Abstract
One neuropathological feature of schizophrenia is a diminished number of dendritic spines in the prefrontal cortex and hippocampus. The neuregulin 1 (Nrg1) system is involved in the plasticity of dendritic spines, and chronic stress decreases dendritic spine densities in the prefrontal cortex and hippocampus. Here, we aimed to assess whether Nrg1 deficiency confers vulnerability to the effects of adolescent stress on dendritic spine plasticity. We also assessed other schizophrenia-relevant neurobiological changes such as microglial cell activation, loss of parvalbumin (PV) interneurons, and induction of complement factor 4 (C4). Adolescent male wild-type (WT) and Nrg1 heterozygous mice were subjected to chronic restraint stress before their brains underwent Golgi impregnation or immunofluorescent staining of PV interneurons, microglial cells, and C4. Stress in WT mice promoted dendritic spine loss and microglial cell activation in the prefrontal cortex and the hippocampus. However, Nrg1 deficiency rendered mice resilient to stress-induced dendritic spine loss in the infralimbic cortex and the CA3 region of the hippocampus without affecting stress-induced microglial cell activation in these brain regions. Nrg1 deficiency and adolescent stress combined to trigger increased dendritic spine densities in the prelimbic cortex. In the hippocampal CA1 region, Nrg1 deficiency accentuated stress-induced dendritic spine loss. Nrg1 deficiency increased C4 protein and decreased C4 mRNA expression in the hippocampus, and the number of PV interneurons in the basolateral amygdala. This study demonstrates that Nrg1 modulates the impact of stress on the adolescent brain in a region-specific manner. It also provides first evidence of a link between Nrg1 and C4 systems in the hippocampus.
Keywords: schizophrenia, microglia, parvalbumin, restraint, prefrontal cortex, amygdala
Introduction
Schizophrenia arises due to a complex interaction between genetic and environmental factors during critical early periods of neurodevelopment.1 Dendritic spines are small protrusions found on dendrites that harbor most excitatory synaptic connections in the brain. One neuropathological feature of schizophrenia is a diminished number of dendritic spines in the prefrontal cortex and hippocampus which is thought to arise due to augmented synaptic pruning during adolescence.2 Stress increases the risk of developing schizophrenia and when applied to rodents decreases dendritic spine densities in the prefrontal cortex and hippocampus.3–5 The Nrg1-ErbB system is involved in the plasticity of dendritic spines and stress alters Nrg1 and ErbB receptor expression, therefore it is likely Nrg1 and stress may interact to influence dendritic spine plasticity.6–9
Nrg1 deficiency confers vulnerability to the effects of stressors via dysregulation of glucocorticoid signaling.10–13 We have shown that Nrg1 heterozygous mice (Nrg1 HET mice) were more sensitive than wild-type (WT) mice to the effects of a mild restraint stress paradigm on dendritic morphology parameters which was associated with impaired sensorimotor gating development and a hypoactive HPA axis.13,14 Unfortunately, the mild restraint stress paradigm we utilized in this study was insufficient to cause dendritic spine regression in WT mice, an important background on which to assess the impact of Nrg1 deficiency. Thus in the present study, we utilized a restraint stress paradigm that reliably causes dendritic spine atrophy in rodents.3,15
We additionally sought to examine whether Nrg1 deficiency moderates other stress-induced neurobiological changes of relevance to schizophrenia, such as loss of parvalbumin (PV) GABAergic interneurons, microglial cell activation and induction of complement factor 4 (C4). These changes may also play a role in mediating the interaction of Nrg1 deficiency and stress on dendritic spine plasticity. To illustrate, ablation of ErbB4 in PV interneurons reduces dendritic spine density on neighbouring pyramidal neurons.16–19 Further, stress activates microglia, the brain’s immune cells, and there is emerging evidence that these cells influence dendritic spine dynamics.20–23 C4 has an emerging role in the pathogenesis of schizophrenia and has been postulated to contribute to excessive synaptic pruning by triggering microglia-mediated engulfment of dendritic spines.24–26 The current study therefore aims to assess whether Nrg1 deficiency confers vulnerability to the effects of adolescent stress on pyramidal neuron dendritic spine density and arborization, PV interneurons, microglial cell activation, and C4 expression in stress-responsive brain regions.
Methods
Mice
Adolescent (PND 35–56) male Nrg1 HET mice (C57BL/6JArc background strain) and WT littermates were used.27 The generation of the mice, genotyping, and housing conditions of the mice was the same as described in Chohan et al.13 All research and animal care procedures were approved by the University of Sydney’s Animal Ethics Committee in agreement with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Experimental Design
The first 2 cohorts of mice were randomly allocated to 1 of 4 experimental groups using a 2 × 2 design of genotype (WT vs Nrg1 HET mice) and stress (stress vs nonstressed homecage controls). In the first cohort, the specific number of mice were: (1) WT–no stress (WT NS), n = 7; (2) WT–stress (WT S), n = 6; (3) Nrg1 HET–no stress (Nrg1 HET NS), n = 7; and (4) Nrg1 HET-stress (Nrg1 HET S), n = 6. In the second cohort: (1) WT NS, n = 8; (2) WT S, n = 9; (3) Nrg1 HET NS, n = 6; and (4) Nrg1 HET NS, n = 8. From postnatal day (PND) 36 onward, mice were subjected to 6 h of restraint stress per day for 21 days until PND 5728 as previously described.12 All animals were sacrificed 24 h following their final restraint session. Mice from the first cohort were sacrificed by cervical dislocation and were used for Golgi staining and dendritic morphology analysis. The second cohort was anaesthetized with isoflurane and perfused transcardially with paraformaldehyde for immunofluorescence studies. A third cohort of unstressed male WT (n = 8) and Nrg1 HET mice (n = 10) (average age 5 months) were used to further examine the impact of partial genetic deletion of Nrg1 on C4 mRNA expression in the hippocampus using qPCR. A detailed description of dendritic morphology, microglial cell, C4, and PV quantification, and qPCR procedures can be found in the supplementary material.
Statistical Analysis
Statistical analyses were performed using SPSS (IBM) or Statview (SAS Institute, Inc.) software. Most data were analyzed using 2-factor ANOVA with genotype and stress conditions as between subjects factors and, when appropriate, a 3-factor ANOVA with genotype and stress conditions as between subjects factors, and dendritic order as a within subjects factor. Planned Bonferroni corrected comparisons were conducted when significant interaction effects were observed using the following comparisons (WT no stress vs Nrg1 HET no stress; WT stress vs Nrg1 HET stress; WT no stress vs WT stress; and Nrg1 HET no stress vs Nrg1 HET stress). Fold changes in C4 mRNA determined by qPCR analysis of WT and Nrg1 HET mice were analyzed using an unpaired Student’s t-test. Due to the violation of assumptions of ANOVA for fourth order prelimbic (PrL) dendritic spine density, and of C4 mean intensity data in the PrL, infralimbic (IL), CA1, and CA3 regions, the Aligned Rank Transform procedure was performed.29,30 All summarized data are reported as mean + SEM. The results of all analyses were deemed significant at P < .05.
Results
Partial Genetic Deletion of Nrg1 Promoted Idiosyncratic Dendritic Spine Densities in Response to Adolescent Stress in a Brain Region-Specific Manner
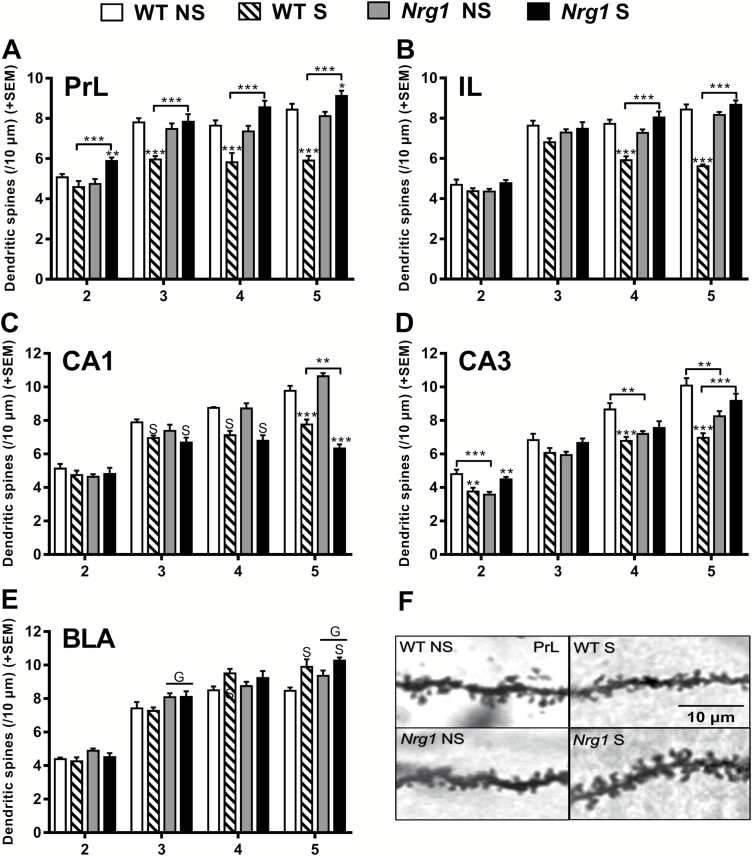
In the PrL cortex, adolescent restraint stress decreased dendritic spine density in WT mice, but increased dendritic spine density in Nrg1 deficient mice (figure 1A). Chronic adolescent stress dramatically reduced dendritic spine densities in WT mice compared to controls in third, fourth, and fifth order dendrites of the PrL cortex. However, chronic stress provoked the opposite effect in Nrg1 HET mice, as stressed Nrg1 HET mice exhibited increased dendritic spine densities compared with homecage Nrg1 HET controls on second order and fifth order apical dendrites. In addition, stressed Nrg1 HET mice displayed increased dendritic spine densities compared to stressed WT mice across orders 2–5. In the IL cortex, Nrg1 deficiency promoted resilience to the effects of chronic adolescent stress on dendritic spine density (figure 1B). Chronic adolescent restraint stress dramatically reduced spine densities in WT mice but not Nrg1 HET mice on fourth and fifth order dendrites of the IL cortex. Stressed Nrg1 HET mice exhibited greater spine density compared to stressed WT mice on fourth and fifth order apical dendrites, whereas no differences were seen between control Nrg1 HET and WT mice.
Fig. 1.
The effects of Nrg1 deficiency and stress on dendritic spine densities of apical dendrites across branching orders 2–5. (A) PrL region, (B) IL region, (C) CA1 region, (D) CA3 region, and (E) BLA region. (F) Exemplars of dendritic spines from the PrL region. Two-factor repeated measures ANOVA on spine densities across orders 2–5 of apical dendrites showed a significant effect of Nrg1 genotype (PrL: F1,22 = 32.30, P < .0001, IL: F1,22 = 36.78, P < .0001, CA1: F1,20 = 5.74, P < .05, BLA: F1,20 = 8.60, P < .001), stress (PrL: F1,22 = 4.70, P < .05, IL: F1,22 = 23.57, P < .0001, CA1: F1,20 = 214.78, P < .0001, CA3: F1,20 = 7.95, P < .05, BLA: F1,20 = 7.26, P < .05) and a Nrg1 genotype by stress interaction in most regions (PrL: F1,22 = 56.24, P < .0001, IL: F1,22 = 91.12, P < .0001, CA1: F1,20 = 5.21, P < .05, CA3: F1,20 = 51.69, P < .0001). A genotype and order interaction was observed in the medial prefrontal cortex but not in other regions (PrL: F3,66 = 3.12, P < .05, IL: F3,66 = 8.59, P < .0001). Stress by order interactions were observed across all regions (PrL: F3,66 = 4.47, P < .01, IL: F3,66 = 5.52, P < .01, CA1: F3,60 = 18.32, P < .0001, CA3: F3,60 = 3.95, P < .05, BLA: F3,60 = 5.61, P < .01) and genotype by stress by order interactions were observed in all regions except the BLA (PrL: F3,66 = 3.10, P < .05, IL: F3,66 = 8.22, P = .0001, CA1: F3,60 = 4.55, P < .001, CA3: F3,60 = 4.87, P < .001). When analyzing all of the branch orders separately using 2-factor ANOVA, significant Nrg1 genotype by stress interactions were observed across orders 2–5 in the PrL (order 2: F1,22 = 12.54, P < .01; order 3: F1,22 = 17.44, P < .001; order 4: F1,22 = 20.45, P < .001 and order 5: F1,22 = 71.99, P < .0001), orders 3, 4, and 5 in the IL (order 3: F1,22 = 4.73, P < .05; order 4: F1,22 = 33.18, P < .0001 and order 5: F1,22 = 96.51, P < .0001), order 5 in the CA1 region (F1,22 = 17.91, P < .001), across each individual order in the CA3 region (order 2: F1,20 = 24.06, P < .0001; order 3: F1,20 = 6.93, P < .05; order 4: F1,19 = 13.84, P < .001; and order 5: F1,19 = 30.61, P < .0001). Significant effects of stress across orders 3, 4, and 5 were observed in the CA1 region (order 3: F1,22 = 8.98, P < .01; order 4: F1,22 = 47.56, P < .0001 and order 5: F1,22 = 84.00, P < .0001) and on fourth and fifth order dendrites in the BLA (F1,21 = 5.93, P < .05, F1,20 = 15.38, P < .001, respectively). There was a significant effect of genotype on dendritic orders 3 and 5 in the BLA (F1,21 = 6.85, P < .05; F1,20 = 4.58, P < .05, respectively). S = main effect of stress and G = main effect of genotype. Bonferroni post hoc analyses: **P < .01, ***P < .001.
In the CA1 region of the hippocampus, Nrg1 deficiency enhanced stress-induced reductions in dendritic spine density (figure 1C). Chronic adolescent stress reduced spine density in both Nrg1 HET and WT mice compared to controls on fifth order dendrites. However, stressed Nrg1 HET mice displayed significantly reduced spine density compared to stressed WT mice on fifth order dendrites. Nrg1 deficiency engendered an idiosyncratic response to chronic adolescent stress on dendritic spine density in apical dendrites of CA3 pyramidal neurons (figure 1D). Chronic adolescent stress decreased dendritic spine density in WT mice on second order dendrites, while increasing the density in Nrg1 HET mice. Further, stressed WT mice showed reduced spine density compared to controls on order 4 and 5 dendrites, although stress did not significantly alter dendritic spine densities in Nrg1 HET mice. Stressed Nrg1 HET mice displayed greater dendritic spine density compared to stressed WT mice on fifth order dendrites. Control Nrg1 HET mice displayed reduced dendritic spine densities relative to WT controls on dendrite orders 2, 4, and 5.
Nrg1 deficiency did not produce any robust moderation of stress-induced changes in dendritic spine density in the BLA (figure 1E). Adolescent stress increased dendritic spine densities on fourth and fifth order dendrites in both Nrg1 HET and WT mice. Nrg1 deficiency increased dendritic spine density on the third and fifth order dendrites of the BLA.
Partial Genetic Deletion of Nrg1 Did Not Influence Adolescent Stress-Induced Alterations in Apical Dendrite Length and Complexity in the Mouse Brain
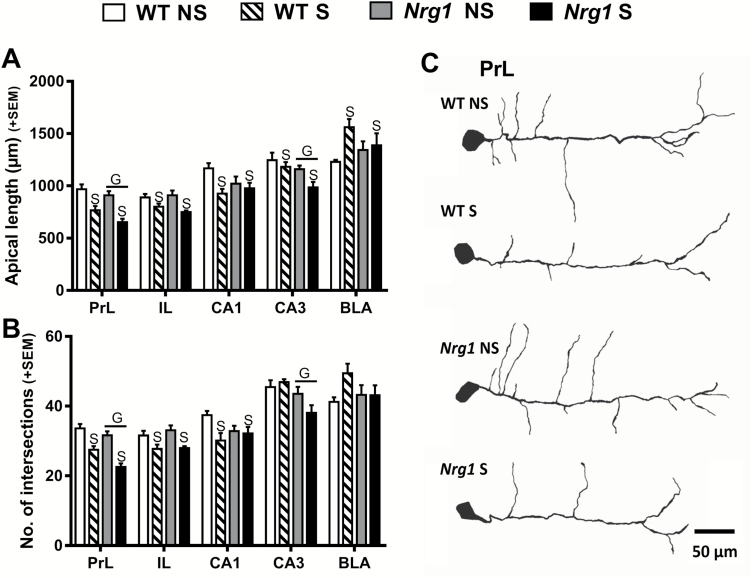
Unlike for dendritic spines, Nrg1 deficiency did not influence adolescent stress-induced alterations in the gross morphological measures of apical dendrite length and complexity (figure 2). Adolescent stress-reduced apical dendrite lengths in the PrL, IL, CA1, and CA3 regions (figure 2A). Nrg1 HET mice displayed significantly shorter apical dendrite lengths in the PrL and CA3 regions (figure 2A). In contrast to the other regions, adolescent stress significantly increased apical dendrite length in the BLA (figure 2A). Dendritic complexity, as evidenced by the number of intersections, mirrored the effects observed on apical dendrite lengths. Adolescent stress decreased intersections in the PrL, IL, and CA1 regions (figure 2B). Nrg1 hypomorphic mice had a reduction in intersections compared to WT mice in both the PrL and CA3 regions (figure 2B).
Fig. 2.
The effects of Nrg1 deficiency and stress on apical dendrite morphology. (A) Cumulative apical dendrite length. (B) Number of intersections as determined by Sholl analysis. (C) Exemplars of traced neurons from the PrL region. Two-factor ANOVA of cumulative apical dendritic lengths in the PrL, IL, CA1, and CA3 regions revealed stress-induced reductions in these regions (main effects of stress: F1,22 = 31.90, P < .0001; F1,20 = 11.72, P < .01, and F1,22 = 6.56, P < .05; F1,21 = 4.67, P < .05, respectively). Nrg1 HET mice displayed significantly shorter apical dendrite lengths in the PrL and CA3 regions (main effect of genotype: F1,22 = 5.02, P < .05, and F1,21 = 6.72, P < .05, respectively). Stress significantly increased apical dendrite length in the BLA (main effect of stress: F1,22 = 6.56, P < .05). Two-factor ANOVA showed a main effect of stress on intersections in the PrL, IL, and CA1 regions (F1,22 = 49.69, P < .0001; F1,20 = 12.39, P < .01; F1,22 = 5.52, P < .05, respectively). Two-factor ANOVA revealed a reduction in intersections in Nrg1 hypomorphic mice compared to WT mice in both the PrL and CA3 regions (main effects of genotype: F1,22 = 11.33, P < .001; F1,21 = 7.66, P < .05, respectively). S = main effect of stress, and G = main effect of genotype.
Nrg1 Deficiency Did Not Influence Adolescent Stress-Induced Microglial Cell Activation in the Medial Prefrontal Cortex or Hippocampus
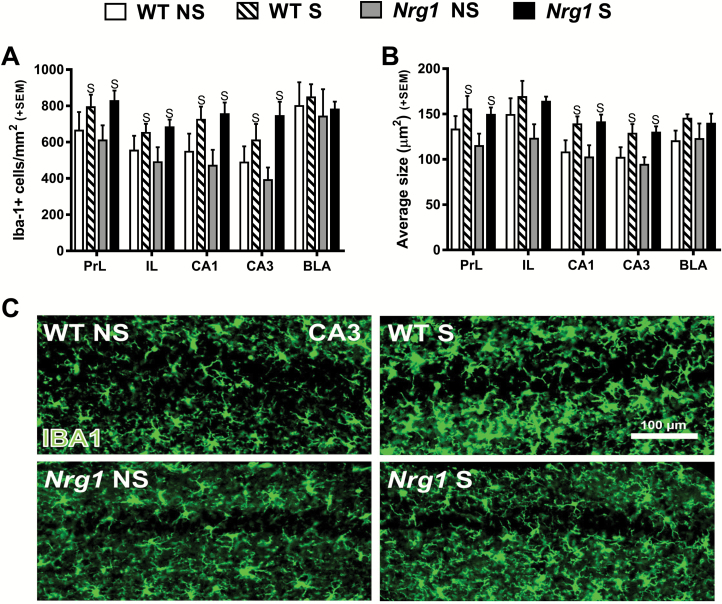
As expected adolescent restraint stress increased the number of microglia in the PrL and IL of the medial prefrontal cortex, and CA1 and CA3 regions of the hippocampus (figure 3A). Adolescent restraint stress also significantly increased microglial cell size in the PrL, CA1, and CA3 regions (figure 3B). However, Nrg1 deficiency did not influence stress-induced alterations in these parameters. Nrg1 genotype, nor stress, altered the number or the size of microglia in the BLA (figures 3A and 3B). Collectively these results show that in WT mice, stress-induced microglial cell activation correlated with the dendritic spine regression observed in the medial prefrontal cortex and hippocampus (figure 1). However, unlike for dendritic spines, Nrg1 deficiency did not influence stress-induced activation of microglial cells in these regions.
Fig. 3.
The effects of Nrg1 deficiency and stress on microglial cell density and size. (A) Number of Iba-1 positive cells per mm2. (B) Average size of counted cells. (C) Representative images from the CA3 region. Two-factor ANOVA showed adolescent restraint stress increased the number of microglial cells in the PrL, IL, CA1, and CA3 regions (main effects of stress: F1,25 = 4.51, P < .05; F1,25 = 4.70, P < .05; F1,25 = 7.55, P < .05; F1,25 = 7.68, P < .05, respectively). Adolescent restraint stress also significantly increased microglial cell size in the PrL, CA1, and CA3 regions (main effects of stress: F1,25 = 4.41, P < .05; F1,25 = 9.60, P < .005; and F1,25 = 9.68, P < .005, respectively). S = main effect of stress.
Partial Genetic Deletion of Nrg1 Increased Neuronal Cell Body Expression of Complement Factor C4 in the Hippocampus
Nrg1 deficiency uncoupled the association of stress-induced dendritic spine regression and microglial cell activation that was observed in WT mice in the prefrontal cortex and hippocampus. It might be that Nrg1 deficiency then impaired the chemical signaling from microglia to neurons which triggers synaptic atrophy. One proposed chemical mediator for microglial engulfment of dendritic spines is C4, which has recently been shown to be increased in schizophrenia brain.24,26 We therefore hypothesized that the concomitant stress-induced microglia cell activation and dendritic spine regression observed in WT mice might be accompanied by a stress-induced increase in C4 expression. Further, we speculated that adolescent stress-induced C4 expression might not be observed in Nrg1 hypomorphic mice. Punctate staining of C4 was observed in the cell bodies of neurons in the PrL and IL cortices, CA1 and CA3 regions and the BLA (figures 4A and B). Analysis of mean C4 immunofluorescence intensity revealed no significant effects of Nrg1 genotype, stress, or their interaction in the PrL or IL cortices, nor in the CA3 region (supplementary table 1). Surprisingly, Nrg1 hypomorphic mice displayed increased C4 expression in both the stratum radiatum and stratum pyramidale of the CA1 region compared to WT mice (figure 4C). To further investigate this effect, we performed qPCR to measure C4 mRNA in the hippocampus of adult WT and Nrg1 HET mice. This experiment showed that partial genetic deletion of Nrg1 reduced hippocampal C4 mRNA expression by approximately 25% (figure 4D).
Fig. 4.
The effects of Nrg1 deficiency and stress on C4 expression. (A) Iba-1/C4 stained hippocampal overview with subregions of CA1 and CA3 demarcated. (B) Representative images from the CA1 (p). (C) Region average mean C4 fluorescence intensity. Two-factor ANOVA showed Nrg1 hypomorphic mice displayed increased C4 expression in both the S Rad and S Py of the CA1 region compared to WT mice (main effect of genotype: F1,25 = 5.78, P < .05; and; F1,25 = 4.38, P < .05, respectively). A significant Nrg1 genotype by stress interaction was observed on C4 intensity in the BLA (2-factor ANOVA: F1,25 = 4.61, P < .05). (D) Expression of C4 mRNA in the hippocampus of unstressed adult WT and Nrg1 HET mice. A Student’s t-test revealed a significant decrease in hippocampal C4 mRNA expression in Nrg1 HET mice compared to WT mice: t(16) = 2.66, *P < .05 S Or; stratum oriens, S Py; stratum pyramidale (p), S Rad; stratum radiatum (r), S Mol; stratum moleculare, AU; arbitrary units, G = main effect of genotype.
Nrg1 and Stress Increased PV Expression and PV+ Interneuron Cell Number in the Hippocampus
An alternative means for Nrg1 to influence adolescent stress-induced dendritic spine regression is via the converging influence of Nrg1 and stress on PV+ interneurons.31–33 We also measured PV expression as it is lower in schizophrenia brain, and decreased interneuron PV expression has functional behavioral consequences and facilitates pyramidal neuronal activity.34,35 Neither Nrg1 deficiency nor chronic adolescent stress altered PV+ interneuron cell number within the IL, PrL, or CA1 regions. However, adolescent stress increased the number of PV+ interneurons in the CA3 region (table 1, representative images in supplementary figure 1). Nrg1 HET mice displayed an increase in PV+ cell number in the BLA and higher PV expression in the CA1 region than WT mice.
Table 1.
The Effects of Nrg1 Deficiency and Stress on PV+ Cell Number and PV Intensity
| WT NS | WT S | Nrg1 NS | Nrg1 S | ||
|---|---|---|---|---|---|
| PV+ cell count cells/mm2 | |||||
| PrL | 99.55 (12.08) | 90.9 (16.56) | 99.55 (9.49) | 116.87 (15.99) | |
| IL | 134.18 (9.04) | 131.78 (22.57) | 117.05 (20.13) | 171.51 (21.95) | |
| CA1 | 33.00 (6.62) | 41.33 (7.39) | 44.00 (12.2) | 35.50 (7.15) | |
| CA3 | 84.00 (10.48) | 104.00 (17.13) | 70.40 (19.82) | 114.00 (4.72) | S |
| BLA | 48.16 (12.22) | 65.56 (8.89) | 85.27 (14.35) | 84.42 (12.89) | G |
| PV cellular intensity AU | |||||
| PrL | 111.66 (5.95) | 122.63 (5.53) | 119.29 (8.21) | 120.53 (3.94) | |
| IL | 124.33 (8.1) | 139.9 (8.12) | 128.07 (8.71) | 144.14 (9.48) | |
| CA1 | 137.64 (9.22) | 141.6 (9.4) | 167.22 (10.01) | 157.23 (11.06) | G |
| CA3 | 141.56 (10.67) | 148.9 (12.58) | 167.37 (13.32) | 174.75 (14.75) | |
| BLA | 97.13 (6.63) | 105.77 (1.96) | 97.31 (7.75) | 107.1 (6.84) | |
Note: Two-factor ANOVA showed stress increased the number of PV+ interneurons in the CA3 region (main effect of stress: F1,26 = 5.15, P < .05). Nrg1 HET mice displayed an increase in PV+ cell number in the BLA (main effect of genotype F1,26 = 5.28, P < .05). Nrg1 HET mice displayed higher PV expression than WT mice in the CA1 region (main effect of genotype: F1,26 = 4.75, P < .05). Data are presented as means + SEM. (AU) arbitrary units. S, main effect of stress; G, main effect of genotype.
Discussion
Overall our results further reinforce that Nrg1 deficiency promotes an idiosyncratic response of the brain to adolescent stress and that interplay exists between Nrg1-ErbB receptor and stress systems.7,11,13 The current study demonstrated Nrg1 deficiency altered dendritic spine plasticity in response to adolescent stress exposure. Chronic adolescent stress in WT mice enhanced dendritic spine loss and microglial cell activation in the prefrontal cortex and the hippocampus. However, Nrg1 deficiency rendered mice resilient to stress-induced dendritic spine loss in the medial prefrontal cortex and the CA3 region of the hippocampus without affecting microglial cell activation in these brain regions. In the CA1 region of the hippocampus, Nrg1 deficiency accentuated the dendritic spine loss promoted by stress without enhancing stress-induced microglial cell activation.
We previously demonstrated Nrg1 deficiency and adolescent stress combined to increase dendritic spine density in the PrL cortex.13 This finding was replicated here using a more severe restraint stress paradigm, however, a more complex picture emerged when considering other brain regions: Nrg1 deficiency and adolescent stress triggered increased dendritic spine densities in the PrL, while conversely, Nrg1 deficiency exaggerated stress-induced dendritic spine regression in the CA1 region of the hippocampus. The region-dependency of the Nrg1 and stress interaction on dendritic spine plasticity might be partly explained by the differential effects of stress on the Nrg1-ErbB system across brain regions. Recent studies show that stress increases Nrg1-ErbB4 signaling in the prefrontal cortex but decreases it in the hippocampus, as stress increased Nrg1 expression in the prefrontal cortex but not in the hippocampus, while increasing ErbB4 phosphorylation in the prefrontal cortex and decreasing it in the hippocampus.6,36 Given the important role of Nrg1 signaling in dendritic spine turnover,8,37 we hypothesize that Nrg1-Erb signaling is enhanced in the prelimbic cortex but decreased in the CA1 region of Nrg1 HET mice undergoing adolescent stress.
We also sought to explore whether the ability of Nrg1 deficiency to modulate adolescent stress-induced dendritic spine losses might involve PV+ inhibitory interneurons, as targeted deletion of the ErbB4 receptor on these cells results in a loss of dendritic spines on neighboring pyramidal neurons.16–18 Previous studies showed that stress reduced the number of PV+ interneurons in the hippocampus.32,33,38 Surprisingly, here we found adolescent stress to increase the number of PV+ interneurons in the hippocampus. This apparent incongruence may be explained by the development period tested, as here stress was applied during adolescence whereas prior studies assessed the actions of stress in adulthood. The ventral section of the hippocampus containing the CA3 region, shows a marked increase in PV cell number during adolescence,39 which may render this brain region particularly vulnerable to the effects of stress. In any case, Nrg1 deficiency did not modulate the effects of adolescent stress on PV+ interneurons, providing no evidence for these cells playing a role in the Nrg1-stress interaction on dendritic spine density.
Prior studies have well-established that stress triggers microglial cell activation and dendritic spine regression in various brain regions.20,22,40 Here, in WT mice microglial cell activation in the medial prefrontal cortex and hippocampus correlated with dendritic spine losses in these regions. However, such a correlation was not observed in the amygdala where the stress-induced increase in dendritic spine density was associated with no change in microglial cell number or morphology. We therefore hypothesize that dendritic spine turnover in the prefrontal cortex and hippocampus may require microglial cell activation, but microglial cell activation is not involved in dendritic spine plasticity in the amygdala. Interestingly, our results show that Nrg1 deficiency uncouples the relationship between microglia cell activation and dendritic spine loss in the prefrontal cortex and hippocampus. Nrg1 signaling was not necessary for adolescent stress-induced microglial cell activation, as Nrg1 deficiency did not alter the observed increase in number and size of microglial cells following adolescent stress. However, it may be that Nrg1 deficiency perturbs chemical signaling between stress-activated microglial cells and dendritic spines, thus altering dendritic spine turnover. For instance, stress may trigger microglial cell release of Nrg1 or some other chemical mediator which is a necessary signal for dendritic spine regression. We hypothesized that Nrg1 deficiency may have impaired C4 signaling, given its emerging role in microglia cell mediated dendritic spine engulfment.24 However, we could not demonstrate any uniform relationship between C4 expression and stress-induced dendritic spine loss and microglial cell activation across brain regions. Inconsistent with our hypothesis, adolescent stress did not increase C4 expression.
Another possibility is that Nrg1 deficiency regulates stress-induced microglial cell release of inflammatory cytokines which are known to influence dendritic spine remodeling.21,41 ErbB receptors are present on microglial cells, and stress increases Nrg1 expression in these cells.42,43 In addition, Nrg1 application to cultured microglial cells alters their release of inflammatory mediators.44,45 In a prior study, adolescent stress decreased IL-1β expression in the prefrontal cortex, while increasing TNF-α expression in the hippocampus in Nrg1 HET mice but not WT mice.46 We therefore hypothesise that Nrg1 may have opposing effects on stress-induced microglial cell release of cytokines in the prefrontal cortex and the hippocampus. In the prefrontal cortex, Nrg1 deficiency may reduce microglial cell release of cytokines and reduce dendritic spine growth, whereas in the hippocampus Nrg1 deficiency may increase microglial cell release of cytokines and therefore favor dendritic spine regression.
The divergent effects of the combination of Nrg1 deficiency and adolescent stress on dendritic spine density in different brain regions also beg the question of their functional significance at both a brain network and behavioral level. While dendritic spine losses would more intuitively translate into a network function deficit, an increase in dendritic spine density might also have detrimental functional consequences. Indeed, increased cortical and hippocampal dendritic spine densities have been observed in mouse models of mental retardation and autism which have reinforced the view that an optimal number of dendritic spines are required for normal synaptic function.47–49 Indeed, the current evidence appears to favor an interpretation of enhanced sensitivity rather than greater resilience of Nrg1 HET mice to adolescent stress exposure.13,46 For example, Nrg1 deficiency conferred vulnerability to adolescent stress-induced behavioral deficits such as working memory impairments and PPI deficits.13,46 Both working memory and sensorimotor gating are impaired in schizophrenia, underscoring that Nrg1 and adolescent stress interactions may have relevance to improving our understanding of these aspects of the disorder. Future investigations might attempt to translate the current findings to humans by observing whether NRG1 SNPs interact with stress to trigger cognitive deficits that are accompanied by alterations in gray matter volume in the prefrontal cortex and hippocampus. One prior human study suggests the interaction may also bare relevance to positive symptoms of schizophrenia, as a NRG1 polymorphism conferred vulnerability to unusual thoughts triggered by psychosocial stress.50
We serendipitously discovered that Nrg1 deficiency increased C4 protein expression in the CA1 region of the hippocampus and decreased C4 mRNA in the whole hippocampus, thus providing first evidence of a link between Nrg1 and C4 systems in the brain.24,51 That Nrg1 deficiency resulted in opposing effects on C4 mRNA and protein may simply reflect a transcriptional homeostatic counteradaptation to the upregulation of C4 protein in the hippocampus of Nrg1 HET mice. However, future research is required to elucidate the exact biological relationship between Nrg1 and complement systems. C4 is contained within the MHC locus which has the strongest association to schizophrenia to date and C4 is upregulated in schizophrenia brain.24,52 That increased C4 protein expression was found in the hippocampus of Nrg1 HET mice further strengthens the face, predictive and construct validity of this genetic animal model of schizophrenia.12,53,54 C4 promoted synaptic elimination in the mouse brain24 which might explain the long-established observation that schizophrenia patients have greater synaptic pruning during adolescence.55,56 The CA1 region of the hippocampus was the only region in which Nrg1 deficiency enhanced stress-induced dendritic spine loss, so it is possible that the increased C4 protein expression promoted by Nrg1 deficiency conferred vulnerability to this. Exploring the role of the immune system in the interaction of Nrg1 and C4 in the brain provides an obvious pathway for future mechanistic investigations. Activation of the complement system triggers inflammation, whereas Nrg1 is anti-inflammatory, implying a counter regulatory relationship between Nrg1 and C4 signaling.44,57 Altered cytokine profiles in the plasma, spleen, and brain have been demonstrated in Nrg1 HET mice,46,58 which may then impact upon C4 expression.59,60 It is noteworthy, that in humans a loss-of-function mis-sense mutation in NRG1 increased plasma levels of numerous autoimmune and inflammatory markers.61 Interestingly, there was a tendency for complement factor 1q, a precursor to C4, to be upregulated in individuals carrying the mutation.
The current study also made some additional noteworthy discoveries with respect to the effects of Nrg1 deficiency alone on neurobiology. We report for the first time that Nrg1 HET mice display reduced dendritic spine density and complexity in the CA3 region of the hippocampus. This further supports numerous studies showing that Nrg1 maintains normal dendritic morphology.8,37 Here, we also show for the first time that Nrg1 deficiency increased the number of PV+ interneurons in the BLA which correlated with increased dendritic spine density in this region. These results are consistent with prior observations that Nrg1 HET mice have a dysfunctional amygdala, eg, these mice have elevated anandamide concentrations in the amygdala and display impaired fear conditioning.12,62,63
In conclusion, this study reinforces the notion that partial genetic deletion of Nrg1 modulates the impact of stress on the adolescent brain, and particularly alters stress-induced dendritic spine regression but not microglial cell activation. We also report for the first time that Nrg1 deficiency increases the expression of C4 in the brain, providing first evidence of a link between neuregulin and complement signaling.
Funding
University of Sydney Bridging Grant (to J.C.A.); Brain & Behaviour Research Foundation Young Investigator Award (to J.C.A).
Supplementary Material
Acknowledgments
The authors report no biomedical interest or potential conflicts of interest.
References
- 1. Gomes FV, Grace AA. Adolescent stress as a driving factor for schizophrenia development—a basic science perspective. Schizophr Bull. 2017;43:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett MR, Farnell L, Gibson WG. Fiber pathway pathology, synapse loss and decline of cortical function in schizophrenia. PLoS One. 2013;8:e60518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radley JJ, Rocher AB, Rodriguez A et al. . Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. [DOI] [PubMed] [Google Scholar]
- 6. Dang R, Cai H, Zhang L et al. . Dysregulation of neuregulin-1/ErbB signaling in the prefrontal cortex and hippocampus of rats exposed to chronic unpredictable mild stress. Physiol Behav. 2016;154:145–150. [DOI] [PubMed] [Google Scholar]
- 7. Brydges NM, Seckl J, Torrance HS, Holmes MC, Evans KL, Hall J. Juvenile stress produces long-lasting changes in hippocampal DISC1, GSK3ß and NRG1 expression. Mol Psychiatry. 2014;19:854–855. [DOI] [PubMed] [Google Scholar]
- 8. Barros CS, Calabrese B, Chamero P et al. . Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011;95:275–300. [DOI] [PubMed] [Google Scholar]
- 10. Chesworth R, Yulyaningsih E, Cappas E, Arnold J, Sainsbury A, Karl T. The response of neuregulin 1 mutant mice to acute restraint stress. Neurosci Lett. 2012;515:82–86. [DOI] [PubMed] [Google Scholar]
- 11. Taylor SB, Taylor AR, Markham JA, Geurts AM, Kanaskie BZ, Koenig JI. Disruption of the neuregulin 1 gene in the rat alters HPA axis activity and behavioral responses to environmental stimuli. Physiol Behav. 2011;104:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke DJ, Stuart J, McGregor IS, Arnold JC. Endocannabinoid dysregulation in cognitive and stress-related brain regions in the Nrg1 mouse model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;72:9–15. [DOI] [PubMed] [Google Scholar]
- 13. Chohan TW, Boucher AA, Spencer JR et al. . Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice. Schizophr Bull. 2014;40:1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chohan TW, Nguyen A, Todd SM, Bennett MR, Callaghan P, Arnold JC. Partial genetic deletion of neuregulin 1 and adolescent stress interact to alter NMDA receptor binding in the medial prefrontal cortex. Front Behav Neurosci. 2014;8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yin DM, Sun XD, Bean JC et al. . Regulation of spine formation by ErbB4 in PV-positive interneurons. J Neurosci. 2013;33:19295–19303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun Y, Ikrar T, Davis MF et al. . Neuregulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron. 2016;92:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazzari P, Paternain AV, Valiente M et al. . Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. [DOI] [PubMed] [Google Scholar]
- 19. Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl). 2016;233:1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith BL, Schmeltzer SN, Packard BA, Sah R, Herman JP. Divergent effects of repeated restraint versus chronic variable stress on prefrontal cortical immune status after LPS injection. Brain Behav Immun. 2016;57:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22:1442–1454. [DOI] [PubMed] [Google Scholar]
- 24. Sekar A, Bialas AR, de Rivera H et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. The complement system: a gateway to gene–environment interactions in schizophrenia pathogenesis. Mol Psychiatry. 2017;22:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sellgren CM, Sheridan SD, Gracias J, Xuan D, Fu T, Perlis RH. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Mol Psychiatry. 2017;22:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology (Berl). 2007;192:325–336. [DOI] [PubMed] [Google Scholar]
- 28. Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI’11), Vancouver, British Columbia, May 07–12, 2011. Association for Computing Machinery, New York, NY; 2011. [Google Scholar]
- 30. Brzozowska NI, Smith KL, Zhou C et al. . Genetic deletion of P-glycoprotein alters stress responsivity and increases depression-like behavior, social withdrawal and microglial activation in the hippocampus of female mice. Brain Behav Immun. 2017;65:251–261. [DOI] [PubMed] [Google Scholar]
- 31. Shepard R, Coutellier L. Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol Neurobiol. 2017;1–12. https://link.springer.com/journal/12035/onlineFirst/page/20 [DOI] [PubMed] [Google Scholar]
- 32. Filipović D, Zlatković J, Gass P, Inta D. The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. [DOI] [PubMed] [Google Scholar]
- 33. Czéh B, Varga ZK, Henningsen K, Kovács GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25:393–405. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wöhr M, Orduz D, Gregory P et al. . Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry. 2015;5:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen YH, Lan YJ, Zhang SR et al. . ErbB4 signaling in the prelimbic cortex regulates fear expression. Transl Psychiatry. 2017;7:e1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cahill ME, Remmers C, Jones KA, Xie Z, Sweet RA, Penzes P. Neuregulin1 signaling promotes dendritic spine growth through kalirin. J Neurochem. 2013;126:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. [DOI] [PubMed] [Google Scholar]
- 39. Caballero A, Diah KC, Tseng KY. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus. 2013;23:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franklin TC, Wohleb ES, Zhang Y, Fogaça M, Hare B, Duman RS. Persistent Increase in microglial RAGE contributes to chronic stress-induced priming of depressive-like behavior. Biol Psychiatry. 2018;83:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. [DOI] [PubMed] [Google Scholar]
- 42. Xiang Y, Liu T, Yang H et al. . NRG1-ErbB signalling promotes microglia activation contributing to incision-induced mechanical allodynia. Eur J Pain. 2015;19:686–694. [DOI] [PubMed] [Google Scholar]
- 43. Ikawa D, Makinodan M, Iwata K et al. . Microglia-derived neuregulin expression in psychiatric disorders. Brain Behav Immun. 2017;61:375–385. [DOI] [PubMed] [Google Scholar]
- 44. Simmons LJ, Surles-Zeigler MC, Li Y, Ford GD, Newman GD, Ford BD. Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. J Neuroinflammation. 2016;13:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dimayuga FO, Ding Q, Keller JN, Marchionni MA, Seroogy KB, Bruce-Keller AJ. The neuregulin GGF2 attenuates free radical release from activated microglial cells. J Neuroimmunol. 2003;136:67–74. [DOI] [PubMed] [Google Scholar]
- 46. Desbonnet L, O’Tuathaigh C, Clarke G et al. . Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene × environment interaction. Brain Behav Immun. 2012;26:660–671. [DOI] [PubMed] [Google Scholar]
- 47. Thomazeau A, Lassalle O, Iafrati J et al. . Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a. J Neurosci. 2014;34:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horling K, Schlegel G, Schulz S et al. . Hippocampal synaptic connectivity in phenylketonuria. Hum Mol Genet. 2015;24:1007–1018. [DOI] [PubMed] [Google Scholar]
- 49. Henderson C, Wijetunge L, Kinoshita MN et al. . Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci Transl Med. 2012;4:152ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kéri S, Kiss I, Seres I, Kelemen O. A polymorphism of the neuregulin 1 gene (SNP8NRG243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:418–420. [DOI] [PubMed] [Google Scholar]
- 51. Mostaid MS, Mancuso SG, Liu C et al. . Meta-analysis reveals associations between genetic variation in the 5′ and 3′ regions of Neuregulin-1 and schizophrenia. Transl Psychiatry. 2017;7:e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spencer JR, Darbyshire KM, Boucher AA et al. . Novel molecular changes induced by Nrg1 hypomorphism and Nrg1-cannabinoid interaction in adolescence: a hippocampal proteomic study in mice. Front Cell Neurosci. 2013;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karl T, Arnold JC. Schizophrenia: a consequence of gene–environment interactions?Front Behav Neurosci. 2014;8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ebdrup BH, Glenthøj B, Rasmussen H et al. . Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. [DOI] [PubMed] [Google Scholar]
- 58. Desbonnet L, Cox R, Tighe O et al. . Altered cytokine profile, pain sensitivity, and stress responsivity in mice with co-disruption of the developmental genes Neuregulin-1×DISC1. Behav Brain Res. 2017;320:113–118. [DOI] [PubMed] [Google Scholar]
- 59. Blanchong CA, Chung EK, Rupert KL et al. . Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int Immunopharmacol. 2001;1:365–392. [DOI] [PubMed] [Google Scholar]
- 60. Yu CY, Chung EK, Yang Y et al. . Dancing with complement C4 and the RP-C4-CYP21-TNX (RCCX) modules of the major histocompatibility complex. Prog Nucleic Acid Res Mol Biol. 2003;75:217–292. [DOI] [PubMed] [Google Scholar]
- 61. Marballi K, Quinones MP, Jimenez F et al. . In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J Mol Med (Berl). 2010;88:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807. [DOI] [PubMed] [Google Scholar]
- 63. Bennett MR, Arnold J, Hatton SN, Lagopoulos J. Regulation of fear extinction by long-term depression: the roles of endocannabinoids and brain derived neurotrophic factor. Behav Brain Res. 2017;319:148–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.