen TM, et al. Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am J Psychiatry. 2017;174:990–998. [DOI] [PubMed] [Google Scholar]
- 24. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199:281–288. [DOI] [PubMed] [Google Scholar]
- 25. Dickson RA, Dalby JT, Williams R, Warden SJ. Hospital days in clozapine-treated patients. Can J Psychiatry. 1998;43:945–948. [DOI] [PubMed] [Google Scholar]
- 26. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hennessy S, Bilker WB, Knauss JS, et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelly DL, McMahon RP, Liu F, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry. 2010;71:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Modai I, Hirschmann S, Rava A, et al. Sudden death in patients receiving clozapine treatment: a preliminary investigation. J Clin Psychopharmacol. 2000;20:325–327. [DOI] [PubMed] [Google Scholar]
- 30. Pridan S, Swartz M, Baruch Y, Tadger S, Plopski I, Barak Y. Effectiveness and safety of clozapine in elderly patients with chronic resistant schizophrenia. Int Psychogeriatr. 2015;27:131–134. [DOI] [PubMed] [Google Scholar]
- 31. Ringbäck WG, Berglund M, Lindström EA, Nilsson M, Salmi P, Rosén M. Mortality, attempted suicide, re-hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden—a register-based study. Pharmacoepidemiol Drug Saf. 2014;23:290–298. [DOI] [PubMed] [Google Scholar]
- 32. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2017; pii: S0920-9964(17)30762-4. [DOI] [PubMed] [Google Scholar]
- 33. Mela M, Depiang G. Clozapine’s effect on recidivism among offenders with mental disorders. J Am Acad Psychiatry Law. 2016;44:82–90. [PubMed] [Google Scholar]
- 34. Schulte PF, Bocxe JT, Doodeman HJ, van Haelst IM, Cohen D. Risk of new-onset diabetes after long-term treatment with clozapine in comparison to other antipsychotics in patients with schizophrenia. J Clin Psychopharmacol. 2016;36:115–119. [DOI] [PubMed] [Google Scholar]
- 35. Taylor DM, Douglas-Hall P, Olofinjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case–control comparison with risperidone long-acting injection. Br J Psychiatry. 2009;194:165–167. [DOI] [PubMed] [Google Scholar]
- 36. Gaertner I, Gaertner HJ, Vonthein R, Dietz K. Therapeutic drug monitoring of clozapine in relapse prevention: a five-year prospective study. J Clin Psychopharmacol. 2001;21:305–310. [DOI] [PubMed] [Google Scholar]
- 37. Khan AA, Ashraf A, Baker D, et al. Clozapine and incidence of myocarditis and sudden death—long term Australian experience. Int J Cardiol. 2017;238:136–139. [DOI] [PubMed] [Google Scholar]
- 38. Lee J, Bies R, Bhaloo A, Powell V, Remington G. Clozapine and anemia: a 2-year follow-up study. J Clin Psychiatry. 2015;76:1642–1647. [DOI] [PubMed] [Google Scholar]
- 39. Munro J, O’Sullivan D, Andrews C, Arana A, Mortimer A, Kerwin R. Active monitoring of 12,760 clozapine recipients in the UK and Ireland. Beyond pharmacovigilance. Br J Psychiatry. 1999;175:576–580. [DOI] [PubMed] [Google Scholar]
- 40. Srivastava S, Agarwal A, Sharma M. A three-year naturalistic follow-up of patients receiving clozapine: report from India. Int J Psychiatry Clin Pract. 2002;6:167–171. [DOI] [PubMed] [Google Scholar]
- 41. Davis MC, Fuller MA, Strauss ME, Konicki PE, Jaskiw GE. Discontinuation of clozapine: a 15-year naturalistic retrospective study of 320 patients. Acta Psychiatr Scand. 2014;130:30–39. [DOI] [PubMed] [Google Scholar]
- 42. Lindstrom LH. A retrospective study on the long-term efficacy of clozapine in 96 schizophrenic and schizoaffective patients during a 13-year period. Psychopharmacology. 1989;99 (suppl):S84–S86. [DOI] [PubMed] [Google Scholar]
- 43. Rimón R, Kuoppasalmi K, Naukkarjnen H, Lang S, Sandqvist A, Leinonen E. Clozapine decreases the level of anxiety and aggressive behaviour in patients with therapy-refractory schizophrenia. Nord J Psychiatry. 1994;48:315–320. [Google Scholar]
- 44. DE Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stringhini S, Carmeli C, Jokela M, et al. ; LIFEPATH Consortium. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. 2017;389:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Hert M, Correll CU, Cohen D. Do antipsychotic medications reduce or increase mortality in schizophrenia? A critical appraisal of the FIN-11 study. Schizophr Res. 2010;117:68–74. [DOI] [PubMed] [Google Scholar]
- 48. Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided?J Clin Psychiatry. 2013;74:603–613; quiz 613. [DOI] [PubMed] [Google Scholar]
- 49. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277–288. [DOI] [PubMed] [Google Scholar]
- 50. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. [DOI] [PubMed] [Google Scholar]
- 51. Kane JM. Clozapine reduces all-cause mortality. Am J Psychiatry. 2017;174:920–921. [DOI] [PubMed] [Google Scholar]
- 52. Bachmann CJ, Aagaard L, Bernardo M, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51. [DOI] [PubMed] [Google Scholar]
- 53. Nielsen J, Dahm M, Lublin H, Taylor D. Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol. 2010;24:965–971. [DOI] [PubMed] [Google Scholar]
- 54. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simon GE, Stewart C, Yarborough BJ, et al. Mortality rates after the first diagnosis of psychotic disorder in adolescents and young adults. JAMA Psychiatry. 2018;75:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.