Introduction
In 2012 vismodegib (Erivedge), an oral hedgehog pathway inhibitor, was approved by the US Food and Drug Administration for the treatment of locally advanced or metastatic basal cell carcinoma (BCC).1 Marketing authorization, however, does not account for long-term obstacles as seen in daily clinical practice. For example, one dilemma is the positioning of vismodegib in the treatment of giant, locally advanced BCC, especially regarding its position as part of a palliative and curative treatment. Another issue is how to deal with tumor resistance to vismodegib in giant, locally advanced BCC in a specific group of patients who avoid health care, often because of fear of surgery or radiotherapy. Gaining insight in the treatment process of these patients is therefore urgently needed to improve decision making in these complex cases. We report on 3 patients treated with vismodegib for giant, locally advanced BCC and 1 patient treated for a metastasized BCC to illustrate and discuss the possibilities and limitations of vismodegib treatment in clinical practice.
Patients
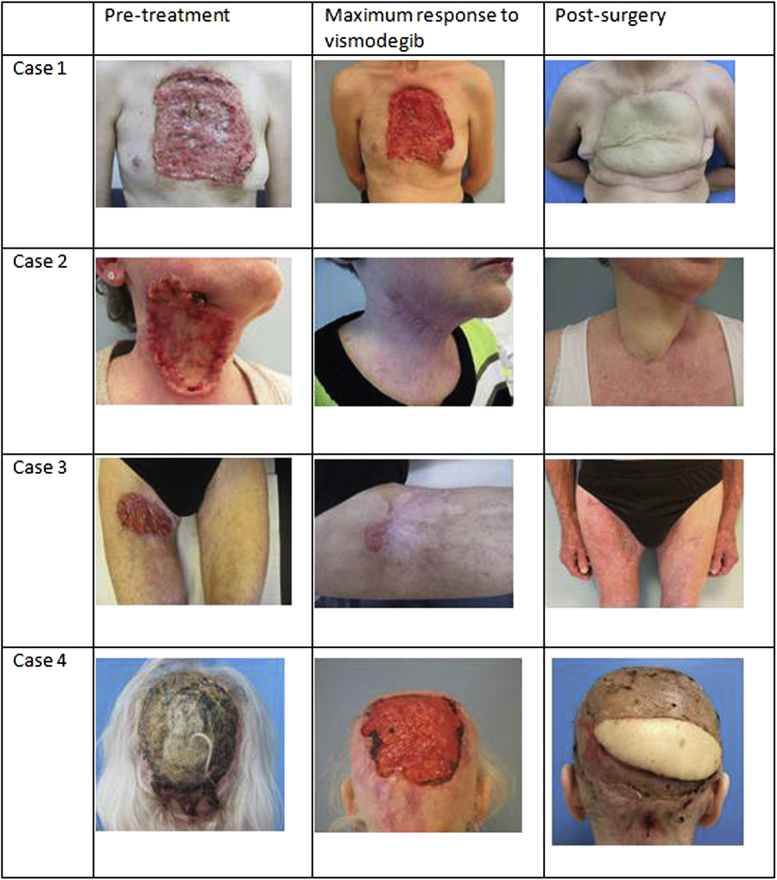
A 59-year-old woman presented with an 18-year history of a morpheaform BCC on her chest. When she ultimately sought medical attention, she reported pain, bleeding, drainage of wound fluid, and weight loss. Physical examination found an ulcerating mass of 14 × 17 cm covering the anterior chest wall (Fig 1, case 1). Initially, she refused any radiologic examinations.
Fig 1.
Cases 1-4. Clinical images of 4 patients with giant, locally advanced BCC; before, during, and after treatment with vismodegib and extensive surgery.
The patient was included in the international vismodegib phase II multicenter safety study (NCT01367665).2 Initially the tumor responded, but after 9 months vismodegib was discontinued because of clinical progression (Fig 1, case 1). At that time, she accepted radiologic investigation. The computed tomography (CT) scan displayed an extensive tumor process of the soft tissue of the anterior chest wall with invasion of the sternum without signs of metastatic disease. Subsequently the tumor, surrounding scar tissue, and 1 cm of healthy-appearing skin were excised including an en bloc subtotal sternectomy. The defect was closed with a polypropylene mesh and a free vascularized latissimus dorsi flap. Histopathologic examination of the BCC found that it was incompletely removed at the deep plane of the superior resection margin, which was located against the thoracic cavity.
The second patient was a 67-year-old otherwise healthy woman presenting with a 9- × 6-cm painful BCC located on the right side of the neck, which she had been covering up for more than 10 years (Fig 1, case 2). There was a palsy of the marginal mandibular branch of the facial nerve. An orthopantomogram and magnetic resonance imaging did not show tumor invasion in the mandible and/or deep soft tissues.
The tumor initially responded to vismodegib, but after about 8 months of the treatment, several persisting nodules located centrally in the tumor started to grow again (Fig 1, case 2). A second magnetic resonance imaging scan showed a remarkable increase in size of the BCC, whereupon vismodegib was discontinued.
Under general anaesthesia, the tumor, including surrounding scar tissue and 1 cm of healthy-appearing skin, was completely excised in 2 sessions. The tissue was processed using staged margin-controlled techniques with permanent sections to completely assess all margins. After the first stage, only the deep plane of the resection still showed BCC, which was completely removed after the second resection. All vital nerves of the neck were spared, and the patient had no postoperative functional hindrance (Fig 1, case 2).
The third patient was a 72-year-old man with a BCC localized on the anterior surface of the proximal right thigh present for 5 years. On physical examination the patient had an ulcerated, indurated 16- x 10-cm tumor with inguinal lymphadenopathy and lymphedema in the whole leg (Fig 1, case 3). Radiologic investigation found tumor invasion in the underlying muscle with para-femoral extension and encasement of the right femoral artery and a pathologic inguinal lymph node.
The patient was administrated vismodegib as a neoadjuvant treatment. After 4 months, treatment was discontinued because of a pneumonia. Clinically, both the tumor size (2 × 3 cm) and the edema had reduced (Fig 1, case 3). However, although imaging confirmed reduction of the cutaneous part of the tumor, there was no evident reduction of the deeper component. Subsequently, the tumor was widely excised including an en bloc inguinal and para-iliac lymph node dissection. Histopathologic examination found that the upper-lateral plane was marginally free (1-2 mm), the deep resection margins were free of tumor, and 4 of 6 lymph nodes were positive for BCC. The proximal part of the femoral and greater saphenous vein had to be resected because of tumor invasion, after which the femoral vein was reconstructed using a venous patch (Fig 1, case 3).
Finally, a 76-year-old woman was referred with the complaint of headaches and an ulcerating and bleeding BCC on the posterior scalp. The BCC was incompletely excised at another hospital 6 years before the referral, after which she avoided medical care because of dissatisfaction (Fig 1, case 4).
A CT scan found destruction of the occipital bone caused by tumor invasion. After initial improvement on vismodegib during the first months, the tumor clinically stabilized (Fig 1, case 4). After 15 months of treatment, 3 biopsies were taken, which all found chronic ulcerating inflammation with no signs of malignancy. A follow-up CT scan, however, found increased destruction of the occipital as well as the parietal bone.
Because of the progressive bone destruction, the ulcer (including 2 cm of healthy-appearing skin), the underlying skull bone, and dura mater were resected and reconstructed (Fig 1, case 4). Histopathologic examination found BCC in all resection margins.
Discussion
These cases illustrate several issues in which vismodegib is applied in clinical practice: selection of complex patients, resistance to vismodegib, and determining resection margins after discontinuation of vismodegib.
In daily practice, the available traditional curative treatment options and the costs and side effects related to vismodegib will likely result in selecting patients whose tumor significantly exceeds the minimal diameter of 10 mm used within registration studies.3, 4 Because BCC is already one of the most mutated cancers,5 and tumor size is probably correlated with tumor multiclonality, the likelihood of resistance developing from selection of specific tumor clones is therefore plausible in clinical practice. The presented cases also suggest that vismodegib resistance seems to occur more often in the deep tumor planes involving bone or cartilage with suboptimal blood perfusion, resulting in lower vismodegib tissue levels.
Another practical issue after partial response to vismodegib is defining appropriate resection margins. To take into account the risk of discontinuous growth after vismodegib therapy, we decided to surgically remove the residual tumor including surrounding scar tissue with 1- to 2-cm margins. Consequently, this approach did not lead to a smaller surgical defect compared with surgical treatment of the BCCs without vismodegib. However, histologic examination with a complete margin-controlled processing technique showed in our 4 cases that all lateral margins were actually free of tumor, which suggests that smaller lateral resection margins may be considered. A small open-label clinical trial investigating vismodegib neoadjuvant to surgery for high-risk BCC suggested a reduction of the surgical defect of 27% after treatment for at least 3 months. However, this study did not evaluate the occurrence of skip lesions, and 1 of 8 patients had a recurrence after a mean follow-up of 22 months.6, 7 Nevertheless, in 2 of 4 patients, the deep resection margins were yet incomplete, suggesting that vismodegib insufficiently affects the deep tumor planes, and adequate deep margins are still needed (Table I).
Table I.
Characteristics and outcomes of 4 patients treated with vismodegib
| Patient | M/F | Age, y | Tumor location | Tumor presence, y | Metastatic disease (Y/N) | Treatment history | Tumor size t = 0 (cm) | Tumor size t = 12 weeks (% reduction)∗ | Treatment duration (mo) and reason for discontinuation | Tumor size at maximum reduction (% reduction)† | Total follow-up after surgery (mo) and signs of recurrence (Y/N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | Chest wall | 18 | N | Radiotherapy/brachytherapy childhood (of unknown kind) | 14 × 17 | 14 × 15 (11.7) | 9, tumor progression | 14 × 14 (17.6) | 37, N |
| 2 | F | 67 | Right neck | 10 | N | — | 6 × 9 | 5.5 × 6 (38.9) | 8, tumor progression | 1 × 1.5 (97.2) | 41, N |
| 3 | M | 72 | Ventral right upper leg | 5 | Y | — | 6 × 10 | 2 × 3 (90) | 4, pneumonia | 2 × 3 (90) | 40, N |
| 4 | F | 76 | Posterior scalp | 6 | N | Incomplete excision at another hospital 6 years prior | 10.5 × 11.3 | 8.5 × 10.3 (26.2) | 4, tumor progression | 7.5 × 9.3 (41.2) | 29, N |
Percent of reduction at t = 12 weeks was calculated by 1- (tumor size t = 12 weeks/tumor size t = 0).
Percent of reduction at t = max reduction was calculated by 1-(tumor size at maximum reduction/tumor size t = 0).
Furthermore, the issue of health care–avoiding behavior in patients with these complex BCCs should not be forgotten. All of the patients we described had specific reasons for avoiding medical care. However, the threshold for consenting with imaging and finally surgery was lowered first by showing these patients that improvement was possible and second through establishing a trustworthy patient-physician relationships via intensive continuous personal care. The 4 patients we describe in this report illustrate that it is often necessary to combine vismodegib with surgery to achieve curation. It is interesting for future studies to evaluate how often combination therapies are applied, whether vital structures can be saved in this way, and if smaller resection sizes are possible.
This report shows very relevant issues with vismodegib that have not been addressed in registration studies. Additional evidence is needed, focussing on appropriate indications, treatment patterns, effectiveness of vismodegib, and outcomes in advanced and metastatic BCC.
Acknowledgments
We thank J. Slob for his careful revision of the English language in this article.
Footnotes
Funding sources: Three patients were extracted from the STEVIE trial and Roche was asked for approval. Roche was not involved in the preparation, writing and data interpretation of this manuscript.
Conflicts of interest: Drs Roodbergen, Wakkee, Baerveldt, and de Haas participated as (sub)investigators in the STEVIE trial. The authors had no relevant financial interest. The rest of the authors have no conflicts to disclose.
References
- 1.US Food and Drug Administration Vismodegib. 2012. https//www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- 2.Sekulic A., Migden M.R., Basset-Seguin N. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17(1):332. doi: 10.1186/s12885-017-3286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekulic A., Migden M.R., Oro A.E. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang A.L., Solomon J.A., Hainsworth J.D. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60–69. doi: 10.1016/j.jaad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Pleasance E.D., Cheetham R.K., Stephens P.J. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463(7278):191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ally M.S., Aasi S., Wysong A. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71(5):904–911.e901. doi: 10.1016/j.jaad.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Kwon G.P., Ally M.S., Bailey-Healy I. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC) J Am Acad Dermatol. 2016;75(1):213–215. doi: 10.1016/j.jaad.2016.02.1235. [DOI] [PubMed] [Google Scholar]