Introduction
Although it is unquestionable that monoclonal gammopathy causes occlusive nonvasculitic vasculopathy,1 it is not clear yet, if it can cause immunoglobulin-mediated leukocytoclastic vasculitis (LcV) and how vigorously both conditions should be treated in monoclonal gammopathy of unclear significance (MGUS).
In the recent nomenclature of cutaneous vasculitides (addendum to the CHCC2012),2 vasculitis in MGUS is mentioned as a possible, so-far unproven entity. Previous case reports did not sufficiently or explicitly distinguish it from occlusive vasculopathy. Adding to this dilemma is a lack of consensus with oncologists if or when chemotherapies are justified in MGUS with a paraprotein-associated disease.
We provide evidence by means of 2 cases of MGUS that monoclonal gammopathy can cause primary immunoglobulin-mediated LcV and that its oncologic treatment abolishes it.
Case reports
Case 1
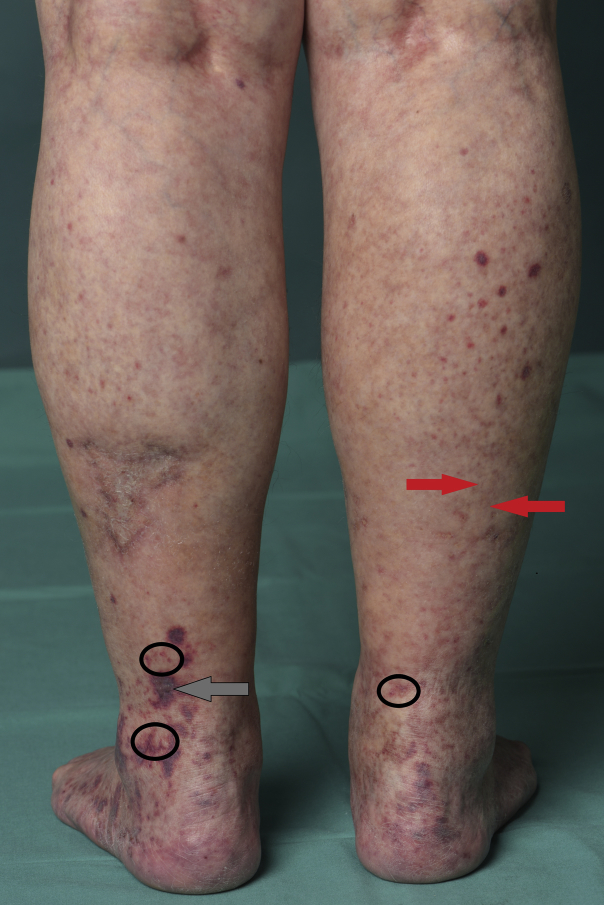
A 75-year-old woman presented with maculopapular purpura and erythematous (inflammatory) retiform purpura3 on her lower legs (Fig 1, black circles). She reported recurrences almost every other week for 4 years, unless she treated them with prednisolone (at least 12.5 mg). On examination we also observed areas with blanchable livedo on her lower legs, thighs, and forearms (Fig 1, red arrow) and purpura with little erythema (grey arrow).
Fig 1.
Lower legs with palpable and macular purpura and erythematous retiform purpura (black circles) (typical for IgA or IgG/IgM vasculitis, especially when on dependent extremities), purpura with only little erythema (grey arrow), and areas with blanchable livedo racemosa (red arrow) (both compatible with occlusive vasculopathy and/or with other forms of vasculitis).
Ten years earlier, she was successfully treated for nodal marginal zone lymphoma (CHOP regimen [cyclophosphamide, doxorubicin (Adriamycin), vincristine (Oncovin), prednisolone] and radiation) and had uneventful follow-up examinations until 2 years ago.
Because of the palpable and erythematous retiform purpura, one could suspect immune complex vasculitis in terms of IgA or IgG/IgM vasculitis.2 Because of the livedo reticularis one could suspect also anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, cryoglobulinemic, or rheumatoid vasculitis.3 Because of livedo and perhaps some lesions with only slightly erythematous purpura, one could suspect occlusive vasculopathy. Workup yielded no signs of systemic vasculitis (no dysmorphic erythrocytes, negative hemoccult). Physical examination and electroneurography found a motor axonal polyneuropathy (PNP). ANCA, antinuclear antibodies, and rheumatoid factor were negative, but we detected monoclonal gammopathy (IgM type λ) in conjunction with cryoglobulinemia type 1 (both had been absent at times of marginal zone lymphoma and follow-up examinations). We initiated an oncologic workup, which included bone marrow biopsy and led to a diagnosis of MGUS with no signs of plasmocytoma or recurring lymphoma.
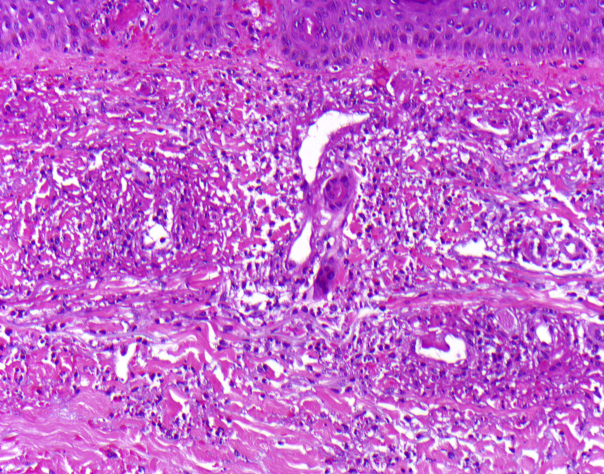
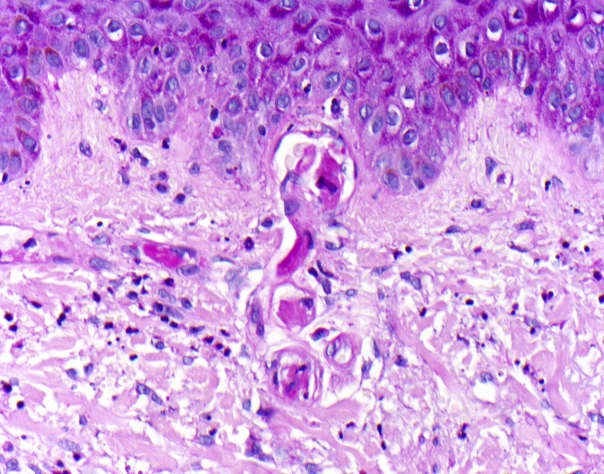
A biopsy of a purpuric papule surrounded by livedo found 2 histologic diagnoses: (1) neutrophilic infiltrates around postcapillary venules with leukocytoclasia, fibrinoid degeneration of vessel walls, and erythrocyte extravasation, but no thrombosed vessels, thus, unequivocally, leukocytoclastic vasculitis (Fig 2) and (2) at the periphery, periodic acid–Schiff (PAS)-positive thrombi in the capillaries of the papillary dermis, indicative of thrombosing vasculopathy (Fig 3).
Fig 2.
Center of biopsy (case 1) with typical histologic signs of leukocytoclastic vasculitis. (Hematoxylin-eosin stain; original magnification: ×200.)
Fig 3.
In the periphery signs of thrombosing vasculopathy with PAS-positive thrombi in the capillaries of the papillary body PAS staining. (Original magnification: ×400.)
Immunofluorescence found IgG, IgM and C3 at the vessel walls, but no IgA. We therefore diagnosed (1) IgG/IgM-positive vasculitis and (2) occluding vasculopathy, both caused by IgM gammopathy/type I cryoglobulinemia in MGUS.
Because of the incapacitating symptoms, we started treatment with rituximab and bendamustine every 4 weeks. After 7 months gammopathy/cryoglobulinemia, occluding vasculopathy, and cutaneous vasculitis had disappeared, and PNP had improved clinically (in terms of reduced tingling sensation and improved sensitivity). The patient has undergone follow-up for 2.5 years.
Case 2
A 50-year-old man presented with solitary hemorrhagic papules and maculae (maculopapular purpura) on the lower legs, forearms, and trunk and generalized livedo reticularis. Furthermore, a symmetrical sensory axonal PNP was diagnosed clinically and by electroneuronography. Urine analysis and hemoccult were normal, cryoglobulins, ANCA, antinuclear antibodies, or rheumatoid factor were absent, but there was κ light chain IgA paraproteinemia. Bone marrow biopsy was compatible with MGUS. Punch biopsy from a fresh purpuric papule (surrounded by livedo) found leukocytoclastic vasculitis without intravascular thrombi and vascular IgA deposits (as there was no histologic correlate to the surrounding livedo, the latter may be caused by unphysiologic vasodilation or higher blood viscosity).
Because in cases of an immunoglobulin-mediated vasculitis temporally defined lesions can be elicited by injecting histamine intracutaneously in clinically uninvolved skin,4, 5 we analyzed punch biopsies taken 1 hour after injection of histamine. They showed signs of an early LcV and vascular deposition of C3 (an indication for immune complex–mediated activation of complement), but no occlusive vasculopathy. A second histamine wheal was left for clinical observation and revealed, after 6 hours, a vasculitic purpuric papule. So histamine induced histologically and clinically LcV in this patient with monoclonal gammopathy, similarly as in patients with IgA- or with other forms of IgG/IgM-vasculitis.5, 6 Yet, the clinical picture was not compatible with skin-limited or systemic cutaneous IgA vasculitis,2 because there was an absence of predilection for lower legs and a relative paucity of palpable purpura.
Therefore, we made a diagnosis of immune-complex vasculitis associated with monoclonal IgA gammopathy in addition to a diagnosis of generalized livedo (and PNP), also in conjunction with monoclonal IgA gammopathy.
Because of PNP and relapsing LcV, chemotherapy with lenalidomide was initiated. It led to normalization of the light chains, reduction of livedo, complete and enduring disappearance of palpable purpura, and stabilization of PNP in terms of halted progress with less burning or tingling sensation.
Discussion
These 2 cases demonstrate that paraproteinemia can cause small vessel vasculitis because:
-
1.
Biopsies from early purpuric maculopapules of both patients presented with typical histologic signs of small vessel vasculitis and without thrombosis of the vessel lumen. Additionally, histologic signs of occlusive vasculopathy were seen in case 1, albeit only at the periphery of the zones with LcV. This is important because type I cryoglobulinemia usually does not form immune complexes (in contrast to mixed cryoglobulinemia) but rather causes disturbed circulation because of high viscosity or cold-induced, thrombosing vasculopathy. Gammopathy causes similar disturbances but without aggravation by cold.
-
2.
The class of the deposited immunoglobulins corresponded with the class of paraprotein (IgM in case 1, IgA in case 2).
-
3.
Because late stages of occlusive vasculopathy may include infiltrates in vessel walls, and later stages of vasculitis may entail intravascular thrombosis, it is not always possible to clearly distinguish both diseases from each other histologically. Therefore, we not only biopsied morphologically early vasculitic lesions, such as partially blanchable, purpuric maculopapules, but also carefully looked for signs of vasculopathy in areas of LcV and provided evidence for an immunoglobulin-mediated vasculitis in case 2 when we demonstrated its inducibility by injection of histamine.
-
4.
Clinically, both patients showed the typical clinical signs of immune-mediated small vessel vasculitis, that is, palpable round and erythematous (inflammatory) retiform purpura, albeit without predilection for lower legs in case 2. On the other hand, they also showed signs of an occluding vasculopathy in case 1, that is, livedo and partially only slightly erythematous retiform purpura. It is known from type II cryoglobulinemia that a vasculitic reaction may well elicit or aggravate an occluding (cryoglobulinemic) vasculopathy and vice versa.
-
5.
Oncologic treatments lead to disappearance of both paraproteinemia and vasculitis.
Previous reports on occurrence of LcV in MGUS6, 7, 8 did not sufficiently differentiate LCV from vasculopathy, especially because occluding vasculopathy may entail an inflammation that resembles LcV. Yet, we think that our careful analysis indicates elicitation of a primary LcV in these, admittedly rare, cases. In contrast to immune complexes, monoclonal immunoglobulins per se do not attract and bind neutrophils. Yet, it is conceivable that under certain circumstances they bind to epitopes on serum proteins, thus forming precipitable immune complexes or to epitopes on vessel walls (which could explain a lack of predilection for the dependent, lower legs, otherwise a typical sign for IgA vasculitis).
We conclude that monoclonal gammopathy induces immunoglobulin-mediated LcV, which differs from cutaneous IgA vasculitis and other immune-mediated LcV.2, 3
Because of the hitherto unacknowledged existence of gammopathy-related vasculitis, there are no reports on its therapy, only on type I cryoglobulinemia.8, 9, 10 Although MGUS is usually not treated, but followed,4 we show that oncologic therapy is justified and can be efficacious when patients suffer from vasculopathy or vasculitis, similarly as in PNP or glomerulonephritis.
Footnotes
Funding sources: This work was partially supported by a grant of the EADV project 2014-028 on nomenclature and diagnostic criteria of cutaneous vasculitis.
Conflicts of interest: None disclosed.
References
- 1.Llamas-Velasco M., Alegría V., Santos-Briz Á. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637–662. doi: 10.1097/DAD.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 2.Sunderkotter C.H., Zelger B., Chen B.K.R. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018;70:171–184. doi: 10.1002/art.40375. [DOI] [PubMed] [Google Scholar]
- 3.Piette W.W., Stone M.S. A cutaneous sign of IgA-associated small dermal vessel leukocytoclastic vasculitis in adults (Henoch-Schönlein purpura) Arch Dermatol. 1989;125(1):53–56. [PubMed] [Google Scholar]
- 4.Gower R.G., Mitchell Sams W., Thorne E.G. Leukocytoclastic vasculitis; sequential appearance of immunoreactants and cellular changes in serial biopsies. J Invest Dermatol. 1977;69(5):477–484. doi: 10.1111/1523-1747.ep12511631. [DOI] [PubMed] [Google Scholar]
- 5.Braverman I.M., Yen A. Demonstration of immune complexes in spontaneous and histamine-induced lesions and in normal skin of patients with leukocytoclastic angiitis. J Invest Dermatol. 1975;64(2):105–112. doi: 10.1111/1523-1747.ep12510321. [DOI] [PubMed] [Google Scholar]
- 6.Bayer-Garner I.B., Smoller B.R. Leukocytoclastic (small vessel) vasculitis in multiple myeloma. Clin Exp Dermatol. 2003;28(5):521–524. doi: 10.1046/j.1365-2230.2003.01324.x. [DOI] [PubMed] [Google Scholar]
- 7.Sampson A., Callen J.P. Thalidomide for type 1 cryoglobulinemic vasculopathy. Arch Dermatol. 2006;142(8):972–974. doi: 10.1001/archderm.142.8.972. [DOI] [PubMed] [Google Scholar]
- 8.Pittelkow M.R., Su W.P. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25:21–27. doi: 10.1016/0190-9622(91)70168-2. [DOI] [PubMed] [Google Scholar]
- 9.Harel S., Mohr M., Jahn I. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168(5):671–678. doi: 10.1111/bjh.13196. [DOI] [PubMed] [Google Scholar]
- 10.Terrier B., Launay D., Kaplanski G. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res. 2010;62(12):1787–1795. doi: 10.1002/acr.20318. [DOI] [PubMed] [Google Scholar]