Abstract
In addition to the assessment of local alterations of specific brain regions, the investigation of entire networks with in vivo neuroimaging techniques has gained increasing attention. In general, connectivity analysis refers to the investigation of links between brain regions, with the aim to characterize their interactions and information transfer. These may represent or relate to different physiological characteristics (structural, functional, or metabolic information) and can be calculated across different levels of granularity (2 regions vs whole brain). In this article, we provide an overview of different connectivity analysis approaches with interpretations and limitations as well as examples in pharmacological imaging and clinical applications. Structural connectivity obtained from diffusion MRI enables the reconstruction of neuronal fiber tracts. These physical links represent major constraints of functional connections, which are in turn defined as correlations between signal time courses. In addition, molecular connectivity approaches based on PET imaging enable the assessment of interregional associations of metabolic demands and neurotransmitter systems. Application of these approaches in clinical investigations has demonstrated novel alterations in various neurological and psychiatric disorders on a network level. Future work should aim for the combined assessment of multiple imaging modalities and to establish robust biomarkers for clinical use. These advancements will further improve the biological interpretation of connectivity metrics and networks of the human brain.
Keywords: connectivity, diffusion tensor imaging, graph theory, network, positron emission tomography, resting-state fMRI
Introduction
The possibility to assess human brain structure and function in vivo has made significant contributions to the clinical characterization of numerous psychiatric and neurological disorders. Imaging techniques like magnetic resonance imaging (MRI), positron emission tomography (PET), and electroencephalography are among the most used modalities. As such, they have substantially increased our knowledge about the maturation of the brain, normal physiological processes, and pathological alterations thereof. Traditionally, the focus has been on local effects and changes in certain brain regions like tumors or atrophy, alterations in blood flow and metabolism, as well as the up- or downregulation of receptors and transporters of neurotransmitter systems. Particularly with the use of functional MRI (fMRI), cognitive functions and processing of certain emotions were often ascribed to specific areas. As the brain, however, comprises far more connections than regions, namely 1014 synaptic connections vs 1010 neurons, the investigation of these links has always been of great interest. Invasive histological approaches such as cell staining and the injection of labeled agents for tract tracing have previously been used for the assessment of anatomical networks, and these represent the very basis for the nowadays widespread connectivity analyses. One example is given by the CoCoMac database (Kötter, 2004), which combines a multitude of anatomical tract tracing studies into a comprehensive data set of physical connections within the primate brain.
It is now well established that not a single brain area but rather the concert of several anatomically and/or functionally interconnected regions is responsible for the diverse processing of external and internal stimuli as well as response and adaptation. The study of connectivity thus aims to model and understand how such a coordinated interplay and the interactions between brain regions form neuronal networks, which in turn maintain efficient information processing. Although current in vivo brain imaging approaches cannot give a complete description of the connectome at a cellular level, these techniques are well suited to investigate brain networks at the macro-scale. The present article aims to provide an overview of common connectivity analysis strategies derived from data such as structural and functional imaging. We also highlight further aspects of connectivity obtained from PET imaging as well as multimodal combinations of these approaches. Following a brief description of each technique as well as their advantages, interpretations, and limitations, several examples with respect to applications in clinical and neuropharmacological research will be provided.
Connectivity Levels and Graph Theory
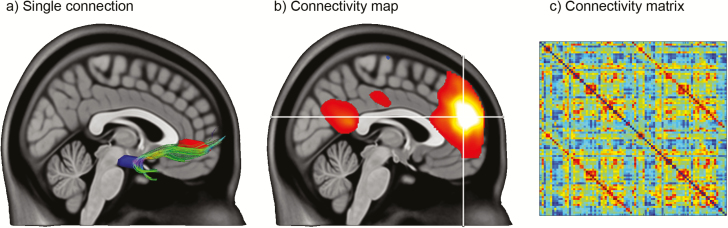
Connectivity analyses based on imaging data can be carried out on multiple levels of detail. The most specific analysis is between 2 a priori chosen brain regions yielding, for example, a single estimate of connection strength or a fiber pathway (seed-to-seed connectivity; Figure 1a). If one is, however, interested in the relation of a certain region (i.e., a seed region) compared with the rest of the brain, a connectivity map can be constructed (seed-to-voxel connectivity; Figure 1b). A more generalized approach covers the assessment of whole-brain connectivity. This can be achieved by setting up a connectivity matrix, where each matrix entry represents the connectivity between any 2 regions (Figure 1c). The approach enables the specific assessment of within- and between-network connections, for example, to investigate if pathological alterations are limited to a certain network, multiple networks independently, or may be specifically ascribed to between-network communications (Kaiser et al., 2015). Such connectivity matrices can then be further used for statistical testing, for example, with network-based statistics (Zalesky et al., 2010), or they may be subject to network analysis using graph theory. While the former approach directly operates on raw connectivity matrices, the appearance of the connectivity matrix regarding sparsity, weighting, and directionality is important for the latter. Usually, weak links are discarded since spurious connections may obscure the topology of the network (Rubinov and Sporns, 2010), though the importance of few weak ties has also been highlighted (Gallos et al., 2012). Weighted connections often provide a measure of “connectivity strength,” but as this in itself can raise difficulties regarding the interpretation (see structural connectivity), matrices are often binarized after thresholding. Regarding the directionality of connections, this can often only be achieved by invasive tract-tracing or with dynamic causal modeling. Graph theory enables the extraction of network summary metrics like node degree and network efficiency reflecting the connection strength of a single region and the interconnectedness of the entire network, respectively. These have in turn been related to intelligence scores (Li et al., 2009; Ryman et al., 2016) and cognitive performance (Hampson et al., 2006; Giessing et al., 2013), and alterations were identified in various psychiatric populations (Lord et al., 2012). A particularly interesting approach is to study network economy and the simulation of network perturbations (Bullmore and Sporns, 2012; Hahn et al., 2015a). This allows to assess cost-efficiency tradeoffs (Achard and Bullmore, 2007) and modelling of putative pathological mechanisms by inducing “lesions” in brain networks (Fornito et al., 2013; Gollo et al., 2018). Although these examples indicate that graph metrics may indeed capture physiologically relevant information, a recent study suggested caution for one of the most prominent findings, namely the association between intelligence and brain network efficiency (Kruschwitz et al., 2018). Thus, several limitations should be considered when evaluating whole-brain connectivity. These include potential pitfalls when constructing a connectivity matrix (e.g., definition of nodes and edges), during the analysis (thresholding, multiple comparisons, and null models), or when interpreting the results (when whole-brain summary measures are actually caused by local alterations) (Fornito et al., 2013). For a detailed description on graph theory and the corresponding outcome metrics (Rubinov and Sporns, 2010), please see previous seminal review articles (Sporns et al., 2004; Bullmore and Sporns, 2009; Fornito et al., 2013).
Figure 1.
Different levels of connectivity. (a) A single structural connection is shown between 2 a priori-defined brain regions: the uncinated fasciculus, connecting the amygdala (blue) with the frontal cortex (red, regions schematically drawn). (b) Functional connectivity map with respect to a particular seed region, the prefrontal cortex (illustrated by the crosshair). (c) Whole-brain connectivity matrix derived from an anatomical atlas. Each matrix entry reflects the connectivity of 2 brain regions as shown in a. Each column (and row) of the connectivity matrix represents a connectivity map as given in b.
Structural Connectivity
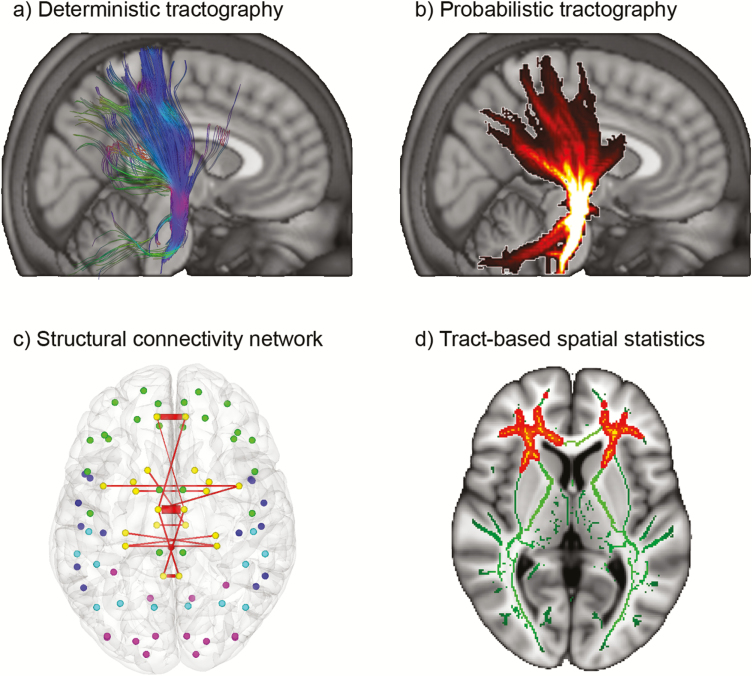
At the structural level, diffusion weighted imaging approaches such as diffusion tensor and spectrum imaging offer the possibility to reconstruct the anatomical/physical links between brain regions. This is based on the anisotropic diffusion of water along white matter fiber tracts (for a recent review, see Shi and Toga, 2017). Reconstruction of fiber pathways is usually done with deterministic or probabilistic algorithms. The former reconstructs tracts based on the preferential diffusion direction and hence yields streamlines with equal weights forming a single tract (Figure 2a). In contrast, the latter approach models the uncertainty of local fiber directions. As a result, probabilistic tractography provides various possible fiber pathways with an estimate of the robustness of each tract against noise (Figure 2b). For both approaches, great effort has been carried out to relate connectivity metrics to actual connectivity strength. Using invasive tract tracing results as reference, a moderate association was obtained for a deterministically defined number of streamlines (van den Heuvel et al., 2015), which was however 2-fold higher for probabilistic indices (Donahue et al., 2016). On the other hand, for whole-brain connectivity matrices, the high specificity obtained with deterministic approaches seems more important than the high sensitivity inherent to probabilistic tractography (Zalesky et al., 2016). Another interesting aspect is given by the interpretation of the commonly used metric of fractional anisotropy. Using the postmortem approach CLARITY, a relationship was found with immunofluorescence-labeled myelin basic protein for commissural tracts with coherent fiber organization (Chang et al., 2017). This association with fractional anisotropy was, however, not confirmed by in vivo MRI-based estimation of myelin content (Arshad et al., 2017). Furthermore, it should be noted that the term white matter “integrity” may be used for disease-related changes in fractional anisotropy but can be misleading for interpretations related to cognitive performance or learning (Jones et al., 2013). More generally, changes in diffusion metrics may reflect various biological aspects. These include myelination, axon diameter, packing density, and fiber organization but also changes in astrocytes and vascularization (Takahashi et al., 2002; Zatorre et al., 2012). Although these cannot be disentangled by diffusion MRI, preclinical studies have provided further insight into the underlying mechanisms, showing that learning-induced changes in white matter fractional anisotropy are related to changes in myelin staining (Blumenfeld-Katzir et al., 2011; Sampaio-Baptista et al., 2013). Moreover, the introduction of neurite orientation dispersion and density imaging may provide an even more specific surrogate of microstructure, which can still be obtained in a reasonable acquisition time (Zhang et al., 2012). Another issue that should be considered is that of crossing fibers within the brain, especially when considering that up to 90% of white matter voxels contain multiple fiber orientations (Tournier et al., 2011). Thus, dedicated algorithms that are able to reconstruct also nondominant tracts (Behrens et al., 2007) should be employed when investigating these brain regions.
Figure 2.
Structural connectivity obtained from diffusion tensor imaging. (a) Deterministic tractography with the seed region defined in the cerebral peduncle yields the corticospinal tract. Each streamline gets the same weight; color coding reflects the direction (blue, inferior-superior; green, anterior-posterior; red, left-right). (b) Probabilistic tractography of the same tract as in a, with each streamline reflecting the probability of how robustly a pathway can be reconstructed in the presence of noise (bright color: higher probability). (c) Evaluation of whole-brain structural network parameters showed increased interhemispheric connectivity in male-to-female transsexual subjects particularly subcortical-cortical connections, which where distinct from male and female controls (reprinted by permission from Oxford University Press) (Hahn et al., 2015b). (d) Tract-based spatial statistics (TBSS) enables a regional assessment of white matter microstructure. In contrast to c, the local mean diffusivity of transsexuals showed a transition from the biological sex to the actual gender identity (Kranz et al., 2014).
Structural connectivity investigations greatly improved our understanding of brain organization and the impact of its wiring. For instance, using graph metrics, the posterior part of the default mode network was identified as structural core, emphasizing its importance in functional integration (Hagmann et al., 2008). Furthermore, sex differences in behavioral abilities seemed to be at least partly related to differences in intra- and interhemispheric wiring of the brain, with connections optimized for coordinated motor abilities and social skills in men and women, respectively (Ingalhalikar et al., 2014). This finding further extends to differences in transsexual subjects with network parameters revealing distinct characteristics (Hahn et al., 2015b) (Figure 2c), whereas regional values of fractional anisotropy mostly reflect the transition from the biological sex to the actual gender identity (Kranz et al., 2014) (Figure 2d). Further clinical examples include prediction of MDD response to selective serotonin reuptake inhibitors (SSRIs), which was achieved by modeling a specific tract between the midbrain raphe and amygdala (Delorenzo et al., 2013). Such a specific application of tractography to model certain tracts also facilitated the individual planning of electrode placement in deep-brain stimulation, leading to markedly high response rates in treatment-resistant depression (Schlaepfer et al., 2013). Other examples include automated computation of tract profiles in bipolar disorder with the advantage to investigate regionally specific differences of white matter pathways (Sprooten et al., 2016) as well as normative databases and atlases of major tracts that may serve as reference for comparison with individual patient data (Oishi et al., 2011; Figley et al., 2017).
We would like to note that the approach of tract-based spatial statistics (Smith et al., 2006) (Figure 2d) is not a connectivity analysis in the strict sense, but merely a voxel-wise evaluation of skeletonized white matter using a sophisticated spatial normalization algorithm. Still, the approach has been widely used and yielded interesting results in clinical investigations. For instance, an increased sensitivity to detect alterations in patients with major depression was demonstrated compared with the nonskeletonized voxel-based approach (Bergamino et al., 2017). Furthermore, tract-based spatial statistics results yielded differences between responders and nonresponders to ketamine (Vasavada et al., 2016) and after electroconvulsive therapy (Lyden et al., 2014) in the cingulum bundle.
Another approach to investigate structural connectivity networks is that of computing structural covariance networks, for example, with voxel-based morphometry data (He et al., 2007; Bassett et al., 2008). Here, a whole-brain connectivity matrix (Figure 1c) is compiled by calculating correlations of gray matter volumes between region pairs across an entire sample. This is based on the strong correlation of local MRI metrics for anatomically connected regions (Mechelli et al., 2005; Lerch et al., 2006). Although the technique indeed relates to biologically relevant information (Evans, 2013), structural covariance networks do not fully reflect tractography-based connections (Gong et al., 2012). The main limitations of the approach are that only a single connectivity matrix is obtained for an entire sample (but first attempts to resolve this have been introduced; Foster-Dingley et al., 2016) and the indirect information of the underlying connectivity pattern.
Functional Connectivity
Functional connectivity computed from resting-state fMRI is by far the most-used approach to assess brain networks. This was first described by Biswal and colleagues for the motor system (Biswal et al., 1995) and required another 10 years until it experienced a dramatic increase in its use, for example with the 1000 functional connectomes project (Biswal et al., 2010). The approach is based on the calculation of statistical metrics of signal time courses between brain regions (mostly correlation analysis, but also coherence or phase-locking) (Figure 3a). Hence, functional connectivity is highly time dependent and connectivity patterns may change even within few milliseconds, which can be captured by electroencephalography or magnetoencephalography. In contrast, structural connections are stable at shorter time scales of minutes but may be subject to changes at longer intervals, such as after prolonged training (Zatorre et al., 2012) or treatment (see above). Functional connectivity “at rest” implies the acquisition of spontaneous fluctuations in brain activity (Fox et al., 2005), which in turn means that subjects are awake but do not perform a certain task with common instructions to “let thoughts come and go freely” or “do not think of anything in particular” (Smith et al., 2013). Studying brain activity in the absence of a task, or historically the deactivation during task performance also identified with PET (Raichle et al., 2001), led to the robust identification of the default mode and various other brain networks (Yeo et al., 2011). These functional networks can also be obtained during anesthesia (Liu et al., 2017) or deep sleep, though characterized by decoupling (Horovitz et al., 2009) and specific network changes related to varying levels of arousal and consciousness (Tagliazucchi and van Someren, 2017). In addition to these networks, functional connectivity has been widely used to refine the parcellation of the human cortex (Glasser et al., 2016; Gordon et al., 2016), offering highly homogeneous areas and identification of individual neuroanatomical fingerprints.
Figure 3.
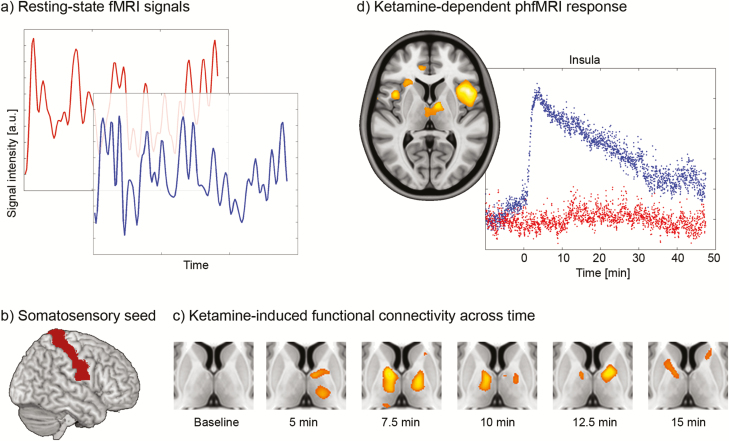
Functional connectivity obtained from functional MRI. (a) Resting-state signal time course of 2 different brain regions from the default mode network (red, posterior cingulate cortex; blue, medial prefrontal cortex). The temporal correlation of these signals is used as index of functional connectivity. (b) Anatomical seed regions of large cortical regions (here the somatosensory cortex) can be used to map small thalamic nuclei. (c) Using the seed region in b, changes in functional connectivity following an acute i.v. challenge of ketamine compared with placebo reflect those seen in schizophrenia patients (reprinted by permission from Springer) (Hoflich et al., 2015). (d) Pharmacological functional MRI (fMRI) shows that neuronal activation followed that of ketamine plasma levels in the insula, anterior cingulate, and thalamus. Scatterplot shows an exemplary time course of the insula with ketamine and placebo response in blue and red, respectively (reprinted by permission from Oxford University Press) (Hoflich et al., 2017).
Functional connectivity is usually computed by seed-based correlation analyses or independent component analysis, each with different aspects to keep in mind such as seed selection bias or the optimal number of independent components to be estimated (Cole et al., 2010). Regardless of the used method, data preprocessing is an essential step for functional connectivity. It is important to account for potentially confounding signals such as scanner drifts and artifacts or those with physiological origin, including motion, respiratory, and cardiovascular noise. Common approaches to minimize their influence are motion scrubbing (Power et al., 2012), nuisance regression against motion parameters, and signals obtained from separately acquired data (Glover et al., 2000) or white matter and cerebrospinal fluid signals (Weissenbacher et al., 2009), as well as bandpass filtering. For specific details, the reader is referred to seminal previous work (Murphy et al., 2013; Shirer et al., 2015; Bright et al., 2017; Caballero-Gaudes and Reynolds, 2017). Of note, motion may represent an issue that requires thorough attention, especially when investigating children, the elderly, or certain clinical populations (Power et al., 2012). Another issue that may require further investigation in the future is to include white matter signal in the nuisance regression due to similar activation in gray and white matter (Courtemanche et al., 2018; Ding et al., 2018). Global signal regression, on the other hand, has been demonstrated to introduce potentially spurious anticorrelations (Weissenbacher et al., 2009; Cole et al., 2010) and renders the interpretation of group comparisons difficult (Saad et al., 2012) and is therefore rarely used anymore.
Functional connectivity also enables a dynamic assessment over short time scales and in response to pharmacological treatment. It has been demonstrated that functional connectivity is not a static phenomenon, but connections may rapidly switch between repeatedly occurring states (Hutchison et al., 2013; Calhoun et al., 2014) and these are hierarchically organized in time (Vidaurre et al., 2017). The evaluation of such dynamic states has also revealed novel aspects of mental disorders. For instance, combining topological properties of static and dynamic connectivity differentiated major depression patients with and without suicidal ideation (Liao et al., 2018), and dynamic connectivity may even have the potential to outperform the static connectivity analysis as, for example, in posttraumatic stress disorder (Jin et al., 2017).
As a limitation, and similar to structural connectivity assessed with diffusion imaging, functional connectivity is nondirectional, that is, more sophisticated models are required for inference on the direction of information flow. This can, however, be used as an advantage for certain investigations. It is particularly difficult to define small subcortical structures and nuclei of the brainstem and the thalamus, although their anatomical projections are well known. Using the above characteristic of nondirectionality, one can use large cortical projection areas as seed regions to functionally (or anatomically) (Behrens et al., 2003) identify thalamic nuclei. The approach has been proven useful in an investigation of schizophrenia patients. Where previous studies only suggested general thalamic dysfunction in these patients, the approach showed that alterations specifically include thalamic connections to the prefrontal, motor, and somatosensory cortices (Woodward et al., 2012). Using i.v. application of ketamine in healthy subjects as a model for schizophrenia, similar changes were observed for the thalamic projections to the somatosensory cortex (Figure 3b–c) (Hoflich et al., 2015), emphasizing the involvement of the NMDA receptor and potential secondary glutamatergic effects in schizophrenia.
Resting-state fMRI has also been used to model acute drug effects during the measurement (pharmaco fMRI). Although most of the work to date has not employed connectivity analyses per se, this may represent an interesting future opportunity to study drug effects on a network level (Schwarz et al., 2007; Hoflich et al., 2015). Current applications have already demonstrated great insight into the association between brain response and neurotransmitter system actions specific to pharmacological effects. These include studies of amphetamine and SSRIs in rodents (Schwarz et al., 2007) as well as human investigations of SSRIs (McKie et al., 2005) and ketamine (Deakin et al., 2008). The latter has also received particular interest due to its rapid antidepressant effects (Zarate et al., 2006) with models derived from previous data (De Simoni et al., 2013) or directly from ketamine plasma levels (Höflich et al., 2017). Regardless of the model, all of these investigations identified important nodes of ketamine action such as the thalamus and insula as well as a negative response in the subgenual anterior cingulate cortex (Figure 3d). This is in line with a large body of literature showing the importance of the subgenual anterior cingulate and its connectivity in the pathophysiology (Murrough et al., 2016) and treatment of major depression (Dunlop et al., 2017). In this context, functional connectivity has been successfully used to model antidepressant effects of ketamine (Scheidegger et al., 2012) and to predict treatment response to psilocybin (Carhart-Harris et al., 2017). Interestingly, these 2 treatment agents elicited decreased and increased functional connectivity, respectively, which may be related to their different modes of action via NMDA and serotonin-2A receptors. Another therapy option for treatment-resistant patients is given by electroconvulsive therapy, which showed changes predominantly in various regions of the default mode and executive control networks (Perrin et al., 2012; Mulders et al., 2016; Wang et al., 2018). Particularly interesting findings have also been obtained for invasive (deep-brain stimulation) and noninvasive brain stimulation (transcranial magnetic and transcranial direct current stimulation), showing that different effective sites for both stimulation types were part of the same functional networks (Fox et al., 2014). Moreover, functional connectivity between different transcranial magnetic stimulation sites and the subgenual anterior cingulate cortex predicted its effectiveness in depression (Fox et al., 2012). These findings demonstrate the usefulness of connectivity analyses in clinical populations, further improving patient care and treatment success. This is supported by recent advances to use functional connectivity for individual prediction of behavioral or clinical scores (Shen et al., 2017), identification of patient subtypes in major depression, as well as response to transcranial magnetic stimulation (Drysdale et al., 2017).
Similar to the above stimulation techniques, functional connectivity changes can also be provoked by mood induction paradigms, where data acquisition is carried out right after specific stimulation (Figueroa et al., 2017; Krause et al., 2018). These applications are likely to yield important clinical information since they are between resting-state and task-specific connectivity. Furthermore, task-relevant connectivity can be obtained during continuous stimulation or task performance rather than afterwards (Shirer et al., 2012; Hahn et al., 2018), which may capture stimulation effects even more precisely. Although connectivity estimates from conventional task fMRI block designs also gained attention (Fair et al., 2007; Braun et al., 2015), these may include potential difficulties in the interpretation due to the alternation of rest and task. Such designs exhibit high-frequency components not seen in continuous resting-state and hence also significant differences (Ganger et al., 2015), which needs to be kept in mind when investigating patient populations (Loitfelder et al., 2014).
Molecular Connectivity
Analogous to structural covariance networks (see Structural Connectivity above), it is possible to compute interregional associations based on molecular information from PET imaging. The approach reaches back to early work in the 1980s and is based on the observation that brain regions with similar metabolic demands are also functionally coupled (Macko et al., 1982; Horwitz et al., 1984). Using the radioligand [18F]FDG has led to important insights into the brain’s organization on a metabolic level (Lee et al., 2008). Interestingly, direct comparison between networks identified on the basis of glucose metabolism and functional connectivity revealed mixed findings, either with high overlap (Savio et al., 2017) or divergence (Di and Biswal, 2012) especially for the default mode network. The approach has further been used, for example, in Alzheimer’s disease showing reduced metabolic connectivity (Morbelli et al., 2012) but also the potential of cognitive reserve in bilingual patients with increased connectivity despite more pronounced hypometabolism (Perani et al., 2017). Furthermore, metabolic networks exhibited a compensatory mechanism in the nonaffected hemisphere in unilateral temporal lobe epilepsy (Vanicek et al., 2016), which parallels findings of functional connectivity (Bettus et al., 2009).
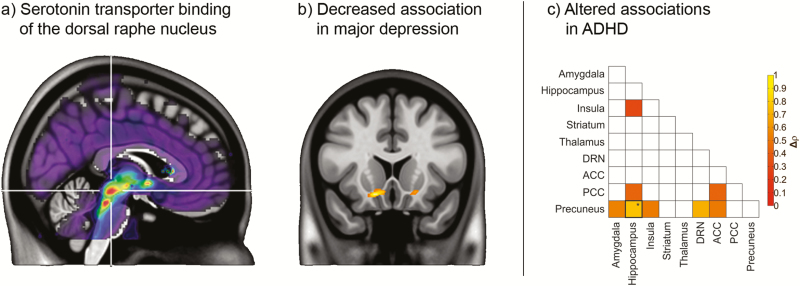
The technique has also been extended to a neurotransmitter level using known anatomical connections. For instance, serotonergic neurons have their cell bodies in the raphe nuclei, projecting to almost the entire brain. There, 2 key players of serotonergic neurotransmission and cell firing are the serotonin-1A receptor and transporter (Figure 4a), exhibiting an autoinhibitory function (Evans et al., 2008) and regulating extracellular serotonin (Tao et al., 2000), respectively. Such changes in cell firing, and presumably serotonin release, may in turn affect the expression of binding sites in cortical and subcortical regions. Hence, the well-known neurotransmitter pathways can be used as a priori hypothesis to map the association of serotonergic binding proteins between the raphe nuclei and projections areas. This approach has shown a strengthened association of the serotonin-1A receptor between the dorsal raphe nucleus and the amygdala and hippocampus after SSRI administration (Hahn et al., 2010), complementing regional changes in patients with anxiety disorder after treatment (Spindelegger et al., 2009) on a network level. Similarly, in patients with major depression, an altered association of the serotonin transporter was found specifically in the ventral striatum (Hahn et al., 2014) (Figure 4b), suggesting a serotonergic involvement (Kranz et al., 2010) of diminished reward processing in these patients.
Figure 4.
Molecular connectivity obtained from PET imaging. (a–b) Interregional associations were assessed on the basis of known serotonergic projections. (a) Serotonin transporter (SERT) binding of the dorsal raphe nucleus (indicated by crosshair) was used as seed region. (b) Decreased SERT associations between the dorsal raphe and the ventral striatum were observed in patients with major depression (reprinted by permission from Wiley) (Hahn et al., 2014). (c) The approach was further extended to various SERT-rich brain regions, showing a decreased association between the precuneus and hippocampus in patients with attention deficit/hyperactivity disorder (reprinted by permission from Wiley) (Vanicek et al., 2017).
Similar to connectivity mappings of glucose metabolism, interregional associations of neurotransmitter systems have also been calculated across several regions and entire brain maps (Bose et al., 2011). For instance, longitudinal characterization of serotonin transporter occupancy induced by SSRIs showed increased correlations in networks of the anterior cingulate cortex and the insula in patients with major depression (James et al., 2017). The usefulness of such an investigation was also evident in attention deficit/hyperactivity disorder with altered interregional associations between the precuneus and hippocampus despite no difference in regional serotonin transporter binding (Vanicek et al., 2017) (Figure 4c). This links changes of the default mode network in these patients (Castellanos et al., 2008; Sudre et al., 2017) to a specific serotonergic contribution. Further expanding the approach to a whole-brain investigation, it has been suggested that interregional correlations of amyloid-β binding may be used to assess disease progression in Alzheimer’s disease (Son et al., 2015; Pereira et al., 2018) and novel relationships between the serotonin and opioid neurotransmitter systems were revealed in healthy subjects (Tuominen et al., 2014).
As mentioned in the section of structural covariance networks, the main limitation of the method is that inference can only be drawn at the group level as covariance matrices cannot be obtained for individual subjects. Hence, the observed associations may reflect molecular connections only indirectly. In this context, the term “connectivity” may be used with care when compared with that of other modalities and should not be used synonymously with, for example, structural connectivity. While molecular connectivity of glucose metabolism has been well characterized, the biological interpretation for interregional associations, especially whole-brain applications, of other radioligands may require further investigations. Another limitation of the approach is that correlated noise between regions may introduce falsely increased correlations. Furthermore, the potential issue of differences in input functions or nonspecific binding should be considered, especially when performing group comparisons, for example, with random permutations. Randomly mixing 2 groups with different average regional values may introduce inflated correlations for random permutations, which will finally yield false negative results.
Mulitmodal Combinations
A particular appealing approach is given by the combination of various imaging modalities to study connectivity from different complementary perspectives. Among these, structure-function relationships have been investigated most intensively. Functional connections can theoretically be obtained independent of (i.e., without) direct anatomical/structural connections, since the former are usually calculated as correlations. However, missing structural connections markedly affect functional ones, probably best described in surgical sections of the corpus callosum (Johnston et al., 2008; Roland et al., 2017), demonstrating that physical links represent important constraints of functional connectivity. As such, major resting-state networks are connected by well-known anatomical links (van den Heuvel et al., 2009). On the other hand, functional connectivity can also result from indirect anatomical connections (Greicius et al., 2009), which underlines that structural and functional connections do not resemble in a simple one-to-one mapping (Ajilore et al., 2013). Furthermore, functional connectivity is subject to a larger variability across subjects than structural connectivity (Chamberland et al., 2017). More importantly, functional connectivity is most similar to the underlying anatomy during anesthesia, whereas wakefulness is characterized by rich functional configurations that deviate from structural connections (Barttfeld et al., 2015). These issues may explain why previous assessments of structure-function relationships with simply correlation analysis showed varying degrees of associations (Hagmann et al., 2008; Brown et al., 2012) and more sophisticated models are required to predict brain function from the underlying anatomy. Examples include nonlinear neuronal mass models (Honey et al., 2009), structurally-derived measures of network communication (Goñi et al., 2014), and anatomically weighted functional connectivity (Bowman et al., 2012). Structure-function relationships have also been investigated for specific brain regions and networks such as the medial temporal lobe (Shah et al., 2018), angular gyrus and intraparietal sulcus (Uddin et al., 2010), supplementary motor area (Johansen-Berg et al., 2004), posterior cingulate cortex (Khalsa et al., 2014), and the thalamus (D. Zhang et al., 2010), demonstrating the usefulness of combined analyses, for example, to refine parcellation of brain regions as well as their interactions.
In addition to the combined assessment of brain structure and function, it is of further importance to evaluate which other physiological characteristics such as neurotransmitter distribution may affect or constrain connectivity. For instance, it has been demonstrated that brain regions with overall higher excitatory than inhibitory receptor expression showed higher functional connectivity (van den Heuvel et al., 2016). Specific associations have also been reported for the serotonin-1A receptor subtype with distinct influence on the posterior default mode network, depending on its effect with respect to autoinhibition on serotonergic cell firing or local inhibition (Hahn et al., 2012). Similar associations have been reported for the anterior part of this network with dopamine D1/D2 receptors (Nagano-Saito et al., 2009), the cathechol-O-methyltransferase genotype (Liu et al., 2010), and gamma aminobutyric acid during emotional processing (Northoff et al., 2007). This multitude of interactions draws a complex picture of how functional connectivity is regulated in healthy subjects. Beyond that, altered associations between neurotransmitter binding proteins and functional connectivity have been reported in several mental disorders. Recent examples include an opposite correlation of the serotonin transporter in mild cognitive impairment (Barrett et al., 2017) and of serotonin-2A receptor gene variants in posttraumatic stress disorder (Miller et al., 2016) compared with controls.
Another interesting approach is to investigate the relationship between functional connectivity and metabolic demands (Tomasi et al., 2013; Aiello et al., 2015; Nugent et al., 2015; Passow et al., 2015; Soddu et al., 2016). It is however worth mentioning that most work evaluated this association with a focus on spatial dependency (i.e., across brain regions), hence, reporting correlations with high significance, but this was sometimes reached by using a vast amount of voxels in the brain. In contrast, investigations across subjects showed a different picture with lower associations (Tomasi et al., 2013; Aiello et al., 2015). The latter is also in line with a partial mismatch regarding the activated regions and connectivity during stimulation in rodents (Wehrl et al., 2013). Despite a similar lack of correlation between glucose metabolism and functional connectivity in humans, regional metabolic changes during task performance were accompanied by changes in functional connectivity and white matter microstructure of the corresponding connections, identifying specific brain networks involved in stimulus processing (Hahn et al., 2018). Combining local metabolic information with functional connectivity may further enable to draw inferences on hierarchical information processing (bottom-up vs top-down) between brain regions (Riedl et al., 2016).
Finally, genetic variants have been shown to be associated with brain connectivity. For instance, global graph metrics are considerably heritable (Sinclair et al., 2015), and functional connectivity differences between adolescents and adults are dependent on genetic variations (Meyer et al., 2016). Moreover, functional connectivity is reflected by transcriptional variation of gene expression specifically for genes enriched in the upper cortical layers (Krienen et al., 2016) and for those involved in ion channel and synaptic activity (Richiardi et al., 2015). The influence of genetic variations on brain connectivity has also been shown in clinical populations, where the genetic risk profile for schizophrenia correlates with functional connectivity (Rashid et al., 2019) and serotonin 2A receptor gene variants moderate default mode connectivity in posttraumatic stress disorder (Miller et al., 2016). As in most studies using imaging genetics, a high sample size is required to demonstrate the often small effects sizes of genetic variants. Thus, the approach may particularly benefit from the recently growing data sharing initiatives.
Conclusions and Future Directions
To summarize, connectivity approaches provide important information on the organization of the human brain on a network level. Investigating the connections and interactions between brain regions offers additional knowledge that cannot be obtained from regional analyses alone. The different connectivity methods contain complementary information about brain structure and function as well as metabolic and molecular insights. That is, structural methods offer delineation of physical neuronal pathways, which has been successfully used as guide for individual neurosurgery plans for deep brain stimulation (Schlaepfer et al., 2013). On the other hand, functional connectivity reflects the correlation between signal time courses across brain regions. Resting-state connectivity represents a particularly promising approach for clinical use due to the rather simple data acquisition. That is, no complex paradigms or stimulation are required and there is no bias induced by task performance; however, potential pitfalls in data processing should be taken into consideration (Shirer et al., 2015; Bright et al., 2017). As the assessment of dynamic functional connectivity is a growing field, an important aspect of future work will be to harmonize procedures to capture such temporal variations. For instance, these include the computation of connectivity (instantaneous phase synchrony, independent component analysis, sliding window with challenges regarding the window type, length, and overlap), choice of appropriate null models for validation, and interpretation of different brain states (Keilholz et al., 2017; Preti et al., 2017). Such advancements will improve the reproducibility of dynamic connectivity and thus the discrimination of patient populations. Finally, molecular approaches based on PET imaging provide information on interregional associations of metabolic demands and neurotransmitter systems. The latter technique may be particularly useful to further investigate the complex regulation of functional connectivity by different neurotransmitters. These techniques may be particularly useful to determine the underlying neurotransmitter actions of the otherwise unspecific functional connectivity obtained from BOLD imaging. Further advances in this direction are important to improve the biological interpretation of the different connectivity approaches.
With respect to clinical applications, alterations of brain connectivity have been successfully demonstrated in various patient populations and in response to pharmacological treatments. A major objective of future work should be to establish robust biomarkers to aid clinical diagnose and choice of therapy. Most studies focus on a single imaging modality; however, the multimodal assessment such as the dissociations between structural and functional connectivity may reveal novel pathological aspects of psychiatric and neurological disorders (Vega-Pons et al., 2016). Furthermore, a recent study reporting connectivity-based subtypes of major depression (Drysdale et al., 2017) could not be replicated (Dinga et al., 2018). Such attempts for replication and methodological developments for individual prediction (Shen et al., 2017) represent important advancements to enable the future use of connectivity metrics in clinical settings.
Statement of Interest
Without any relevance to this work, S.K. received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier; R.L. received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. A.H. declares no conflict of interest.
References
- Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. Plos Comput Biol 3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello M, Salvatore E, Cachia A, Pappatà S, Cavaliere C, Prinster A, Nicolai E, Salvatore M, Baron JC, Quarantelli M (2015) Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage 113:111–121. [DOI] [PubMed] [Google Scholar]
- Ajilore O, Zhan L, Gadelkarim J, Zhang A, Feusner JD, Yang S, Thompson PM, Kumar A, Leow A (2013) Constructing the resting state structural connectome. Front Neuroinform 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M, Stanley JA, Raz N (2017) Test-retest reliability and concurrent validity of in vivo myelin content indices: myelin water fraction and calibrated T1 w/T2 w image ratio. Hum Brain Mapp 38:1780–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Workman CI, Sair HI, Savonenko AV, Kraut MA, Sodums DJ, Joo JJ, Nassery N, Marano CM, Munro CA, Brandt J, Zhou Y, Wong DF, Smith GS (2017) Association between serotonin denervation and resting-state functional connectivity in mild cognitive impairment. Hum Brain Mapp 38:3391–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S (2015) Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A 112:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A (2008) Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between humanthalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamino M, Kuplicki R, Victor TA, Cha YH, Paulus MP (2017) Comparison of two different analysis approaches for DTI free-water corrected and uncorrected maps in the study of white matter microstructural integrity in individuals with depression. Hum Brain Mapp 38:4690–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M (2009) Decreased basal fmri functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, et al. (2010) Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y (2011) Diffusion MRI of structural brain plasticity induced by a learning and memory task. Plos One 6:e20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, Grasby PM, Turkheimer FE, Murthy V (2011) Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology 36:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman FD, Zhang L, Derado G, Chen S (2012) Determining functional connectivity using fmri data with diffusion-based anatomical weighting. Neuroimage 62:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, Meyer-Lindenberg A, Bassett DS (2015) Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A 112:11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MG, Tench CR, Murphy K (2017) Potential pitfalls when denoising resting state fmri data using nuisance regression. Neuroimage 154:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Rudie JD, Bandrowski A, Van Horn JD, Bookheimer SY (2012) The UCLA multimodal connectivity database: a web-based platform for brain connectivity matrix sharing and analysis. Front Neuroinform 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2012) The economy of brain network organization. Nat Rev Neurosci 13:336–349. [DOI] [PubMed] [Google Scholar]
- Caballero-Gaudes C, Reynolds RC (2017) Methods for cleaning the BOLD fmri signal. Neuroimage 154:128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adalı T (2014) The chronnectome: time-varying connectivity networks as the next frontier in fmri data discovery. Neuron 84:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, Tanner M, Kaelen M, McGonigle J, Murphy K, Leech R, Curran HV, Nutt DJ (2017) Psilocybin for treatment-resistant depression: fmri-measured brain mechanisms. Sci Rep 7:13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP (2008) Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 63:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland M, Girard G, Bernier M, Fortin D, Descoteaux M, Whittingstall K (2017) On the origin of individual functional connectivity variability: the role of white matter architecture. Brain Connect 7:491–503. [DOI] [PubMed] [Google Scholar]
- Chang EH, Argyelan M, Aggarwal M, Chandon TS, Karlsgodt KH, Mori S, Malhotra AK (2017) The role of myelination in measures of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 147:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF (2010) Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche MJ, Sparrey CJ, Song X, MacKay A, D’Arcy RCN (2018) Detecting white matter activity using conventional 3 tesla fmri: an evaluation of standard field strength and hemodynamic response function. Neuroimage 169:145–150. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164. [DOI] [PubMed] [Google Scholar]
- Delorenzo C, Delaparte L, Thapa-Chhetry B, Miller JM, Mann JJ, Parsey RV (2013) Prediction of selective serotonin reuptake inhibitor response using diffusion-weighted MRI. Front Psychiatry 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, Stephenson S, Williams SC, Mehta MA (2013) Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage 64:75–90. [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB; Alzheimer’s Disease Neuroimaging Initiative (2012) Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fmri networks. Brain Connect 2:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Huang Y, Bailey SK, Gao Y, Cutting LE, Rogers BP, Newton AT, Gore JC (2018) Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc Natl Acad Sci U S A 115:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinga R, Schmaal L, Penninx B, van Tol MJ, Veltman D, van Velzen L, van der Wee N, Marquand A (2018) Evaluating the evidence for biotypes of depression: attempted replication of Drysdale et al. 2017. bioRxiv. doi: 10.1101/416321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue CJ, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Behrens TE, Dyrby TB, Coalson T, Kennedy H, Knoblauch K, Van Essen DC, Glasser MF (2016) Using diffusion tractography to predict cortical connection strength and distance: A quantitative comparison with tracers in the monkey. J Neurosci 36:6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, et al. (2017) Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Rajendra JK, Craighead WE, Kelley ME, McGrath CL, Choi KS, Kinkead B, Nemeroff CB, Mayberg HS (2017) Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry 174:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. (2013) Networks of anatomical covariance. Neuroimage 80:489–504. [DOI] [PubMed] [Google Scholar]
- Evans AK, Reinders N, Ashford KA, Christie IN, Wakerley JB, Lowry CA (2008) Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol 590:136–149. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE (2007) A method for using blocked and event-related fmri data to study “resting state” functional connectivity. Neuroimage 35:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley TD, Mortazavi Moghadam B, Bhullar N, Kornelsen J, Courtney SM, Figley CR (2017) Probabilistic white matter atlases of human auditory, basal ganglia, language, precuneus, sensorimotor, visual and visuospatial networks. Front Hum Neurosci 11:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CA, Mocking RJT, van Wingen G, Martens S, Ruhé HG, Schene AH (2017) Aberrant default-mode network-hippocampus connectivity after sad memory-recall in remitted-depression. Soc Cogn Affect Neurosci 12:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M (2013) Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage 80:426–444. [DOI] [PubMed] [Google Scholar]
- Foster-Dingley JC, Hafkemeijer A, van den Berg-Huysmans AA, Moonen JE, de Ruijter W, de Craen AJ, van der Mast RC, Rombouts SA, van der Grond J (2016) Structural covariance networks and their association with age, features of cerebral small-vessel disease, and cognitive functioning in older persons. Brain Connect 6:681–690. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A (2012) Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A (2014) Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A 111:E4367–E4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallos LK, Makse HA, Sigman M (2012) A small world of weak ties provides optimal global integration of self-similar modules in functional brain networks. Proc Natl Acad Sci U S A 109:2825–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganger S, Hahn A, Küblböck M, Kranz GS, Spies M, Vanicek T, Seiger R, Sladky R, Windischberger C, Kasper S, Lanzenberger R (2015) Comparison of continuously acquired resting state and extracted analogues from active tasks. Hum Brain Mapp 36:4053–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET (2013) Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci 33:5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC (2016) A multi-modal parcellation of human cerebral cortex. Nature 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D (2000) Image-based method for retrospective correction of physiological motion effects in fmri: RETROICOR. Magn Reson Med 44:162–167. [DOI] [PubMed] [Google Scholar]
- Gollo LL, Roberts JA, Cropley VL, Di Biase MA, Pantelis C, Zalesky A, Breakspear M (2018) Fragility and volatility of structural hubs in the human connectome. Nat Neurosci 21:1107–1116. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Chen ZJ, Evans AC (2012) Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage 59:1239–1248. [DOI] [PubMed] [Google Scholar]
- Goñi J, van den Heuvel MP, Avena-Koenigsberger A, Velez de Mendizabal N, Betzel RF, Griffa A, Hagmann P, Corominas-Murtra B, Thiran JP, Sporns O (2014) Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A 111:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE (2016) Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008) Mapping the structural core of human cerebral cortex. Plos Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien LK, Mitterhauser M, Kasper S (2010) Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci 30:14482–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Wadsak W, Windischberger C, Baldinger P, Höflich AS, Losak J, Nics L, Philippe C, Kranz GS, Kraus C, Mitterhauser M, Karanikas G, Kasper S, Lanzenberger R (2012) Differential modulation of the default mode network via serotonin-1A receptors. Proc Natl Acad Sci U S A 109:2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Haeusler D, Kraus C, Höflich AS, Kranz GS, Baldinger P, Savli M, Mitterhauser M, Wadsak W, Karanikas G, Kasper S, Lanzenberger R (2014) Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp 35:3857–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Sladky R, Ganger S, Windischberger C, Kasper S, Lanzenberger R (2015a) Individual diversity of functional brain network economy. Brain Connect 5:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Küblböck M, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Winkler D, Kasper S, Windischberger C, Swaab DF, Lanzenberger R (2015b) Structural connectivity networks of transgender people. Cereb Cortex 25:3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Gryglewski G, Nics L, Rischka L, Ganger S, Sigurdardottir H, Vraka C, Silberbauer L, Vanicek T, Kautzky A, Wadsak W, Mitterhauser M, Hartenbach M, Hacker M, Kasper S, Lanzenberger R (2018) Task-relevant brain networks identified with simultaneous PET/MR imaging of metabolism and connectivity. Brain Struct Funct 223:1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006) Brain connectivity related to working memory performance. J Neurosci 26:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17:2407–2419. [DOI] [PubMed] [Google Scholar]
- Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Windischberger C, Saria A, Kasper S, Winkler D, Lanzenberger R (2015) Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. Int J Neuropsychopharmacol 18. pii: pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Ganger S, Spies M, Windischberger C, Kasper S, Winkler D, Lanzenberger R (2017) Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct 222:1533–1542. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009) Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH (2009) Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A 106:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI (1984) Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab 4:484–499. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C (2013) Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014) Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GM, Baldinger-Melich P, Philippe C, Kranz GS, Vanicek T, Hahn A, Gryglewski G, Hienert M, Spies M, Traub-Weidinger T, Mitterhauser M, Wadsak W, Hacker M, Kasper S, Lanzenberger R (2017) Effects of selective serotonin reuptake inhibitors on interregional relation of serotonin transporter availability in major depression. Front Hum Neurosci 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, Hu X, Deshpande G (2017) Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp 38:4479–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A 101:13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, Shimony JS, Snyder AZ, Raichle ME (2008) Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci 28:6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015) Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz S, Caballero-Gaudes C, Bandettini P, Deco G, Calhoun V (2017) Time-resolved resting-state functional magnetic resonance imaging analysis: current status, challenges, and new directions. Brain Connect 7:465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP (2014) The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. Neuroimage 102 Pt 1:118–127. [DOI] [PubMed] [Google Scholar]
- Kötter R. (2004) Online retrieval, processing, and visualization of primate connectivity data from the cocomac database. Neuroinformatics 2:127–144. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R (2010) Reward and the serotonergic system. Neuroscience 166:1023–1035. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C, Kasper S, Lanzenberger R (2014) White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci 34:15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause AL, Colic L, Borchardt V, Li M, Strauss B, Buchheim A, Wildgruber D, Fonagy P, Nolte T, Walter M (2018) Functional connectivity changes following interpersonal reactivity. Hum Brain Mapp 39:866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Ge T, Buckner RL, Sherwood CC (2016) Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A 113:E469–E478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz JD, Waller L, Daedelow LS, Walter H, Veer IM (2018) General, crystallized and fluid intelligence are not associated with functional global network efficiency: a replication study with the human connectome project 1200 data set. Neuroimage 171:323–331. [DOI] [PubMed] [Google Scholar]
- Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, Lee MC (2008) Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging 35:1681–1691. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC (2006) Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31:993–1003. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T (2009) Brain anatomical network and intelligence. PLoS Comput Biol 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Li J, Duan X, Cui Q, Chen H, Chen H (2018) Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum Brain Mapp 39:4105–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T (2010) Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J Neurosci 30:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lauer KK, Ward BD, Roberts CJ, Liu S, Gollapudy S, Rohloff R, Gross W, Xu Z, Chen G, Binder JR, Li SJ, Hudetz AG (2017) Fine-grained parcellation of brain connectivity improves differentiation of states of consciousness during graded propofol sedation. Brain Connect 7:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loitfelder M, Pinter D, Langkammer C, Jehna M, Ropele S, Fazekas F, Schmidt R, Enzinger C (2014) Functional connectivity analyses using emulated and conventional resting-state data: parts versus the whole story. Brain Connect 4:842–848. [DOI] [PubMed] [Google Scholar]
- Lord A, Horn D, Breakspear M, Walter M (2012) Changes in community structure of resting state functional connectivity in unipolar depression. Plos One 7:e41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, Woods RP, Narr KL (2014) Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry 4:e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko KA, Jarvis CD, Kennedy C, Miyaoka M, Shinohara M, Sololoff L, Mishkin M (1982) Mapping the primate visual system with [2-14C]deoxyglucose. Science 218:394–397. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, Deakin JF (2005) Neuronal effects of acute citalopram detected by pharmacomri. Psychopharmacology (Berl) 180:680–686. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ (2005) Structural covariance in the human cortex. J Neurosci 25:8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BM, et al. (2016) Oppositional COMT val158met effects on resting state functional connectivity in adolescents and adults. Brain Struct Funct 221:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Sperbeck E, Robinson ME, Sadeh N, Wolf EJ, Hayes JP, Logue M, Schichman SA, Stone A, Milberg W, McGlinchey R (2016) 5-HT2A gene variants moderate the association between PTSD and reduced default mode network connectivity. Front Neurosci 10:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli S, Drzezga A, Perneczky R, Frisoni GB, Caroli A, van Berckel BN, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Sambuceti G, Pagani M, Salmon E, Nobili F (2012) Resting metabolic connectivity in prodromal Alzheimer’s disease. A European Alzheimer Disease Consortium (EADC) project. Neurobiol Aging 33:2533–2550. [DOI] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Pluijmen J, Schene AH, Tendolkar I, Beckmann CF (2016) Default mode network coherence in treatment-resistant major depressive disorder during electroconvulsive therapy. J Affect Disord 205:130–137. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA (2013) Resting-state fmri confounds and cleanup. Neuroimage 80:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, Averill C, Jia-Wei Yang G, Wong E, Tang CY, Krystal JH, Iosifescu DV, Charney DS (2016) Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp 37:3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Liu J, Doyon J, Dagher A (2009) Dopamine modulates default mode network deactivation in elderly individuals during the tower of London task. Neurosci Lett 458:1–5. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P (2007) GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fmri. Nat Neurosci 10:1515–1517. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Martinez A, D’Alfonso A, Zarate CA, Theodore WH (2015) The relationship between glucose metabolism, resting-state fmri BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab 35:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Huang H, Yoshioka T, Ying SH, Zee DS, Zilles K, Amunts K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans AC, van Zijl PC, Mazziotta JC, Mori S (2011) Superficially located white matter structures commonly seen in the human and the macaque brain with diffusion tensor imaging. Brain Connect 1:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow S, Specht K, Adamsen TC, Biermann M, Brekke N, Craven AR, Ersland L, Grüner R, Kleven-Madsen N, Kvernenes OH, Schwarzlmüller T, Olesen RA, Hugdahl K (2015) Default-mode network functional connectivity is closely related to metabolic activity. Hum Brain Mapp 36:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Farsad M, Ballarini T, Lubian F, Malpetti M, Fracchetti A, Magnani G, March A, Abutalebi J (2017) The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer’s dementia. Proc Natl Acad Sci U S A 114:1690–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Strandberg TO, Palmqvist S, Volpe G, van Westen D, Westman E, Hansson O; Alzheimer’s Disease Neuroimaging Initiative (2018) Amyloid network topology characterizes the progression of Alzheimer’s disease during the predementia stages. Cereb Cortex 28:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C (2012) Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A 109:5464–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti MG, Bolton TA, Van De Ville D (2017) The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage 160:41–54. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, et al. (2019) A framework for linking resting-state chronnectome/genome features in schizophrenia: a pilot study. Neuroimage 184:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi J, et al. ; IMAGEN consortium (2015) BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science 348:1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Utz L, Castrillón G, Grimmer T, Rauschecker JP, Ploner M, Friston KJ, Drzezga A, Sorg C (2016) Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc Natl Acad Sci U S A 113:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JL, Snyder AZ, Hacker CD, Mitra A, Shimony JS, Limbrick DD, Raichle ME, Smyth MD, Leuthardt EC (2017) On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A 114:13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Ryman SG, Yeo RA, Witkiewitz K, Vakhtin AA, van den Heuvel M, de Reus M, Flores RA, Wertz CR, Jung RE (2016) Fronto-parietal gray matter and white matter efficiency differentially predict intelligence in males and females. Hum Brain Mapp 37:4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, Kleim J, Sibson NR, Bannerman D, Johansen-Berg H (2013) Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci 33:19499–19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Küpper H, Kellmeyer P, Kümmerer D, Klöppel S, Glauche V, Lange R, Mader W, Feess D, Timmer J, Weiller C (2010) Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage 49:3187–3197. [DOI] [PubMed] [Google Scholar]
- Savio A, Fünger S, Tahmasian M, Rachakonda S, Manoliu A, Sorg C, Grimmer T, Calhoun V, Drzezga A, Riedl V, Yakushev I (2017) Resting-state networks as simultaneously measured with functional MRI and PET. J Nucl Med 58:1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E (2012) Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. Plos One 7:e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA (2013) Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 73:1204–1212. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Reese T, Bifone A (2007) In vivo mapping of functional connectivity in neurotransmitter systems using pharmacological MRI. Neuroimage 34:1627–1636. [DOI] [PubMed] [Google Scholar]
- Shah P, Bassett DS, Wisse LEM, Detre JA, Stein JM, Yushkevich PA, Shinohara RT, Pluta JB, Valenciano E, Daffner M, Wolk DA, Elliott MA, Litt B, Davis KA, Das SR (2018) Mapping the structural and functional network architecture of the medial temporal lobe using 7T MRI. Hum Brain Mapp 39:851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT (2017) Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc 12:506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Toga AW (2017) Connectome imaging for mapping human brain pathways. Mol Psychiatry 22:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Jiang H, Price CM, Ng B, Greicius MD (2015) Optimization of rs-fmri pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage 117:67–79. [DOI] [PubMed] [Google Scholar]
- Sinclair B, Hansell NK, Blokland GA, Martin NG, Thompson PM, Breakspear M, de Zubicaray GI, Wright MJ, McMahon KL (2015) Heritability of the network architecture of intrinsic brain functional connectivity. Neuroimage 121: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. ; WU-Minn HCP Consortium (2013) Resting-state fmri in the human connectome project. Neuroimage 80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soddu A, Gómez F, Heine L, Di Perri C, Bahri MA, Voss HU, Bruno MA, Vanhaudenhuyse A, Phillips C, Demertzi A, Chatelle C, Schrouff J, Thibaut A, Charland-Verville V, Noirhomme Q, Salmon E, Tshibanda JF, Schiff ND, Laureys S (2016) Correlation between resting state fmri total neuronal activity and PET metabolism in healthy controls and patients with disorders of consciousness. Brain Behav 6:e00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SJ, Kim J, Seo J, Lee JM, Park H; ADNI (2015) Connectivity analysis of normal and mild cognitive impairment patients based on FDG and pib-PET images. Neurosci Res 98:50–58. [DOI] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, Moser U, Holik A, Pezawas L, Kletter K, Kasper S (2009) Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry 14:1040–1050. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004) Organization, development and function of complex brain networks. Trends Cogn Sci 8:418–425. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Barrett J, McKay DR, Knowles EE, Mathias SR, Winkler AM, Brumbaugh MS, Landau S, Cyr L, Kochunov P, Glahn DC (2016) A comprehensive tractography study of patients with bipolar disorder and their unaffected siblings. Hum Brain Mapp 37:3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre G, Szekely E, Sharp W, Kasparek S, Shaw P (2017) Multimodal mapping of the brain’s functional connectivity and the adult outcome of attention deficit hyperactivity disorder. Proc Natl Acad Sci U S A 114:11787–11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, van Someren EJW (2017) The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle. Neuroimage 160:55–72. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Hackney DB, Zhang G, Wehrli SL, Wright AC, O’Brien WT, Uematsu H, Wehrli FW, Selzer ME (2002) Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A 99:16192–16196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB (2000) Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther 294:571–579. [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND (2013) Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A 110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen L, Nummenmaa L, Keltikangas-Järvinen L, Raitakari O, Hietala J (2014) Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Hum Brain Mapp 35:1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V (2010) Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex 20:2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE (2009) Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, de Reus MA, Feldman Barrett L, Scholtens LH, Coopmans FM, Schmidt R, Preuss TM, Rilling JK, Li L (2015) Comparison of diffusion tractography and tract-tracing measures of connectivity strength in rhesus macaque connectome. Hum Brain Mapp 36:3064–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Scholtens LH, Turk E, Mantini D, Vanduffel W, Feldman Barrett L (2016) Multimodal analysis of cortical chemoarchitecture and macroscale fmri resting-state functional connectivity. Hum Brain Mapp 37:3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanicek T, Hahn A, Traub-Weidinger T, Hilger E, Spies M, Wadsak W, Lanzenberger R, Pataraia E, Asenbaum-Nan S (2016) Insights into intrinsic brain networks based on graph theory and PET in right- compared to left-sided temporal lobe epilepsy. Sci Rep 6:28513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanicek T, Kutzelnigg A, Philippe C, Sigurdardottir HL, James GM, Hahn A, Kranz GS, Höflich A, Kautzky A, Traub-Weidinger T, Hacker M, Wadsak W, Mitterhauser M, Kasper S, Lanzenberger R (2017) Altered interregional molecular associations of the serotonin transporter in attention deficit/hyperactivity disorder assessed with PET. Hum Brain Mapp 38:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada MM, Leaver AM, Espinoza RT, Joshi SH, Njau SN, Woods RP, Narr KL (2016) Structural connectivity and response to ketamine therapy in major depression: a preliminary study. J Affect Disord 190:836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Pons S, Olivetti E, Avesani P, Dodero L, Gozzi A, Bifone A (2016) Differential effects of brain disorders on structural and functional connectivity. Front Neurosci 10:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D, Smith SM, Woolrich MW (2017) Brain network dynamics are hierarchically organized in time. Proc Natl Acad Sci U S A 114:12827–12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang L, Zhang H, Bai T, Cheng L, Tian Y, Wang K (2018) Functional reorganization of intra- and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp 39:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrl HF, Hossain M, Lankes K, Liu CC, Bezrukov I, Martirosian P, Schick F, Reischl G, Pichler BJ (2013) Simultaneous PET-MRI reveals brain function in activated and resting state on metabolic, hemodynamic and multiple temporal scales. Nat Med 19:1184–1189. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009) Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S (2012) Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, van den Heuvel MP, Breakspear M (2016) Connectome sensitivity or specificity: which is more important? Neuroimage 142:407–420. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME (2010) Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 20:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016. [DOI] [PubMed] [Google Scholar]