Abstract
Auditory verbal hallucinations (AVHs) are a core symptom of schizophrenia, and resistant to antipsychotic medication in a substantial proportion of patients. This study aimed to investigate the neural correlates of AVHs in schizophrenia patients and its response to a modified continuous theta-burst stimulation (cTBS) by transcranial magnetic stimulation. In a cross-sectional experiment, resting-state functional magnetic resonance images were collected from 31 AVH schizophrenia patients, 26 non-AVH schizophrenia patients, and 33 sex-/age-matched healthy controls (HCs). Functional connectivity strength (FCS) maps were compared among groups by 1-way analysis of variance (ANOVA). In a longitudinal experiment, 16 and 11 AVH patients received real and sham cTBS treatment for 15 days, respectively. Notably, this was not a randomized control trail. Changes in AVH and FCS were analyzed by 2-way ANOVA and 2-sample t-test, respectively. In the cross-sectional experiment, comparison of FCS maps identified 8 clusters among groups, but only one cluster (in left cerebellum) differed significantly in AVH patients compared to both HCs and non-AVH patients. In the longitudinal experiment, the real cTBS group showed a greater improvement in symptoms and a larger FCS decrease in left cerebellum than the sham group. Pearson’s correlation analysis indicated that baseline FCS of the overlapping cerebellum cluster (between the cross-sectional and longitudinal findings) was negatively correlated with symptom improvement in the real treatment group. These findings emphasize the role of the left cerebellum in both the pathophysiology and clinical treatment of AVHs in schizophrenia patients.
Keywords: functional connectivity strength, cerebellum, longitudinal, schizophrenia, auditory verbal hallucinations
Introduction
Auditory verbal hallucinations (AVHs) are a characteristic symptom of schizophrenia, affecting approximately 60%−80% of patients.1 Although most patients are responsive to antipsychotic pharmacotherapy, a substantial number (~25%) are treatment-resistant and continue to experience AVHs. Thus, alternative therapies are urgently needed to reduce the severity and frequency of AVHs experienced by these patients.
AVH refers to the experience of perceiving speech in the absence of corresponding external stimuli.2 Despite numerous studies using a variety of approaches,3–6 the exact mechanisms by which AVHs arise spontaneously from intrinsic brain activity remains unclear. To address this issue, recent studies have examined the spontaneous functional connectivity in schizophrenia patients with AVH using resting-state functional magnetic resonance imaging (rs-fMRI).5,7 Such studies have given rise to the “resting state hypothesis of AVH,” which posits that anomalies in intrinsic brain connectivity and ensuing activity generate AVHs.8 Indeed, compared to schizophrenia patients without AVH (non-AVH), AVH patients exhibited distinct intrinsic cortico-subcortical connectivity patterns9–11 and interhemispheric circuits.12–14 Particular attention has been paid to functional connectivity with the left temporo-parietal junction (TPJ),13,15,16 because the TPJ may be an effective repetitive transcranial magnetic stimulation (rTMS) target for AVH patients.17–19 Local activity and global network properties have also been investigated by amplitude of low-frequency fluctuations20 and graph theory.21 To exclude potential confounding variables (ie, variations in medication history and severity of symptoms), the neural correlates of AVH have also been examined by comparing nonpsychotic individuals with and without AVH.22–24 Such rs-fMRI studies yielded useful information to enhance an understanding of the neurobiological mechanisms underlying AVH. However, most of them were cross-sectional6,25,26 and rarely associated with novel therapies, such as rTMS.
rTMS is a well-established, noninvasive technique that induces long-lasting changes in excitatory and inhibitory activity (aftereffects) of the target network depending on the frequency and temporal pattern of stimulation. It has been applied for the treatment of many neurological and psychiatric disorders.27 Hoffman and colleagues28,29 reported the possible efficacy of inhibitory rTMS on AVH in schizophrenia patients, and its frequency protocol (1-Hz rTMS over the left TPJ) was adopted in most subsequent studies. However, the clinical efficacy of this protocol was not consistently supported.30–33 In most of these studies, the TPJ was defined according to the international 10/20 system of electroencephalography electrode placement. Given the anatomical variability of the human brain, this coarse localization method may prove less accurate and efficient than image-based navigation approaches.18,34,35 Several studies have also tested continuous theta-burst stimulation (cTBS) for AVH treatment,36–38 since this paradigm exhibited more powerful inhibitory aftereffects than 1-Hz rTMS in the motor system.39 However, a randomized trial found that the efficacy of cTBS was not significantly higher than placebo treatment.33 This negative finding may be attributable to the stimulation parameters, which would be improved in the current study by using a longer stimulation regimen, precise target localization, and an optimized inter-session interval (ISI = 30 min).40
This study compared the resting-state brain function of AVH schizophrenia patients, non-AVH schizophrenia patients, and healthy controls (HCs) to provide a context for a longitudinal rTMS experiment. The researchers hypothesized that a modified cTBS protocol, compared to sham rTMS, may significantly alleviate AVH symptoms by remodeling the abnormal brain function observed in the cross-sectional experiment.
Materials and Methods
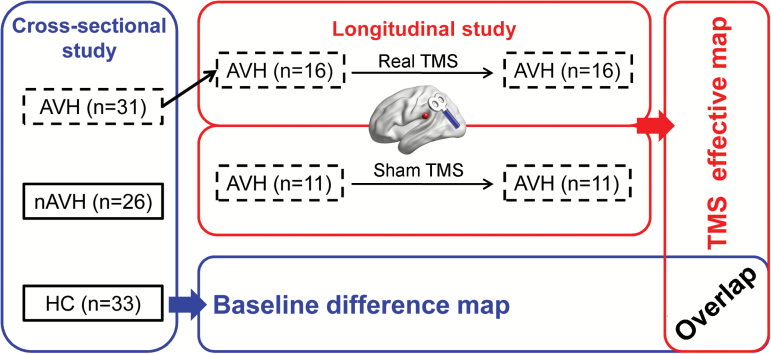
This study is composed of cross-sectional and longitudinal experiments. All participants provided written informed consent before experiments. The cross-sectional experiment investigated the neural correlates of AVH by comparing FCS maps among AVH, non-AVH, and HC participants. The longitudinal experiment tested the clinical efficacy of a modified rTMS protocol and its underlying neural mechanism by rs-fMRI. Finally, a spatial overlap map was produced, utilizing the fMRI findings of both experiments, to evaluate the rTMS mechanism in terms of baseline abnormality (figure 1). Notably, the longitudinal rTMS experiment was not a randomized control trail, although both real and sham treatment were performed in this part.
Fig. 1.
Experiment design and analytic strategy. Sixteen of 31 AVH patients in the cross-sectional study received real rTMS treatment. After rTMS treatment, 11 AVH patients were recruited to represent a control group in the longitudinal study. Notably, their imaging data were acquired using different scanners (albeit the same brand) than the other groups. The “overlap” represents a spatial overlap map of significant clusters from cross-sectional and longitudinal studies.
Participants
A total of 57 patients diagnosed with refractory schizophrenia at the Anhui Mental Health Center (Hefei, China) were consecutively enrolled in this study (supplementary tables E1–E3). The study protocol was reviewed and approved by the Medical Ethics Committee of Anhui Medical University, Hefei, China. All participants satisfied the following inclusion criteria: (1) diagnosis of schizophrenia using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID-IV), (2) were taking stable doses of psychotropic medication for at least 8 weeks before the study, and (3) verbal intelligence quotient >85 as measured utilizing a Chinese version of the National Adult Reading Test. Exclusion criteria were as follows: (1) history of significant head trauma or neurological disorders, (2) alcohol or drug abuse, (3) focal brain lesions on T1- or T2-weighted fluid-attenuated inversion-recovery magnetic resonance images, (4) recent aggression or other forms of behavioral dysfunction, (5) head motion exceeding 3 mm in translation or 3° in rotation during rs-fMRI scanning, or (6) Hamilton Anxiety Rating Scale or Hamilton Depression Rating Scale score >7. Patients were further classified into 2 groups: those reporting AVHs of spoken speech at least 5 times per day during the preceding 8 weeks (AVH group, n = 31) and patients who had not experienced AVHs (or olfactory, gustatory, tactile hallucination) since the diagnosis of schizophrenia or within 5 years before scans (non-AVH group, n = 26). The most commonly positive syndromes of the non-AVH group were delusional disorder (n = 15), impulsion (n = 10), and restlessness (n = 8). All AVH patients were refractory, which may be defined as the experience of persistent daily hallucinations without remission despite antipsychotic medication administered at an adequate dosage for at least 12 weeks.
With regard to AVH patients participating in the longitudinal rTMS study, additional exclusion criteria included (1) age <18 years, (2) non-removable metal objects in or around head, or (3) prior history of seizure or history in first degree relatives. Finally, 16 AVH patients provided their consent for real rTMS treatment. After the end of the rTMS treatment experiment, the researchers realized that a sham–control would be necessary to exclude the placebo effect of rTMS. According to the primary results of real stimulation (see Results), a sample of 7 participants proved large enough to identify the treatment effect (alpha = 0.05, beta = 0.8). Fortunately, an interim inspection of another ongoing study (Randomized Clinical Trail [RCT] number: NCT02863094) allowed the researchers to collect data from 11 refractory AVH patients (meeting the inclusion and exclusion criteria of this study) who had received sham rTMS. Thus, their data were utilized in the sham–control group in the current study. Thirty-three HCs with no history of neurological or psychiatric illnesses were randomly recruited from the local community. This group exhibited no gross abnormalities on brain MR images.
MRI Data Acquisition
Magnetic resonance images were acquired from 2 scanners of the same type (3.0T, Discovery GE750w, General Electric). One scanner was used for the sham treatment group, and the other for the real treatment group and cross-sectional experiment. Details were outlined in supplementary materials.
Neuro-navigated rTMS
The AVH patients made 17 study-related visits to the hospital. On the first and last visits, the researchers acquired MRI data and assessed neuropsychological conditions and clinical symptoms. From the 2nd to 16th day, participants received 3 daily sessions of cTBS treatment delivered using a MagStim Rapid2 stimulator (Magstim Company Ltd.) with a 70-mm air-cooled figure-of-eight coil. One session of cTBS was 40 seconds in duration and consisted of 3-pulse bursts at 50 Hz repeated every 200 milliseconds (5 Hz) until a total of 600 pulses was reached.39 To achieve cumulative aftereffects, this protocol was repeated 3 times and (1800 pulses in total) separated by two 15-minute breaks (controlled by a stopwatch) in line with previous methodological studies.40,41 The researchers delivered cTBS at 80% of the resting motor threshold (RMT)38 or the highest intensity the stimulator could deliver for this protocol (50% of maximum output). The RMT was determined at each visit according to a 5-step procedure.42
The stimulation target, the left TPJ, was defined as a sphere of 6-mm radius centered at Montreal Neurological Institute (MNI) coordinates [−51, −31, 23].13,16 This target was transformed into each participant’s T1 space by applying an inverse matrix produced during T1 segmentation in SPM (www.fil.ion.ucl.ac.uk/spm) and TMStarget software.43 Then, each individual’s target was imported into a frameless neuronavigation system (Visor 2.0, Advanced Neuro Technologies). The coil was held tangentially to the skull pointing forward, with the center over the target sphere.
Patients in the sham control group received the same rTMS protocol and treatment duration as the real rTMS group. The only difference was the usage of a sham coil (Magstim Company Ltd.) that produced a similar feeling on the participant’s scalp as the real coil but did not induce a current in the cortex.
Clinical Symptom and Neuropsychological Assessments
Clinical symptoms of all patients were graded according to the Positive and Negative Syndrome Scale (PANSS). In addition, all patients and HCs were evaluated using standardized neuropsychological tests (supplementary tables E1 and E2). For AVH patients participating in the rTMS study, the primary outcome was the Auditory Hallucination Rating Scale (AHRS), which would be administered with other measures on the 1st and 17th visits.
MRI Data Processing
Functional image processing was carried out using the DPARSF (http://rfmri.org)44 and SPM (www.fil.ion.ucl.ac.uk/spm) toolkits. The preprocessing included (1) deleting the first 5 volumes; (2) slice timing and realignment; (3) co-registering T1 to functional images; (4) normalizing T1 to the MNI space and segmenting it into gray matter, white matter, and cerebrospinal fluid (spatial resolution: 3 × 3 × 3); (5) smoothing images with a 4-mm isotropic Gaussian kernel; (6) filtering temporal bandpass (0.01–0.1 Hz), and regressing out 15 nuisance signals (global mean, white matter, cerebrospinal fluid signals, and 24 head-motion parameters.45 Subsequently, Pearson’s correlations were conducted between the time series of all pairs of voxels to construct a whole-brain matrix for each participant. Finally, functional connectivity strength (FCS) defined as the sum of the coefficients between a given voxel and all other voxels, was then standardized by dividing by the average whole-brain FCS value. To eliminate voxels with weak correlations attributable to signal noise, the analysis was restricted to positive correlations (r > .25, P < .001).46,47
Statistical Analysis
For the cross-sectional study, demographic characteristics, neuropsychological scores, and imaging features were compared between the 3 groups by one-way analysis of variance (ANOVA). Notably, outliers in neuropsychological scores were identified before ANOVA by nonlinear regression analyses in GraphPad Prism.48 The Bonferroni or Tamhane’s test was used for pair-wise post hoc comparisons. Clinical characteristics were compared between patient groups using 2-sample t-tests. In the longitudinal study, the change in clinical symptom was analyzed by 2-way (group by time) ANOVA. Since the MRI data of real and sham groups were acquired using different scanners, comparisons of pre- or post-treatment data between the groups largely reflect the systematic error of scanners rather than brain functional changes. However, the pre- to post-treatment alteration is comparable, since the scanner effect could be well controlled by the within center subtraction (post- minus pre-state). Thus, the FCS changes following treatment were compared between groups by 2-sample t-test. All voxel-based imaging analyses were corrected by Gaussian Random Field (GRF) theory (cluster-defined threshold, P < .05, cluster-level corrected P < .05).
Complementary Experiments
First, target-to-whole brain functional connectivity was used as a secondary measure. Second, longitudinal data of 2 HC groups from 2 scanning sites were utilized to clarify whether the MRI findings for the TMS effect were influenced by scanner. Third, we re-computed the FCS for correlations >0 to test the robustness of the findings. Fourth, all image-based statistical analyses were corrected for multiple comparisons using Threshold-Free Cluster Enhancement in FSL software (corrected P < .05).49 See details of these experiments in supplementary materials.
Results
Demographic, Clinical, and Neuropsychological Assessments at Baseline
There were no differences in age, education, and sex ratio among the 3 groups (supplementary table E1). Four neuropsychological tests (digit span [backward], Stroop word test, trail making B, and recognition) showed significant group differences, and post hoc analyses indicated that both patient groups showed decreased performance on the Stroop word test and trail making B test compared to HCs; digit span [backward] was abnormal in non-AVH but not AVH patients (supplementary table E1). The AVH patients had higher PANSS positive scores than the non-AVH patients (supplementary table E1). There were no significant differences in baseline estimations between AVH patients in the real and sham cTBS groups (supplementary table E2). Medication information of patients in TMS group (n = 27) was listed in supplementary table E3.
FCS at Baseline
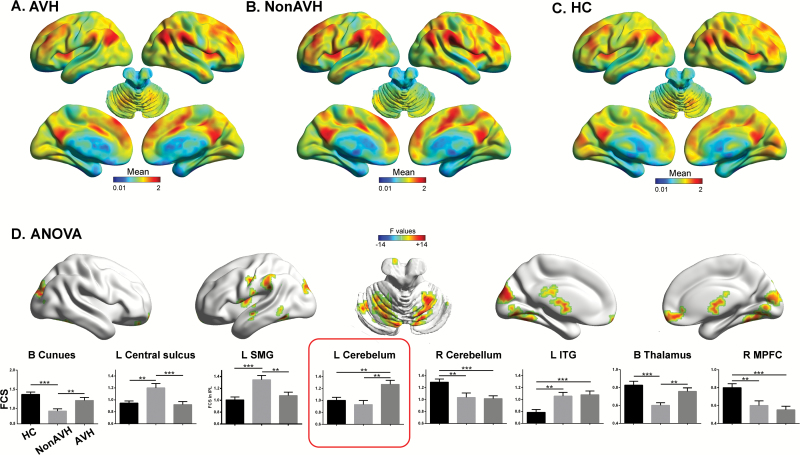
One-way ANOVA indicated significant differences in FCS among AVH (figure 2A), non-AVH (figure 2B), and HC (figure 2C) groups in the bilateral cuneus, left central sulcus, left inferior temporal gyrus (ITG), bilateral cerebellum, left supramarginal gyrus (SMG), bilateral thalamus, and right medial prefrontal cortex (MPFC, supplementary table E4; figure 2D, corrected P < .05). Post hoc analyses on these clusters are summarized in supplementary table E4 and figure 2D. No significant difference was found in frame-wise head motion50 among the 3 groups (F = 1.21, P = .30).
Fig. 2.
One-way analysis of variance of functional connectivity strength (FCS) maps in the cross-sectional experiment. Mean maps of FCS are illustrated for schizophrenia patients with auditory verbal hallucinations (AVHs) (A), non-AVH patients (B), and healthy controls (C). Significant clusters were found in 8 brain areas (D). Post hoc findings are illustrated by bar graphs (E; error bars indicate SEM; **P < .01, ***P < .001). L, left; ITG, inferior temporal gyrus; MPFC, medial prefrontal gyrus; R, right; SMG, supramarginal gyrus.
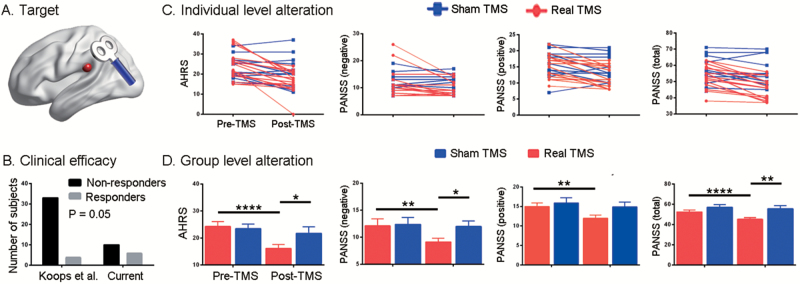
rTMS Improved Clinical Symptoms But Not Cognitive Function
According to a recent double-blind randomized trial,33 this study defined rTMS responders as participants who showed ≥25% decrease in AHRS. Six of 16 participants in the real group, and 3 of 11 patients in the sham group were deemed responders. Fisher’s exact test did not reveal a significant difference in the responder/nonresponder ratio between groups (P = .69). However, the ratio for the real group was higher (P = .05; figures 3A and 3B) than that found in a previous rTMS study (4 responders in 37 subjects).33 Two-way repeated ANOVA indicated a significant group × time interaction effect for clinical symptoms but not for cognitive functions (supplementary table E2; figures 3C and 3D). Specifically, the AHRS (t = −5.66, P <.0001), negative PANSS (t = −3.75, P = .002), positive PANSS (t = −5.25, P < .0001), and total PANSS (t = −6.17, P < .0001) scores (figures 3C and 3D) significantly decreased following real treatment. Negative PANSS consisted of 3 dimensions (emotion, behavior, and thought), and behavior factor was the most responsive one to the treatment (paired t = 3.93, P = .001, supplementary table E5). Compared to the sham group, the real group showed lower scores in AHRS (t = −2.13, P = .04), negative PANSS (t = −2.48, P = .02), and total PANSS (t = −3.30, P = .003), but similar positive PANSS (t = 0, P > .99, figures 3C and 3D) at the end of treatment.
Fig. 3.
Clinical efficacy of 15-days’ repetitive transcranial magnetic stimulation (rTMS) treatment on the left temporal parietal junction (A). Bar graph (B) indicates a higher responder/nonresponder ratio in the current study than in Koops et al.33 The symptom improvements after real and sham treatment are illustrated at both the individual (C) and group (D) level. Notably, there is no outlier in the symptom measures. Error bars indicate SEM. *P < .05, **P < .01, ****P < .0001.
rTMS Modified Functional Connectivity
The frame-wise head motion before and after treatment50 did not differ in either the real rTMS (t = 0.90, P = .39) or sham (t = 0.51, P = .62) group. Although the image datasets from the real and sham cTBS groups were obtained from 2 scanners, the alterations after rTMS were comparable between groups (see Complementary Experiments for the demonstration). Paired t-tests (post- vs pre-TMS) were first performed for both groups (voxel P < .05, voxel number > 10; unthresholded map in figures 4A and 4B). Voxels surviving in either group constituted a mask for comparing the functional alterations between groups by 2-sample t-tests. Compared to the sham group, the real group showed increased FCS in the bilateral inferior occipital gyrus (IOG) and right post-central gyrus, and decreased FCS in the left MPFC and left cerebellum (supplementary table E6, figure 4C; corrected P < .05). Notably, significant alterations of the left cerebellum were found in the real cTBS group (t = −3.1, P = .008) but not in the sham group (t = 1.8, P = .1) (supplementary table E6, figure 4C).
Fig. 4.
Functional connectivity strength (FCS) maps in the longitudinal experiment. FCS alterations after real (A) and sham (B) rTMS treatment are illustrated by unthresholded t maps. Regions showing different alterations between real and sham groups were identified by 2-sample t-test (C), and the bar graphs indicate functional alterations of these regions within both groups. Error bars indicate SEM. *P < .05, **P < .01, ***P < .001. Cereb., cerebellum; L, left; IOG, inferior occipital gyrus; MPFC, medial prefrontal gyrus; R, right; post-CG, post-central gyrus.
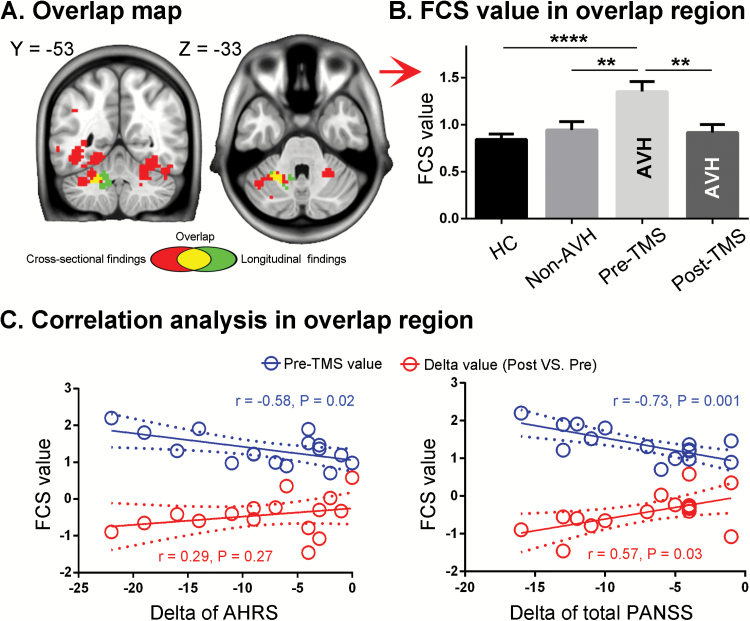
Spatial Overlap and Correlation With Symptom Improvement
The baseline binary result map (figure 2D) overlapped with that of the longitudinal experiment (figure 4C) at the left cerebellum (figure 5A). The FCS value of the overlapping cluster was extracted from HC, non-AVH patients, and 16 AVH patients before and after real cTBS treatment. One-way ANOVA (F2,72 = 0.74, P = .0003) and post hoc analyses indicated that the left cerebellum cluster showed higher FCS in AVH patients before treatment than either non-AVH patients (t = 2.89, P = .006) or HCs (t = 4.66, P < .0001; figure 5B). Following rTMS treatment, FCS in the left cerebellum significantly decreased (t = 3.44, P = .004; figure 5B).
Fig. 5.
Analysis of the cerebellum cluster identified by both cross-sectional and longitudinal experiments. The overlap area (A) showed significantly higher FCS in AVH patients than non-AVH patients and HCs at baseline, and significantly decreased FCS after real rTMS treatment (B). The pretreatment FCS value in the overlap area negatively correlated with symptom improvement in both AHRS and PANSS total scores (C). The FCS decrease in the overlap area after real treatment positively correlated with an improvement in total PANSS scores but not in AHRS (C).
Since clinical symptoms were not significantly improved in the sham group (supplementary table E2, figure 3), the researchers performed a correlation analysis only for the real group. The average FCS value in the overlapping cerebellum cluster at baseline showed a negative correlation with both AHRS (r = −0.58, P = .02) and total PANSS improvement (r = −.73, P = .001), but not negative (r = −.27, P = .07) or positive (r = −.43, P = .10) PANSS changes (post- minus pre-rTMS, negative change indicating improvement; figure 5C). The FCS change in the overlapping cluster showed a positive correlation with the total PANSS improvement (r = .57, P = .03), but not with AHRS (r = .29, P = .27), negative PANSS changes (r = .19, P = .48), or positive PANSS changes (r = .38, P = .14) (figure 5C).
Complementary Experiments
All the 4 experiments confirmed the important role of left cerebellum in schizophrenia as the main text. See details in the supplementary table E7 and supplementary figures E1–E7.
Discussion
From a functional connectivity perspective, this study revealed the neuronal correlates of AVH in schizophrenia by a cross-sectional experiment involving patients exhibiting AVHs, patients without AVHs, and matched HCs. Among the clusters identified in the baseline ANOVA, only the cluster in the left cerebellum differed significantly in AVH patients vs the other groups. Importantly, FCS within this cluster was modulated by real rTMS treatment but not the sham condition, and the baseline value was negatively correlated with clinical symptom improvement.
The cerebellum is connected to the cerebral cortex via a cortico-cerebellar-thalamic-cortical circuit. Its involvement in AVH has been demonstrated by meta-analyses of functional activation experiments,51,52 though inconsistencies were also found.53,54 Dynamic fMRI analysis further indicated the activation of the left cerebellum prior to the occurrence of AVHs, suggesting a “trigger” role of the cerebellum.55,56 At a cellular level, the crucial role of the cerebellum in schizophrenia has been systemically reviewed.57 Cerebellar Purkinje cells discriminate specific input conditions, such as variation in patterns of auditory input, through synaptoplastic processes including long-term potentiation and depression. In schizophrenia patients, the cerebellum fails to perform these error detection functions. As a result, input information from the auditory cortex without an external stimulus may be misinterpreted as “external” rather than internal, leading to the experience of AVHs.9,57 Decreased FCS within the cerebellum following treatment may reflect an attempt to rebalance inhibitory and excitatory transmission, which may result in an appropriate perception of inner speech.4 Both AVH and non-AVH patients showed similar alterations in the right cerebellum, right MPFC, and left ITG compared to HCs, which may underlie common symptoms of schizophrenia. On the other hand, non-AVH patients showed abnormalities in the bilateral cuneus, bilateral thalamus, left central sulcus, and left SMG compared to both AVH patients and HCs, which may be related to the unique symptoms of non-AVH patients. However, none of these clusters survived the fourth validation analysis. As such, their biological meaning should be interpreted with care.
The researchers found significant symptom improvement after cTBS treatment, and the responder/nonresponder ratio was higher than in Koops et al.33 This improved outcome may be due to optimized stimulation parameters, such as ISI, longer treatment and more sessions/day.40,41 Psychological factor may also contribute to this difference, since patients in our real group knew they would received real treatment, while patients in Koops et al33 did not know their group allocation. Our findings suggest that the left cerebellum constitutes an important neural correlate for this clinical improvement. The left cerebellum has directly structural connectivity with the right rather than left cortices, such as the stimulation target. Thus, future rTMS studies stimulating the right TPJ may produce stronger aftereffect in both neuroimaging and clinical efficacy. Moreover, the negative correlation between symptom improvement and the pretreatment FCS value in the left cerebellum suggests that the baseline FCS may be used to screen patients sensitive to rTMS treatment.
Analyses of neuropsychological tests indicated that both patient groups showed decreased performance on the Stroop word and trail making B test compared to HCs; a deficit was found in the digit span [backward] of non-AVH patients. These findings derived from neuropsychological tests were in line with previous studies that reported cognitive deficits in schizophrenia patients.58–60 Importantly, no cognitive difference was found between non-AVH and AVH groups. This finding suggests that extraneous factors were well-balanced between groups. Although rTMS could modulate cognitive function and neural circuitry,43,61 no cognitive improvement or deterioration was found in this study after treatment. This finding may be attributed to the fact that the current cTBS parameters, especially with regard to the target, were specifically designed for AVH alleviation rather than any particular cognitive function improvement.
Although the findings are encouraging, 3 limitations should be mentioned. First, the rTMS experiment was not a randomized control trial. The sham group was recruited after the real group, and their instructions were slightly different. The real rTMS group was told that they would receive a novel treatment through magnetic stimulation, but its clinical efficacy was still in controversial. The sham group was part of a real RCT experiment, and the patients were instructed that they would be assigned to the real or sham group randomly. Thus, both real and sham group in this study would not have extremely high or no expectation on the treatment. Additionally, the sham stimulation was performed by a placebo coil, which induced quite similar sensory on scalp as the real one, but actually no induced current within the brain. Thus, the patients hardly knew whether they were receiving real or sham stimulation. For both group, the clinical syndrome was estimated by an experimenter blinded to the group allocation of patients. In all, the real and sham group had similar expectation before treatment and experienced similar stimulation procedures. To further exclude the effect of the design flaws on our findings, we strongly suggest a real RCT investigation. Second, the real and sham groups were not acquired by the same scanner, which was not an ideal design. For cross-sectional comparison, the results may be affected by the systematic error between scanners. But for comparison of longitudinal alteration, as our complementary experiment indicated, no significant scanner effect was found in the left cerebellum area. Third, this study did not control for the possible confounding effects of AVHs during rs-fMRI data acquisition. A similar problem exists for rs-fMRI studies on epilepsy, though a previous study demonstrated that internal events do not exert substantial effects on brain function.62
This rs-fMRI study revealed abnormally high FCS of the left cerebellum in schizophrenia patients with AVH. This abnormality as well as the AVH symptom could be significantly decreased through the administration of a modified cTBS treatment. Furthermore, the improvement in AVHs after rTMS treatment may be predicted by baseline FCS in the left cerebellum. In summary, these findings emphasize the role of the left cerebellum in both the pathophysiology and clinical treatment of AVH in schizophrenia patients.
Supplementary Material
Acknowledgment
This study was funded by the Natural Science Foundation of China (91432301, 31571149, 81171273, and 91232717 to K.W., 31771222 to F.Y., 81771456 to C.Z.), the China Postdoctoral Foundation (2017M612057 to G.J.J.), the Doctoral Foundation of Anhui Medical University (XJ201532 to G.J.J.), the Youth Talent Support Plan of Anhui Medical University (to G.J.J.), the National Basic Research Program of China (2015CB856405, 2012CB720704, and 2011CB707805 to K.W.), the Science Fund for Distinguished Young Scholars of Anhui Province (1808085J23 to Y.T.), and the Anhui Collaborative Innovation Centre of Neuropsychiatric Disorders and Mental Health.
References
- 1. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49. [DOI] [PubMed] [Google Scholar]
- 2. Aleman A, de Haan EH. On redefining hallucination. Am J Orthopsychiatry. 1998;68:656–659. [DOI] [PubMed] [Google Scholar]
- 3. Allen P, Modinos G, Hubl D et al. . Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jardri R, Hugdahl K, Hughes M et al. . Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain?Schizophr Bull. 2016;42:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alderson-Day B, Diederen K, Fernyhough C et al. . Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr Bull. 2016;42:1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev. 2015;55:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127:202–214. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolland B, Amad A, Poulet E et al. . Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 2015;41:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui LB, Liu K, Li C et al. . Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22. [DOI] [PubMed] [Google Scholar]
- 12. Chang X, Xi YB, Cui LB et al. . Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci Rep. 2015;5:11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67:912–918. [DOI] [PubMed] [Google Scholar]
- 14. Gavrilescu M, Rossell S, Stuart GW et al. . Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med. 2010;40:1149–1158. [DOI] [PubMed] [Google Scholar]
- 15. Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44:725–731. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman RE, Hawkins KA, Gueorguieva R et al. . Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman RE, Hampson M, Wu K et al. . Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17:2733–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IE. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry. 2014;76:101–110. [DOI] [PubMed] [Google Scholar]
- 20. Hare SM, Ford JM, Ahmadi A et al. ; Functional Imaging Biomedical Informatics Research Network. Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr Bull. 2017;43:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu J, Wang C, Liu F, Qin W, Li J, Zhuo C. Alterations of functional and structural networks in schizophrenia patients with auditory verbal hallucinations. Front Hum Neurosci. 2016;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diederen KM, Neggers SF, de Weijer AD et al. . Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol Med. 2013;43:1685–1696. [DOI] [PubMed] [Google Scholar]
- 23. van Lutterveld R, Diederen KM, Otte WM, Sommer IE. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum Brain Mapp. 2014;35:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Weijer AD, Neggers SF, Diederen KM et al. . Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum Brain Mapp. 2013;34:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong D, Wang Y, Chang X, Chen X, Luo C, Yao D. Common and diagnosis-specific fractional anisotropy of white matter in schizophrenia, bipolar disorder, and major depressive disorder: evidence from comparative voxel-based meta-analysis. Schizophr Res. 2018;193:456–458. [DOI] [PubMed] [Google Scholar]
- 26. Dong D, Wang Y, Jia X et al. . Abnormal brain activation during threatening face processing in schizophrenia: a meta-analysis of functional neuroimaging studies. Schizophr Res. 2017; pii: S0920-9964(17)30708-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Lefaucheur JP, André-Obadia N, Antal A et al. . Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150–2206. [DOI] [PubMed] [Google Scholar]
- 28. Hoffman RE, Boutros NN, Berman RM et al. . Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol Psychiatry. 1999;46:130–132. [DOI] [PubMed] [Google Scholar]
- 29. Hoffman RE, Gueorguieva R, Hawkins KA et al. . Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58:97–104. [DOI] [PubMed] [Google Scholar]
- 30. Slotema CW, Blom JD, de Weijer AD et al. . Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2011;69:450–456. [DOI] [PubMed] [Google Scholar]
- 31. Moseley P, Alderson-Day B, Ellison A, Jardri R, Fernyhough C. Non-invasive brain stimulation and auditory verbal hallucinations: new techniques and future directions. Front Neurosci. 2015;9:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffman RE, Wu K, Pittman B et al. . Transcranial magnetic stimulation of Wernicke’s and right homologous sites to curtail “voices”: a randomized trial. Biol Psychiatry. 2013;73:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koops S, van Dellen E, Schutte MJ, Nieuwdorp W, Neggers SF, Sommer IE. Theta burst transcranial magnetic stimulation for auditory verbal hallucinations: negative findings from a double-blind-randomized trial. Schizophr Bull. 2016;42:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sommer IE, de Weijer AD, Daalman K et al. . Can fMRI-guidance improve the efficacy of rTMS treatment for auditory verbal hallucinations?Schizophr Res. 2007;93:406–408. [DOI] [PubMed] [Google Scholar]
- 35. Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S. Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res. 2009;113:77–83. [DOI] [PubMed] [Google Scholar]
- 36. Poulet E, Brunelin J, Ben Makhlouf W, D’Amato T, Saoud M. A case report of cTBS for the treatment of auditory hallucinations in a patient with schizophrenia. Brain Stimul. 2009;2:118–119. [DOI] [PubMed] [Google Scholar]
- 37. Kindler J, Homan P, Flury R, Strik W, Dierks T, Hubl D. Theta burst transcranial magnetic stimulation for the treatment of auditory verbal hallucinations: results of a randomized controlled study. Psychiatry Res. 2013;209:114–117. [DOI] [PubMed] [Google Scholar]
- 38. Plewnia C, Zwissler B, Wasserka B, Fallgatter AJ, Klingberg S. Treatment of auditory hallucinations with bilateral theta burst stimulation: a randomized controlled pilot trial. Brain Stimul. 2014;7:340–341. [DOI] [PubMed] [Google Scholar]
- 39. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. [DOI] [PubMed] [Google Scholar]
- 40. Nettekoven C, Volz LJ, Kutscha M et al. . Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Volz LJ, Benali A, Mix A, Neubacher U, Funke K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul. 2013;6:598–606. [DOI] [PubMed] [Google Scholar]
- 42. Schutter DJ, van Honk J. A standardized motor threshold estimation procedure for transcranial magnetic stimulation research. J ECT. 2006;22:176–178. [DOI] [PubMed] [Google Scholar]
- 43. Ji GJ, Yu F, Liao W, Wang K. Dynamic aftereffects in supplementary motor network following inhibitory transcranial magnetic stimulation protocols. Neuroimage. 2017;149:285–294. [DOI] [PubMed] [Google Scholar]
- 44. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. [DOI] [PubMed] [Google Scholar]
- 46. Buckner RL, Sepulcre J, Talukdar T et al. . Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol. 2010;6:e1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 51. Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113–123. [DOI] [PubMed] [Google Scholar]
- 52. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 55. Hoffman RE, Pittman B, Constable RT, Bhagwagar Z, Hampson M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br J Psychiatry. 2011;198:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diederen KM, Neggers SF, Daalman K et al. . Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry. 2010;167:427–435. [DOI] [PubMed] [Google Scholar]
- 57. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mendrek A, Kiehl KA, Smith AM, Irwin D, Forster BB, Liddle PF. Dysfunction of a distributed neural circuitry in schizophrenia patients during a working-memory performance. Psychol Med. 2005;35:187–196. [DOI] [PubMed] [Google Scholar]
- 59. Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 60. Keefe RS. Cognitive deficits in patients with schizophrenia: effects and treatment. J Clin Psychiatry. 2007;68 (Suppl 14):8–13. [PubMed] [Google Scholar]
- 61. Wang JX, Rogers LM, Gross EZ et al. . Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ji GJ, Yu Y, Miao HH, Wang ZJ, Tang YL, Liao W. Decreased network efficiency in benign epilepsy with centrotemporal spikes. Radiology. 2017;283:186–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.