Abstract
Aims/introduction
We compared the diagnostic performance and correlation between salivary, serum and capillary blood glucose of diabetes and non-diabetes patients. Early detection of diabetes mellitus (DM) contributes to the prevention of complications and management.
Materials and methods
This case-control study was conducted among a total of 138 participants comprising 79 newly diagnosed diabetes patients (cases) and 59 non-diabetes patients (controls). Fasting salivary glucose (FSLG), fasting serum glucose (FSEG) levels and fasting capillary whole blood glucose (FCWBG) level were assayed for each participant.
Results
The mean FSLG, FSEG and FCWBG levels were significantly higher among the cases compared to controls (p < 0.0001). There was a significant mean difference between the levels of FSLG vs. FSEG (p < 0.0001) and FSLG vs. FCWBG (p < 0.0001) but not levels of FSEG vs. FCWBG (p > 0.05) in both cases and controls. A positive correlation was observed between FSLG and FSEG (r = 0.89; p < 0.0001) and FCWBG (r = 0.87; p < 0.0001). At the cut-off value >6.8 mmol/l for FSEG, a sensitivity of 99%, specificity of 100.0% and area under the curve (AUC) of 98.8% was observed for predicting DM while a sensitivity of 80%, specificity of 95% and AUC of 91.0% was observed for FSLG at a cut-off value >0.5 mmol/l. At the cut-off value >6.9 mmol/l for FCWBG, a sensitivity of 100.0%, specificity of 100.0% and AUC of 100.0% was observed for predicting DM.
Conclusion
Fasting salivary glucose (FSLG) levels increased with increasing blood glucose levels. However, it does not generate enough diagnostic and predictive accuracy compared to capillary whole blood glucose which less invasive.
Keywords: Medicine, Metabolism
1. Introduction
Diabetes Mellitus (DM) is a metabolic disorders characterised by relative or absolute insufficiency of insulin secretion and or concomitant resistance to insulin [1]. The world prevalence of diabetes mellitus among general adults was 6.4% in 2010, affecting 285 million adults, and is expected to increase to 7.7% by 2030, affecting 439 million adults [2]. This indicates that, there will be a 69% increase in numbers of adults with DM between 2010 and 2030 [2].
Early diagnosis of type II DM has been suggested as an important strategy to lower the incidence of this disease worldwide [3].
To date, urine and blood tests are widely and readily available for screening, prognosis, diagnosis and monitoring DM across the globe [4]. However, the estimation of glucose levels using urine and blood samples are associated with several challenges. For instance, urine glucose positivity cannot be detected in early stages but at advanced stage of DM [4]. On the other hand, venous blood sampling is more invasive while capillary blood sampling can be slightly invasive, thus making patients feel inconvenience and uncomfortable. Additionally, blood collection using needles or lancets disturb daily life, cause nervousness and are difficult to do in long-term diabetes patients due to development of finger lumps, risk of infection and poor peripheral circulation [5]. There is the need to use a more convenient and comfortable sampling procedure which is non-invasive, reliable and requires less expertise.
From the diagnostic point of view, the use of saliva samples in glucose estimation have been well-documented and recommended [6, 7, 8]. Human saliva reflects the body's health and can indicate local and systemic alterations such that the components of saliva can be related to the metabolic state of the individual [9]. Not only can it be used for large scale screening program but it's also non-invasive, and does not require specialised skills. Recent studies have focused on the development of saliva-based tests for screening or monitoring systemic diseases, including diabetes mellitus [10, 11]. Several studies have found an association between salivary glucose and blood glucose [12, 13, 14, 15].
Although few studies conducted in Sub-Saharan Africa (SSA) estimated salivary glucose for the diagnosis and monitoring of diabetes mellitus, it has not been studied in the Ghanaian population, to the best of our knowledge. In addition, evaluation of diagnostic and predictive performance of fasting salivary glucose levels with blood glucose levels has not been studied in together among DM patients in a Ghanaian population. This study compared the diagnostic performance and correlation of salivary glucose to serum glucose and capillary blood glucose among diabetes patients and non-diabetes patients in a Ghanaian population.
2. Materials and Methods
2.1. Study design/study site
This was a case-control study conducted at the Methodist Faith Healing Hospital in the Afigya Kwabre of the Ashanti region, Ghana. This hospital is a referral hospital for almost 20 health facilities in the district including clinics, Community-based Health Planning and Services (CHPS) centers, maternity homes and private hospitals. Participants for this study were recruited from September 2016 to March 2017.
2.2. Ethical consideration
Approval for this study was obtained from the Institutional Review Board (IRB) of the University of Cape Coast and the Ethic Committee of the Methodist Faith Healing Hospital. Written informed consents was obtained from all participants. Confidentiality of all participants was assured.
2.3. Study population/participants selection
A total of 138 participants aged 30–79 years who had fasted for 8–12 h prior to sample collection were recruited for this study. Seventy-nine (79) newly diagnosed diabetic patients were recruited as cases and age-and-sex matched 59 healthy non-diabetes voluntary blood donors were recruited as controls. Demographic information was obtained from participants after written informed consent and ethical approval. All eligible participants were adequately informed of procedures, risk and benefits involved.
Pregnant women, patients who had taken any medication the morning before blood sampling, mentally unstable and patients who were very ill to comply with procedures of testing were excluded.
2.4. Fasting capillary whole blood glucose (FCWBG) measurement
Capillary whole blood was obtained from the index finger of all participants. The patient's finger was pricked with a disposable needle and a drop of blood was collected by applying slight pressure to the finger. The blood (2μL) was placed on a reactive test strip for glucose and the concentration of glucose was determined using a glucometer (an ACCU-CHEK ACTIVE glucometer strip). Briefly, glucose in the blood sample is converted to gluconolactone by glucose dehydrogenase in the strip. This releases two (2) electrons that react with a coenzyme (PQQ) electron acceptor. The resultant reaction creates a harmless electrical current which is interpreted as http://www.scialert.net/asci/result.php?searchin=Keywords&cat=&ascicat=ALL&Submit=Search&keyword=blood+glucose blood glucose on the screen of the glucometer. The blood glucose was expressed in milli mole per liter (mmol/L). According to the manufacturer, the test can accurately measure glucose levels from 0.3 mmol/L to 33.3 mmol/L [16].
2.5. Fasting serum and saliva glucose measurements
Three (3) milliliter (ml) fasting venous blood was drawn from all participants in the morning by trained personnel after 8–12 h overnight fast. Simultaneously, unstimulated whole saliva samples were collected into clean plastic saliva collection containers using the spitting method as described by Wang et al. [17]. The unstimulated whole saliva and blood samples were centrifuged at 3000rpm for 10 minutes and the supernatants were obtained for estimation of glucose. Reagent for glucose estimation were purchased from ELITech Diagnostic (Envoy 500 Glucose Reagent Kits by ELITech Group). Fasting salivary and serum glucose levels was estimated spectrophotometrically using the automatic clinical chemistry analyser (Mindray 3000 automated chemistry analyzer). Glucose determination was based on endpoint glucose oxidase peroxidase (GOD-POD) method [16].
2.6. Statistical analysis
Data was entered in an excel worksheet and analyzed using the statistical package for social sciences (SPSS) version 24.0. Independent sample t-test was used for comparison between two means while one-way ANOVA followed by Bonferroni multiple comparison test was used for comparison between more than two means. Chi square analysis was performed for comparison between categorical variables. Data was expressed as means ±SD, and frequency (percentage). Receivers operating characteristics (ROC) analysis was employed to determine the sensitivity and specificity of salivary, serum and whole blood glucose test while correlation coefficient were performed to determine the relationship between salivary serum and whole blood glucose in study population. p < 0.05 were considered statistically significant.
3. Results
The mean age of the participants was 53.61 years and a higher proportion (26.1%) were 60–69 years. There was no significantly different between the mean ages of the diabetes compared to non-diabetes mellitus participants (53.65 ± 12.30 vs. 53.56 ± 13.33years; p = 0.969). Most (58%) of the participants were females compared to males (42.0%). The means of levels of FSLG, FSEG and FCWBG were significantly higher among the diabetes patients compared to non-diabetes patients (p < 0.001) Table 1.
Table 1.
General characteristics of study participants.
| Parameters | Total |
Diabetics |
Non-diabetics |
p-value |
|---|---|---|---|---|
| (n = 138) | (n = 79) | (n = 59) | ||
| Age (years) | 53.61 ± 12.70 | 53.65 ± 12.30 | 53.56 ± 13.33 | 0.969 |
| Age group | 0.661 | |||
| 30–39 | 25 (18.1) | 13 (16.5) | 12 (20.3) | |
| 40–49 | 33 (23.9) | 20 (25.3) | 13 (22.0) | |
| 50–59 | 31 (22.5) | 19 (24.1) | 12 (20.3) | |
| 60–69 | 36 (26.1) | 21 (26.6) | 15 (25.4) | |
| 70–79 | 13 (9.4) | 6 (7.6) | 7 (11.9) | |
| Sex | 0.944 | |||
| Male | 58 (42.0) | 33 (41.8) | 25 (42.4) | |
| Female | 80 (58.0) | 46 (58.2) | 34 (57.6) | |
| FSEG (mmol/l) | 11.16 ± 6.99 | 15.83 ± 5.75 | 4.90 ± 1.23 | <0.0001 |
| FSLG (mmol/l) | 0.67 ± 0.45 | 0.92 ± 0.43 | 0.32 ± 15.70 | <0.0001 |
| FCWBG (mmol/l) | 11.06 ± 6.84 | 15.63 ± 5.62 | 5.64 ± 1.21 | <0.0001 |
Values are presented as frequency (percentage), and mean ± SD. FSEG: fasting serum glucose; FSLG: fasting salivary glucose; FCWBG: fasting capillary whole blood glucose.
As shown in Fig. 1a and b, the mean FSEG and FCWBG were significantly higher compared to salivary glucose in both the cases (p < 0.0001) and controls (p < 0.0001). However, no significant difference was found between mean FSEG and FCWBG concentrations in both groups (p > 0.05) (Fig.1a and b).
Fig.1.
Comparison of glucose concentrations in serum, saliva and capillary whole blood among cases and controls. Fig. 1a (Cases); Fig. 1b (Control).
As shown in Table 2, There was a significant mean difference and a strong effect size between the FSEG and FSLG and that between the FCWBG and FSLG levels in both cases (p < 0.0001) and control participants (p < 0.0001). There was no statistically significant mean difference between FSEG and FCWBG levels in both diabetes (bias = 0.160; p = 0.974) and non-diabetes patients (bias = -0.005; p = 0.978). The effect size (Cohen d) between the mean difference in the FSEG and FCWBG concentration was significantly smaller in both diabetic and non-diabetes patients.
Table 2.
Mean difference (Bias) in glucose concentration between the various samples among the diabetics and non-diabetics.
| Samples | Mean Difference (Bias) | CI | Effect Size (Cohen's d) | p-value |
|---|---|---|---|---|
| FSEG vs. FSLG | ||||
| DM | 14.91 | 13.16–16.65 | 1.78 | <0.0001 |
| Non-DM | 4.62 | 4.19–5.05 | 1.76 | <0.0001 |
| FSEG vs. FCWBG | ||||
| DM | 0.16 | −1.54–1.91 | 0.01 | 0.974 |
| Non-DM | -0.005 | −0.43–0.42 | 0.001 | 0.978 |
| FCWBG vs. FSLG | ||||
| DM | 14.7 | 12.96–16.44 | 1.79 | <0.0001 |
| Non-DM | 4.63 | 4.20–5.06 | 1.76 | <0.0001 |
DM = Diabetes Mellitus, CI = Confidence Interval; FSEG: fasting serum glucose; FSLG: fasting salivary glucose; FCWBG: fasting capillary whole blood glucose.
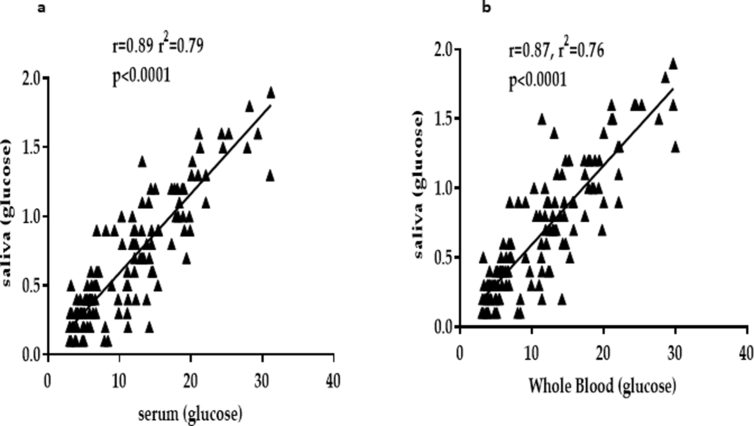
A strong and significant positive correlation between FSLG and FSEG (r = 0.890, p < 0.0001) (Fig. 2a) and FCWBG concentration (r = 0.870, p < 0.0001) (Fig. 2b) among participants.
Fig. 2.
Correlation of saliva with serum and capillary whole blood glucose concentrations among study participants. Fig. 1a (correlation between salivary glucose and serum glucose levels); Fig. 1b (correlation between salivary glucose and capillary whole blood glucose levels).
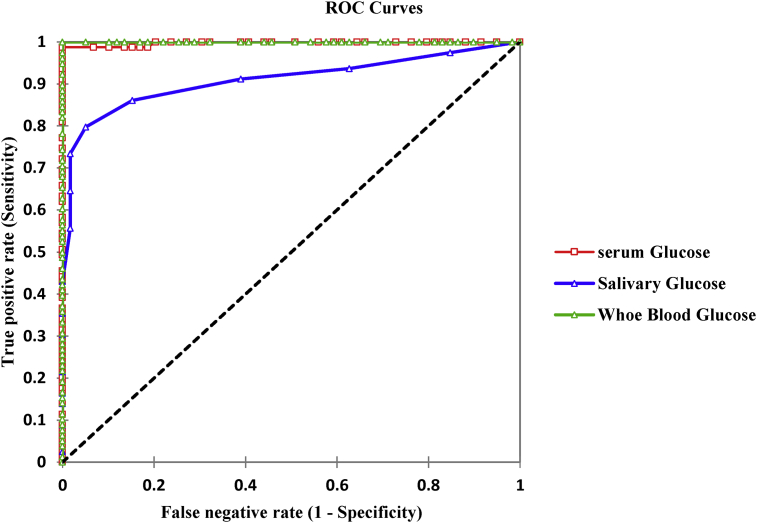
As shown in Fig. 3, the area under the curve (AUC) obtained for the diagnosis and identification of participants with diabetes mellitus was 99.8% for FSEG, 91.0% for FSLG and 100.0% for FCWBG. These percentage accuracies were statistically significant (p < 0.0001).
Fig. 3.
ROC curves for salivary, serum and capillary whole blood glucose concentrations for diagnosis of diabetes mellitus.
Table 3 shows the diagnostic performance of salivary, serum and capillary whole blood glucose concentrations for DM diagnosis. After an ROC analysis, a cut-off (threshold) value ≥0.5mmol, a sensitivity of 80.0% and a specificity of 95.0% for FSLG was obtained for the prediction and identification of patients with DM. Conversely, at a threshold value ≥6.8 mmol/L, sensitivity of 99.0% and specificity of 100.0% for FSEG and a threshold value ≥6.9 mmol/L, sensitivity of 100.0% and specificity of 100.0% for FCWBG was obtained for the prediction and identification of patients with DM.
Table 3.
Diagnostic performance of salivary, serum and whole blood glucose concentrations for diabetes diagnosis.
| Markers | Sensitivity | Specificity | TP | TN | FP | FN | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| FSEG (mmol/l) | 99.8(99.2–100.0) | ||||||
| >6.7 | 99.0% | 100.0% | 78 | 55 | 4 | 1 | |
| >6.8 | 99.0% | 100.0% | 78 | 59 | 0 | 1 | |
| >7.1 | 97.0% | 100.0% | 77 | 59 | 0 | 2 | |
| FSLG (mmol/l) | 91.0(88.0–94.0) | ||||||
| >0.4 | 86.0% | 85.0% | 50 | 50 | 9 | 11 | |
| >0.5 | 80.0% | 95.0% | 63 | 56 | 3 | 16 | |
| >0.6 | 73.0% | 98.0% | 58 | 56 | 1 | 21 | |
| FCWBG (mmol/l) | 100.0(99.0–100.0) | ||||||
| >6.8 | 100.0% | 95.0% | 78 | 56 | 3 | 0 | |
| >6.9 | 100.0% | 100.0% | 78 | 59 | 0 | 0 | |
| >7.1 | 97.0% | 100.0% | 76 | 59 | 0 | 2 |
AUC: Area Under Curve, TP: True Positive, TN: True Negative, FP: False Positive, FN: False Negative, CI: Confidence Interval; FSEG: fasting serum glucose; FSLG: fasting salivary glucose; FCWBG: fasting capillary whole blood glucose.
4. Discussion
Diabetic mellitus is a global chronic condition associated with a huge metabolic nightmare. Early identification is a key to diagnosis and management to avert future cardiovascular events [3]. The use of saliva as a non-invasive measure of glucose levels among diabetes patients is gaining most stands despite the widely used routine blood glucose estimations. For the first time in a Ghanaian population we compared the diagnostic performance, and the correlation between fasting glucose levels in the saliva, serum and whole blood among diabetes and non-diabetes patients.
In agreement with other studies, the intriguing but expected findings of this present study were the significantly increased mean levels of FSLG, FSEG and FCWBG in diabetes patients compared to non-diabetes controls. Particularly for FSLG and FSEG, case-control studies among Indian population [9, 12, 13], have reported an increased level in diabetes compared to non-diabetes controls. In general, increased levels of blood glucose in type II diabetes mellitus (T2DM) has been associated with insulin resistance and large amount of glucose becomes available in blood and saliva. The probable mechanism that underpins the increased salivary glucose among diabetes patients has commonly been due to modifications in permeability of glandular tissues, which results in basement membrane abnormalities of parotid gland and oversecretion of glucose from the parotid duct [18]. In an experimental diabetic rat model the ultrastructure of parotid gland cells had swollen intracytoplasmic mitochondria without visible cristae [19].
Alterations in the salivary flow and composition of saliva in diabetes patients have been reported in previous studies [20, 21]. In this present study, FSEG and FCWBG levels were significantly higher compared to salivary glucose while there was no statistically significant difference between FSEG compared to FCWBG levels in both diabetes and non-diabetes mellitus controls. This concur with several case-control studies among the Indian [9, 12, 13], and Chinese population [17]. The mechanisms which underlie the lower levels of glucose in saliva compared to that in serum and capillary whole blood among diabetes patients in this present study is not self-understood however, this could be influenced by the water intake, the environment factor, mood and anti-diabetic medications.
A significantly positive correlation between salivary glucose and blood glucose has been reported by several studies [12, 13, 14, 15]. This is consistent with the findings of this present study, which also found a strong positive and significant correlation between FSLG and FSEG as well as FCWBG. This indicates that FSLG may serve as an alternative and reliable measure of blood glucose levels. The coefficient of determination explained that FSLG levels contributed to about 80% of the variations in FSEG and FCWBG levels (Fig. 2). Increase in salivary glucose with increasing serum and capillary glucose could be explained by several pathways. For instance, high blood glucose levels has been linked to increased advanced glycosylation end (AGEs) products formation, and other by-products, which increases the permeability by chemically binding to extracellular matrix proteins to cause basement membrane alteration and endothelial dysfunction [12]. The increased passage of glucose from the blood into the saliva occurs as a result of an increased permeability caused by other glucose product, such as diacylglycerol, sorbitol, and fructose-6-phosphate, which are formed during chronic high glucose levels [12]. A study reported that the increased permeability of basement membrane of parotid glands may result to an increased leakage of smaller molecules like glucose into whole saliva through gingival crevices [22].
Based on the strong significant correlations observed between salivary glucose and blood glucose concentrations, we further evaluated its diagnostic and predictive performance. At ≥0.5 mmol/l for FSLG, sensitivity and specificity of 80.0% and 95.0% respectively were recorded at an overall accuracy of 91% in this present study. In a matched case-control study by Shashikanth et al. [23] among diabetes patients in an Indian population, using salivary glucose yielded a 100% sensitivity, 78% specificity and 89% accuracy (area under the curve). Compared to our study, the area under the curve (accuracy) reported by Shashikanth and colleagues was similar. However, there were disparities in the percentage sensitivity and specificity in this present study compared to that reported by Shashikanth et al. [23], which we speculate could probably due to difference in fasting time, sample size, and different in sample estimation among other factors. The previous study by Shashikanth et al. [23] recruited smaller sample participants and took samples at 2 h postprandial while we used a larger sample and collected 8 to12 h fasting samples. Further studies are needed to evaluate if a difference exist between salivary glucose levels 2 h post-prandial and 8 to 12 h fasting glucose levels.
In this present study the diagnostic performance of blood glucose levels, that is, FSEG and FCWBG were similar with a threshold range of ≥6.8–6.9 mmol/l, sensitivity range of 99–100.0% and a specificity range of 100-100%. The higher diagnostic yield observed for both FSEG and FCWBG in the diagnosis and identification of diabetes in this present study is as expected. This makes blood glucose measure of DM more reliable compared to salivary glucose. The probable explanation could be due to the increased levels of glucose in blood compared to saliva observed in this study. However, the diagnostic accuracies and specificities as well as correlations yielded for FSLG, FSEG and FCWBG are comparable suggesting that salivary glucose could be used in addition to serum and capillary blood glucose for diagnosis and monitoring diabetes. However, when utilizing salivary glucose to monitor diabetes patients’, factors such as water intake, the environment factor, mood and anti-diabetes medication must be considered. Although, sampling saliva is non-invasive and can easily be obtained without expertise compared to blood glucose sampling, there is the need to improve the sensitivity of saliva for glucose determination by using a high throughput assay method. Developing more sensitive methods for salivary glucose determination will add to knowledge and increase innovative approach to diagnosis and self-management of DM.
Aside the interesting findings of this present study, there are some limitation that would need improvement for further studies. Firstly, subject population size was small and thus further studies will need a large sample to validate the findings of this study. Secondly, the measurement of fasting capillary blood glucose was different from salivary and serum glucose, which might introduce the methodology difference, hence further studies is needed to correct this limitation by using the same method estimation. Although our findings are consistent with the findings of several published articles, we recommend evaluation of salivary glycated hemoglobin (HbA1c) in comparison with salivary glucose levels 2 h post-prandial in a large population Cohort study.
In conclusion, fasting salivary glucose level increased with increases in serum and capillary blood glucose levels. However, from the diagnostic point of view, fasting salivary glucose does not yield enough diagnostic and predictive accuracy compared to serum and capillary whole blood glucose. Meanwhile salivary glucose can be used in addition to blood glucose as a potentially useful non-invasive tool to monitor glycemic control among patients with type 2 diabetes mellitus if its method of estimation is improved.
Declarations
Author contribution statement
Richard K.D. Ephraim, Enoch Odame Anto, Emmanuel Acheampong, Richmond B. Barnie, Ambrose Asare: Conceived and designed the experiments; Performed the experiments; Analysed and interpreted the data; Wrote the paper.
Linda Ahenkorah-Fondjo, Samuel Asamoah Sakyi: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors wish to thank clinicians and laboratory staffs of the Methodist Faith Healing Hospital for their support during participant's recruitment and sample assays.
References
- 1.Petersmann A., Nauck M., Muller-Wieland D., Kerner W., Muller U.A., Landgraf R. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 2018;126:406–410. doi: 10.1055/a-0584-6223. [DOI] [PubMed] [Google Scholar]
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Ding Y., Tanaka Y., Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014;11:1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan D.M. Diabetes: advances in diagnosis and treatment. Jama. 2015;314:1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 5.Mascarenhas P., Fatela B., Barahona I. Effect of diabetes mellitus type 2 on salivary glucose--a systematic review and meta-analysis of observational studies. PLoS One. 2014;9:e101706. doi: 10.1371/journal.pone.0101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladgotra A., Verma P., Raj S.S. Estimation of salivary and serum biomarkers in diabetic and non diabetic patients - a comparative study. J. Clin. Diagn. Res. 2016;10:Zc56–Zc61. doi: 10.7860/JCDR/2016/19135.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshmi P.V., Sridevi E., Sai Sankar A.J., Manoj Kumar M.G., Sridhar M., Sujatha B. Diagnostic perspective of saliva in insulin dependent diabetes mellitus children: an in vivo study. Contemp. Clin. Dent. 2015;6:443–447. doi: 10.4103/0976-237X.169844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smriti K., Pai K.M., Ravindranath V., Gadicherla S., Pentapati K.C. Salivary glucose as a diagnostic marker for diabetes mellitus. J Diabetes Sci Technol. 2016;10:991–992. doi: 10.1177/1932296816637619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhanya M., Hegde S. Salivary glucose as a diagnostic tool in Type II diabetes mellitus: a case-control study. Niger. J. Clin. Pract. 2016;19:486–490. doi: 10.4103/1119-3077.183314. [DOI] [PubMed] [Google Scholar]
- 10.Rathnayake N., Akerman S., Klinge B., Lundegren N., Jansson H., Tryselius Y. Salivary biomarkers for detection of systemic diseases. PLoS One. 2013;8:e61356. doi: 10.1371/journal.pone.0061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaffe T., Cooper-White J., Beyerlein P., Kostner K., Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin. Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 12.Patel B.J., Dave B., Dave D., Karmakar P., Shah M., Sarvaiya B. Comparison and correlation of glucose levels in serum and saliva of both diabetic and non-diabetic patients. J. Int. Oral Health. 2015;7:70–76. [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S., Nayak M.T., Sunitha J.D., Dawar G., Sinha N., Rallan N.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Pathol. 2017;21:334–339. doi: 10.4103/jomfp.JOMFP_222_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurysta C., Bulur N., Oguzhan B., Satman I., Yilmaz T.M., Malaisse W.J. Salivary glucose concentration and excretion in normal and diabetic subjects. J. Biomed. Biotechnol. 2009;2009:430426. doi: 10.1155/2009/430426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sashikumar R., Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:706–711. doi: 10.1016/j.tripleo.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Owiredu W., Amegatcher G., Amidu N. Precision and accuracy of three blood glucose meters: accu-chek advantage, one touch horizon, and sensocard. J. Med. Sci. 2009;9:185–193. [Google Scholar]
- 17.Wang B., Du J., Zhu Z., Ma Z., Wang S., Shan Z. Evaluation of parotid salivary glucose level for clinical diagnosis and monitoring type 2 diabetes mellitus patients. BioMed Res. Int. 2017;2017:2569707. doi: 10.1155/2017/2569707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadashetti V., Baad R., Malik N., Shivakumar K.M., Vibhute N., Belgaumi U. Glucose level estimation in diabetes mellitus by saliva: a bloodless revolution. Rom. J. Intern. Med. 2015;53:248–252. doi: 10.1515/rjim-2015-0032. [DOI] [PubMed] [Google Scholar]
- 19.Caldeira E.J., Camilli J.A., Cagnon V.H. Stereology and ultrastructure of the salivary glands of diabetic Nod mice submitted to long-term insulin treatment. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:930–937. doi: 10.1002/ar.a.20236. [DOI] [PubMed] [Google Scholar]
- 20.Lasisi T.J., Fasanmade A.A. Salivary flow and composition in diabetic and non-diabetic subjects. Niger. J. Physiol. Sci. 2012;27:79–82. [PubMed] [Google Scholar]
- 21.Yavuzyilmaz E., Yumak O., Akdoganli T., Yamalik N., Ozer N., Ersoy F. The alterations of whole saliva constituents in patients with diabetes mellitus. Aust. Dent. J. 1996;41:193–197. doi: 10.1111/j.1834-7819.1996.tb04855.x. [DOI] [PubMed] [Google Scholar]
- 22.Dodds M.W., Yeh C.K., Johnson D.A. Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Community Dent. Oral Epidemiol. 2000;28:373–381. doi: 10.1034/j.1600-0528.2000.028005373.x. [DOI] [PubMed] [Google Scholar]
- 23.Shashikanth M., Shambulingappa P. Comparison of serum glucose and salivary glucose in diabetic patients. J. Indian Acad. Oral Med. Radiol. 2008;20:9. [Google Scholar]