Abstract
Background
Acquired epidermal growth factor receptor (EGFR) T790M mutation is the primary resistance mechanism to first-generation EGFR tyrosine kinase inhibitors (TKIs) used in advanced, EGFR mutation-positive non-small-cell lung cancer (NSCLC). Available data, predominantly in Asian patients, suggest that this mutation is also the major cause of resistance to the irreversible ErbB family blocker, afatinib. For EGFR T790M-positive patients who progress on EGFR TKI therapy, osimertinib is an effective treatment option. However, data on osimertinib use after afatinib are, to date, scarce.
Objective
To identify the prevalence of EGFR T790M mutations in predominantly Caucasian patients with stage IV EGFR mutation-positive NSCLC who progressed on afatinib, and to investigate the subsequent response to osimertinib.
Patients and Methods
In this single-center, retrospective analysis, EGFR T790M mutation status after afatinib failure was assessed using liquid biopsy and tissue rebiopsy. EGFR T790M-positive patients subsequently received osimertinib.
Results
Sixty-seven patients received afatinib in the first-, second-, or third-line (80.6%, 14.9%, and 4.5%, respectively). After afatinib failure, the T790M mutation was identified in 49 patients (73.1%). Liquid biopsy and tissue rebiopsy were concordant in 79.4% of cases. All patients with T790M-positive tumors received osimertinib (73.5% after first-line afatinib); 37 (75.5%) of these had an objective response (complete response: 22.4%; partial response: 53.1%). Response rate was independent of T790M copy number.
Conclusion
EGFR T790M mutation is a major mechanism of acquired resistance to afatinib. Osimertinib confers high response rates after afatinib failure in EGFR T790M-positive patients and its use in sequence potentially allows extended chemotherapy-free treatment.
Key Points
| Approximately three quarters of patients with EGFR mutation-positive NSCLC who progressed following treatment with afatinib had the EGFR T790M mutation. | |
| In this real-world setting, all afatinib-treated patients who developed the EGFR T790M mutation were subsequently treated with osimertinib. | |
| Targeted treatment with afatinib followed by osimertinib results in pronounced responses and provides the potential for long-term chemotherapy-free treatment. |
Introduction
For patients with advanced non-small-cell lung cancer (NSCLC) and activating epidermal growth factor receptor (EGFR) mutations, EGFR tyrosine kinase inhibitors (TKIs) are standard first-line treatment [1]. First- (gefitinib, erlotinib), second- (afatinib, dacomitinib), and third-generation (osimertinib) EGFR TKIs are currently approved in this setting and have demonstrated robust clinical activity. Recent head-to-head data have demonstrated that the second-generation irreversible ErbB family blockers, afatinib and dacomitinib, and the third-generation irreversible EGFR wild-type sparing TKI, osimertinib, achieve superior clinical outcomes over reversible first-generation TKIs [2–6]. Nevertheless, progression inevitably occurs with all EGFR TKIs. It is therefore important that tumors are characterized for further molecular aberrations at the time of acquired resistance, so subsequent therapy can be tailored. Liquid biopsy is a strategy for tumor genotyping that is now commonly used to assess the presence of aberrations at the time of progression [7, 8]. Although various underlying mechanisms of acquired resistance to first- and second-generation EGFR TKIs have been described, the ‘gatekeeper’ EGFR T790M mutation, which arises from acquisition of a single recurrent missense mutation within exon 20, is the most common mechanism [9, 10].
Osimertinib is selective for both EGFR-sensitizing mutations and the EGFR T790M-resistance mutation. Based on the phase III AURA3 trial, in which 94% of patients had received first-line treatment with a first-generation EGFR TKI and 7% had been pretreated with afatinib, osimertinib was approved for the second-line treatment of patients with an EGFR T790M mutation following progression on or after EGFR TKI therapy [1, 11]. Consequently, it is imperative that patients are screened for the EGFR T790M mutation at the point of acquired resistance. Furthermore, it is important to assess the relative frequency of acquired EGFR T790M resistance mutations in patients treated with first- and second-generation EGFR TKIs, as this information may drive the selection of first-line treatment.
To date, analyses of the frequency of acquired EGFR T790M mutation have mainly been conducted in patients who received first-generation EGFR TKIs, with prevalence rates of 49–69% [9, 10, 12]. However, available data from studies undertaken in Taiwanese [13] or Japanese patients [14], and a predominantly Asian population [12] suggest that development of the EFGR T790M mutation may also be the major mechanism of resistance to afatinib (43–68% of patients). Data on the prevalence of the EGFR T790M mutation after afatinib failure, and subsequent response to osimertinib, are lacking in Caucasian patients. As the mechanism of action of afatinib differs from that of reversible EGFR TKIs, evidence of similar resistance mechanisms across these agents is essential. Additionally, it is important to demonstrate that osimertinib is as effective a treatment option after afatinib failure as it is after erlotinib and gefitinib. We therefore conducted a retrospective analysis in patients who progressed on afatinib, with the aim of identifying the prevalence of EGFR T790M mutations in this setting, and the subsequent responses of these patients to osimertinib.
Patients and Methods
Study Design and Patient Population
This single-center, retrospective analysis included all patients with stage IV adenocarcinoma of the lung and activating EGFR mutations who progressed on afatinib treatment between April 2015 and August 2018 at the Department of Respiratory and Critical Care Medicine of the Otto Wagner Hospital, Vienna. Exclusion criteria included lack of liquid biopsy, negative liquid biopsy that was not followed by rebiopsy, and therapies administered between afatinib and osimertinib treatment for patients with the T790M mutation.
The patients included in this analysis had been treated with afatinib in the first-, second-, or third-line setting; patients who received afatinib in the second- and third-line settings after a first generation TKI were included previously in a named patient use program. Some patients (n = 33) had been included in a previous study [8].
EGFR mutation status was determined using liquid biopsy only, or liquid biopsy followed by tissue rebiopsy if the liquid biopsy result was negative for T790M and the patient was fit enough to undergo rebiopsy. Patients with the EGFR T790M mutation received subsequent osimertinib.
All procedures in studies involving human participants, which were approved and overseen by an ethics committee at the Otto Wagner Hospital, were performed in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For retrospective studies, formal consent is not required. Patient written informed consent to this study was obtained before implementation of the liquid biopsies.
Liquid Biopsy
Liquid biopsy was performed as previously described [8]. Briefly, blood samples were collected in ethylenediaminetetraacetic acid-containing vacutainer tubes and processed within 2 h of collection. Cell-free plasma DNA was extracted using QIAamp circulating nucleic acid kits (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The patient mutation status was determined using droplet digital PCR (QX-100™ Droplet Digital™ PCR system; Bio-Rad, Hercules, CA, USA).
Assessment and Statistical Analysis
The patient and tumor characteristics monitored included age, sex, race, smoking status, presence of brain metastases, type of EGFR mutation at baseline (prior to any systemic treatment for stage IV adenocarcinoma of the lung), and treatment prior to afatinib. Tumor responses to afatinib and osimertinib were assessed by centralized radiologic review based on imaging methods, according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and institutional guidelines. The objective response rate (ORR) was defined as the combined percentages of patients with complete responses (CRs) and partial responses (PRs). Treatment probabilities were calculated using the Kaplan-Meier method. T790M copy number was estimated based on the read-out of the droplet digital PCR assay. Comparison of response according to T790M copy number was done by one-factorial analysis of variance (ANOVA). All analyses were performed using GraphPad Prism version 7 software. Data cut-off was August 31, 2018.
Results
Patient Characteristics
Overall, 67 patients were included in this analysis. Patient baseline characteristics are summarized in Table 1. Most patients were Caucasian (92.5%) and received first-line afatinib (80.6%). Nine patients (13.4%) had received prior treatment with a first-generation EGFR TKI, one (1.5%) had received platinum-based chemotherapy, and three (4.5%) had received both. Fifty-five patients (82.1%) had tumors with either an exon 19 deletion or an L858R mutation.
Table 1.
Demographic and clinical characteristics of patients at baseline
| Characteristic | Patient group | |
|---|---|---|
| All | Acquired T790M | |
| Total, n (%) | 67 (100) | 49 (73.1) |
| Median age, years (range) | 67 (36–87) | 70 (44–87) |
| Sex, n (%) | ||
| Female | 49 (73.1) | 39 (79.6) |
| Male | 18 (29.6) | 10 (20.4) |
| Race, n (%) | ||
| Caucasian | 62 (92.5) | 45 (91.8) |
| Asian | 5 (7.5) | 4 (8.2) |
| Smoking status, n (%) | ||
| Never-smokers | 49 (73.1) | 38 (77.6) |
| Ex-smokers | 13 (19.4) | 8 (16.3) |
| Smokers | 5 (7.5) | 3 (6.1) |
| Brain metastases, n (%) | 14 (20.9) | 9 (18.4) |
| EGFR mutation at baseline (prior to any systemic therapy), n (%) | ||
| Exon 19 deletion | 38 (56.7) | 30 (61.2) |
| L858R | 17 (25.4) | 14 (28.6) |
| G719X | 3 (4.5) | 1 (2.0) |
| L861Q | 4 (6.0) | 1 (2.0) |
| Other | 5 (7.5)a | 3 (6.1)b |
| Treatment prior to afatinib, n (%) | ||
| First-generation EGFR TKIc | 9 (13.4) | 9 (18.4) |
| Platinum-based chemotherapyd | 1 (1.5) | 1 (2.0) |
| Chemotherapy and EGFR TKIe | 3 (4.5) | 3 (6.1) |
| Afatinib treatment line, n (%) | ||
| First | 54 (80.6) | 36 (73.5) |
| Second | 10 (14.9) | 10 (20.4) |
| Third | 3 (4.5) | 3 (6.1) |
aExon 20 insertions (n = 2), L858R/G719X (n = 1), G719X/S786I (n = 1), G719X/L861Q (n = 1)
bExon 20 insertion (n = 1), L858R/G719X (n = 1), G719X/S786I (n = 1)
cErlotinib (n = 1) and gefitinib (n = 8)
dCarboplatin/pemetrexed (n = 1)
eCisplatin/pemetrexed followed by erlotinib (n = 1), gefitinib followed by cisplatin/pemetrexed/bevacizumab (n = 1), gefitinib followed by pemetrexed (n = 1)
Prevalence of Acquired T790M Mutation
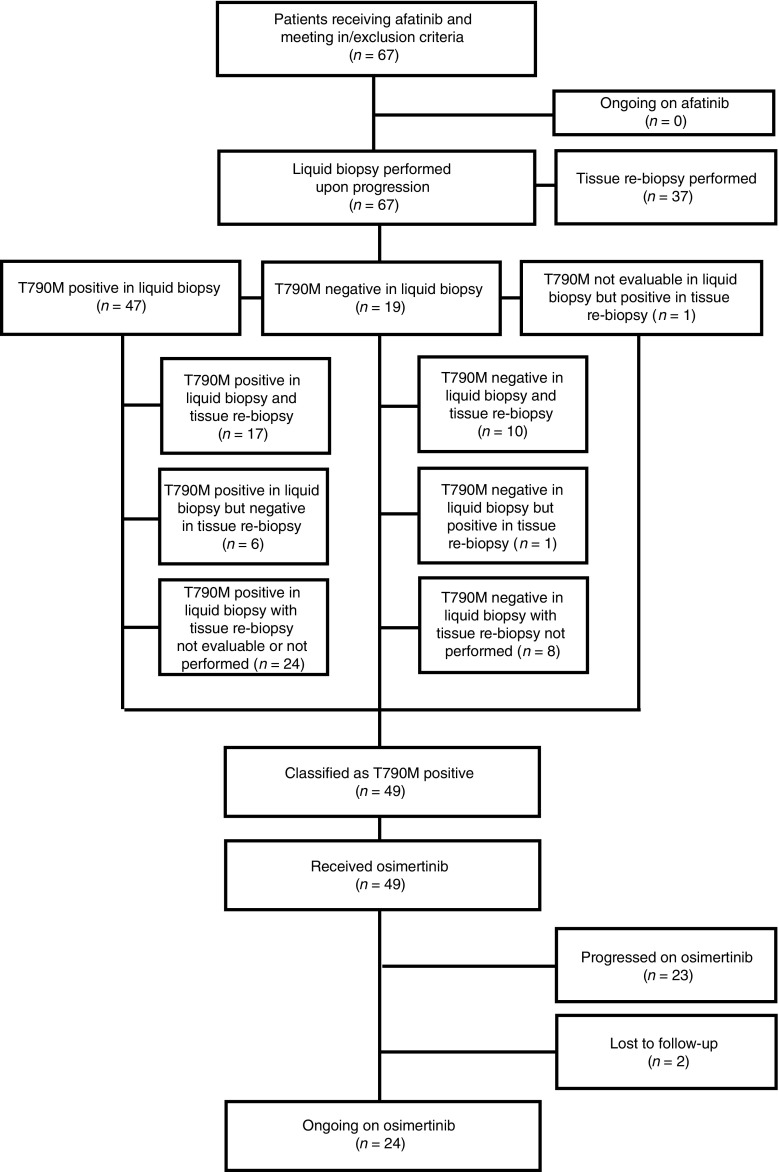
Forty-nine (73.1%) patients tested positive for the EGFR T790M mutation after afatinib treatment (Fig. 1); baseline characteristics of these patients were comparable to the overall cohort (Table 1). Rebiopsy was conducted in 37 patients. In three cases, test results for either liquid biopsy or tissue rebiopsy were not evaluable. Of the remaining cases the rebiopsy results were concordant with liquid biopsy results in 27 (79.4%) cases (Fig. 1). Six patients had a T790M-negative rebiopsy but positive liquid biopsy and one patient was only T790M-positive on rebiopsy. Rebiopsy identified the presence of small-cell lung cancer histology in two of the 37 patients who were re-tested; both tumors were EGFR T790M negative. Of the 54 patients who received first-line afatinib, 36 (66.7%) tested positive for T790M.
Fig. 1.
Patient disposition chart
Response to Afatinib
The ORR obtained with afatinib was 92.5% (CR: 19.4%; PR: 73.1%; Table 2) in the overall cohort and 93.9% (CR: 26.5%; PR: 67.3%) in the patients who acquired the EGFR T790M mutation. Among the 54 patients who received first-line afatinib, the ORR was 92.6% (CR: 18.5%; PR: 74.1%) in all patients and 94.5% in those patients who acquired the EGFR T790M mutation (CR: 27.8%; PR: 66.7%). Patients with an EGFR mutation other than exon 19 deletion or L858R (N = 14) showed a comparable ORR of 85.7% (CR: 7.1%; PR: 78.6%, stable disease (SD): 7.1%, progressive disease (PD): 7.1%).
Table 2.
Tumor response to afatinib
| Treatment line | ||||
|---|---|---|---|---|
| Any | First | |||
| All patients (N = 67) | Acquired T790M (n = 49) | All patients (n = 54) | Acquired T790M (n = 36) | |
| Response, n (%) [95% CI] | ||||
| Overall | 62 (92.5) | 46 (93.9) | 50 (92.6) | 34 (94.5) |
| Complete | 13 (19.4) [9.9–28.8] | 13 (26.5) [14.2–38.9] | 10 (18.5) [8.2–28.9] | 10 (27.8) [13.1–42.4] |
| Partial | 49 (73.1) [62.5–83.7] | 33 (67.3) [54.2–80.5] | 40 (74.1) [62.4–85.8] | 24 (66.7) [51.3–82.1] |
| Stable disease | 2 (3.0) [−1.1–7.0] | 2 (4.1) [−1.5–9.6] | 1 (1.9) [−1.7–5.4] | 1 (2.8) [−2.6–8.1] |
| Duration, n (%) [95% CI] | ||||
| ≥6 months | 59 (88.1) [80.3–95.8] | 44 (89.8) [81.3–98.3] | 47 (87.0) [78.1–96.0] | 32 (88.9) [78.6–99.1] |
| ≥12 months | 34 (50.7) [38.8–62.7] | 24 (49.0) [35.0–63.0] | 29 (53.7) [40.4–67.0] | 19 (52.8) [36.5–69.1] |
| ≥18 months | 15 (22.4) [12.4–32.4] | 13 (26.5) [14.2–38.9] | 12 (22.2) [11.1–33.3] | 10 (27.8) [13.1–42.4] |
| ≥24 months | 5 (7.5) [1.1–13.8] | 5 (10.2) [1.7–18.7] | 4 (7.4) [0.4–14.4] | 4 (11.1) [0.8–21.4] |
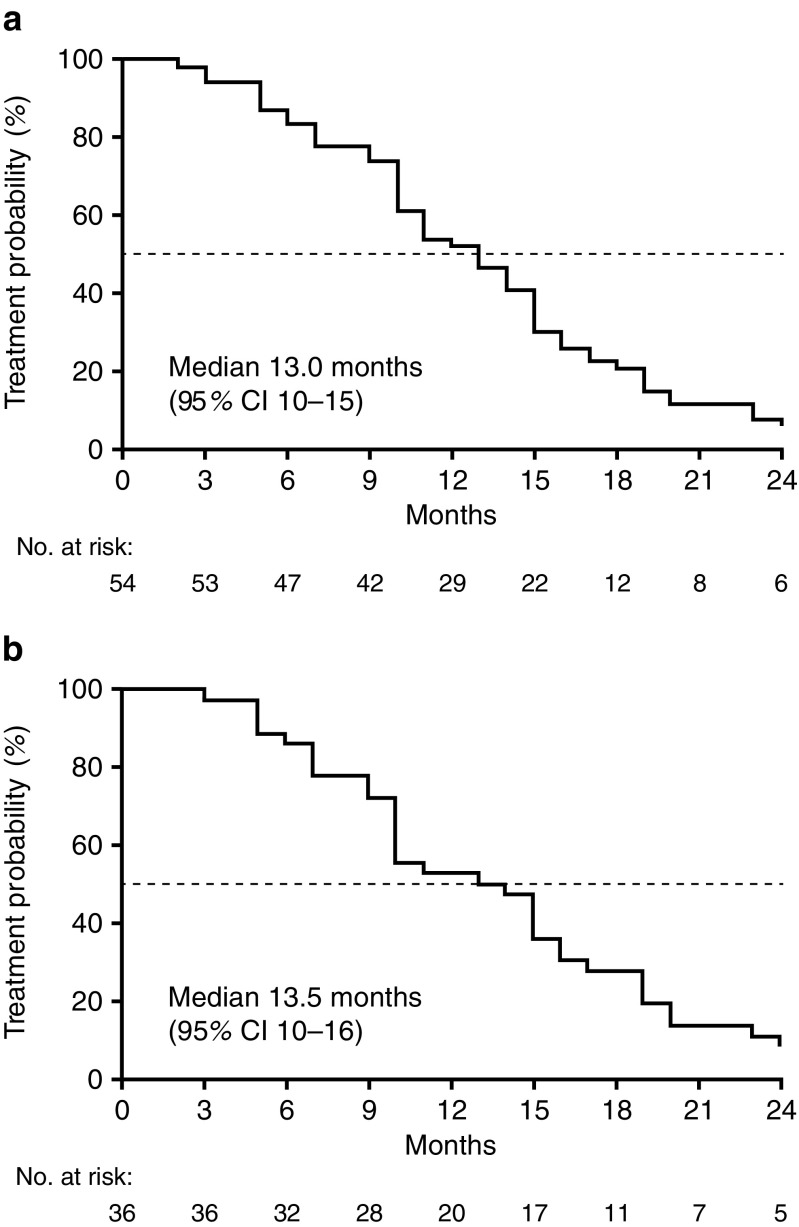
Figure 2 shows Kaplan–Meier curves for time on treatment (ToT) with afatinib in patients who received it as first-line therapy (n = 54) and those who received first-line afatinib and tested positive for T790M (n = 36). Median ToT was 13.0 months (95% CI: 10–15) and 13.5 months (95% CI: 10–16), respectively. Median ToT with afatinib in the overall cohort (n = 67) was 12.0 months (95% CI: 10–15). Five (7.5%) patients achieved a long-term response to afatinib of ≥24 months. All five of these patients developed the T790M mutation and four received first-line afatinib. Achievement of long-term response to afatinib in this analysis was independent of baseline EGFR mutation type (as listed in Table 1). In the T790M-negative and positive groups, four (22.2%) and zero patients were treated beyond progression, respectively.
Fig. 2.
Time on afatinib treatment. a Patients who received first-line afatinib; b patients who received first-line afatinib and tested positive for T790M
Response to Osimertinib
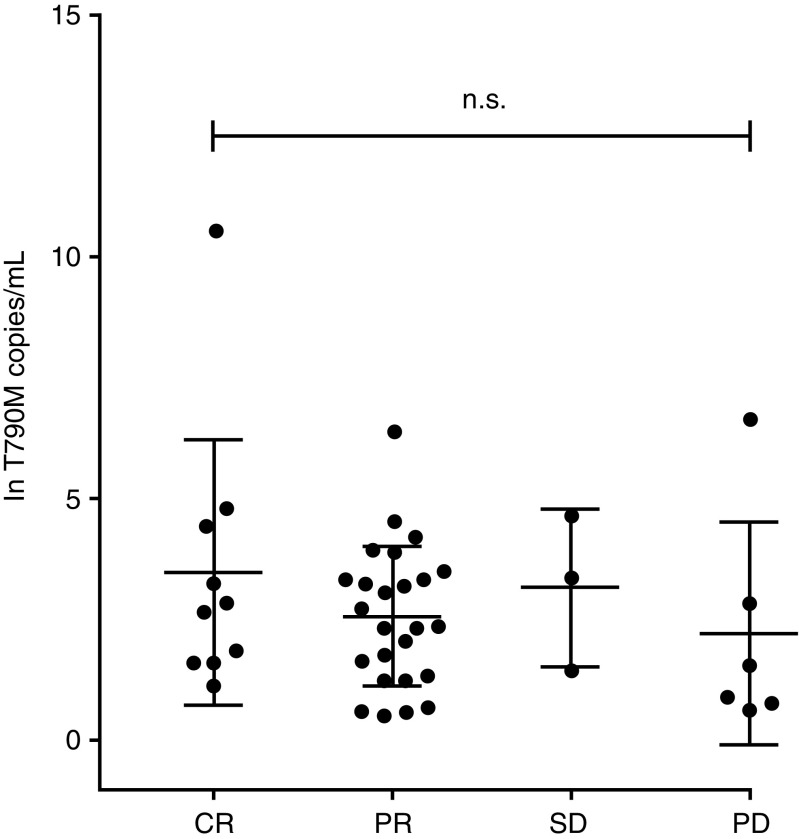
All 49 patients who had EGFR T790M-positive tumors after progression on afatinib received osimertinib. The ORR was 75.5% (CR: 22.4%; PR: 53.1%; Table 3) in all patients, and 77.8% (CR: 19.4%; PR: 58.3%) in patients who had received first-line afatinib. For patients who received afatinib in later lines (N = 13) ORR was 69.3% (CR: 30.8%; PR: 38.5%, SD: 7.7%, PD: 15.4%, NE: 7.7%). There was no significant correlation between T790M copy number and response to osimertinib (Fig. 3).
Table 3.
Tumor response to osimertinib
| Afatinib treatment line | ||
|---|---|---|
| Any (N = 49) | First (n = 36) | |
| Response, n (%) | ||
| Overall | 37 (75.5) | 28 (77.8) |
| Complete | 11 (22.4) | 7 (19.4) |
| Partial | 26 (53.1) | 21 (58.3) |
| Stable disease | 3 (6.1) | 2 (5.6) |
| Progressive disease | 6 (12.2) | 4 (11.1) |
| Not evaluable | 3 (6.1) | 2 (5.6) |
Fig. 3.
Response to osimertinib with respect to T790M copy number
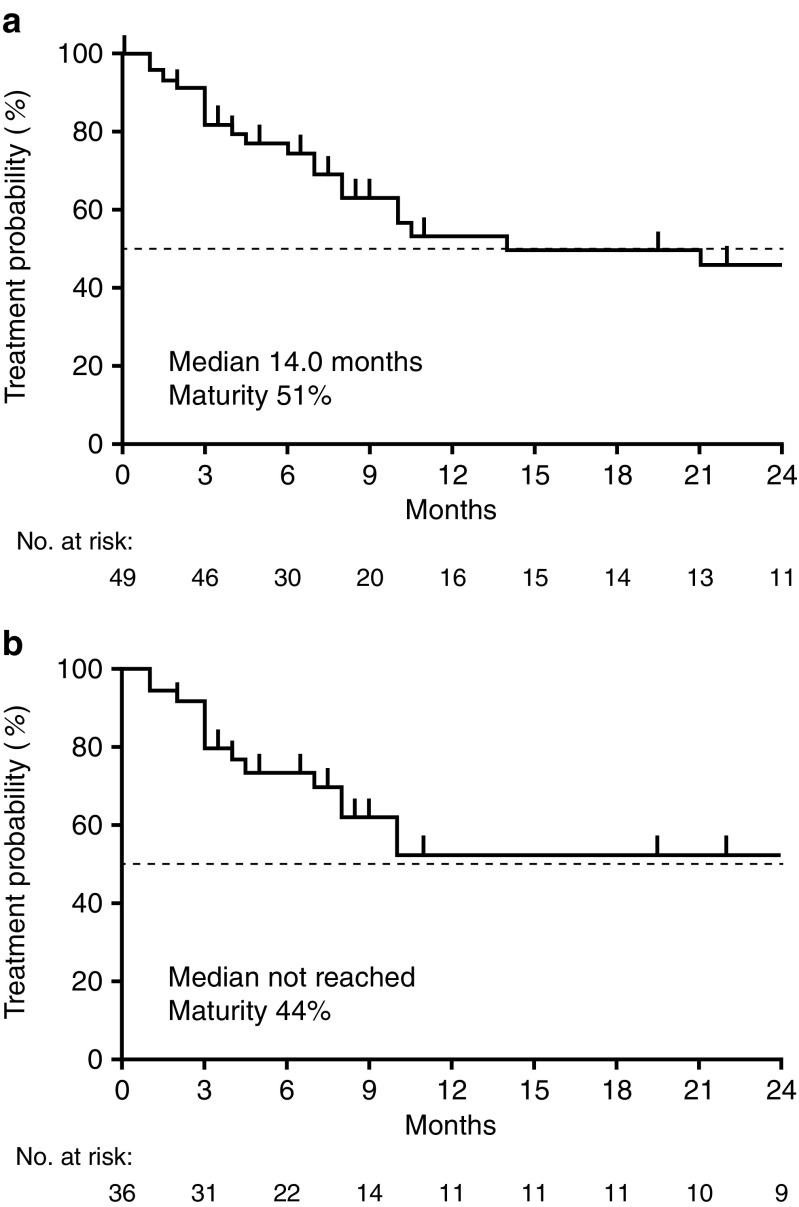
Median duration of osimertinib treatment was 14.0 months overall, and was not reached in patients who received first-line afatinib (Fig. 4). The data are still immature. At data cut-off, osimertinib treatment was ongoing in 49% of patients (n = 24); 29% of these patients initiated osimertinib treatment less than 6 months prior to the time of analysis.
Fig. 4.
Time on osimertinib treatment. a All patients who received osimertinib; b patients who received osimertinib following first-line afatinib
Of the patients who discontinued osimertinib, 47.8% received at least one line of subsequent treatment, chemotherapy being the most commonly used option (Table 4).
Table 4.
Treatment post osimertinib
| Additional lines of treatment, n (%) | |
| 0 | 12 (52.2) |
| 1 | 6 (26.1) |
| 2 | 1 (4.3) |
| 3 | 4 (17.4) |
| Type of treatment, n (%) | |
| Chemotherapy | 8 (72.7) |
| Immunotherapy | 3 (27.3) |
| EGFR TKI | 3 (27.3) |
| Radiotherapy | 3 (27.3) |
Discussion
To our knowledge, this study represents the largest real-world analysis of EGFR T790M prevalence following progression on afatinib in predominantly Caucasian patients with EGFR mutation-positive NSCLC. The results demonstrate that the EGFR T790M mutation is the most common mechanism of acquired resistance in the 54 patients who received first-line afatinib (66.7% of cases). These findings are largely consistent with the rates of EGFR T790M reported following treatment with first-generation EGFR TKIs (49–73%) [8–12, 15, 16] or afatinib in predominantly Asian patients (43–68%) [12–14]. In our overall cohort of 67 patients who received afatinib in the first-, second-, or third-line, the rate of acquired EGFR T790M mutation was 73.1%; emergence of the mutation did not appear to correlate with baseline characteristics. However, it was not possible to define when the resistance mutations emerged in the 18.4% of patients who had received prior treatment with erlotinib or gefitinib, as T790M testing took place only after afatinib failure had occurred, and not before initiation of afatinib treatment in the majority of patients. Consequently, these mutations could have arisen at the time of failure of first-generation EGFR TKI therapy.
Of note, the droplet digital PCR method used in this study has the advantage of higher sensitivity compared to alternative methods. In 21 T790M-positive patients, the number of identified T790M copies/ml was below 10 and therefore well below the limit of detection of the widely-used cobas EGFR Mutation Test version 2.0. The higher rate of T790M positivity following afatinib in the current study (73%) compared with previous studies (43–68%) [12–14] might be attributable to the higher sensitivity of droplet digital PCR compared with other methods. Droplet digital PCR may, for example, detect cases where the T790M allele is present in only a small proportion of tumor cells. Importantly, T790M allele frequency did not appear to influence response rate, indicating that patients with subclonal T790M-positive tumors may still benefit from second-line treatment with osimertinib. We did not monitor other molecular resistance mechanisms to afatinib therapy due to the lack of approved drugs targeting these mechanisms.
The use of tissue biopsy to determine EGFR T790M mutation status in patients progressing following initial treatment with an EGFR TKI can be problematic, as some patients may refuse or be ineligible for biopsy, or progression may have occurred at an inaccessible site. In one analysis of EGFR T790M mutation status among 24 patients who progressed following afatinib, only 14 patients (58%) underwent tissue biopsy at the time of progression, and only 11 samples were sufficient for molecular analysis [17]. Consequently, use of liquid biopsy maximizes the number of patients for whom EGFR T790M mutation status can be determined and who are therefore able to benefit from subsequent treatment with osimertinib. Additionally, liquid biopsy may identify patients with EGFR T790M mutation who would not otherwise be able to access osimertinib because of a false-negative tissue biopsy result, caused by the biopsied site not being representative of all metastatic sites [7]. However, a relative lack of sensitivity for EGFR mutations has been reported for some current liquid biopsy assays compared with tissue biopsy [18]. In our analysis, both liquid biopsy and tissue rebiopsy were used to determine EGFR T790M mutation status, with a concordance rate of approximately 80%. Importantly, all patients included in this analysis received a valid test result for T790M, allowing those who tested positive to receive subsequent treatment with osimertinib. Based on these data, and encouraging results in other studies [7, 15, 19, 20], it appears that liquid biopsy represents an important technical advance in the context of EGFR T790M testing.
In our study, the ORR achieved with afatinib was above 90% in both the overall cohort and the EGFR T790M-positive patient group, with high CR rates (19.4% and 26.5%, respectively); response was independent of baseline characteristics. These high response rates might have been influenced by the single site nature of our study. The ORR achieved with osimertinib after afatinib in patients with an EGFR T790M mutation was also high (75.5%), with 22.4% having CRs and 53.1% having PRs. These findings are similar to those in the AURA3 trial [11], in which an ORR of 71% was achieved; most patients (94%) had received erlotinib or gefitinib as first-line treatment. Although duration of response data is immature in our study (49% of patients were still on treatment at the cutoff date), the median ToT with osimertinib was 14 months overall and not reached in patients who received first-line afatinib.
In patients who progressed on osimertinib and were eligible for subsequent therapy, the most commonly-used option by far was chemotherapy (72.7%). Other studies have reported promising outcomes in patients treated with sequential osimertinib after afatinib. In a retrospective analysis of the LUX-Lung 3, 6, and 7 trials, 37 patients were identified who received subsequent osimertinib following discontinuation of afatinib, mostly in the ≥third-line setting [21]. In these patients, median time on osimertinib treatment was 20.2 months (95% CI: 12.8–31.5) and median OS for osimertinib had not been reached after >4 years’ follow-up. A recent multicenter observational study across ten countries assessed outcomes in 204 patients who received sequential osimertinib after first-line afatinib. In these patients, overall median ToT was 27.6 months, with particularly promising ToT in Asian patients (46.7 months) and patients with an EGFR Del19 mutation (30.3 months) [22]. Finally, a recent phase II trial in 111 T790M-positive patients demonstrated that PFS and response rate were significantly better in patients treated with sequential afatinib and osimertinib versus sequential erlotinib/gefitinib and osimertinib [23].
This study has a number of limitations due to its retrospective nature. First, it was potentially subject to selection bias as only patients who progressed during a defined period of time were included. Second, time points for radiographic assessments were not standardized. For this reason, we assessed ToT rather than the more stringent endpoint of progression-free survival (PFS) as a time-to-event variable, which included patients who were treated beyond progression, a practice that is not proven to provide additional clinical benefit. However, it is noteworthy that no patients received treatment beyond progression in the T790M-positive group. This reflects the availability of an effective subsequent targeted treatment option, osimertinib, in this setting following progression and the molecular confirmation of T790M. Four patients in the T790M negative group were treated beyond progression.
Recent data from the phase III FLAURA study have shown that first-line osimertinib significantly improves PFS compared with first-generation EGFR TKIs, while demonstrating a similar safety profile and lower rate of serious adverse events [4], thus suggesting that osimertinib will be broadly used as first-line treatment. However, as mechanisms of resistance to osimertinib are diverse, and are predominantly independent of EGFR [24], subsequent treatment options post-osimertinib are mostly limited to the chemotherapy at the moment. Therefore, given the high rate of EGFR T790M observed after afatinib treatment, and the high efficacy of osimertinib after afatinib failure observed in this, and other, studies [21], sequential afatinib followed by osimertinib might represent a valid chemotherapy-free regimen that could be offered to patients. Future investigations on the ideal treatment sequence of EGFR TKIs for patients with EGFR mutation-positive NSCLC are clearly warranted.
Conclusions
In patients with stage IV NSCLC and sensitizing EGFR mutations who progress on treatment with the irreversible ErbB family blocker afatinib, the EGFR T790M mutation is present in the majority of cases and is thus the clear main mechanism of acquired resistance. Liquid biopsy can be used to determine T790M mutation status, which maximizes the number of patients who are able to receive subsequent treatment with the third-generation EGFR TKI, osimertinib. Notwithstanding the retrospective nature of this study, osimertinib appears to provide high response rates when administered after afatinib, potentially enabling prolonged chemotherapy-free treatment.
Funding
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Katharine Williams and Lynn Pritchard of GeoMed, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript. Open access publication of this article was funded by Boehringer Ingelheim.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest
Maximilian J. Hochmair has received honoraria from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, and Roche and had consulting or advisory roles with Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Roche. Anna Buder has received honoraria from AstraZeneca. Helmut Prosch has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche and has received a research grant from Boehringer Ingelheim. Wolfgang Hilbe has received advisory board and lecture fees from Boehringer Ingelheim. Agnieszka Cseh and Richard Fritz are employees of Boehringer Ingelheim. Martin Filipits has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Ratiopharm, and Roche. Sophia Schwab, and Otto C. Burghuber declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Footnotes
To view enhanced digital features for this article go to 10.6084/m9.figshare.7270556.
Maximilian J. Hochmair and Anna Buder contributed equally to this work.
References
- 1.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 2.Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 3.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 4.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2017;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 5.Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;14(11):1117–1132. doi: 10.2217/fon-2017-0636. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh V. Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol. 2018;10:1758834017753338. doi: 10.1177/1758834017753338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2(8):1014–1022. doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buder A, Hochmair MJ, Schwab S, Bundalo T, Schenk P, Errhalt P, et al. Cell-free plasma DNA-guided treatment with osimertinib in patients with advanced EGFR-mutated NSCLC. J Thorac Oncol. 2018;13(6):821–830. doi: 10.1016/j.jtho.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 13.Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ, Yang PC, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7(11):12404–12413. doi: 10.18632/oncotarget.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka K, Nosaki K, Otsubo K, Azuma K, Sakata S, Ouchi H, et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget. 2017;8(40):68123–68130. doi: 10.18632/oncotarget.19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reckamp KL, Melnikova VO, Karlovich C, Sequist LV, Camidge DR, Wakelee H, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016;11(10):1690–1700. doi: 10.1016/j.jtho.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campo M, Gerber D, Gainor JF, Heist RS, Temel JS, Shaw AT, et al. Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol. 2016;11(11):2022–2026. doi: 10.1016/j.jtho.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Qin A, Ramnath N. The "liquid biopsy" in non-small cell lung cancer - not quite ready for prime time use. Transl Cancer Res. 2016;5(suppl 4):S632–S6S5. doi: 10.21037/tcr.2016.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahama T, Sakai K, Takeda M, Azuma K, Hida T, Hirabayashi M, et al. Detection of the T790M mutation of EGFR in plasma of advanced non-small cell lung cancer patients with acquired resistance to tyrosine kinase inhibitors (West Japan oncology group 8014LTR study) Oncotarget. 2016;7(36):58492–58499. doi: 10.18632/oncotarget.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Chen R, Wang S, Zhong J, Wu M, Zhao J, et al. Quantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLC. PLoS One. 2014;9(11):e110780. doi: 10.1371/journal.pone.0110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequist L, Wu Y, Schuler M, Kato T, Yang JC, Tanaka H, et al. Subsequent therapies post-afatinib among patients (pts) with EGFR mutation-positive (EGFRm+) NSCLC in LUX-lung (LL) 3, 6 and 7. Ann Oncol. 2017;28(suppl 5):v460–vv96. [Google Scholar]
- 22.Hochmair MJ, Morabito A, Hao D, Yang C-T, Soo RA, Yang JC-H, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018;14(27):2861–2874. doi: 10.2217/fon-2018-0711. [DOI] [PubMed] [Google Scholar]
- 23.Tamiya M, Tamiya A, Suzuki H, Nakahama K, Taniguchi Y, Kunimasa K, et al. Which is better EGFR-TKI followed by osimertinib between afatinib and gefitinib/erlotinib? Ann Oncol. 2018;29(suppl 8):viii493–viii547. [Google Scholar]
- 24.Le X, Puri S, Negrao MV, Nilsson M, Robichaux JP, Boyle TA, et al. Landscape of EGFR -dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018. 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.