Highlights
-
•
Conventional fractionated radiotherapy (RT) is predominantly used for the palliation of symptomatic metastatic disease.
-
•
Hypofractionated stereotactic RT is increasingly adopted for the treatment of locally recurrent and oligometastatic disease.
-
•
High-dose radiation seems to have an immunogenic effect in patients with renal cell cancer.
-
•
Combinations of ablative RT with immunotherapies are promising approaches that might improve outcomes.
Abstract
Purpose
To review the role of radiotherapy (RT) in the treatment of renal cell cancer (RCC) in the curative and palliative setting.
Content
Details related to the clinical outcomes of primary, preoperative, postoperative and palliative RT are discussed, along with a presentation of the established role of surgery and systemic therapy. An overview of data derived from mono- and multi-institutional trials is provided.
Conclusion
Radiotherapy has been shown to provide good symptom palliation and local control in RCC depending on the dose that can be delivered. There is emerging data suggesting that with the use of high-precision RT methods the indication spectrum of RT can be exploited covering different clinical situations particularly for unresectable local recurrences and oligometastatic disease.
1. Introduction
Renal cell cancer (RCC) accounts for 2–3% of all cancers and over 90% of kidney cancers among adults with a worldwide incidence of about 300.000 new cases per year during the last decade. It is typically diagnosed in the seventh decade of life, with a median age at diagnosis of 65 years, however it represents also 1.4% of all renal tumors in patients younger than 4 years [1]. There is a strong gender preponderance, with incidence rates in men approximately twice that of women. In addition to gender disparities, there is also a notable variability in RCC incidence across racial and ethnic groups with the incidence rate being the highest in North America and Scandinavia and the lowest in Asia and South America [2]. Even though the reason for the higher incidence in developed countries and in men is not fully investigated, several risk factors have been implicated for this disparity including cigarette smoking, excess body weight, end-stage renal disease, acquired cystic kidney disease and treatment with phenacetin-containing analgetics as well as occupational exposures to trichloroethylene (TCE) [3].
With regard to genetic susceptibility, inherited RCC is known to occur in a number of familial cancer syndromes, most notably the von Hippel-Lindau syndrome and hereditary papillary RCC. A recent meta-analysis of small case-control studies showed a greater than twofold risk among individuals having a first-degree relative diagnosed with kidney cancer [4]. At this, RCC risk has been evaluated in relation to a number of common genetic variants identifying genes that may be relevant for carcinogenesis including GSTM1, GSTT1, GSTP1 and NAT 2. Although the GST genes and NAT 2 generally have not been linked to RCC risk, associations with tobacco smoke [5] or exposure to TCE [6] have been shown to vary among subgroups defined by genotype status. However, the results of these studies have yet to be validated considering that large genome-wide association studies of RCC risk are not available at this time.
As for treatment outcomes, the TNM-stages correlate with prognosis [7]. Patients with stage I disease have a 5-year disease specific survival (DSS) of about 80–95% and patients with stage II of around 75%. For patients with stage III RCC, 5-year DSS is about 50–60% and for stage IV disease of less than 10% with a median overall survival of 10–15 months [8]. At this, prognosis estimation has been enhanced by modifications to RCC staging in association with features based on the Fuhrman histologic classification system [9].
2. General management principles
About three quarters of people with RCC present with localized disease, and definitive local treatment remains the gold standard for managing patients with no evidence of distant metastasis. The role of routine adjuvant radiotherapy (RT) in the management of RCC is not established in patients with localized disease after gross total resection. In patients at high risk for local failure with positive margins and lymph node involvement, postoperative RT may be considered. Primary irradiation is not routinely used for RCC given its wide spectrum of radiosensitivity [10]. However, early results by high-precision RT methods suggest good local control rates for primary RCC in patients unable to undergo nephrectomy. As for systemic therapy, there is at present no indication for the use of adjuvant systemic treatment for RCC following gross total resection of the kidney with curative intent.
For the management of metastatic RCC, established practice is to perform cytoreductive nephrectomy, with or without local treatment of metastases (metastasectomy or stereotactic body radiotherapy, SBRT), before starting systemic treatment. In analogy, RT has an indication for symptom palliation and local control for unresectable local recurrences or metastatic disease.
3. Surgical therapy
The widespread use of abdominal imaging with computed tomography, magnetic resonance imaging, and ultrasound has propagated the detection of early stage RCC that is usually performed for symptoms unrelated to RCC. Currently, over half of all RCC lesions are discovered incidentally and the majority are diagnosed as localized disease [11]. Since the only possible curative treatment for RCC is complete surgical resection, careful risk-benefit counseling is required as the goals of treatment and complication profile are varied for the different oncosurgical procedures available.
3.1. Localized disease
The current surgical management for localized RCC include partial nephrectomy (PN) and radical nephrectomy (RN) with PN as the recommended treatment of choice, when technically feasible, and RN reserved for larger tumors that are central in location and adjacent to hilar structures. Partial nephrectomy is also recommended for genetic disorders such as von Hippel-Lindau syndrome which predispose to RCC and where repeated surgical treatments are needed. It is performed using open, laparoscopic, or robotic techniques with main benefit the preservation of nephrons leading to a decreased risk of renal insufficiency, which is associated with secondary morbidity and mortality-causing events [12].
Even though complete tumour excision by PN is preferred in healthy individuals, both the American Urological Association and European Association of Urology (EAU) list in their guidelines RN as a viable treatment option for early stage RCC where PN is not technically feasible [13], [14]. Although RN can include resection of hilar lymph nodes or regional lymph node dissection (LND), it has not been proven that LND prolongs survival in localised disease [15].
Cryoablation and radiofrequency ablation are two less invasive treatment options which should be offered to elderly patients with small, incidentally found, renal cortical lesions. Due to the lack of adequate oncological follow-up and several drawbacks (e.g., limitation in terms of tumor location, accuracy of post-ablation biopsy, need for frequent imaging), ablative methods are reserved for patients unfit for surgery [14].
3.2. Locally advanced disease
The definition of locally advanced RCC is typically defined as ≥T3 in the absence of distant metastasis. As in localized disease, the oncologic goals of surgery in locally advanced RCC are identical, i.e. to provide the greatest survival benefit with the lowest morbidity possible. Due to the varying scope of locally advanced RCC, open, laparoscopic, and robotic techniques have been described [13], [14].
The benefit of LND for patients with locally advanced disease is not clearly defined. However, it is hypothesized that LND may benefit higher-stage tumors and/or tumors with adverse pathological features such as high-grade differentiation, presence of sarcomatoid features, and histologic tissue necrosis. The presence of these risk factors can help to determine which patients may benefit from an extensive LND [16].
3.3. Metastatic disease
Approximately 20% of RCC patients present with metastatic disease at diagnosis. For this group, two phase III randomized clinical trials reported a statistically significant survival benefit when RN was combined with systemic immunotherapy independent of performance status and site of metastatic spread [17], [18]. Although these two trials used immunotherapies, they have continued to motivate cytoreductive nephrectomy (CN) in the contemporary targeted therapy era. Even though prospective studies are not available, retrospective data demonstrate that CN has an additional benefit in prolonging patient survival compared to systemic targeted therapy alone [19]. For patients developing metastases after prior nephrectomy followed by a disease-free interval, metastasectomy or SBRT should be considered given that many retrospective studies show longer survival in patients who received local treatment of (oligo)metastatic disease than those without [20], [21], [22], [23], [24].
4. Systemic therapy
Systemic therapy is mainstream for the treatment of patients with metastatic RCC. If patients are unlikely to benefit from metastasectomy, systemic therapy is initiated. If prior nephrectomy has not been performed at the time of systemic therapy and if CN is not indicated because of poor patient condition, tumor biopsy should be considered because histological subtype influences the choice of agents. At present, there is no indication to support the use of adjuvant systemic therapy for RCC following resection of the kidney with curative intent [25].
There is a variety of molecularly targeted and immunotherapeutic agents for the therapy of metastatic RCC including tyrosine kinase inhibitors (TKIs) that inhibit signaling by vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) such as sunitinib and sorafenib; multikinase TKIs that inhibit signaling for VEGFR, PDGFR, and c-kit, such as pazopanib or axitinib; monoclonal antibodies to VEGFR such as bevacizumab as well as M-TOR inhibitors such as everolimus and temsirolimus. The most common criteria used for therapy decisions are the Motzer criteria, which classify patients into different risk categories (favorable, intermediate, and poor risk). Based on recent evidence from prospective clinical trials, treatment of naive patients with clear cell histology and good/intermediate risk includes sunitinib, bevacizumab plus interferon-alpha, and pazopanib. The use of cytokines, including interleukin-2 or sorafenib, is an alternative treatment option. When patients are at poor risk, temsirolimus, sunitinib, sorafenib or pazopanib can be used. After the failure of first-line treatment, sorafenib, pazopanib, or axitinib is recommended for patients with cytokine refractory. When patients are at poor risk, nivolumab or cabozantinib is recommended with axitinib, sorafenib or everolimus as alternatives. Third-line therapy after nivolumab has not been determined [26].
For patients with non-clear cell histology there is currently no standard care. However, small prospective trials and subgroup analyses from larger trials suggest that these patients may benefit from treatment with everolimus, sorafenib, pazopanib or temsirolimus [26].
5. Radiation therapy
The spectrum of radiosensitivity in RCC is wide, but it is not a radioresistant disease. There is a developing rationale with emerging data suggesting that the apparent radioresistance of RCC can be overcome with the use of higher dose per fraction treatments usually delivered by new high-precision RT methods such as volumetric modulated arc therapy (VMAT) or SBRT [10]. Experiences with improvement in local control for patients treated with stereotactic radiosurgery (SRS) for renal cell brain metastases have led to growing interests in stereotactic RT of RCC for patients unsuitable for surgery. Several prospective trials tested SBRT as primary treatment for localised or locally recurrent RCC reporting promising rates of local control and acceptable toxicity [27], [28], [29], [30]. Nonetheless, at present there is still insufficient evidence to recommend a consensus view for the optimal dose/fractionation, technique, or delivery system.
The use of intraoperative RT (IORT) in patients with RCC, particularly in those with locally recurrent and/or advanced-stage non-metastatic disease, has also been reported. Data from one of the largest series, consisting of 98 patients from nine different institutions, revealed that the 5-year disease-specific and disease-free survival rates compare favourably to those of patients in surgical series without use of IORT [31]. However, given the paucity of data available on the outcomes following this approach, intraoperative irradiation for RCC should still be considered experimental. When assessing the overall body of evidence for use of external beam RT in the definitive management of patients with primary RCC, IORT seems to have only limited utility [32].
There is no current evidence for adjuvant RT after nephrectomy on the basis of two negative ‘old’ randomized trials [33], [34] which are characterized by several limitations in trial design and methodology. Those included inappropriate case selection, subtherapeutic RT regimes and inadequate patient numbers. Furthermore, treatment morbidity was substantially high while the RT techniques used have now been superseded by modern delivery systems such as VMAT. Newer retrospective analyses likewise failed to show a survival benefit for postoperative irradiation [35], [36].
Renal cell cancers rarely invade adjacent organs and large tumors tend to displace and compress adjacent tissue. However, if tumor directly invades contiguous tissues complete resection may not be feasible. Neoadjuvant irradiation can potentially downsize the tumor increasing the likelihood of an adequate surgical resection. The only two available prospective clinical trials failed to show a benefit for preoperative RT of locally advanced RCC in terms of overall survival [37], [38].
Palliative RT is an effective treatment for palliation of symptomatic metastatic RCC. Several prospective studies have reported excellent symptomatic response and local control rates for palliative irradiation of skeletal, soft-tissue and brain deposits using mainly SBRT [39], [40], [41] but also conventional 3D-conformal RT [42].
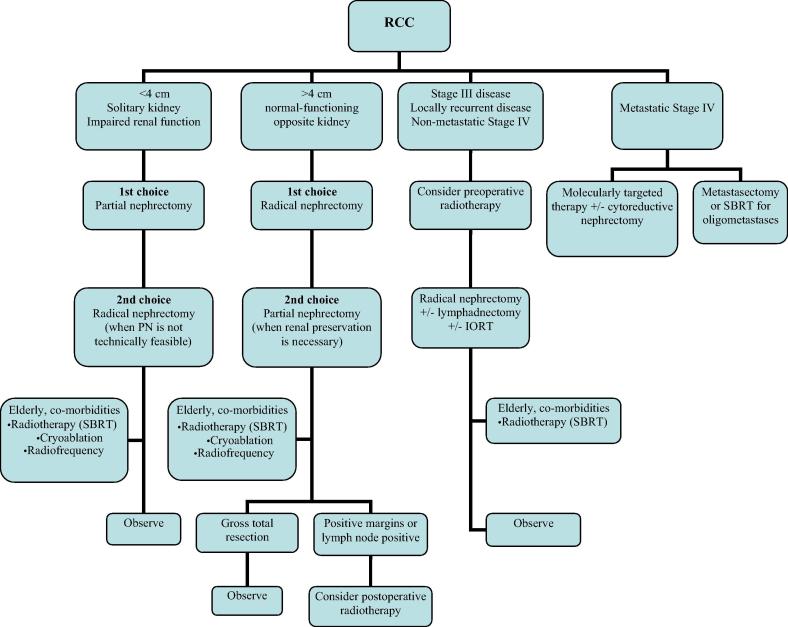
Fig. 1 presents an algorithm based on the current Mayo Clinic philosophy on diagnosis and treatment of RCC [43] considering the implementation of RT and local ablative methods for the primary site treatment in various clinical settings. Patients with stage I-II disease are candidates for PN or RN depending on tumour size, need for renal preservation and surgical feasibility. If positive surgical margins or lymph node involvement is present at nephrectomy, consideration should be given to postoperative RT. For stage III or locally recurrent disease, preoperative RT may downsize tumour burden to facilitate surgery. Maximal resection should be planned at 4–6 weeks after completion of preoperative RT. In this concept it may be noted that in contrary to the Mayo approach, european guidelines do not discuss neoadjuvant or postoperative RT as part of a multimodal treatment algorithm [13], [24]. Stereotactic body radiotherapy and local ablative treatment methods can be considered for patients who are medically inoperable or unwilling to undergo nephrectomy. Patients who present with metastatic disease are primarily candidates for molecularly targeted treatment. Cytoreductive nephrectomy can be considered to palliate symptoms, to enhance the effects of systemic therapy, or in patients with favorable prognostic factors such as oligometastatic disease.
Fig. 1.
Treatment algorithm for renal cell cancer.
6. Combination of radiation therapy and immunotherapy
The role of immunotherapy drugs, particularly checkpoint inhibitors, is rapidly evolving in the management of RCC having shown a significant survival benefit in the treatment of metastatic disease [44], [45], [46]. Based on preclinical and clinical data, a particularly promising approach is the combination of RT and immunotherapy to augment the local efficacy of RT as well as to allow for improved and more durable systemic responses with immunotherapy [47]. In this context, RT plays an important role in the potentiation and modulation of tumor immunity. It has the potential to convert immunologically ‘cold’ tumors into ‘hot’ tumors by a combination of distinct mechanisms including: (a) increasing tumor immunogenicity via the upregulation of antigenic expression, antigen processing, major histocompatibility molecules, and co-stimulatory signals; (b) overcoming an immunosuppressive tumor microenvironment by shifting the cytokine balance in favor of immunostimulation (e.g. by increasing the production of immunostimulatory cytokines) and (c) recruiting antigen-presenting and immune-effector cells to the tumor microenvironment [48], [49], [50], [51].
Although several mechanisms have been elucidated to account for the ability of RT to influence tumor immunity, it has been sidelined in the treatment of RCC, partly due to disappointing pre-clinical and randomized trial results that utilized conventionally fractionated treatment schedules and outdated RT techniques. These studies suggested that RCC was inherently radioresistant and fostered nihilism amongst clinicians [44]. Technical advances as well as a greater understanding of the radiobiology of RCC have allowed RT to re-emerge as a promising treatment option. Extreme hypofractionated RT with fractional doses ≥6 Gy activates different apoptosis pathways compared to conventional dose-fractionation schemes, resulting in translocation of ASMase and formation of pro-apoptotic ceramide, which is critical in the realization of tumor kill for vascular malignancies such as RCC [10], [52]. Conventional RT causes oxygen-dependent DNA damage and P53-mediated programmed cell death, which allows amassing of pro-angiogenic factors, and ongoing viability of the vascular endothelium. The effectiveness of high fractional doses are supported by cell survival curve studies including human RCC cell lines which show the α/β-ratio of RCC to be relatively low (between 3 and 7 Gy) and therefore likely more sensitive to hypofractionated RT [53], [54].
Few trial results evaluating the combined use of RT and immunotherapy in the genitourinary setting are available. A phase III trial by Kwon et al. [55] evaluated the use of ipilimumab versus placebo following bone-directed EBRT in 799 patients with metastatic castration-resistant prostate cancer who had progressed on prior docetaxel. Radiotherapy consisted of a single fraction of 8 Gy to one to five areas per investigator discretion. With a median follow-up of 9 months, there was numerically improved median OS (11.2 vs 10.0 months), but this difference was not statistically significant (p = 0.053). The primary analysis revealed that the proportional hazard assumption was violated, and in an exploratory piecewise hazard model, the hazard ratio for OS decreased over time, favoring the ipilimumab group at later time points. This benefit seemed to be greatest in patients with favorable prognostic features, including an absence of visceral metastases. Additionally, patients treated with ipilimumab had improved progression-free survival (median 4 vs 3.1 months; p < 0.0001). Table 3 depicts ongoing clinical trials evaluating the combination of RT with immunotherapy in patients with RCC [47].
Table 3.
Clinical trials combining immunotherapy with radiotherapy in renal cell cancer.
| Study | Eligibility | Design | Intervention | Institution |
|---|---|---|---|---|
| NCT01896271 | Metastatic ccRCC | Phase II | SABR + IL-2 | University of Texas Southwestern Medical Center (USA) |
| NCT03065179 | Metastatic ccRCC | Phase II | SBRT + nivolumab + ipilimumab | University of Texas Southwestern Medical Center (USA) |
| NCT02781506 | Metastatic ccRCC | Phase II | SABR + nivolumab | University of Texas Southwestern Medical Center (USA) |
| NCT02855203 | Metastatic ccRCC | Phase I/II | SABR + pembrolizumab | Peter MacCallum Cancer Centre (Australia) |
| NCT03050060 | Metastatic RCC, melanoma, or NSCLC | Phase II | IGRT + nelfinavir + (pembrolizumab or nivolumab or atezolizumab) | University of Washington (USA) |
| NCT02318771 | Recurrent/metastatic HNSCC, RCC, melanoma or NSCLC | Phase I | RT + pembrolizumab | Thomas Jefferson University (USA) |
| NCT02599779 | Metastatic RCC | Phase II | SBRT + pembrolizumab | Sunnybrook Health Sciences Centre (Canada) |
| NCT03149159 | Metastatic ccRCC | Phase II | SBRT + nivolumab + ipilimumab | Medical University of South Carolina (USA) |
| NCT02864615 | Metastatic RCC | Phase 1b | SBRT + (VEGFR inhibitor or mTOR inhibitor or checkpoint inhibitor) | Cornell University (USA) |
| NCT03469713 | Metastatic RCC | Phase II | SBRT + nivolumab | Gruppo Oncologico Italiano di Ricerca Clinica (Italy) |
| NCT03226236 | Metastatic RCC | Phase II | RT + IL-2 + dendritic cell vaccine | Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (Italy) |
ccRCC, clear cell renal cell carcinoma; HNSCC, head and neck squamous cell carcinoma; IGRT, image-guided radiation therapy.
IL-2, interleukin-2; mTOR, mammalian target of rapamycin; NSCLC, non-small cell lung carcinoma; RCC, renal cell cancer.
RT, radiation therapy; SABR, stereotactic ablative radiosurgery; SBRT, stereotactic body radiation therapy; VEGFR, vascular endothelial growth factor receptor.
7. Outcome and prognosis
7.1. Outcomes of radical nephrectomy vs partial nephrectomy
Only one randomized EORTC trial is available comparing postoperative survival between RN and PN for early-stage renal cancer. Patients randomized to either PN or RN showed a10-year OS of 75.7% for PN compared to 81.1% for RN with no statistical significant difference. The 10-year progression rate for PN was 4.1% and 3.3% for RN, likewise without statistical significant difference [56]. Non-randomized studies as well as large Cancer Database analyses matched for age, tumor size, and year of surgery, report mainly 5-year OS rates of 78–89% for PN and 74–86% for RN in the treatment of both localised and locally advanced disease [57], [58], [59], [60] with cancer specific survival of 78 ± 10% for PN and 74 ± 3% for RN in the treatment of locally advanced disease [61].
The benefit of PN is the preservation of nephrons leading to a decreased risk of renal insufficiency, as renal insufficiency is associated with secondary morbidity and mortality-causing events. In a retrospective series of 662 patients, the probability of freedom from new-onset renal insufficiency after PN was 80% versus 35% after RN, with RN identified as an independent risk factor for new-onset renal insufficiency [62]. Likewise, in the EORTC trial by van Poppel et al. approximately 85.7% of patients who underwent RN had renal dysfunction with an estimated glomerular filtration rate <60 ml/min, compared to 64.7% of patients after PN [56].
7.2. Primary irradiation
Technological advances in image guidance and motion management in patients receiving RT have facilitated the application of SBRT to the treatment of patients with primary RCC. The non-invasive approach enabled by SBRT provides advantages compared with alternative local ablative techniques. These include a lack of specific size limitations for the primary tumour and the ability to treat tumours at any location within the affected kidney. SBRT appears to be an attractive approach in patients with complex renal lesions, in which complete tumor resection might otherwise be required but whose suitability for surgery is borderline [32].
No data from randomized trials evaluating the efficacy of SBRT in patients with primary RCC are currently available. A systematic review published in 2012 consisted of 10 studies comprising 126 patients with inoperable RCC treated with SBRT. Three studies were prospective in design and seven were retrospective. The weighted local control rate obtained across all studies was 92.9% with a weighted rate of grade ≥3 toxicities of 3.8% [32]. More recent prospective trial and retrospective studies have continued to indicate high short-term and medium-term rates of local control that are typically >90% with low toxicity rates [27], [28], [29], [30], [63], [64], [65]. The main acute toxicities reported in the literature are nausea and fatigue, followed by radiation dermatitis and enteritis. Severe toxicities include renal toxicities, duodenal ulceration and skin toxicities in <5% of patients. Table 1 lists recent prospective trials and retrospective series on SBRT for primary RCC.
Table 1.
Recent prospective trials and retrospective series on stereotactic body radiotherapy for primary renal cell cancer.
| Study | n | Marginal dose (Gy) | Outcomes | Toxicities |
|---|---|---|---|---|
| Pham et al. [28] | 20 | 26 Gy in 1 fraction 42 Gy in 3 fractions |
Follow–up duration for toxicity reporting of 6 months | Grade 1–2 toxicities in 60% of patients; no grade ≥ 3 toxicities |
| Ponksy et al.[30] | 19 | 48 Gy in 4 fractions | Median follow–up of 13.7 months | Grade 2 toxicities in 5.2% of patients; grade 3–4 toxicities in 15.8% of patients |
| Siva et al. [27] | 33 | 26 Gy in 1 fraction 42 Gy in 3 fractions |
Crude local control 97%; 2-year local control 100%; 2 year OS 92% at median follow-up of 24 months | Grade 1–2 toxicities in 78% of patients; grade 3 toxicities in 3% of patients |
| Staehler et al. [29] | 30 | 25 Gy in 1 fraction | Crude local control 98% at median follow-up of 28.1 months | Grade 1–2 toxicities in 13% of patients |
| McBride et al.[65] | 15 | Median 33 Gy in 3 fractions | Crude local control 87% at median follow-up of 36.7 months | 1 grade 3 toxicity (renal); 7 acute grade 1 toxicities |
| Chang et al. [63] | 16 | 30–40 Gy in 5 fractions | Crude local control 100% at median follow-up of 19 months | 1 grade 2 acute toxicity; 2 grade 4 late toxicities |
| Gilson et al. [64] | 33 | Median 40 Gy in 5 fractions | Crude local control 94% at mean follow-up of 17 months | NS |
n, number of patients; NS, not stated.
7.3. Preoperative irradiation
Two prospective clinical trials evaluating the role of neoadjuvant irradiation have been conducted. Van der Werf-Messing [38] reported a series of 126 patients treated between 1965 and 1972 randomized to nephrectomy alone versus low-dose preoperative RT (30 Gy in 15 fractions for 3 weeks) followed by nephrectomy. Survival was considerably better at 18 months with the use of preoperative irradiation. However, there was no difference in 5-year OS. Juusela et al. [37] conducted a prospective, randomized study of preoperative irradiation followed by nephrectomy in 38 patients versus RN alone in 44 patients. Patients treated with preoperative RT received 2.2 Gy per day to a total dose of 33 Gy and had a 47% 5-year OS, compared with 63% for patients treated with nephrectomy alone. Furthermore, subgroup analysis failed to reveal any specific group of patients that benefited from preoperative radiotherapy. Accordingly, interest in the use of preoperative radiotherapy waned following these results.
7.4. Adjuvant irradiation
Around the same time that data investigating neoadjuvant RT were published, similar randomized trials were being conducted in the postoperative setting. Finney et al. [33] published data from a randomized group of patients with high-risk disease who had either positive surgical margins or inferior vena cava involvement that underwent RN either with (n = 52) or without (n = 48) subsequent RT. No improvement in OS was observed with use of RT. In another randomized study, Kjaer et al. [34] investigated the efficacy of adjuvant RT using a dose of 50 Gy in 20 fractions for patients with stage II-III RCC. The overall relapse rate was 48% not differing significantly between patients in either arm. In addition, 44% of gastrointestinal toxicities affecting the stomach, duodenum and liver were observed, as well as a mortality rate of 20% among patients in the RT arm. Makarewicz et al. [66] analyzed retrospectively 186 patients with locally advanced RCC that underwent RN either without (n = 72) or with (n = 114) RT of median dose of 50.0 Gy. For all patients, the 5-year overall and disease-free survival rates were 36.2% and 30.5%, respectively. Non-significant difference was observed in terms of 5-year overall and disease-free survival between the group of patients with postoperative RT and without, 37.9%/29.5% vs. 35.5%/31.3%. A meta-analysis by Tunio et al. [67] including data from seven studies revealed an improvement in the extent of local control with use of postoperative RT without any survival benefit. Five of the seven studies included were retrospective with the two above mentioned of prospective design. Overall, the analysis highlighted the substantial limitations of the various studies including the use of non-conformal RT techniques, inappropriate dosing, and outdated technology, concluding the need for multi-institutional trials to investigate the additional benefits of adjuvant RT regarding OS along with targeted therapy. In conclusion, the role of routine postoperative RT in the management of RCC is not established in patients with localized disease after gross total resection. However, the risk of local failure is higher in patients with positive margins and lymph node involvement, and postoperative RT may be considered. Table 2 lists prospective trials and retrospective series on adjuvant RT for non-metastatic RCC [68], [69].
Table 2.
Prospective trials and retrospective series on adjuvant radiotherapy for renal cell cancer.
| Study | n | Treatment | Radiotherapy dose (Gy)/Fractions | 5-year survival (%) |
|---|---|---|---|---|
| Finney et al. (33) | 49 51 |
N N + RT |
55/25 | 51 45 |
| Kjaer et al. (34) | 33 32 |
N N + RT |
55/20 | 62 38 |
| Stein et al. (69) | 63 56 |
N N + RT |
46/23 | 40 50 |
| Kao et al. (68) | 12 12 |
N N + RT |
45/25 | 62* 75* |
| Makarewicz et al. (66) | 72 114 |
N N + RT |
Median 50 Gy/NS | 38 30 |
| Ulutin et al. (35) | 14 26 |
N N + RT |
46–50/NS | 20 70 |
N, Nephrectomy; n, number of patients; NS, not stated; RT, radiation therapy. *Disease-free survival.
7.5. Palliative irradiation
In the palliative setting, conventional RT can be used to alleviate pain, control the severity of neurological symptoms or to ameliorate haematuria. The outcomes of a number of retrospective studies and a few prospective trials indicate response rates of >50% among patients with metastatic RCC receiving conventionally fractionated RT [32]. Lee et al. [42] reported the outcomes of a multicentre phase II trial, in which 83% of patients had site-specific pain relief after receiving conventional RT at a dose of 30 Gy in 10 fractions, with a median response duration of 3 months. Hugeinin et al. [70] treated 90 patients with metastatic melanoma or RCC within a nonrandomized study with 5 × 4 Gy or 10 × 3 Gy. Relief of pain from bone lesions was observed in 26 of 40 cases, with a duration of response of 2.4 months, corresponding to 57% of the remaining lifetime. Freedom from symptoms in patients treated for impending neurological complications from metastases to the brain, spine, or nerve plexus was documented for 86–100% of their lifetime. Thus, conventional palliative RT has a well-defined role in controlling the localized symptoms of advanced RCC.
Concerning the efficacy of stereotactic RT in patients with metastatic RCC, rates of local control are typically exceptional compared with those provided by conventionally fractionated RT. The findings of a systematic review and meta-analysis published in 2015 indicate that SRS provides a weighted local control rate of 92%, with median OS durations ranging from 6.7 to 25.6 months. These data encompass 1301 patients with >3433 treated metastases. The reported incidences of grade 3–4 toxicities ranged from 0% to 6% of patients. Similarly, for patients with extracranial RCC metastases treated using SBRT, the conclusions of this systematic review indicate a weighted local control rate of 89%, with median OS durations ranging from 11.7 to 22 months. Reported rates of grade 3–4 toxicities ranged from 0% to 4% [23]. Overall, intracranial SRS and extracranial SBRT seem to be highly effective and safe for the control of metastases in patients with RCC. In addition, SRS of the surgical cavity after complete resection of brain oligometastases has been shown to significantly lower local recurrence compared with observation alone without the decline in cognitive function observed with whole-brain RT (WBRT). Thus, the use of SRS after brain metastasis resection should be considered one of the standards of care as a less toxic alternative to WBRT [71], [72].
However, intra- and extracranial stereotactic techniques are likely to be of limited utility in patients with widely disseminated disease with inadequate responses to systemic therapy. Similarly, patients with severely symptomatic disease might instead benefit from conventional RT or metastasectomy [32].
8. Conclusion
Treatment for patients with RCC has dramatically changed during the last decades. As for the surgical treatment option, development of the laparoscopic and robotic-based procedure provides a great benefit for patients in terms of less invasive surgery. As for systemic therapy, introduction of targeted therapy has prolonged the survival of patients with metastatic RCC compared to that of the cytokine era. In addition, immune checkpoint inhibitors have recently introduced a major paradigm shift in sequential therapy. Radiotherapy has been shown to provide good symptom palliation and local control in RCC depending on the dose that can be delivered. There is emerging data showing that with the use of high-precision methods, such as SBRT, unresectable local recurrences or oligometastatic disease can successfully be treated with durable local control and low toxicity. Additionally, an emerging awareness of the capacity of high-dose radiation to stimulate an immune response has resulted in combinations of stereotactic RT with immunotherapy-based approaches and future advances will likely result from the integration of systemic targeted agents and immunotherapies with high-dose radiation. This approach might be implemented in early stage as well as advanced or metastatic settings. In early stage disease, immunotherapy could be utilized after definitive therapy to prevent recurrences, including metastases. In the advanced and metastatic setting, the addition of RT to immunotherapy may potentiate the generation of antitumor immune responses, which could treat existing metastases as well as prevent future metastases.
Conflict of interest notification
The authors would like to ensure that no financial support has been received in conjunction with the generation of the current submission and none of the authors has any personal or institutional financial interest in drugs or materials described in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.01.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ridge C.A., Pua B.B., Madoff D.C. Epidemiology and staging of renal cell carcinoma. Semin Intervent Radiol. 2014;31(1):3–8. doi: 10.1055/s-0033-1363837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungberg B., Campbell S.C., Choi H.Y. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Chow W., Dong L.M., Devesa S.S. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clague J., Lin J., Cassidy A. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18(3):801–807. doi: 10.1158/1055-9965.EPI-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza J.C., Ziogas A., Largent J. Gene-environment interactions in renal cell carcinoma. Am J Epidemiol. 2001;153(9):851–859. doi: 10.1093/aje/153.9.851. [DOI] [PubMed] [Google Scholar]
- 6.Charbotel B., Gad S., Caiola D. Trichloroethylene exposure and somatic mutations of the VHL gene in patients with Renal Cell Carcinoma. J Occup Med Toxicol. 2007;2:13. doi: 10.1186/1745-6673-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Jonasch E., Gao J., Rathmell W.K. Renal cell carcinoma. BMJ. 2014;349 doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker A., Hickmann D., Hansen J. Critical analysis of a simplified Fuhrman grading scheme for prediction of cancer specific mortality in patients with clear cell renal cell carcinoma–Impact on prognosis. Eur J Surg Oncol. 2016;42(3):419–425. doi: 10.1016/j.ejso.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 10.de Meerleer G., Khoo V., Escudier B. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15(4):e170–e177. doi: 10.1016/S1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 11.Sankineni S., Brown A., Cieciera M. Imaging of renal cell carcinoma. Urol Oncol. 2016;34(3):147–155. doi: 10.1016/j.urolonc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Lane B.R., Campbell S.C., Gill I.S. 10-year oncologic outcomes after laparoscopic and open partial nephrectomy. J Urol. 2013;190(1):44–49. doi: 10.1016/j.juro.2012.12.102. [DOI] [PubMed] [Google Scholar]
- 13.Ljungberg B., Bensalah K., Canfield S. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S., Uzzo R.G., Allaf M.E. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198(3):520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 15.Marchioni M., Bandini M., Pompe R.S. The impact of lymph node dissection and positive lymph nodes on cancer-specific mortality in contemporary pT2-3 non-metastatic renal cell carcinoma treated with radical nephrectomy. BJU Int. 2017 doi: 10.1111/bju.14024. [DOI] [PubMed] [Google Scholar]
- 16.Bekema H.J., MacLennan S., Imamura M. Systematic review of adrenalectomy and lymph node dissection in locally advanced renal cell carcinoma. Eur Urol. 2013;64(5):799–810. doi: 10.1016/j.eururo.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Flanigan R.C., Salmon S.E., Blumenstein B.A. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 18.Mickisch G.H., Garin A., van Poppel H. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 19.Heng D.Y.C., Wells J.C., Rini B.I. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704–710. doi: 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Dabestani S., Marconi L., Hofmann F. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15(12):e549–e561. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 21.Naito S., Kinoshita H., Kondo T. Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: a multicenter study of 556 patients. Urology. 2013;82(4):846–851. doi: 10.1016/j.urology.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Bamias A., Escudier B., Sternberg C.N. Current clinical practice guidelines for the treatment of renal cell carcinoma: a systematic review and critical evaluation. Oncologist. 2017;22(6):667–679. doi: 10.1634/theoncologist.2016-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kothari G., Foroudi F., Gill S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: a systematic review. Acta Oncol. 2015;54(2):148–157. doi: 10.3109/0284186X.2014.939298. [DOI] [PubMed] [Google Scholar]
- 24.Muller A., van Oorschot B., Micke O. German S3 guideline for renal cell carcinoma: presentation and discussion of essential aspects for the radiation oncologist. Strahlenther Onkol. 2018;194(1):1–8. doi: 10.1007/s00066-017-1185-y. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y., Li S., Jia Z. Adjuvant therapy for locally advanced renal cell carcinoma: a meta-analysis and systematic review. Urol Oncol. 2017 doi: 10.1016/j.urolonc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B., Porta C., Schmidinger M. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v58–v68. doi: 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]
- 27.Siva S., Pham D., Kron T. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int. 2017;120(5):623–630. doi: 10.1111/bju.13811. [DOI] [PubMed] [Google Scholar]
- 28.Pham D., Thompson A., Kron T. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys. 2014;90(5):1061–1068. doi: 10.1016/j.ijrobp.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Staehler M., Bader M., Schlenker B. Single fraction radiosurgery for the treatment of renal tumors. J Urol. 2015;193(3):771–775. doi: 10.1016/j.juro.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Ponsky L., Lo S.S., Zhang Y. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117(1):183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Paly J.J., Hallemeier C.L., Biggs P.J. Outcomes in a multi-institutional cohort of patients treated with intraoperative radiation therapy for advanced or recurrent renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2014;88(3):618–623. doi: 10.1016/j.ijrobp.2013.11.207. [DOI] [PubMed] [Google Scholar]
- 32.Siva S., Kothari G., Muacevic A. Radiotherapy for renal cell carcinoma: renaissance of an overlooked approach. Nat Rev Urol. 2017;14(9):549–563. doi: 10.1038/nrurol.2017.87. [DOI] [PubMed] [Google Scholar]
- 33.Finney R. The value of radiotherapy in the treatment of hypernephroma–a clinical trial. Br J Urol. 1973;45(3):258–269. doi: 10.1111/j.1464-410x.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- 34.Kjaer M., Iversen P., Hvidt V. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21(4):285–289. doi: 10.3109/00365598709180784. [DOI] [PubMed] [Google Scholar]
- 35.Ulutin H.C., Aksu G., Fayda M. The value of postoperative radiotherapy in renal cell carcinoma: a single-institution experience. Tumori. 2006;92(3):202–206. doi: 10.1177/030089160609200303. [DOI] [PubMed] [Google Scholar]
- 36.Gez E., Libes M., Bar-Deroma R. Postoperative irradiation in localized renal cell carcinoma: the Rambam Medical Center experience. Tumori. 2002;88(6):500–502. doi: 10.1177/030089160208800613. [DOI] [PubMed] [Google Scholar]
- 37.Juusela H., Malmio K., Alfthan O. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11(3):277–281. doi: 10.3109/00365597709179965. [DOI] [PubMed] [Google Scholar]
- 38.van der Werf-Messing B. Proceedings: carcinoma of the kidney. Cancer. 1973;32(5):1056–1061. doi: 10.1002/1097-0142(197311)32:5<1056::aid-cncr2820320505>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Ghia A.J., Chang E.L., Bishop A.J. Single-fraction versus multifraction spinal stereotactic radiosurgery for spinal metastases from renal cell carcinoma: secondary analysis of Phase I/II trials. J Neurosurg Spine. 2016;24(5):829–836. doi: 10.3171/2015.8.SPINE15844. [DOI] [PubMed] [Google Scholar]
- 40.Altoos B., Amini A., Yacoub M. Local control rates of metastatic Renal Cell Carcinoma (RCC) to thoracic, abdominal, and soft tissue lesions using Stereotactic Body Radiotherapy (SBRT) Radiat Oncol. 2015;10:218. doi: 10.1186/s13014-015-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen S., Dahlke M., Trang N.T. Estimation of the six-month survival probability after radiosurgery for brain metastases from kidney cancer. Anticancer Res. 2015;35(7):4215–4217. [PubMed] [Google Scholar]
- 42.Lee J., Hodgson D., Chow E. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer. 2005;104(9):1894–1900. doi: 10.1002/cncr.21410. [DOI] [PubMed] [Google Scholar]
- 43.William W., Wong J.L.P. Kidney and ureteral carcinoma. In: Gunderson Leonard L., Tepper Joel E., editors. Clinical Radiation Oncology. 4th ed. Elsevier LTD, Oxford; Philadelphia: 2016. [Google Scholar]
- 44.Kothari G., Louie A.V., Pryor D. Stereotactic body radiotherapy for primary renal cell carcinoma and adrenal metastases. Chin Clin Oncol. 2017;6(Suppl 2):S17. doi: 10.21037/cco.2017.06.30. [DOI] [PubMed] [Google Scholar]
- 45.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motzer R.J., Rini B.I., McDermott D.F. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solanki A.A., Bossi A., Efstathiou J.A. Combining immunotherapy with radiotherapy for the treatment of genitourinary malignancies. Eur Urol Oncol. 2018 doi: 10.1016/j.euo.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Demaria S., Golden E.B., Formenti S.C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y., Dong Y., Kong L. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko E.C., Formenti S.C. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918768240. 1758835918768240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichselbaum R.R., Liang H., Deng L. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14 doi: 10.1038/nrclinonc.2016.211. 365 EP. [DOI] [PubMed] [Google Scholar]
- 52.Truman J., Garcia-Barros M., Kaag M. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson D., Hiller L., Gray L. The effect of biological effective dose on time to symptom progression in metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol) 2003;15(7):400–407. doi: 10.1016/s0936-6555(03)00164-x. [DOI] [PubMed] [Google Scholar]
- 54.Ning S., Trisler K., Wessels B.W. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80(12 Suppl):2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 55.Kwon E.D., Drake C.G., Scher H.I. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Poppel H., Da Pozzo L., Albrecht W. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 57.Wang D.C., Plante K., Stewart T. Comparison of survival for partial vs. radical nephrectomy in young patients with T1a renal cell carcinoma treated at commission on cancer-accredited facilities and influence of comorbidities on treatment choice. Urol Oncol. 2017;35(11) doi: 10.1016/j.urolonc.2017.06.056. 660.e9-660.e15. [DOI] [PubMed] [Google Scholar]
- 58.Mir M.C., Derweesh I., Porpiglia F. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71(4):606–617. doi: 10.1016/j.eururo.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 59.Kopp R.P., Mehrazin R., Palazzi K.L. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114(5):708–718. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 60.Zini L., Perrotte P., Capitanio U. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115(7):1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 61.Margulis V., Tamboli P., Jacobsohn K.M. Oncological efficacy and safety of nephron-sparing surgery for selected patients with locally advanced renal cell carcinoma. BJU Int. 2007;100(6):1235–1239. doi: 10.1111/j.1464-410X.2007.07225.x. [DOI] [PubMed] [Google Scholar]
- 62.Huang W.C., Levey A.S., Serio A.M. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang J.H., Cheung P., Erler D. Stereotactic ablative body radiotherapy for primary renal cell carcinoma in non-surgical candidates: initial clinical experience. Clin Oncol (R Coll Radiol) 2016;28(9):e109–e114. doi: 10.1016/j.clon.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Gilson B., Lederman G., Qian G. Int J Radiat Oncol Biol Phys. 2006;66(3):S349. 2249. [Google Scholar]
- 65.McBride S.M., Wagner A.A., Kaplan I.D. A phase 1 dose-escalation study of robotic radiosurgery in inoperable primary renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;87(2):S84. [Google Scholar]
- 66.Makarewicz R., Zarzycka M., Kulinska G. The value of postoperative radiotherapy in advanced renal cell cancer. Neoplasma. 1998;45(6):380–383. [PubMed] [Google Scholar]
- 67.Tunio M.A., Hashmi A., Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol. 2010;21(9):1839–1845. doi: 10.1093/annonc/mdq028. [DOI] [PubMed] [Google Scholar]
- 68.Kao G.D., Malkowicz S.B., Whittington R. Locally advanced renal cell carcinoma: low complication rate and efficacy of postnephrectomy radiation therapy planned with CT. Radiology. 1994;193(3):725–730. doi: 10.1148/radiology.193.3.7972814. [DOI] [PubMed] [Google Scholar]
- 69.Stein M., Kuten A., Halpern J. The value of postoperative irradiation in renal cell cancer. Radiother Oncol. 1992;24(1):41–44. doi: 10.1016/0167-8140(92)90352-u. [DOI] [PubMed] [Google Scholar]
- 70.Huguenin P.U., Kieser S., Glanzmann C. Radiotherapy for metastatic carcinomas of the kidney or melanomas: an analysis using palliative end points. Int J Radiat Oncol Biol Phys. 1998;41(2):401–405. doi: 10.1016/s0360-3016(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 71.Brown P.D., Ballman K.V., Cerhan J.H. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC. 3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahajan A., Ahmed S., McAleer M.F. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.