Introduction
Drug-induced lupus erythematosus (DILE) is a lupus-like syndrome temporally related to continuous drug exposure. DILE can be divided into systemic lupus erythematosus (SLE), subacute cutaneous lupus erythematosus (SCLE) and chronic cutaneous lupus.1 Hydrochlorothiazide was the first drug associated with SCLE in 1985,2 but at least 100 other agents have since been reported to induce/exacerbate SCLE, with terbinafine, tumor necrosis factor (TNF)-α inhibitors, antiepileptics, and proton pump inhibitors, the most frequently associated medications. We present a case of ustekinumab-induced SCLE in a patient being treated for psoriasis.
Case report
A 68-year-old woman with a history of chronic plaque psoriasis was previously treated with narrow-band ultraviolet B, acitretin, and methotrexate. Because of loss of efficacy with previous treatments, she was started on ustekinumab. After her second dose at week 4, generalized itch with a mildly erythematous scaly rash developed, which progressed to florid erythematous plaques on her trunk, limbs, and face over the next 2 weeks. This was associated with lethargy and generalized aches. She had not been started on any other medications.
On examination, there were large annular scaly plaques on her trunk and limbs (Figs 1 and 2). A clinical diagnosis of SCLE was made. Routine blood tests were unremarkable apart from a chronically raised γ-glutamyl transferase level, at 76 U/L (range, 9-48 U/L). Anti-nuclear antibody (ANA) was positive at 1:200 with a speckled pattern. She also had a positive Anti-SSA/Ro, Anti-SSB/La and Anti-Jo1 antibodies. These were negative before starting ustekinumab. Two punch biopsies were performed with histology showing skin with mild hyperkeratosis, a normal granular layer, and focal interface change with necrotic keratinocytes (Fig 3). These findings were consistent with lupus erythematosus. A diagnosis of ustekinumab-induced SCLE was made.
Fig 1.
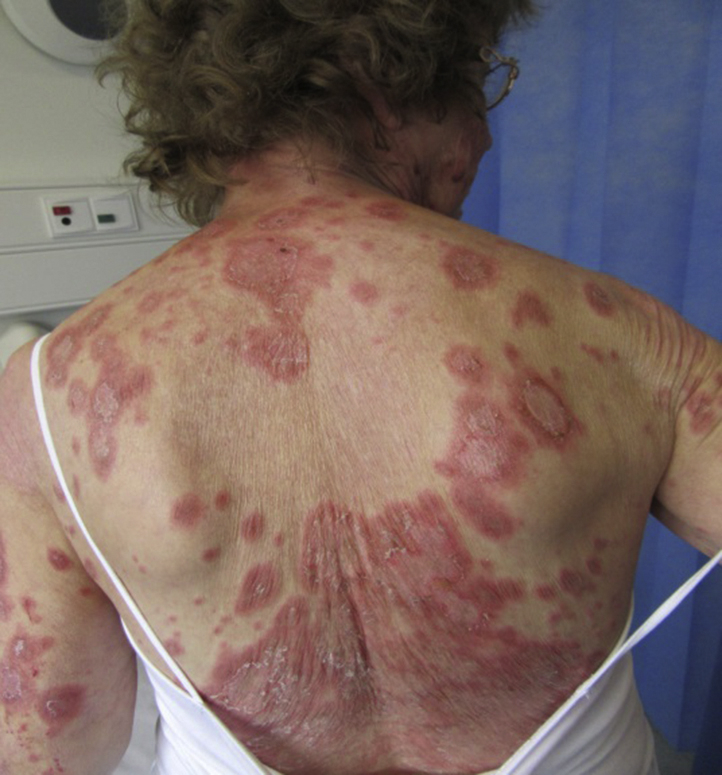
SCLE rash on patient's back after second dose of ustekinumab.
Fig 2.
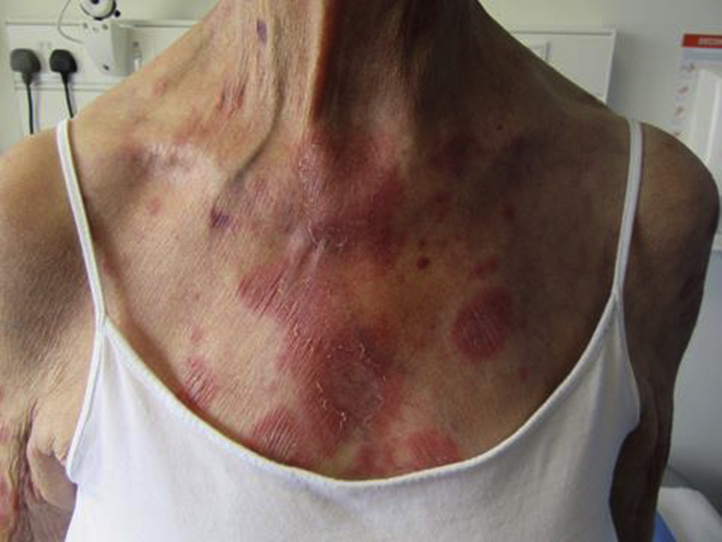
SCLE rash on patient's chest after second dose of ustekinumab.
Fig 3.
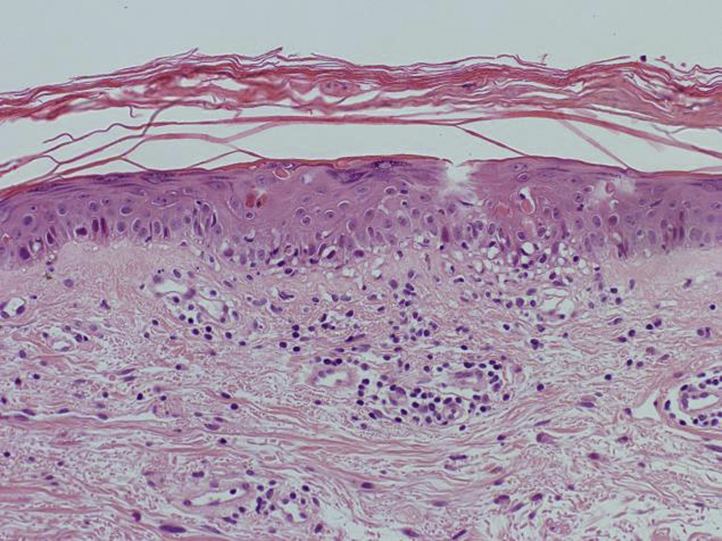
Histology (H&E) of skin biopsy shows features of lupus erythematosus: mild hyperkeratosis, a normal granular layer, and focal interface change with necrotic keratinocytes. (Hematoxylin-eosin stain.)
Her ustekinumab was discontinued and she was prescribed betamethasone valerate ointment, a tapering course of oral corticosteroids, and hydroxychloroquine. She was cautioned about the possibility of hydroxychloroquine exacerbating her psoriasis. Her lupus started to clear within 8 to 10 weeks (Fig 4), but unfortunately, her psoriasis has begun to flare. She is reluctant to start another biologic agent as she is fearful that she may develop a further adverse drug effect, despite our efforts to reassure her otherwise.
Fig 4.
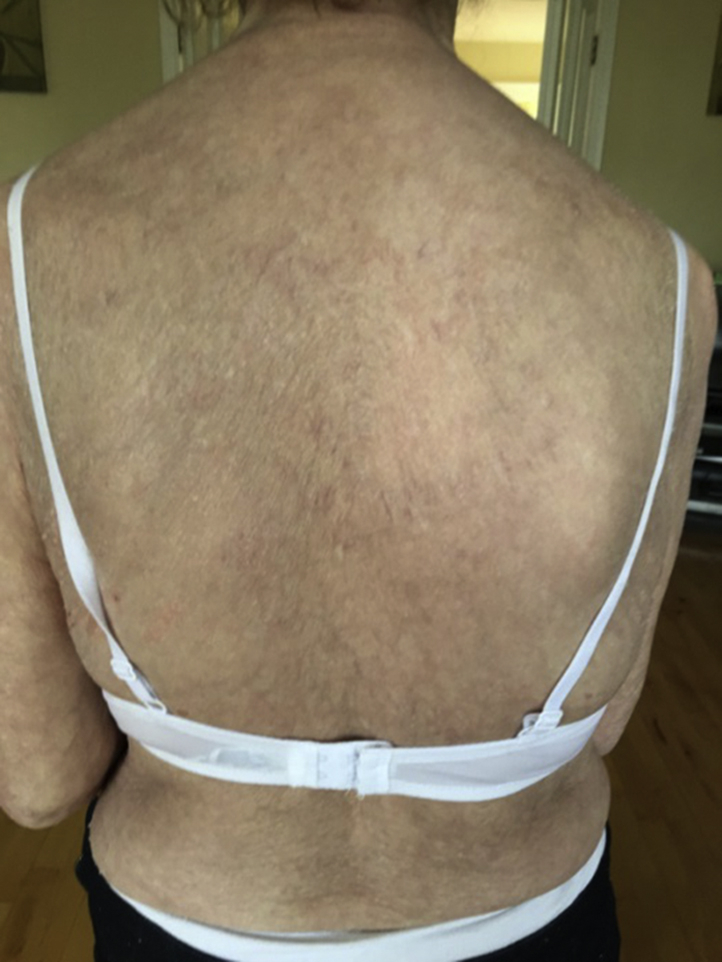
Resolution of subacute cutaneous lupus erythematosus rash 10 weeks after discontinuing ustekinumab.
Discussion
Drug-induced SCLE is a nonscarring, photosensitive dermatosis and the most common form of DILE1 accounting for 20% of all SCLE cases.2 Most patients affected by drug-induced SCLE are female (72%), with a mean age of 58.0 years.1 The duration between drug exposure and onset of skin lesions can range from 3 days to 10 years, and the resolution time after withdrawal of medication ranges between 1 week and 1 year.3
Drug-induced SCLE presents clinically, histopathologically, and immunologically in a manner similar to that of idiopathic SCLE.4 The lesions of SCLE start as erythematosus papules/plaques progressing to widespread annular, polycyclic lesions with central clearing or papulosquamous lesions. SCLE is strongly associated with the anti-Ro/SSA antibody in 70% of cases.5 Sixty percent to 80% display positive ANA and 30% to 50% display the anti-La/SSB antibody, which is almost always seen together with anti-Ro/SSA.5 Considering these antibodies are also associated with Sjögren syndrome, some patients may have features of both conditions.2 It is reported that half of SCLE patients fulfill the 1997 American College of Rheumatology criteria for SLE, with arthritis and arthralgia being the most common symptoms. Severe systemic disease is rare.5
Although biologic agents used in the treatment of psoriasis, such as TNF-α inhibitors, are known to induce a lupus-like syndrome,6 there are also reports of abatacept3 and secukinumab7 inducing SCLE. A thorough literature search did not yield any reports to date of an ustekinumab-induced SCLE.
The pathogenesis of drug-induced lupus is poorly understood, and there are many proposed mechanisms. Idiopathic SCLE is thought to relate to Ro/SSA autoantibody–dependent cell-mediated keratinocyte cytotoxicity.2 Therefore, we propose, that in the case of ustekinumab, a monoclonal antibody to the p40 subunit of interleukin-12 and interleukin-23, it is possible that by inhibiting the function of both of these cytokines, T-cell differentiation is diverted down the alternative pathway of T helper cell 22 production, via interleukin-6, causing increased production of TNF-α, a pro-inflammatory cytokine implicated in the pathogenesis of numerous inflammatory and autoimmune diseases. In fact, immunohistochemical studies in skin biopsies of SCLE patients found that lesional skin tissue displayed a strongly positive distribution of TNF-α, mostly epidermal, whereas no prominent staining was seen in nonlesional skin from the same group or the control group.8 TNF-α is capable of inducing translocation of Ro/SSA and La/SSB autoantigens to the surface of keratinocytes, which then leads to deposition of immunoglobulins and complement at the dermoepidermal junction, causing tissue injury characteristic of SCLE.8
Despite the updated American College of Rheumatology/European League Against Rheumatism 2018 diagnostic guidelines for SLE, criteria for the diagnosis of drug-induced lupus, and more specifically drug-induced SCLE are lacking. However, the consensus is that a diagnosis of drug-induced SCLE can be suspected upon characteristic clinical features combined with a relevant drug history, supported by positive histopathology and anti-Ro/SSA antibodies.5 The mainstay of treatment of DILE is drug discontinuation along with sun protection factor, topical or intralesional corticosteroids, and systemic antimalarials. However, in our patient, the benefit of starting hydroxychloroquine had to be weighed against the risk of it flaring her psoriasis, especially considering she had decided to avoid biologic therapy for the time being and her disease was refractory to 2 prior oral systemic agents.
Given that ustekinumab is one of the first-line biologic agents recommended in the treatment of adult psoriasis, prescribers need to be mindful of this potential adverse drug effect. In addition, we suggest considering testing for ANA before initiation of biologic therapy, particularly in the case of TNF-α inhibitors, which can potentially induce a lupus-like syndrome.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Vedove D.C., Simon J.C., Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents. J Dtsch Dermatol Ges. 2012;10:889–897. doi: 10.1111/j.1610-0387.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe C.G., Henderson C.L., Grau R.H. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465–472. doi: 10.1111/j.1365-2133.2010.10110.x. [DOI] [PubMed] [Google Scholar]
- 3.Tarazi M., Aiempanakit K., Werth V.P. Subacute cutaneous lupus erythematosus and systemic lupus erythematosus associated with abatacept. JAAD Case Rep. 2018;4:698–700. doi: 10.1016/j.jdcr.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grönhagen C.M., Fored C.M., Linder M. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167:296–305. doi: 10.1111/j.1365-2133.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 5.Grönhagen C.M., Nyberg F. Cutaneous lupus erythematosus: an update. Indian Dermatol Online J. 2014;5:7–13. doi: 10.4103/2229-5178.126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunasso A.M., Aberer W., Massone C. Subacute lupus erythematosus during treatment with golimumab for seronegative rheumatoid arthritis. Lupus. 2014;23:201–203. doi: 10.1177/0961203313517153. [DOI] [PubMed] [Google Scholar]
- 7.Wehrmann C., Sondermann W., Körber A. Secukinumab-induced subacute cutaneous lupus erythematosus. Hautarzt. 2018;69:64–66. doi: 10.1007/s00105-017-4071-8. [DOI] [PubMed] [Google Scholar]
- 8.Sandholdt L.H., Laurinaviciene R., Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2014;170:342–351. doi: 10.1111/bjd.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]


