Abstract
Schizophrenia is genetic in origin and associated with a fecundity disadvantage. The deficits in schizophrenia have been attributed to variation related to the human capacity for language or brain laterality. How sex influences the relative connectivity of the 2 hemispheres is a route to understanding these 2 functions. Using resting-state functional magnetic resonance imaging (fMRI) we searched for sex- and hemisphere-specific changes in whole-brain functional-connectivity in multi-site datasets (altogether 672 subjects including 286 patients, all right-handed) in the first-episode schizophrenia (illness duration ≤ 1 year, mostly drug naive) and in chronic stages of schizophrenia (illness duration > 1 year), respectively. We used meta-analyses to integrate data from different sources concerning individuals at the same illness stage. We found first-episode male patients are predominantly left-lateralized in aberrant connectivity with a focus on Broca’s area. Female patients show a lesser degree of lateralization than males, but to the right particularly in orbital frontal cortex. In the chronic stage, the focus of aberrant connectivity shifted from anterior to posterior structures with prominent involvement of the thalamus and pre- and post-central gyri bilaterally and in both sexes. While the “deviant connectivity” is right-sided in both the first-episode and the chronic stages in females, in males there is a shift between stages from the left to the right hemisphere. We hypothesized that the pathophysiology of schizophrenia may lie in the interaction between sex and lateralization, ie, in genetic mechanisms located on the X and Y chromosomes, intrinsic to the evolution of language.
Keywords: resting-state fMRI, whole-brain functional connectivity analysis, sex-effect, lateralization, language, PCDH11XY, sexual selection
Introduction
In 1861, Broca1 recognized that a component of the faculty of articulate speech is localized in the frontal lobes and in the left hemisphere. In a presentation to the Montpellier Medical Society, Dax2 had reported laterality of speech 25 years before and by 1839, Pierre Gratiolet had described the cortical gyri as developing in the frontal lobe on the left before the right, and in the occipital lobe on the right before the left.3 In 1877, Broca4 proposed that asymmetry was specific to the human brain, ie, to say that it defined the species and enabled the faculty of language. That hypothesis has proven controversial.
Within 2 years of Broca’s conjecture, in what was perhaps the first evolutionary hypothesis of the origins of psychosis and in relation to a postmortem study of brain weight Crichton-Browne5 wrote that “it seemed not improbable that the cortical centres that are the last organized, which are the most highly evolved and voluntary, and which are supposed to be located on the left side of the brain, might suffer first in insanity”. An evolutionary theory is required to explain a lifetime prevalence of schizophrenia approaching 1% becoming more uniform across populations as diagnostic criteria are more strictly drawn,6 associated with a fecundity disadvantage7 and in the absence of environmental precipitation. Why does the disorder persist? Why is the genetic variation not selected out?
Differences between the sexes in the onset of schizophrenia have been held to reflect the operation of sexual selection in man.8 Onsets occur throughout the reproductive phase of life with a sex difference—earlier in males—perhaps by as much as 2–3 years depending on the diagnostic criteria, and outcome is generally worse.9 The interaction with laterality may hold the key to etiology. Although there has been research on sex differences in functional lateralization in healthy individuals10–13 and on changes in cerebral lateralization in schizophrenia,14–16 there has been little on the interaction between sex and laterality in relation to resting-state functional connectivity in disease. Though task functional magnetic resonance imaging (fMRI) may reveal more specific information of functional brain laterality in schizophrenia, in the current work, we focused on resting-state fMRI because it allows for a broader sampling of patient populations, and also that the whole-brain network architectures in task-state fMRI have a high level of similarity with those of resting-state fMRI.17
In addition, existing work of laterality of schizophrenia usually calculate the laterality index for a given pair of homologous structures [ie, (left-right)/(left +right)], and focused more on structural fMRI18 than functional fMRI.19,20 In comparison, here we first identified the significantly altered resting state functional connectivity, and then seek the laterality patterns of the altered functional connectivity.
Specifically, in a meta-analysis of resting-state fMRI of a total of 672 subjects we address these issues at the onset (illness duration ≤ 1 year) and with progression of schizophrenia (illness duration> 1 year). In previous work, we identified functional connectivity change in the inferior frontal gyrus in drug naïve first-episode patients, and in the thalamus in chronic schizophrenia,21 without specifying the influence of sex. We formulated the hypothesis that if schizophrenia is “the price that Homo sapiens pays for the faculty of language” the developmental course of the illness should be critically dependent on the sex and laterality of the affected individual.
Materials and Methods
Data Quality Control
To ensure data quality, a control protocol was set up with exclusion criteria as follows: (1) Subjects with poor structural scans, or without complete demographic information and the Positive and Negative Syndrome Scale (PANSS) scores, or age <16 years old. (2) Head movement: subjects with >10% displaced frames in a scrubbing procedure or maximal motion between volumes in each direction >3 mm, and rotation about each axis >3° were excluded. Patients and controls were screened in each dataset so that the total root mean square displacements did not show significant differences. (3) Left-handed subjects were excluded.
Subjects and Data Acquisition
Resting-state functional scans were collected from 814 subjects (428 patients with schizophrenia and 386 healthy control subjects. Our data is comprised of patient and healthy control data from 5 sources: (1) Huaxi hospital in China (163 schizophrenia patients and 150 healthy controls, first-episode)22; (2) Xiangya hospital in China (83 schizophrenia patients and 60 healthy controls)23; (3) The Center for Biomedical Research Excellence (COBRE, 71 patients and 74 controls24; (4) The National Taiwan University Hospital in Taiwan (13 first-episode patients and 56 chronic patients, and 62 healthy controls)25; (5) Nottinghamshire and Leicestershire community-based mental health teams (42 patients and 40 controls).26 After quality control, 672 subjects, involving 286 patients with schizophrenia and 386 controls were recruited; patients’ demographics are provided in table 1. Here, first-episode patients were defined as having illness duration less than a year, while chronic patients were defined as having illness duration longer than a year. Medication details can be found in the supplement data acquisition.
Table 1.
Demographic and Clinical Characteristics of Patient in Datasets 1# to 5#
| Sites | Group | N | Age (Year) | Positive Scale | Negative Scale | General Scale | Duration of Illness (Year) | Medication |
|---|---|---|---|---|---|---|---|---|
| 1# Huaxi (FE) | Male (C) | 80 | 24.7 ± 7.7 | |||||
| Female (C) | 70 | 27.1 ± 9.6 | ||||||
| Male (P) | 53 | 23.2 ± 6.4 | 25.1 ± 6.1 | 19.4 ± 7.8 | 45.7 ± 9.1 | 0.29 ± 0.28 | All drug-naïve | |
| Female (P) | 60 | 25.6 ± 8.8 | 25.7 ± 5.2 | 18.8 ± 6.2 | 49.6 ± 8.5 | 0.19 ± 0.25 | All drug-naïve | |
| 2# Xiangya (FE) | Male (C) | 32 | 26 ± 5.2 | |||||
| Female (C) | 25 | 26.6 ± 6.0 | ||||||
| Male (P) | 26 | 24.6 ± 5.8 | 20.2 ± 8.1 | 23.1 ± 9.7 | 40.0 ± 14.8 | 0.42 ± 0.33 | 14 drug-naïve | |
| Female (P) | 13 | 25.3 ± 8.0 | 18.1 ± 4.4 | 21.1 ± 4.6 | 39.0 ± 10.0 | 0.54 ± 0.34 | 5 drug-naïve | |
| 2# Xiangya (chronic) | Male (C) | 30 | 25.3 ± 4.5 | |||||
| Female (C) | 22 | 25.1 ± 4.4 | ||||||
| Male (P) | 18 | 24.7 ± 5.4 | 21.0 ± 7.3 | 20.0 ± 6.4 | 38.7 ± 10.2 | 2.7 ± 1.1 | 14 drug-naïve | |
| Female (P) | 13 | 25.0 ± 7.2 | 17.1 ± 6.2 | 18.5 ± 5.3 | 35.4 ± 9.6 | 2.9 ± 1.2 | 5 drug-naïve | |
| 3# COBRE (chronic) | Male (C) | 44 | 35.5 ± 11.5 | |||||
| Female (C) | 21 | 32.5 ± 9.3 | ||||||
| Male (P) | 33 | 36.8 ± 14.0 | 14.8 ± 4.2 | 15.1 ± 5.3 | 29.8 ± 8.4 | 17.0 ± 13.5 | Medicated | |
| Female (P) | 9 | 37.3 ± 12.1 | 15.6 ± 6.2 | 13.3 ± 4.7 | 29.6 ± 8.0 | 15.6 ± 9.7 | ||
| 4# Taiwan (FE) | Male (C) | 20 | 27.0 ± 6.0 | |||||
| Female (C) | 30 | 27.2 ± 6.4 | ||||||
| Male (P) | 5 | 21.6 ± 3.5 | 13.8 ± 3.1 | 11.2 ± 1.9 | 28.8 ± 8.4 | ≤1 years | Medicated | |
| Female (P) | 8 | 26.0 ± 8.8 | 17.2 ± 5.7 | 13.0 ± 5.85 | 33.6 ± 9.2 | ≤1 years | ||
| 4# Taiwan (chronic) | Male (C) | 19 | 31.0 ± 7.2 | |||||
| Female (C) | 32 | 32.3 ± 8.7 | ||||||
| Male (P) | 29 | 33.8 ± 8.7 | 10.7 ± 3.4 | 13.9 ± 5.3 | 26.0 ± 8.5 | 8.1 ± 6.2 | Medicated | |
| Female (P) | 23 | 33.1 ± 10.5 | 13.1 ± 5.5 | 13.3 ± 7.3 | 28.5 ± 10.7 | 6.3 ± 7.0 | ||
| 5# Nottingham (chronic) | Male (C) | 23 | 31.5 ± 8.0 | |||||
| Female (C) | 9 | 34.0 ± 9.7 | ||||||
| Male (P) | 22 | 33 ± 9 | Medicated | |||||
| Female (P) | 5 | 33.8 ± 12.8 | ||||||
Note: C, control; P, patients; FE, first episode.
All patients were identified according to the DSM-IV diagnostic criteria by qualified psychiatrists using all available clinical information including a diagnostic interview, clinical case notes, and clinician’s observations. Symptom severity was measured using the PANSS assessment. All healthy subjects were assessed in accordance with DSM-IV criteria as being free of schizophrenia and other axis I disorders, and none had neurological diseases, head trauma, or substance abuse. Written informed consent was obtained from all individual participants, and all of the research procedures and ethical guidelines were approved by the local research ethics committee of the West China Hospital, Second Xiangya Hospital, the Institutional Review Board (IRB) of the Hartford Hospital and Yale University, the IRB of the National Taiwan University Hospital, and National Research Ethics Committee, Derbyshire, UK, respectively, for the above 5 datasets. All subjects underwent resting-state functional MRI scanning for 5–7 minutes. All individuals were asked to remain still, think of nothing while remain awake, with eyes open in subjects of datasets 1#, 2#, and 4#, and eyes closed in datasets 3# and 5#. The imaging acquisition protocols for 5 datasets are provided in the supplementary data acquisition.
Data Preprocessing
All fMRI data were preprocessed by Statistical Parametric Mapping package (SPM8, Wellcome Department for Imaging Neuroscience). The first 10 image volumes of each subject were discarded to allow the fMRI signal to reach a steady state. After slice time correction, the image was realigned and normalized to a standard template (Montreal Neurological Institute) and resampled to 3 × 3 × 3 mm3, and subsequently smoothed with an isotropic Gaussian kernel (FWHM 8 mm).
All fMRI time-series underwent band-pass temporal filtering (0.01–0.08 Hz), nuisance signal removal from ventricles, deep white matter, global mean signal (GMS) and 6 rigid-body motion correction parameters. Considering the concern about the possible influence of excessive movement, we performed the following procedures for motion correction. We first apply 3-dimensional motion correction by aligning each functional volume to the mean image of all volumes and then implemented a careful data scrubbing (see supplemental method S1 for detail). The mean frame-wise displacement (Jenkinson) was computed with a threshold for displacement of 0.5. One preceding and 2 succeeding time points were also deleted. Subjects with >10% displaced frames were excluded due to the high-level of movement. Finally, the mean displacements after scrubbing were used as a covariate in t-tests between control and patient groups. After this, 90 regional time series were extracted by averaging voxel time series within each anatomically defined region (by the Automated-Anatomical-Labeling template27). A discussion of global-signal removal can also be found in supplemental method S1.
Statistical Analysis
Whole-Brain Functional-Connectivity Analysis
Pearson cross-correlations between all pairs of regional BOLD signals were calculated to reflect the functional connectivity between regional pairs. The whole-brain functional network (90 × 90 network with 4005 edges) was then constructed. For each of the 4005 connection, a 2-sample, 2-tailed t-test was performed to identify whether differences between controls and patients were significant for each functional connectivity link in each dataset, with age, sex, root mean square displacements of head movement, and dosage (if the dataset contains both medicated and drug-naïve patients) being regressed out, and Bonferroni correction applied for multiple statistical comparisons (P-threshold = .05/4005).
Sex Effect
To investigate how sex affects alterations in functional connectivity, we compared schizophrenia patients with matched controls of the same sex. That is, we compared male patients with matched male controls, and female patients with matched female controls, to identify sex-specific changes. We also differentiated between first-episode and chronic patients, and analyzed whole brain functional connectivity for each site. The P-value for each change in functional connectivity was then derived by meta-analysis.
Meta-Analysis
To combine results from different imaging centers with patients of the same sex and stage, we used the Lepta–Stouffer z-score method that has been widely used in multi-site gene28 and MRI data analysis.29,30 The P-value of each functional connectivity result from 2-sample t-tests in each center was converted to the corresponding z score as in equation: zi = Φ^ (−1) (1 − pi), where Φ is the standard normal cumulative distribution function and i represent the i site. A combined z score for a functional connectivity was then obtained using the Liptak–Stouffer formula:
which has a standard normal distribution under the null hypothesis; where wi is the square root of the ith dataset. Finally, The Z is transformed into its corresponding P-value and a Bonferroni procedure was used to correct for multiple comparisons (P-threshold = .05/4005).
For first-episode schizophrenia, we integrated results from datasets 1# (first-episode patients), 2# (first-episode patients), and 4# (first-episode patients) for each sex, respectively; For chronic schizophrenia, we integrated datasets 2# (chronic patients), 3# (chronic patients), 4# (chronic patients), and 5# (chronic patients) also for each sex. Note that 2# and 4# contain patients of both first episode and chronic stage. After meta-analysis, we obtained functional connectivity links that are significantly changed in schizophrenia patients compared with matched controls (ie, P < .05/4005).
Patterns of Laterality of Functional Connectivity Changes
After identifying functional connectivity links that are significantly changed in schizophrenia patients through meta-analysis, we then counted the number of altered functional connectivity and relevant brain regions (nodes) in each hemisphere.
The difference between our laterality investigation and traditional laterality studies is that the literatures12 usually calculated the laterality index of a pair of symmetric structure in each subject first and then performed group-level analysis. While in our work, we first identified significant group-level functional connectivity changes, and then examined their laterality patterns.
To attach statistical significance to this statistics, we used 60%-sample permutation approach, ie, we randomly pick 60% of the sample in both the patient and control group. After obtained the significantly changed functional connectivity by integrating results for each relevant dataset through meta-analysis, we count the number of nodes pertinent to the altered functional connectivity in each hemisphere (N_l and N_r, representing the number of affected nodes in left and right hemisphere). We permute for 1000 times, and get the distribution of N_l and N_r. We then evaluate the statistical significance of the following: (1) N_l and N_r are significantly larger than 0; (2) N_l–N_r significantly different from 0 (N_l–N_r > 0 indicated left-laterality, while N_l–N_r < 0 indicated right-laterality).
Correlation With Symptom Scores
Pearson correlations between symptom score and altered functional connectivity were calculated. Meta-analysis was used to combine data from each illness stage. For the first-episode, the results were obtained integrating datasets 1#, 2#, and 4#, and for the chronic stage, by integrating datasets 2#, 3#, 4#, and 5#.
Results
Lateralization of Functional Connectivity Changes in First-Episode Schizophrenia
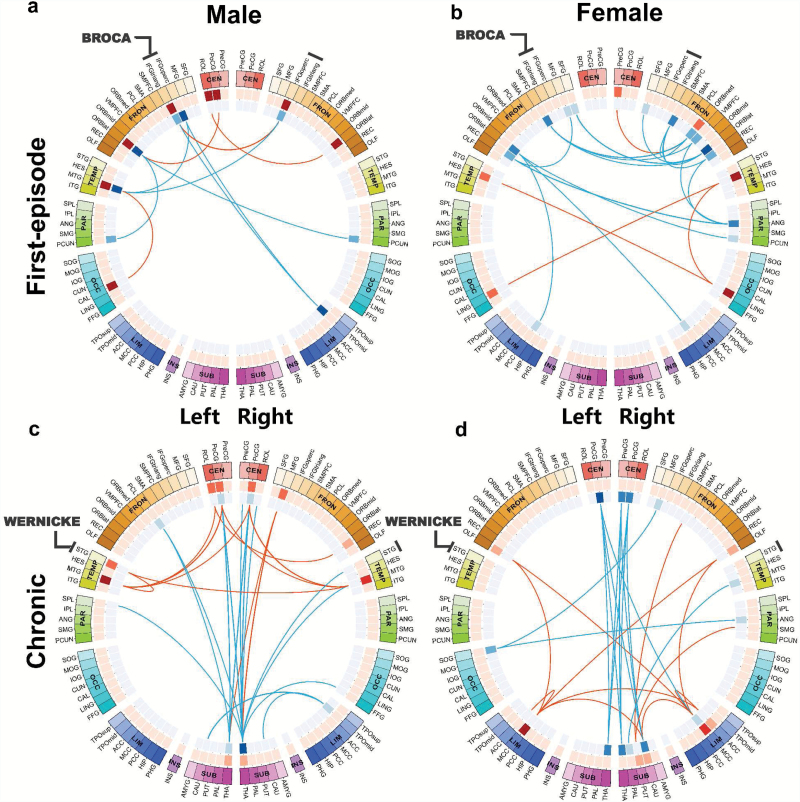
In the first-episode in male patients functional change (number of brain regions, or nodes affected) was more frequent in the left (8 nodes) than right hemisphere (4 nodes), with 60%-sample permutation test showing that Nl–Nr significantly larger than 0 (P < .001, Nl and Nr represent the number of affected nodes in left and right brain hemisphere, respectively). While in female patients it was more frequent in the right (12 nodes) than left (8 nodes), with 60%-sample permutation test showing Nl–Nr significantly smaller than 0 (P = .002). Notably males had changes in connectivity to the components of Broca’s area (Brodmann areas 44, the triangular inferior frontal gyrus, and Brodmann areas 45, the opercular inferior frontal gyrus) in the left hemisphere in 5 of 10 of their deviant connections whereas females had no such connections (figures 1a, 1b and 2, table 2, supplementary table S1; Fisher’s exact test P < .05). Alterations were more widely spread in female patients than in males, including structures on the medial (bilateral superior) and orbital (inferior and superior) frontal and lateral surfaces (the lingual and Heschl’s gyri—both affected bilaterally in first episode female patients; figures 1a, 1b and 2, table 2, supplementary table S1). Notably, the orbital part of inferior frontal gyrus is the only node to be changed in disease in both hemispheres in both males and females at the time of the first episode (supplementary table S1).
Fig. 1.
Sex-specific functional connectivity changes for first-episode (a and b) and chronic (c and d) schizophrenia patients. Meta-analysis is performed combining 3 first-episode datasets (1#, 2#, and 4#) for each sex, and by comparing male patients with matched male controls (a) and comparing female patients with matched female controls (b). Similarly, for chronic schizophrenia. Meta-analysis is performed combining 4 chronic datasets (2#, 3#, 4#, and 5#) for each sex (c for male patients and d for females). The color of the 3 circles (from outside to inside) denotes the 90 different automatic anatomical labeling (AAL) regions (the first circle); the number of increased links (the second circle, deep red means a region has more increased links); the number of decreased links (the third circle, deep blue means a region has more decreased links). Here, the increased/decreased links means the functional connectivity in patients is larger/smaller compared to controls. Broca’s area (inferior frontal gyrus, triangular and opercular part) and Wernicke’s area (superior temporal gyrus) are both marked in the figure.
Table 2.
The Number of Affected Nodes/Connections in First-Episode Patients (a) and in Chronic Patients (b) in Both Hemispheres and for Both Sexes
| Hemisphere | Males | Females | ||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| a. First-episode | ||||
| Total nodes affected | 8 | 4 | 8 | 12 |
| Total connections | 10 | 6 | 11 | 17 |
| Intra-hemispheric | 4 | 0 | 2 | 8 |
| Paired nodes | 3 pairs | 7 pairs | ||
| SFG dorsolateral | ||||
| Precuneus | IFG orbital | |||
| SFG medial | ||||
| IFG opercular | Rectus | |||
| Posterior cingulate | ||||
| IFG triangular | Lingual gyrus | |||
| Heschl gyrus | ||||
| Broca’s area | Left | Right | Left | Right |
| Total nodes | 2 | 1 | 0 | 0 |
| IFG opercular | IFG opercular | |||
| IFG triangular | ||||
| Total connections | 4 | 2 | 0 | 0 |
| b. Chronic | ||||
| Total nodes | 8 | 11 | 6 | 14 |
| Total connections | 15 | 19 | 14 | 21 |
| Intra-hemispheric | 5 | 9 | 3 | 10 |
| Paired nodes | 5 pairs | 5 pairs | ||
| Rolandic operculum | Olfactory cortex | |||
| Postcentral gyrus caudate | Anterior cingulate | |||
| Thalamus | Middle cingulate | |||
| Middle temporal gyrus | Post-central gyrus | |||
| Thalamus | ||||
| Wernicke’s area | Left | Right | Left | Right |
| Total nodes | 1 | 1 | 0 | 0 |
| Total connections | 2 | 1 | 0 | 0 |
Note: The statistical significance of the number of affected nodes: under 60%-sample permutation, for all cases in table 2, Nl and Nr (represent the number of affected nodes in left and right brain hemisphere, respectively) are significantly larger than 0. For first-episode male patients/matched controls, Nl–Nr is significantly larger than 0 (P < .001), ie, left lateralized; for first-episode female patients/matched controls, Nl–Nr is significantly smaller than 0 (P = .002), ie, right lateralized. For chronic male and female patients, Nl–Nr are both significantly smaller than 0 (P = .006 and P < .001, respectively), indicating right lateralization of altered functional connectivity. Total nodes, number of affected structures; total connections, number of connections between nodes; intrahemispheric connections, both affected ends in the same hemisphere; paired nodes, the same node affected in both hemispheres.
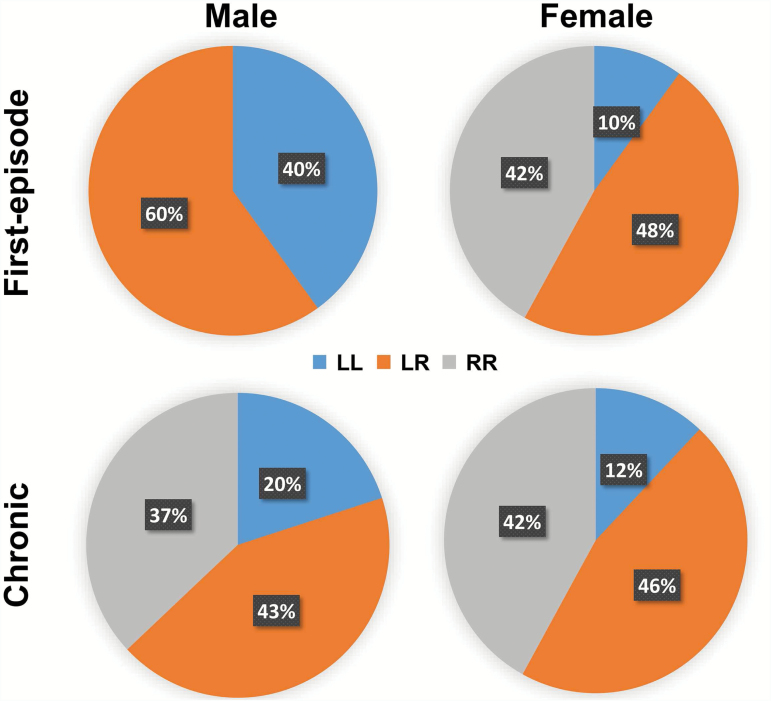
Fig. 2.
Percentage of significantly altered functional connectivity that belong to inter-hemispheric (LR) or intra-hemispheric (left to left: LL; right to right: RR) connections for both sexes in first-episode and chronic schizophrenia patients.
Lateralization of Functional Connectivity Changes in Chronic Schizophrenia
In the chronic stage, functional alterations for patients of both sexes extended posteriorly (figures 1c, 1d and 2, supplementary table S2). Both sexes demonstrate significant change in thalamo-cortical connections, with 5 such connections (bilateral thalamus to bilateral postcentral gyrus and right thalamus to right precentral gyrus; figures 1c, 1d and 2, supplementary table S2) altered in common to patients of both sexes.
As the focus of aberrant connectivity moves posteriorly in both sexes, with the females preceding the males, males who were left-lateralized in the first episode (L: R ratio 8:4 for number of affected nodes) now become right-lateralized (L:R ratio 8:11) in the chronic stage, with 60%-sample permutation test showing Nl–Nr significantly smaller than 0 (P = .006). Female patients still demonstrate a right-laterality in chronic stage, with L: R = 6:14 (60%-sample permutation test showing Nl–Nr significantly smaller than 0 (P < .001).
Figures 1c and d also illustrates a possible sex difference with respect to sensory modality. In males, there is evidence of changed function in the temporal lobe (the superior temporal gyrus on the left and middle gyrus on both sides) in association with the precentral gyrus bilaterally. In females, there is an indication of changed function bilaterally in olfactory pathways in association with the anterior and dorsal cingulate gyrus and the precentral gyrus on both sides.
Sex-Specific Laterality Patterns of Functional Connectivity Change
In first-episode patients, there is a striking difference between the sexes in selectivity of the affected connections by hemisphere. In males, none of the affected connections are confined to the right hemisphere (RR in figure 2). For female patients, half of the connections (9 out of 19) are associated with the right hemisphere, and only 2 out of 19 connections—of the orbital frontal gyrus—are between structures both located in the left hemisphere (LL), figure 2. In the chronic stage, both male and female patients show right laterality in functional connectivity changes, with female patients showing a higher level of right-laterality, figures 1 and 2.
Group by Sex Effect to the Laterality Pattern of Altered Functional Connectivity
To determine if there are group by sex effect to the laterality pattern observed, we performed a group by sex ANOVA analysis for the altered functional connectivity links we identified for both the first-episode and chronic schizophrenia patients. The links with significant sex by group effect (P < .05) were marked in Table S1 (in italic). A reexamination of their laterality patterns for the 4 groups (first-episode male patients, first-episode female patients, chronic male patients, and chronic female patients) still demonstrate the same laterality patterns as we identified previously.
Correlation With Symptom Scores
In first-episode males, functional connectivity between left inferior triangular and right inferior opercular frontal gyri was correlated with delusions (Table S3a). In females, connectivity between the posterior cingulate gyrus and the left inferior orbital frontal gyrus correlated with hostility and poor impulse control (Table S3b). In the chronic state, there were significant clinical correlations involving the thalamus in males (with suspiciousness and anxiety; Table S4a) and in females (including hostility and excitement; Table S4b). At the level of the cortex, the significant connectivity with symptom score was largely with the right hemisphere, but in males with right middle frontal or temporal gyri, whereas in females almost exclusively with right pre- and postcentral gyri.
Some negative findings should be noted. There was little evidence of disturbed connectivity with the insula, amygdale, or hippocampus, subcortical structures in which abnormality in schizophrenia is often invoked. The findings in general relate to functions of the cortex rather than to subcortical structures (apart from the thalamus as noted above).
Discussion
Whatever the origin of the deviation in connectivity in psychosis the factor of sex must be taken into account. With the same diagnosis, females in the first episode had more widespread functional connectivity changes than males and these were more likely to be in the right hemisphere. In males, the changes were less numerous, predominantly left-sided and targeted to Broca’s area. In transition to the chronic condition, the focus of pathology in both sexes shifted from the anterior to the posterior half of the brain, but in males from the left to the right hemisphere, with the focus of aberrant connection moving toward the females from whom they were earlier largely separated by the mid-line. Of particular note, the males who in the first episode had connectivity changes strongly lateralized to the left hemisphere (Left: Right 8:4) in the chronic state had changes lateralized to the right (Left: Right 8:11). The deficit can be characterized as a decrease or loss of lateralization. Symptoms follow the hemispheric pattern of aberrant connectivity. In the first episode, significant change in functional connectivity at the cortical level was predominantly in the left hemisphere particularly in females, see Table S3. In the chronic state such correlations were all but one located in the right hemisphere and associated, respectively, with the mid-temporal and mid-frontal gyri in males and pre- and postcentral gyri in females, Table S4.
Sex Difference in Laterality of Functional Connectivity Changes in Schizophrenia
“Given that schizophrenic illnesses are generally associated with earlier onset and worse outcome in males the more widespread distribution of aberrant connectivity in females requires explanation. One possibility is that (1) cortical complexity goes on developing in an antero-posterior direction into the third and fourth decades of life; (2) a sex difference (with some anterior components greater in males and posterior components greater in females) reflects earlier, faster lateralization in females; who avoid aberrant connections in the frontal lobes that males, developing more slowly, encounter at a younger age with subsequent greater risk of negative symptoms, eg, poverty of speech.”31
Whereas language is often regarded as a left hemisphere function a body of evidence32 is consistent with the concept that it involves both hemispheres. Separate components are located in either hemisphere,25,33 aligned along the antero-posterior sensory-motor axis to confer a 4 quadrant structure on the human brain.34 Phonological components, both motor and sensory and grammar are located in the left hemisphere and semantic components to a greater extent in the right. Language deficits in schizophrenia are more severe in males than females35 and increase in severity from phonology to semantics to grammar (syntax). In contrast, in ill females grammar is least affected.
Sex differences in brain structure have been little studied in psychosis. Only one of 11 meta-analyses36 of magnetic resonance imaging voxel-based morphometry (VBM) took sex systematically into account but this study did so with singular effect. In schizophrenia and bipolar illness, Bora et al37 found that when all studies were included without respect to sex (ie, with the expected excess of early onsets of schizophrenia in males included) there was loss of substance in the insula and anterior cingulate gyrus on both sides together with loss in the region of the hippocampus and para-hippocampal gyrus on the left side. When studies were selected for equality of the sex ratio the pattern of findings was notably different. In schizophrenia, the losses were in the insula and lateral sulcus on the left side and the anterior cingulate gyrus on the right. In bipolar disorder, the losses were in the insula/lateral sulcus on the right and the anterior cingulate on the left. Thus with respect to structure as well as function schizophrenia and bipolar disorder are related to each other on a continuum of laterality, and the continuum interacts with sex.
The Role of Cerebral Dominance in Language and Schizophrenia
Where and what is the cerebral dominance gene? On the basis of genome-wide association studies (GWAS), it is claimed that predisposition to psychosis is highly polygenic and that the genes are predominantly autosomal.38 The present findings indicate that an interaction between sex and laterality of major effect has yet to be accounted for. Following Broca, it has been argued that a gene for cerebral dominance was pivotal to the evolution of Homo sapiens39,40 but the search for a genetic correlate of handedness41–43 or schizophrenia41,44,45 by linkage has yielded inconsistent findings, as also have association studies of handedness.46 The difficulty is exemplified by a micro-array study in which the expression of 3000 genes was assessed at 11 pairs of corresponding sites in the 2 hemispheres in 15 pre- and postnatal epochs of development.47 No consistent asymmetry of expression was observed for any gene at any site or developmental stage.
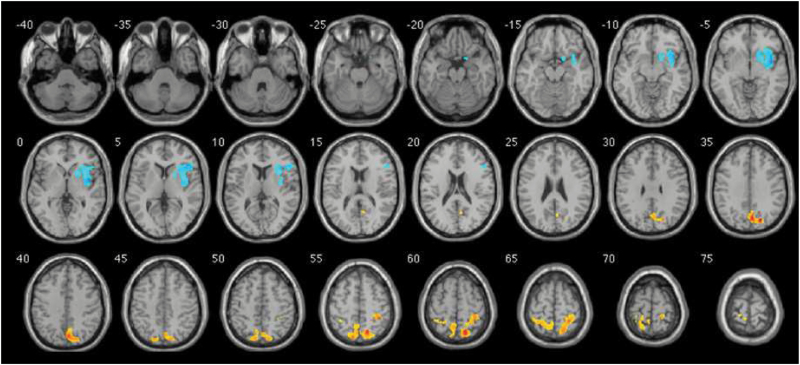
The answer probably lies in observations on sex chromosome aneuploidies.48 Turner’s syndrome individuals with only one X (XO) have spatial deficits (right hemisphere’s deficit), while Klinefelter’s syndrome (XXY) and XXX individuals with an extra X, and XYY individuals, with an extra Y each have language problems (left hemisphere). Thus, it is possible that loss of a sex chromosome (X or Y) impairs the development of the right hemisphere, whereas the presence of an extra X or Y impairs left hemisphere. The hypothesis was proposed that cerebral dominance is carried by a gene pair with homology between X and Y chromosomes.49 In an MRI comparison, frontal lobe deficits in gray matter were found in the XYY syndrome individuals exclusively on the right side (figure 3),50 suggesting that a gene with an influence on anatomical asymmetry is located on the Y chromosome.
Fig. 3.
Distribution of gray matter deficits (blue) in XYY individuals, from Legrange et al,50 with kind permission from the authors. Orange indicates gain of tissue.
These findings direct attention to the Y and its interaction with the X chromosomes in cerebral dominance and also, our observations indicate, in schizophrenia. Whereas the gene-pair in pseudo-autosomal region 1 (PAR1—within which recombination between X and Y sequences maintains strict sequence homology) is shown to be relevant51–53 such a gene would not explain sex differences. An XY homologous gene-pair with the Y homologue within the nonrecombining region49,54 can explain such a difference (as in the absence of recombination the X and Y gene sequences will diverge over evolutionary time) and may explain the genetic predisposition to psychosis.55,56 Finally, it is likely that a solution to both problems lies in the origin of the species57,58: a “species identifier” is created from the pattern of genes on the X chromosome that is protected from inactivation in male meiosis and thus is active on the inactive X in females. This pattern is being formed by the combination of X and Y chromosome changes specified above, and stabilized in the XY body in male meiosis by the speciation gene PRDM9.59 This gene has the capacity to change the motif that localizes sites of recombination across the genome. The theory incorporates the notion of a saltation (a jump or discontinuity) between successive species,56 and depends on the generation of a single sexual dimorphism as the distinctive core of each species.
Strengths and Weaknesses
Some strengths and shortcomings have to be borne in mind. The strength is that we have used the largest dataset yet to report sex-specific functional changes in schizophrenia, and a meta-analytic approach that integrates results from multiple sites. Traditional validation tests whether an observation survives a type I error in multiple datasets. Meta-analysis is more appropriate here as it assesses the effect-size distribution rather than individual observations across arbitrary thresholds. A limitation, however, is that while most first-episode patients are drug-naïve and the result from first-episode populations is minimally confounded by medication, chronic patients are mostly medicated at the time of assessment. We assessed the possible influence of medication by examining medication dose of patients and altered functional connectivity strength differences between medicated and drug-naive patients. For first-episode patients (in dataset 2#), we find no functional connection showing significant correlation with medication dosage. For the chronic stage schizophrenia (dataset 3#), only one connection shows a weak correlation (P = 0.0506, uncorrected) between medication dose of female patients and functional connectivity strength, see Table S5, indicating the influence of medication is limited. It is also beneficial to adopt an individual-based parcellation scheme60,61 for our analysis, considering the individual variability.60 Finally, our hypothesis of the underlying genetic mechanism related to the X and Y chromosomes needs to be scrutinized with further genetic data, which remains to be our future work.
Funding
J.F. is a Royal Society Wolfson Research Merit Award holder, partially supported by the National High Technology Research and Development Program of China (no. 2015AA020507) and the Key Project of Shanghai Science & Technology Innovation Plan (no. 15JC1400101), the National Centre for Mathematics and Interdisciplinary Sciences (NCMIS) of the Chinese Academy of Sciences, and the Key Program of the National Natural Science Foundation of China (no. 91230201). J.Z. is supported by the National Science Foundation of China (NSFC 61573107) and special Funds for Major State Basic Research Projects of China (2015CB856003). T.L. is partly supported by the National Nature Science Foundation of China Key Project (81630030 and 81771446); the National Natural Science Foundation of China/Research Grants Council of Hong Kong Joint Research Scheme (81461168029); the National Key Research and Development Program of the Ministry of Science and Technology of China (2016YFC0904300); 1.3.5 Project for Disciplines of Excellence, West China Hospital of Sichuan University (ZY2016103 and ZY2016203). Q.W. is supported by the National Science Foundation of China (NSFC 81771446). J.W. acknowledges support from Shanghai Science and Technology Committee (14411961400). This work is also supported by the Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, PR China. We are grateful to Department of Psychiatry, National Taiwan University Hospital for sharing with us their data which was collected under the auspices of Taiwan Ministry of Science and Technology, grant number: NSC99-3112-B-002-030.
Supplementary Material
References
- 1. Broca P. Sur le siège de la faculté du langage articulé. Vol Bull. Soc. anat. de Paris. 6 V. Masson; 1865. [Google Scholar]
- 2. Dax M. Lésions de la moitié gauche de l’encéphale coïncident avec l’oublie des signes de la pensée: lu au Congrès méridional tenu à Montpellier en 1836, par le docteur Marc Dax. Gazette hebdomadaire de médecine et de chirurgie 1865;17:259–260. [Google Scholar]
- 3. Harrington A. Medicine, Mind and the Double Brain. Princeton: Princeton University Press; 1989. [Google Scholar]
- 4. Broca P. Rapport sur un memoire de M. Armand de Fleury intitulee: de l’inegalite dynamique des deux hemispheres cerebraux. Bull Acad Med. 1877;6:508. [Google Scholar]
- 5. Crichton-Browne J. On the weight of the brain and its component parts in the insane. Brain. 1879;2:42–67. [Google Scholar]
- 6. Jablensky A, Sartorius N, Ernberg G, et al. . Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. [DOI] [PubMed] [Google Scholar]
- 7. Essen‐Möller E. Mating and fertility patterns in families with schizophrenia. Eugen Q. 1959;6:142–147. [Google Scholar]
- 8. Gould JLG, Gould CGJL, Gould CG.. Sexual Selection. No. 591.56 G6. 1989. [Google Scholar]
- 9. Häfner H, Maurer K, Löffler W, Riecher-Rössler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. [DOI] [PubMed] [Google Scholar]
- 10. Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. [DOI] [PubMed] [Google Scholar]
- 11. Shaywitz BA, Shaywitz SE, Pugh KR, et al. . Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. [DOI] [PubMed] [Google Scholar]
- 12. Wang D, Buckner RL, Liu H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol. 2013;109:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dollfus S, Razafimandimby A, Delamillieure P, et al. . Atypical hemispheric specialization for language in right-handed schizophrenia patients. Biol Psychiatry. 2005;57:1020–1028. [DOI] [PubMed] [Google Scholar]
- 15. Slewa-Younan S, Gordon E, Harris AW, et al. . Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2004;161:1595–1602. [DOI] [PubMed] [Google Scholar]
- 16. Mueller S, Wang D, Pan R, Holt DJ, Liu H. Abnormalities in hemispheric specialization of caudate nucleus connectivity in schizophrenia. JAMA Psychiatry. 2015;72:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada N, Fukunaga M, Yamashita F, et al. . Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 2014;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di X, Kim EH, Chen P, Biswal BB. Lateralized resting-state functional connectivity in the task-positive and task-negative networks. Brain Connect. 2014;4:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li T, Wang Q, Zhang J, et al. . Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng W, Palaniyappan L, Li M, et al. . Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo S, Kendrick KM, Yu R, Wang HL, Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp. 2014;35:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer AR, Ruhl D, Merideth F, et al. . Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp. 2013;34:2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo S, Kendrick KM, Zhang J, et al. . Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. Neuroimage Clin. 2013;2:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 28. Hwang D, Rust AG, Ramsey S, et al. . A data integration methodology for systems biology. Proc Natl Acad Sci USA. 2005;102:17296–17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glahn DC, Laird AR, Ellison-Wright I, et al. . Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savadjiev P, Whitford TJ, Hough ME, et al. . Sexually dimorphic white matter geometry abnormalities in adolescent onset schizophrenia. Cereb Cortex. 2014;24:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beeman M, Chiarello C.. Right hemisphere language comprehension: perspectives from cognitive neuroscience. London: Psychology Press; 2013. [Google Scholar]
- 33. Crow TJ. The Speciation of Modern Homo sapiens. Vol 106 Oxford: Oxford University Press; 2004. [Google Scholar]
- 34. Crow TJ. The nuclear symptoms of schizophrenia reveal the four quadrant structure of language and its deictic frame. J Neurolinguist. 2010;23:1–9. [Google Scholar]
- 35. Walder DJ, Seidman LJ, Cullen N, Su J, Tsuang MT, Goldstein JM. Sex differences in language dysfunction in schizophrenia. Am J Psychiatry. 2006;163:470–477. [DOI] [PubMed] [Google Scholar]
- 36. Crow TJ, Chance SA, Priddle TH, Radua J, James AC. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta-analyses of structural MRI. Psychiatry Res. 2013;210:1232–1244. [DOI] [PubMed] [Google Scholar]
- 37. Bora E, Fornito A, Yücel M, Pantelis C. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychol Med. 2012;42:295–307. [DOI] [PubMed] [Google Scholar]
- 38.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Annett M. In defence of the right shift theory. Percept Mot Skills. 1996;82:115–137. [DOI] [PubMed] [Google Scholar]
- 40. Crow TJ. Sexual Selection, Timing and the Descent of Man: A Theory of the Genetic Origins of Language. Cahiers de Psychologie Cognitive/Current Psychology of Cognition; 1998. [Google Scholar]
- 41. Laval SH, Dann JC, Butler RJ, et al. . Evidence for linkage to psychosis and cerebral asymmetry (relative hand skill) on the X chromosome. Am J Med Genet. 1998;81:420–427. [DOI] [PubMed] [Google Scholar]
- 42. Francks C, Fisher SE, MacPhie IL, et al. . A genomewide linkage screen for relative hand skill in sibling pairs. Am J Hum Genet. 2002;70:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Agtmael T, Forrest SM, Del-Favero J, Van Broeckhoven C, Williamson R. Parametric and nonparametric genome scan analyses for human handedness. Eur J Hum Genet. 2003;11:779–783. [DOI] [PubMed] [Google Scholar]
- 44. Crow TJ. How and why genetic linkage has not solved the problem of psychosis: review and hypothesis. Am J Psychiatry. 2007;164:13–21. [DOI] [PubMed] [Google Scholar]
- 45. DeLisi LE, Shaw S, Sherrington R, et al. . Failure to establish linkage on the X chromosome in 301 families with schizophrenia or schizoaffective disorder. Am J Med Genet. 2000;96:335–341. [DOI] [PubMed] [Google Scholar]
- 46. McManus IC, Davison A, Armour JA. Multilocus genetic models of handedness closely resemble single-locus models in explaining family data and are compatible with genome-wide association studies. Ann N Y Acad Sci. 2013;1288:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pletikos M, Sousa AM, Sedmak G, et al. . Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Orig Artic Ser. 1986;22:293–306. [PubMed] [Google Scholar]
- 49. Crow T. The case for an XY homologous determinant of cerebral asymmetry. Paper presented at: Cytogenetics and Cell Genetics; Switzerland: Karger, 1994;67:393–394. [Google Scholar]
- 50. Lepage JF, Hong DS, Raman M, et al. . Brain morphology in children with 47, XYY syndrome: a voxel- and surface-based morphometric study. Genes Brain Behav. 2014;13:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crow TJ, Ball J, Bloom SR, et al. . Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46:1145–1150. [DOI] [PubMed] [Google Scholar]
- 52. Crow TJ. A re-evaluation of the viral hypothesis: is psychosis the result of reztroviral integration at a site close to the cerebral dominance gene?Br J Psychiatry. 1984;145: 243–253. [DOI] [PubMed] [Google Scholar]
- 53. Crow TJ. Sex chromosomes and psychosis. The case for a pseudoautosomal locus. Br J Psychiatry. 1988;153:675–683. [DOI] [PubMed] [Google Scholar]
- 54. Crow TJ. Sexual selection, Machiavellian intelligence, and the origins of psychosis. Lancet. 1993;342:594–598. [DOI] [PubMed] [Google Scholar]
- 55. Turner JM. Meiotic sex chromosome inactivation. Development. 2007;134:1823–1831. [DOI] [PubMed] [Google Scholar]
- 56. Crow TJ. Schizophrenia as variation in the sapiens-specific epigenetic instruction to the embryo. Clin Genet. 2012;81:319–324. [DOI] [PubMed] [Google Scholar]
- 57. Crow TJ. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31:118–129. [DOI] [PubMed] [Google Scholar]
- 58. Crow TJ. Is schizophrenia the price that Homo sapiens pays for language?Schizophr Res. 1997;28:127–141. [DOI] [PubMed] [Google Scholar]
- 59. Myers S, Bowden R, Tumian A, et al. . Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science. 2010;327:876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang D, Buckner RL, Fox MD, et al. . Parcellating cortical functional networks in individuals. Nat Neurosci. 2015;18:1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mueller S, Wang D, Fox MD, et al. . Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.