Abstract
Introduction
Mismatch negativity (MMN) is a measure of automatic neurophysiological brain processes for detecting unexpected sensory stimuli. This study investigated MMN reduction in patients with schizophrenia and bipolar disorder and examined whether cortical thickness is associated with MMN, for exploratory purposes.
Methods
Electroencephalograms were recorded in 38 patients with schizophrenia, 37 patients with bipolar disorder, and 32 healthy controls (HCs) performing a passive auditory oddball paradigm. All participants underwent T1 structural magnetic resonance imaging scanning to investigate the cortical thickness of MMN-generating regions. Average MMN amplitudes from the frontocentral electrodes were analyzed.
Results
Patients with schizophrenia and bipolar disorder exhibited significantly reduced MMN amplitude compared with HCs. In bipolar disorder, we found intermediate MMN amplitude among the groups. Average MMN and cortical thickness of the right superior temporal gyrus (STG) were significantly negatively correlated in patients with schizophrenia. In patients with bipolar disorder, average MMN was significantly correlated with cortical thickness of the left anterior cingulate cortex and the right STG. MMN showed negative correlations with social and occupational functioning in schizophrenia, and with the Korean auditory verbal learning test for delayed recall in bipolar disorder.
Conclusions
MMN reduction was associated with cortical thinning in frontal and temporal areas in patients, particularly with an auditory verbal hallucination-related region in schizophrenia and emotion-related regions in bipolar disorder. MMN was associated with functional outcomes in schizophrenia, whereas it was associated with neurocognition in bipolar disorder.
Keywords: mismatch negativity, cortical thickness, schizophrenia, bipolar disorder
Introduction
Mismatch negativity (MMN) is an event-related potential (ERP) elicited when a sequence of unattended repetitive sounds is interrupted by a deviant stimulus.1 The major pathology of MMN reduction appears to originate from the dysfunction of the N-methyl-D-aspartate (NMDA) receptor system.2,3 NMDA-receptor-mediated glutamatergic dysfunction may well explain the pathology of both schizophrenia and bipolar disorder,3,4 which explicitly exhibit MMN attenuation.
There are 2 main theories regarding the mechanisms underlying MMN. One of the theories is “sensory memory.”5 According to this theory, standard stimuli make a trace in the memory, and MMN is evoked by deviant stimuli which have incongruent sensory property. This theory has been expanded into “predictive coding”6 and “regularity violation” theories7 which state that MMN can be explained with a prediction error by deviant stimulus. The other theory is “neural adaptation,”8 which explains that repeated standard stimuli lead to adaptation and attenuation of neural activity, whereas neurons regard deviant stimuli as novel, and are less adapted to them.
MMN reduction has been found consistently in schizophrenia across various stages of the disease progress9–11 and interpreted as reflecting impairments in early pre-attentive auditory processing.12,13 Recent research has also shown that bipolar disorder exhibits abnormally decreased MMN.14–16 However, given that earlier research on bipolar disorder reported null findings,17–19 it remains unclear how robust the apparent MMN decrease in bipolar disorder actually are.
The auditory MMN is generally generated in the primary auditory cortex and in adjacent areas of the superior temporal lobe. The frontal areas including the middle and inferior frontal gyrus (IFG) and the anterior cingulate cortex (ACC) are regarded as additional MMN generators.20–27 The frontal generators of the MMN are activated slightly later than the temporal ones. The temporal generators have been associated with auditory feature analysis and deviance detection, and the frontal generators with the involuntary switching of attention towards changes in the auditory environment.24,28 Moreover, it has been reported that functional and anatomical alterations in the regions regarded as MMN generators are related with impaired executive function in schizophrenia, and impaired emotional process and inhibition control in bipolar disorder.29–31
Magnetic resonance imaging (MRI) studies have demonstrated structural brain abnormalities in patients with schizophrenia and bipolar disorder. Several studies recently demonstrated that cortical thinning in the frontal lobe appears to be common in both schizophrenia and bipolar disorder.32–34 These studies suggested that thinning of the frontal lobe may represent a biological feature shared by both disease groups.
There have been few studies investigating the relationship between MMN reduction and brain structural abnormalities in schizophrenia. MMN reduction has been reported to be correlated with gray matter volume reduction in Heschl’s gyrus in the left hemisphere.35 Another study demonstrated that bilateral gray matter reductions in Heschl’s gyrus as well as motor and executive regions of the frontal cortex correlated with reduced MMN amplitudes.36 The latest study showed negative correlations between MMN source activity and cortical thickness in the bilateral inferior frontal gyri.37 For bipolar disorder, there is a lack of research on abnormalities of MMN and brain structures. Furthermore, no previous studies have explored and compared the relationship between MMN and cortical thickness in patients with schizophrenia and bipolar disorder.
Neurocognitive dysfunction and disturbances of social function are well-known features of schizophrenia.38,39 Several studies suggest that neurocognitive dysfunction may be associated with poor social functioning in schizophrenia.40,41 Bipolar disorder has generally been regarded as having a better course and outcome than schizophrenia.42 However, previous studies have revealed that patients with bipolar disorder demonstrate neuropsychological deficits in executive function, verbal learning, and immediate and delayed verbal memory.43,44 Moreover, deficits in social functioning have been reported in patients with bipolar disorder.42
In this study, we aimed to examine the reduction of MMN and its correlation with cortical thickness in patients with schizophrenia and bipolar disorder, for exploratory purposes. In addition, correlations between MMN and neurocognitive and social functioning were also examined. We hypothesized that MMN amplitudes would be reduced in patients with schizophrenia and bipolar disorder compared to those in healthy controls (HCs) and that reduced MMN would show close relationships with the cortical thickness of frontal and temporal areas.
Methods
Participants
A total of 107 subjects between the ages of 20 and 64 years participated in this study. The subjects included patients with schizophrenia (n = 38, age: 43.26 ± 10.9 [range: 21–60]) and bipolar disorder (n = 37, age: 41.00 ± 13.06 [range: 20–63]) as well as HCs (n = 32, age: 43.09 ± 13.16 [range: 23–64]). All patients were assessed for Axis I45 and II46 disorders based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (SCID) by a psychiatrist. Psychiatric symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS) for patients with both diseases47 and the Young Mania Rating Scale (YMRS) for bipolar disorder.48 No patient had a lifetime history of central nervous system disease, alcohol or drug abuse, mental retardation, or head injury with loss of consciousness. Patients with schizophrenia were being treated with atypical antipsychotics, and patients with bipolar disorder with mood-stabilizing agents (lithium, topiramate, lamotrigine, and sodium valproate) with or without atypical antipsychotics. Thirty-two HCs were recruited from the local community through newspapers and flyers. An initial screening interview excluded subjects with identifiable neurological disorders, head injury, or any personal or family history of psychiatric illness. After the initial screening, potential HCs were interviewed using the SCID for Axis II Psychiatric Disorders46 and were excluded if they had any of these disorders.
All subjects signed a written informed consent form approved by the Institutional Review Board of Inje University Ilsan Paik Hospital (July 23, 2015).
Psychological Measures
To evaluate neurocognition, a verbal fluency test49 and the Korean-Auditory Verbal Learning Test (K-AVLT)50 were applied. In the verbal fluency test, subjects state the names of as many animals as possible within 60 seconds. This evaluates verbal production and semantic memory abilities.49 The K-AVLT, which is included in the Rey-Kim Memory Test,50 is a verbal memory test consisting of 5 immediate recall trials (trials 1–5), plus delayed recall and recognition trials. The immediate recall score is the sum of words (trials 1–5) recalled correctly. The delayed recall score indicates the number of words recalled correctly after a delay period of 20 minutes. The delayed recognition score indicates the correctly chosen words in the original list (15 words), spoken by the examiner, among a list of 50 words after delayed recall.
To assess functional outcomes, the Social and Occupational Functioning Assessment Scale (SOFAS)51,52 was applied. The SOFAS was derived as a 1-item rating scale for Axis V, the clinician’s judgment of the overall level of functioning, in the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition. The SOFAS is a global rating of current functioning ranging from 0 to 100, with lower scores representing lower functioning.51,52
In addition, the Hospital Anxiety and Depression Scale (HADS)53,54 was applied to measure symptoms of anxiety and depression. The HADS consisted of 14 items using a 4-point Likert scale (scoring 0–3) where higher scores indicated higher levels of anxiety and depression. The HADS is separated into two 7-item subscales of anxiety and depression.
Auditory Stimuli and Procedures
The subjects were seated in a comfortable chair, in a quiet, shielded room in front of a computer screen (Mitsubishi, 22-inch CRT monitor), and asked to watch a Charlie Chaplin movie without paying attention to auditory stimuli. The auditory stimuli were delivered via MDR-D777 headphones (Sony, Tokyo, Japan) and consisted of sounds at 85 dB SPL and 1000 Hz. Deviant tones lasting 100 ms were presented randomly, interspersed with standard tones lasting 50 ms (probabilities: 10% and 90%, respectively). In total, 750 auditory stimuli were presented with an interstimulus interval of 500 ms. The experiment took about 10 min to complete. The stimuli were generated using E-Prime software (Psychology Software Tools).
Recording and Preprocessing of Electroencephalography
Electroencephalography (EEG) recording was synchronized to stimulus presentation onset by E-Prime. EEG was recorded using a NeuroScan SynAmps amplifier (Compumedics USA) with 64 Ag-AgCl electrodes mounted on a Quik-Cap using an extended 10–20 placement scheme. The ground electrode was placed on the forehead and the physically linked reference electrode was attached to both mastoids. The vertical electrooculogram (EOG) channels were positioned above and below the left eye, and the horizontal EOG channels were recorded at the outer canthus of each eye. The impedance was maintained below 5 kΩ. EEG data were recorded with a 0.1–100 Hz band pass filter at a sampling rate of 1000 Hz.
The recorded EEG data were preprocessed using CURRY 7 (Compumedics USA). Gross artifacts such as movement artifacts were rejected by visual inspection by a trained person with no prior information regarding the data origin. Artifacts related to eye movement or eye blinks were removed using the mathematical procedure implemented in the preprocessing software55 of CURRY 7. The data were filtered using a 0.1–30 Hz bandpass filter and epoched from 100 ms pre-stimulus to 600 ms post-stimulus. The epochs were subtracted from the average value of the pre-stimulus interval for baseline correction. If any remaining epochs contained significant physiological artifacts (amplitude exceeding ± 75 μV) in any site over 62 electrodes, they were excluded from further analysis. Only artifact-free epochs were averaged across trials and subjects for ERP analysis. The MMN wave was generated by subtracting the standard ERP wave from the deviant ones. MMN amplitude was measured as the mean voltage between 130 and 280 ms at 9 electrode sites (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, and C4), because the frontocentral electrodes have shown larger MMN amplitudes.56–58 The time window for MMN amplitudes was based on visual inspection of the grand-averaged waveforms at FCz. The number of epochs of deviant and standard stimuli used for the analysis did not significantly differ among patients with schizophrenia or bipolar disorder and HCs (deviant stimuli: 66.53 ± 6.66 vs 67.49 ± 6.78 vs 68.31 ± 8.28, P = .586, standard stimuli: 594.47 ± 59.08 vs 600.78 ± 58.65 vs 609.66 ± 73.78, P = .611, respectively).
MRI Acquisition and SBM
MRI was performed using a 1.5 T scanner (Magneton Avanto, Siemens). Head motion was minimized with restraining foam pads provided by the manufacturer. High-resolution T1-weighted MRI images were acquired with the acquisition parameters of a 227 × 384 acquisition matrix, a 210 × 250 field-of-view, 0.9 × 0.7 × 1.2 voxel size, a total of 87168 voxels, a TE of 3.42 ms, a TR of 1900 ms, 1.2 mm slice thickness, and a flip angle of 15°.
All images were inspected visually for motion or other artifacts before and after preprocessing. Surface-based morphometry (SBM) analysis was conducted using CAT12 (http://dbm.neuro.uni-jena.de/cat/) implemented in SPM12 (Wellcome Department of Cognitive Neurology). SPM12 tissue probability maps were used for the initial spatial registration. The structural T1 images were regularized with an ICBM East Asian template and normalized using the DARTEL algorithm.59 The images were then segmented into gray matter, white matter, and cerebrospinal fluid.60 Jacobian-transformed tissue probability maps were used to modulate images. The projection-based thickness method was applied to the SBM analysis to estimate the cortical thickness for the left and right hemispheres.61
The cortical thickness of the regions was extracted using the Destrieux atlas, which is the default FreeSurfer atlas. The Destrieux atlas contains 75 cortical areas in each hemisphere, including both gyri and sulci. Segmentation is automatically conducted using probabilistic methods.62 The ROIs of cortical thickness related to the MMN generator were selected based on the results of the following neuroimaging and ERP source-localization studies: the frontal areas, including the ACC, middle frontal gyrus, and IFG, and the temporal areas, including Heschl’s gyrus and the superior temporal gyrus (STG).20–27
Statistical Analysis
A chi-squared test and 1-way ANOVA were used to examine differences in demographic variables among the 3 groups. For further analyses, MMN amplitudes at the 9 electrodes were averaged, which is a more suitable way to represent and interpret the results than using each electrode. A multivariate ANOVA with the 3 groups as between-subjects variable and premorbid IQ as a covariate was used to assess patterns of MMN activity. Post hoc pairwise comparisons using least significant difference (LSD) were conducted between groups. Effect sizes are expressed as partial eta squared (η2).
A partial Pearson’s correlation was conducted between MMN amplitude and other variables including psychological measures and thickness of ROIs, with a 5000-bootstrap resampling technique to correct for multiple correlations in each group. The bootstrap test is a weaker method than Bonferroni test for solving the multiple comparison problem; however, the robustness and stability of the bootstrap test have been recognized by various previous studies.63–65 Further, the bootstrap test has been widely used in EEG analysis.66,67 For the patient groups, a partial Pearson’s correlation was performed to control for body mass index, alcohol consumption, cigarette smoking, the potential effects of medication (equivalent doses of chlorpromazine and sodium valproate68), and duration of illness as covariates. For the control group, a partial Pearson’s correlation was performed to control for body mass index, alcohol consumption, and cigarette smoking as covariates. The significant level was set at P < .05 (2-tailed). Statistical analyses were performed using SPSS 21 (SPSS, Inc.).
Results
Demographic and Psychological Characteristics
Table 1 shows the comparison of demographic and psychological characteristics among patients with schizophrenia and bipolar disorder and HCs. The premorbid IQ was significantly different among the 3 groups; HCs showed a significantly higher premorbid IQ than patients with schizophrenia and bipolar disorder (100.98 ± 10.29 vs 97.79 ± 8.21 vs 107.76 ± 9.59, P < .001). The scores of verbal fluency and the K-AVLT-delayed recall were significantly higher in HCs than in patients with schizophrenia and bipolar disorder (verbal fluency: 15.11 ± 5.17 vs 14.57 ± 5.35 vs 19.03 ± 5.87, P = .002; K-AVLT-delayed recall: 6.14 ± 3.54 vs 7.57 ± 3.60 vs 9.97 ± 2.04, P < .001, respectively). The HADS-anxiety score was significantly lower in HCs than in patients with schizophrenia and bipolar disorder (7.29 ± 3.00 vs 8.46 ± 3.78 vs 5.55 ± 2.25, P = .001). Furthermore, The SOFAS score was significantly lower in schizophrenia than in bipolar disorder (62.71 ± 15.28 vs 70.81 ± 12.28, P = .014).
Table 1.
Demographic Characteristics of all Study Participants
| Schizophreniaa (N = 38) | Bipolar Disorderb (N = 37) | Healthy Controlsc (N = 32) | P | Post hoc (LSD) | |
|---|---|---|---|---|---|
| Age (y) | 43.26 ± 10.99 | 41.00 ± 13.06 | 43.09 ± 13.16 | .686 | |
| Sex | .573 | ||||
| Male | 15 (39.5) | 11 (29.7) | 13 (40.6) | ||
| Female | 23 (60.5) | 26 (70.3) | 19 (59.4) | ||
| Premorbid IQ | 100.98 ± 10.29 | 97.79 ± 8.21 | 107.76 ± 9.59 | <.001 | a < c, b < c |
| Education (y) | 13.29 ± 2.55 | 12.62 ± 2.97 | 13.94 ± 3.71 | .211 | |
| Number of hospitalizations | 2.97 ± 3.64 | 4.06 ± 9.78 | .526 | ||
| Duration of illness (y) | 12.64 ± 8.51 | 9.88 ± 6.95 | .151 | ||
| Onset age (y) | 29.64 ± 10.77 | 31.35 ± 13.05 | .560 | ||
| Dosage of medication (CPZ equivalent, mg) | 376.08 ± 535.90 | 305.51 ± 436.24 | |||
| Dosage of medication (equivalent to sodium valproate dose, mg) | 161.89 ± 488.69 | 767.69 ± 537.49 | |||
| PANSS | |||||
| Positive | 14.61 ± 8.09 | 8.97 ± 1.98 | |||
| Negative | 17.32 ± 6.55 | 9.05 ± 2.70 | |||
| General | 32.26 ± 11.14 | 24.00 ± 6.10 | |||
| Total | 64.18 ± 23.52 | 42.03 ± 8.95 | |||
| YMRS | 6.28 ± 6.71 | ||||
| Verbal fluency | 15.11 ± 5.17 | 14.57 ± 5.35 | 19.03 ± 5.87 | .002 | a < c, b < c |
| KAVLT-delayed recall | 6.14 ± 3.54 | 7.57 ± 3.60 | 9.97 ± 2.04 | <.001 | a < c, b < c |
| HADS-depression | 10.03 ± 3.69 | 10.68 ± 4.42 | 8.68 ± 3.42 | .107 | |
| HADS-anxiety | 7.29 ± 3.00 | 8.46 ± 3.78 | 5.55 ± 2.25 | .001 | a > c, b > c |
| SOFAS | 62.71 ± 15.28 | 70.81 ± 12.28 | .014 | ||
Note: CPZ, chlorpromazine; PANSS, positive and negative syndrome scale; YMRS, young mania rating scale; KAVLT, Korean auditory verbal learning test; HADS, hospital anxiety and depression scale; SOFAS, social and occupational functioning assessment scale; LSD, least significant difference.
MMN
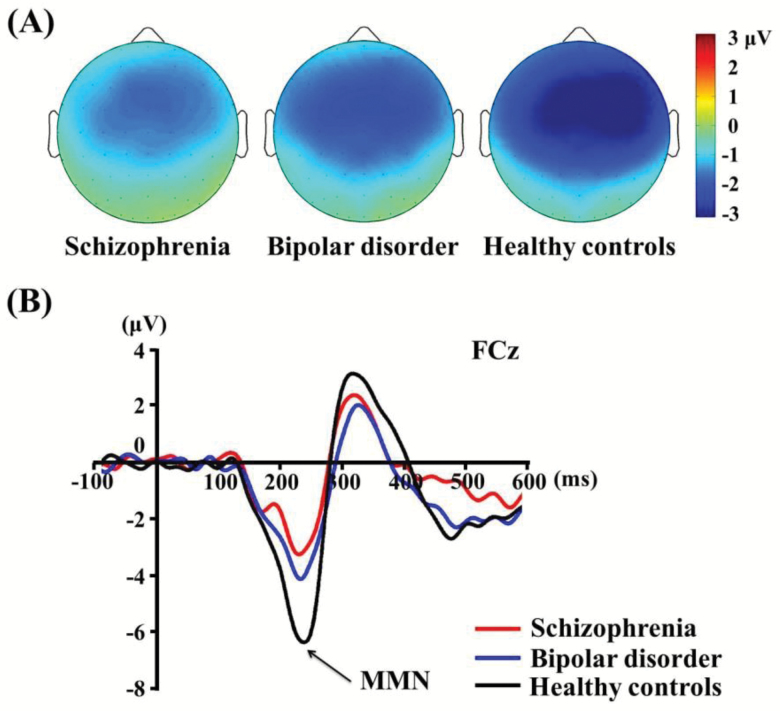
Grand-averaged MMN waveforms and topographical maps for each group are shown in figure 1. All 3 groups exhibited higher MMN activity in frontocentral regions, although patients with schizophrenia and bipolar disorder exhibited clearly reduced MMN amplitudes compared with HCs.
Fig. 1.
(A) Topographic maps of mismatch negativity (MMN), and (B) MMN waveforms at electrode site FCz in schizophrenia, bipolar disorder, and healthy controls.
A multivariate ANOVA revealed significant main effects of group for average MMN (F = 11.803, df = 2, P < .001). Post hoc tests revealed that patients showed significantly smaller MMN amplitude than HCs (table 2). The average MMN amplitudes for all 9 channels were: −1.59 ± 0.92 μV, −2.08 ± 1.35 μV, and −3.09 ± 1.67 μV for schizophrenia, bipolar disorder, and HCs, respectively.
Table 2.
Mean (SD) Amplitude of Mismatch Negativity of Among-Group Differences
| Site (μV) | Schizophreniaa (N = 38) | Bipolar Disorderb (N = 37) | Healthy Controlsc (N = 32) | Effect Size (η2) | P | Post hoc (LSD) |
|---|---|---|---|---|---|---|
| F3 | −1.40 ± 1.03 | −1.95 ± 1.25 | −2.73 ± 1.53 | 0.166 | <.001 | a < c, b < c |
| Fz | −1.82 ± 1.03 | −2.17 ± 1.49 | −3.23 ± 1.72 | 0.161 | <.001 | a < c, b < c |
| F4 | −1.65 ± 1.23 | −2.02 ± 1.56 | −3.30 ± 1.86 | 0.182 | <.001 | a < c, b < c |
| FC3 | −1.56 ± 1.04 | −2.10 ± 1.34 | −2.90 ± 1.63 | 0.154 | <.001 | a < c, b < c |
| FCz | −1.77 ± 0.98 | −2.33 ± 1.51 | −3.42 ± 1.71 | 0.184 | <.001 | a < c, b < c |
| FC4 | −1.61 ± 1.09 | −2.26 ± 1.57 | −3.43 ± 1.71 | 0.221 | <.001 | a < c, b < c |
| C3 | −1.49 ± 1.13 | −1.97 ± 1.26 | −2.75 ± 1.61 | 0.153 | <.001 | a < c, b < c |
| Cz | −1.54 ± 1.08 | −1.97 ± 1.54 | −2.89 ± 1.85 | 0.133 | .001 | a < c, b < c |
| C4 | −1.46 ± 1.05 | −1.98 ± 1.47 | −3.11 ± 1.82 | 0.195 | <.001 | a < c, b < c |
| Average MMN | −1.59 ± 0.92 | −2.08 ± 1.35 | −3.09 ± 1.67 | 0.194 | <.001 | a < c, b < c |
Note: Premorbid IQ was used as a covariate. LSD, least significant difference; MMN, mismatch negativity.
Correlations Between MMN and Psychological Measures
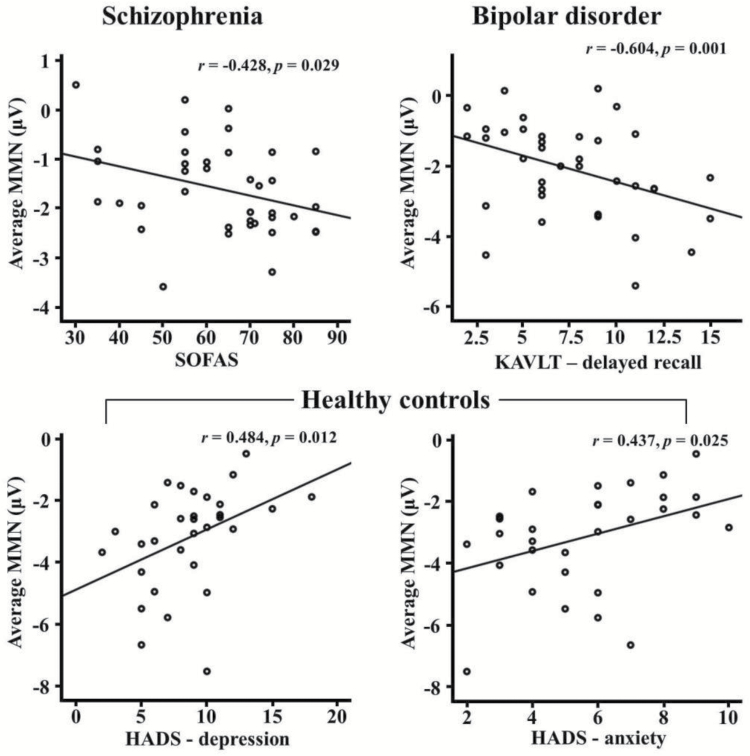
In patients with schizophrenia, there was a significant correlation between average MMN amplitude and SOFAS (r = −.428, P = .029). In bipolar disorder, average MMN amplitude was significantly negatively correlated with the K-AVLT-delayed recall (r = −.604, P = .001). In HCs, there were significant correlations between average MMN amplitudes and HADS-depression/anxiety (HADS-depression: r = .484, P = .012; HADS-anxiety: r = .437, P = .025). Figure 2 shows scatter plots between MMN amplitudes and psychological measures in each group.
Fig. 2.
Correlations between MMN amplitudes and psychological measures in each group. MMN: mismatch negativity; SOFAS: social and occupational functioning assessment scale; KAVLT: Korean auditory verbal learning test; HADS: hospital anxiety and depression scale.
Correlations Between MMN and Cortical Thickness
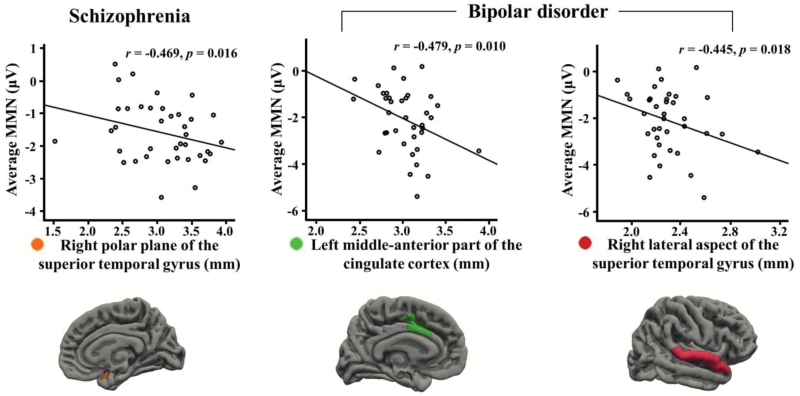
In schizophrenia, average MMN amplitude was significantly negatively correlated with cortical thickness of a region, the right polar plane of the STG (r = −.469, P = .016). In bipolar disorder, average MMN amplitude was significantly negatively correlated with cortical thickness of the left middle anterior part of the cingulate cortex and the right lateral aspect of the STG (r = −.479, P = .010; r = −.445, P = .018, respectively). Figure 3 shows scatter plots between MMN amplitudes and cortical thickness in patients with schizophrenia and bipolar disorder. Comparison between correlations of schizophrenia and bipolar disorder for average MMN amplitude and cortical thickness was provided in supplementary table 1.
Fig. 3.
Correlations between mismatch negativity (MMN) amplitudes and cortical thickness in patients with schizophrenia and bipolar disorder.
Discussion
This study aimed to examine whether MMN was related to cortical thickness in patients with schizophrenia and bipolar disorder, for exploratory purposes. Patient groups exhibited diminished MMN amplitudes that were associated with cortical thinning in frontal and temporal areas. There were significant negative correlations between average MMN and cortical thickness in the right STG of patients with schizophrenia, and in the left ACC and the right STG of patients with bipolar disorder. Furthermore, MMN showed negative correlations with functional outcomes in schizophrenia and with neurocognitive function in bipolar disorder.
Our results reveal that patients with schizophrenia and bipolar disorder show reduced MMN amplitude in the frontocentral region compared with HCs. There was no significant difference in MMN amplitude between schizophrenia and bipolar patient groups. This is consistent with the results of previous studies showing the robust attenuation of duration MMN in patients with schizophrenia.9,69,70 Patients with bipolar disorder have shown mixed results. Although earlier results were negative,17,18,35,71 more recent studies have shown MMN amplitude attenuation in bipolar disorder.15,70,72,73 In such cases, in line with our results, bipolar disorder shows intermediate MMN amplitudes between those found in schizophrenia and in HCs.15,16 Various studies suggest that schizophrenia and bipolar disorder have common etiological factors and pathophysiological pathways, which could indicate that they have overlapping clinical features.74 The overlap or similarity between schizophrenia and bipolar disorder has been found at the neural processing level,15,16 as well as the brain anatomy level,74,75 and even at the genetic and molecular levels.76,77 In this regard, the potential phenomenological overlap between these 2 disorders might explain the intermediate MMN amplitude reduction for patients with bipolar disorder compared to HCs and patients with schizophrenia.
Here, reduced average MMN was correlated with cortical thinning in the right STG in schizophrenia. In bipolar disorder, reduced average MMN was associated with cortical thinning in the left ACC and in the right STG. These results support that MMN alterations could be associated with structural changes in the regions related to MMN generators in patients with schizophrenia and bipolar disorder.
Seol et al37 found negative correlations between MMN current source density strength using magnetoencephalography (MEG) and cortical thickness in the bilateral inferior frontal gyri in schizophrenia. These results were thought to be due to abnormal neuronal pruning, aberrant global networks, and/or neuronal compensation in schizophrenia.37 In addition, Rasser et al36 reported that MMN amplitude was correlated with cortical volume in frontal and temporal areas including the left superior frontal gyrus and the right STG. The STG has been known to be involved in language-related symptoms of schizophrenia including hallucinations and thought disorders.78,79 Reduced white matter volume in the right STG is observed in schizophrenia with auditory verbal hallucination.80 Furthermore, gray matter volume reduction of this region has been demonstrated in schizophrenia with hallucinations.81,82 In addition, previous studies have reported that hallucinations are associated with MMN reduction.12,83,84 These findings corroborate the suggestion that auditory verbal hallucinations compete with incoming external stimuli for finite resources in the auditory cortex,85 resulting in reduced MMN. Our results suggest that MMN attenuation is associated with cortical thinning in the region related to auditory verbal hallucinations in schizophrenia.
In bipolar disorder, ACC abnormalities have been reported in previous studies,86–88 particularly, reduced gray matter volume or cortical thinning in the left ACC.89,90 The ACC is known as an important region in identifying the emotional significance of stimuli and in producing affective states.91,92 Interestingly, right-sided volume reduction or cortical thinning in the STG were found in patients with bipolar disorder.34,93,94 Functional abnormalities in the right STG have been associated with bipolar disorder,95,96 and abnormal responses to emotional prosody.97 Our results suggest that MMN attenuation is associated with cortical thinning in these regions related to emotion in bipolar disorder.
Considering that each region indicates structural and/or functional significance in the corresponding group, our results have important clinical implications in that MMN reduction corresponded to disease-specific neuropathologies in frontal and temporal regions in schizophrenia and bipolar disorder.
In schizophrenia, MMN attenuation was associated with poor social and occupational functioning. Meanwhile, in bipolar disorder, reduced MMN amplitude was associated with poor delayed verbal memory. Light and Braff56 suggested that MMN reduction represents a core neurophysiological dysfunction in patients with schizophrenia that is linked to global impairment in everyday functioning. There has been a well-replicated association between reduced MMN amplitudes and poor social and occupational functioning in schizophrenia.13,27,58,98 In bipolar disorder, MMN showed stronger involvement with cognitive functions than with social functioning, which may be due to less deterioration of social functioning in patients with bipolar disorder than in those with schizophrenia.99 In HCs, the present study showed that MMN reduction was associated with symptom severity in depression and anxiety. Previously, anxiety symptoms were positively correlated with MMN amplitude in healthy volunteers.100 Anxiety, as an alarm system, could influence attention switching and consequently cause the MMN attenuation.101,102 In sum, our results suggest that MMN amplitudes mainly represent social functioning and cognitive functions in schizophrenia and bipolar disorders, respectively.
This study has several limitations. First, most patients were chronic patients and were taking atypical antipsychotics and mood-stabilizing agents. A previous longitudinal study found that cortical thinning was associated with higher cumulative antipsychotic intake in patients with schizophrenia.103 Although we controlled the dosage of medication, future studies are warranted to assess the duration of medication treatment and include drug-naive patients. Second, this study utilized duration deviants and it is thus not appropriate to generalize our conclusions to other types of deviants, such as those of frequency or intensity.
In conclusion, our results demonstrate that MMN alterations are associated with frontotemporal cortical thickness changes in patients with schizophrenia and bipolar disorder, particularly with an auditory verbal hallucination-related region in schizophrenia and emotion-related regions in bipolar disorder. Our results suggest that the conjoint use of structural MRI and ERP could provide important information about the correlated brain regions of ERP components in both diseases. Furthermore, MMN was associated with functional outcomes in schizophrenia, and with neurocognition in bipolar disorder. Replication of these results in future studies, with sufficient statistical power, would be needed to verify the observed tentative trends regarding the associations between MMN, and cortical thickness and psychological measures of patients with schizophrenia and bipolar disorder.
Funding
This work was supported by a grant from the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2018R1A2A2A05018505), and by the Ministry of Science, ICT & Future Planning (NRF-2015M3C7A1028252).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst). 1978;42:313–329. [DOI] [PubMed] [Google Scholar]
- 2. Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci. 1996;93:11962–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. [DOI] [PubMed] [Google Scholar]
- 4. McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Näätänen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005;42:25–32. [DOI] [PubMed] [Google Scholar]
- 6. Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol. 2009;120:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winkler I, Schröger E, Cowan N. The role of large-scale memory organization in the mismatch negativity event-related brain potential. J Cogn Neurosci. 2001;13:59–71. [DOI] [PubMed] [Google Scholar]
- 8. Jääskeläinen IP, Ahveninen J, Bonmassar G, et al. . Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci USA 2004;101:6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 10. Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. [DOI] [PubMed] [Google Scholar]
- 11. Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. [DOI] [PubMed] [Google Scholar]
- 12. Hirayasu Y, Potts GF, O’Donnell BF, et al. . Auditory mismatch negativity in schizophrenia: topographic evaluation with a high-density recording montage. Am J Psychiatry. 1998;155:1281–1284. [DOI] [PubMed] [Google Scholar]
- 13. Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. [DOI] [PubMed] [Google Scholar]
- 14. Andersson S, Barder HE, Hellvin T, Løvdahl H, Malt UF. Neuropsychological and electrophysiological indices of neurocognitive dysfunction in bipolar II disorder. Bipolar Disord. 2008;10:888–899. [DOI] [PubMed] [Google Scholar]
- 15. Jahshan C, Wynn JK, Mathis KI, Altshuler LL, Glahn DC, Green MF. Cross-diagnostic comparison of duration mismatch negativity and P3a in bipolar disorder and schizophrenia. Bipolar Disord. 2012;14:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaur M, Battisti RA, Lagopoulos J, Ward PB, Hickie IB, Hermens DF. Neurophysiological biomarkers support bipolar-spectrum disorders within psychosis cluster. J Psychiatry Neurosci. 2012;37:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catts SV, Shelley AM, Ward PB, et al. . Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. [DOI] [PubMed] [Google Scholar]
- 18. Umbricht D, Koller R, Schmid L, et al. . How specific are deficits in mismatch negativity generation to schizophrenia?Biol Psychiatry. 2003;53:1120–1131. [DOI] [PubMed] [Google Scholar]
- 19. Hall MH, Rijsdijk F, Kalidindi S, et al. . Genetic overlap between bipolar illness and event-related potentials. Psychol Med. 2007;37:667–678. [DOI] [PubMed] [Google Scholar]
- 20. Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- 21. Jemel B, Achenbach C, Müller BW, Röpcke B, Oades RD. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topogr. 2002;15:13–27. [DOI] [PubMed] [Google Scholar]
- 22. Park HJ, Kwon JS, Youn T, et al. . Statistical parametric mapping of LORETA using high density EEG and individual MRI: application to mismatch negativities in schizophrenia. Hum Brain Mapp. 2002;17:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oknina LB, Wild-Wall N, Oades RD, et al. . Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophr Res. 2005;76:25–41. [DOI] [PubMed] [Google Scholar]
- 24. Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–19. [DOI] [PubMed] [Google Scholar]
- 25. Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schröger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage. 2003;20:1270–1282. [DOI] [PubMed] [Google Scholar]
- 26. Schönwiesner M, Novitski N, Pakarinen S, Carlson S, Tervaniemi M, Näätänen R. Heschl’s gyrus, posterior superior temporal gyrus, and mid-ventrolateral prefrontal cortex have different roles in the detection of acoustic changes. J Neurophysiol. 2007;97:2075–2082. [DOI] [PubMed] [Google Scholar]
- 27. Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Röpcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paavilainen P, Mikkonen M, Kilpeläinen M, Lehtinen R, Saarela M, Tapola L. Evidence for the different additivity of the temporal and frontal generators of mismatch negativity: a human auditory event-related potential study. Neurosci Lett. 2003;349:79–82. [DOI] [PubMed] [Google Scholar]
- 29. Kubicki M, Alvarado JL, Westin CF, et al. . Stochastic tractography study of Inferior Frontal Gyrus anatomical connectivity in schizophrenia. Neuroimage. 2011;55:1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. 2012;134:158–164. [DOI] [PubMed] [Google Scholar]
- 31. Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rimol LM, Hartberg CB, Nesvåg R, et al. . Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. [DOI] [PubMed] [Google Scholar]
- 33. Rimol LM, Nesvåg R, Hagler DJ Jr, et al. . Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–560. [DOI] [PubMed] [Google Scholar]
- 34. Knöchel C, Reuter J, Reinke B, et al. . Cortical thinning in bipolar disorder and schizophrenia. Schizophr Res. 2016;172:78–85. [DOI] [PubMed] [Google Scholar]
- 35. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasser PE, Schall U, Todd J, et al. . Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seol JJ, Kim M, Lee KH, et al. . Is there an association between mismatch negativity and cortical thickness in schizophrenia patients?Clin EEG Neurosci. 2017;48:383–392. [DOI] [PubMed] [Google Scholar]
- 38. Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. [DOI] [PubMed] [Google Scholar]
- 39. Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116:403–418. [DOI] [PubMed] [Google Scholar]
- 40. Addington J, Addington D. Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophr Res. 2000;44:47–56. [DOI] [PubMed] [Google Scholar]
- 41. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. [DOI] [PubMed] [Google Scholar]
- 42. Sanchez-Moreno J, Martinez-Aran A, Tabarés-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom. 2009;78:285–297. [DOI] [PubMed] [Google Scholar]
- 43. Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–226. [DOI] [PubMed] [Google Scholar]
- 44. Robinson LJ, Thompson JM, Gallagher P, et al. . A meta- analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. [DOI] [PubMed] [Google Scholar]
- 45. First MB, Gibbon M, Spitzer RL, Williams JB.. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 46. First MB, Gibbon M, Spitzer RL, Benjamin LS.. User’s Guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders: SCID-II. Washington, DC: American Psychiatric Pub; 1997. [Google Scholar]
- 47. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 48. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 49. Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 50. Kim H. Rey-Kim Memory Test. Daegu, Korea: Neuropsychology Press; 1999. [Google Scholar]
- 51. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148–1156. [DOI] [PubMed] [Google Scholar]
- 52. Lee JY, Cho MJ, Kwon JS. Global assessment of functioning scale and social and occupational functioning scale. Korean J Psychopharmacol. 2006;17:122–127. [Google Scholar]
- 53. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 54. Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999;38:289–296. [Google Scholar]
- 55. Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. [DOI] [PubMed] [Google Scholar]
- 56. Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. [DOI] [PubMed] [Google Scholar]
- 57. Şevik AE, Yağcıoğlu AEA, Yağcıoğlu S, Karahan S, Gürses N, Yıldız M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011;130:195–202. [DOI] [PubMed] [Google Scholar]
- 58. Lee SH, Sung K, Lee KS, Moon E, Kim CG. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:213–219. [DOI] [PubMed] [Google Scholar]
- 59. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 60. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 61. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. [DOI] [PubMed] [Google Scholar]
- 62. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 63. Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med. 2005;12:360–365. [DOI] [PubMed] [Google Scholar]
- 64. Ruscio J. Constructing confidence intervals for Spearman’s rank correlation with ordinal data: a simulation study comparing analytic and bootstrap methods. J Mod Appl Stat Methods 2008;7:7. [Google Scholar]
- 65. Pernet CR, Wilcox R, Rousselet GA. Robust correlation analyses: false positive and power validation using a new open source matlab toolbox. Front Psychol. 2012;3:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pernet CR, Chauveau N, Gaspar C, Rousselet GA. LIMO EEG: a toolbox for hierarchical LInear MOdeling of ElectroEncephaloGraphic data. Comput Intell Neurosci. 2011;2011:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim JS, Kim S, Jung W, Im CH, Lee SH. Auditory evoked potential could reflect emotional sensitivity and impulsivity. Sci Rep. 2016;6:37683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ.. Clinical Handbook of Psychotropic Drugs. Gottingenm Lower Saxony, Germany: Hogrefe Publishing; 2017. [Google Scholar]
- 69. Näätänen R, Kähkönen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12:125–135. [DOI] [PubMed] [Google Scholar]
- 70. Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hall MH, Schulze K, Rijsdijk F, et al. . Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39:1277–1287. [DOI] [PubMed] [Google Scholar]
- 72. Takei Y, Kumano S, Maki Y, et al. . Preattentive dysfunction in bipolar disorder: a MEG study using auditory mismatch negativity. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:903–912. [DOI] [PubMed] [Google Scholar]
- 73. Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. Eur Neuropsychopharmacol. 2013;23:1348–1363. [DOI] [PubMed] [Google Scholar]
- 74. Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Curr Opin Psychiatry. 2006;19:165–170. [DOI] [PubMed] [Google Scholar]
- 75. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. [DOI] [PubMed] [Google Scholar]
- 76. Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 2009;1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Matsumoto H, Simmons A, Williams S, et al. . Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am J Psychiatry. 2001;158:1299–1304. [DOI] [PubMed] [Google Scholar]
- 79. Gaser C, Nenadic I, Volz HP, Büchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14:91–96. [DOI] [PubMed] [Google Scholar]
- 80. Plaze M, Paillère-Martinot ML, Penttilä J, et al. . “Where do auditory hallucinations come from?”–A brain morphometry study of schizophrenia patients with inner or outer space hallucinations. Schizophr Bull. 2011;37:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1203–1229. [DOI] [PubMed] [Google Scholar]
- 82. Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61:14–32. [DOI] [PubMed] [Google Scholar]
- 83. Youn T, Park HJ, Kim JJ, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophr Res. 2003;59:253–260. [DOI] [PubMed] [Google Scholar]
- 84. Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’ multi-feature paradigm. Int J Psychophysiol. 2011;81:245–251. [DOI] [PubMed] [Google Scholar]
- 85. Woodruff PW. Auditory hallucinations: insights and questions from neuroimaging. Cogn Neuropsychiatry. 2004;9:73–91. [DOI] [PubMed] [Google Scholar]
- 86. Davanzo P, Thomas MA, Yue K, et al. . Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. [DOI] [PubMed] [Google Scholar]
- 87. Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–1162. [DOI] [PubMed] [Google Scholar]
- 88. Lyoo IK, Kim MJ, Stoll AL, et al. . Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. [DOI] [PubMed] [Google Scholar]
- 89. Sassi RB, Nicoletti M, Brambilla P, et al. . Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. [DOI] [PubMed] [Google Scholar]
- 90. Lyoo IK, Sung YH, Dager SR, et al. . Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. [DOI] [PubMed] [Google Scholar]
- 91. Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150; discussion 151. [DOI] [PubMed] [Google Scholar]
- 92. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. [DOI] [PubMed] [Google Scholar]
- 93. Li M, Cui L, Deng W, et al. . Voxel-based morphometric analysis on the volume of gray matter in bipolar I disorder. Psychiatry Res. 2011;191:92–97. [DOI] [PubMed] [Google Scholar]
- 94. Selvaraj S, Arnone D, Job D, et al. . Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–145. [DOI] [PubMed] [Google Scholar]
- 95. Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. [DOI] [PubMed] [Google Scholar]
- 96. Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. [DOI] [PubMed] [Google Scholar]
- 97. Malhi GS, Lagopoulos J, Ward PB, et al. . Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. [DOI] [PubMed] [Google Scholar]
- 98. Kawakubo Y, Kamio S, Nose T, et al. . Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. [DOI] [PubMed] [Google Scholar]
- 99. Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang W, Zhu SZ, Pan LC, Hu AH, Wang YH. Mismatch negativity and personality traits in chronic primary insomniacs. Funct Neurol. 2001;16:3–10. [PubMed] [Google Scholar]
- 101. Gray JA. The Psychology of Fear and Stress, vol 5 New York, NY: Press Syndicate of the University of Cambridge; 1987. [Google Scholar]
- 102. Naatanen R. Attention and Brain Function. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1992. [Google Scholar]
- 103. van Haren NE, Schnack HG, Cahn W, et al. . Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.