Abstract Abstract
There are many reports describing chromosome structure, organization and evolution within goatgrasses (Aegilops spp.). Chromosome banding and fluorescence in situ hybridization techniques are main methods used to identify Aegilops Linnaeus, 1753 chromosomes. These data have essential value considering the close genetic and genomic relationship of goatgrasses with wheat (Triticumaestivum Linnaeus, 1753) and triticale (× Triticosecale Wittmack, 1899). A key question is whether those protocols are useful and effective for tracking Aegilops chromosomes or chromosome segments in genetic background of cultivated cereals. This article is a review of scientific reports describing chromosome identification methods, which were applied for development of prebreeding plant material and for transfer of desirable traits into Triticum Linnaeus, 1753 cultivated species. Moreover, this paper is a resume of the most efficient cytomolecular markers, which can be used to follow the introgression of Aegilops chromatin during the breeding process.
Keywords: Aegilops , chromosome, banding, fluorescence in situ hybridization (FISH), genomic in situ hybridization (GISH), prebreeding, triticale, wheat
Introduction
There are twenty three species of goatgrasses (Aegilops spp.) (Slageren 1994) and several of them are the closest relatives to wheats (Triticum spp.) (Kilian et al. 2011). The genomic constitution of goatgrasses is wide and include six genomes (D, S, U, C, N and M), which can be organized as diploids, tetraploids or hexaploids. What is more, most polyploid Aegilops Linnaeus, 1753 species are assumed to contain a common (pivotal) subgenome (U or D) while the second - differential genome (or genomes) is (are) much more genetically diversified (Zohary and Feldman 1962; Feldman and Levy 2012; Mirzaghaderi and Mason 2017). The evolution of Aegilops species was also intertwined with speciation of Triticum Linnaeus, 1753 forms (Goncharov 2011). It is reported that hexaploid wheat (Triticumaestivum Linnaeus, 1753; genomes AABBDD) originated through one or more hybridization events between a tetraploid wheat, T.turgidum Linnaeus, 1753 (genomes AABB), with the diploid goatgrass Aegilopstauschii Cosson, 1849 [genomes DD; syn. Triticumtauschii (Cosson,1849) Schmalhausen, 1897; syn. Aegilopssquarrosa auct. non Linnaeus, 1753, Patropyrumtauschii (Cosson, 1849) A. Love, 1984] (Kihara 1924, 1954; McFadden and Sears 1946). More precisely, Aegilopstauschiisubsp.strangulata (Eig, 1929) Tzvelev, 1973, has been accepted to be a donor of D-genome of wheat (Dvořák et al. 1998). Tetraploid wheat originated via hybridization of a species closely related to the extant Aegilopsspeltoides Tausch, 1837 [genomes SS; syn. Sitopsisspeltoides (Tausch, 1837) Á. Löve, 1984; syn. Triticumspeltoides (Tausch, 1837) Grenier, 1890], which contributed the wheat B genome (Sarkar and Stebbins 1956; Dvořák et al. 1993; Feldman and Levy 2012; Salse et al. 2008), with diploid wheat (genomes AA). The most likely donor of A-genome of polyploid wheats is T.urartu Tumanian ex Gandilyan, 1972 (Konarev et al. 1974; Petersen et al. 2006; Golovnina et al. 2009). Some reports describe both genera jointly, as Aegilops-Triticum complex (Li et al. 2015; Ozkan et al. 2003; Zohary and Feldman 1962). A close relationship between the genera Aegilops and Triticum is widely adopted for introducing new genes by interspecific hybridization into cultivated cereals (Ruban and Badaeva 2018). Such introgression forms are important genetic resources for breeding. These kinds of genetic stocks can be used as an interesting plant material to study the expression of alien traits and for mapping particular loci (genes) onto Aegilops chromosomes (Rakszegi et al. 2017).
The ability to distinguish alien chromosomes, which were introduced into a genetic background of an acceptor plant, is the initial step in characterization of introgression lines. The first chromosome identification studies in wheat were done by Sears (1948), who assigned the loci for several agronomic and morphological traits on particular chromosomes and chromosome arms. Later, in 1970s all chromosomes of wheat could be distinguished using the C-banding or N-banding techniques (Gill and Kimber 1974; Iordansky et al. 1978; Endo and Gill 1984; Lukaszewski and Xu 1995). In 1990s, molecular biology protocols were combined with classical cytogenetic techniques to develop the fluorescence in situ hybridization (FISH) method. FISH allows the identification of DNA sequences directly on the chromosomes.
The first molecular probes used for FISH purposes on Aegilops-Triticum chromosomes contained conserved high-copy sequences, such as telomere sequences or 5S and 45S ribosomal RNA genes (Gerlach and Bedbrook 1979; Gerlach and Dyer 1980). The number and distribution of rDNA loci mapped on chromosomes of species belonging to Aegilops-Triticum complex turned out to be invariant. Hence, these probes were often used as markers in evolution and speciation studies, as well as in the evaluation of interspecific divergence (Badaeva et al. 1996a; b; 2002; 2004; 2015). Mukai et al. (1993) used pSc119.2 and pAs1 sequences to identify all 21 chromosome pairs in wheat. Over time a number of cytomolecular markers were developed for the identification of chromosome arms or segments. For example, Cuadrado et al. (2000; 2008) used synthetic oligonucleotides (three base-pair repeats) to detect FISH signals on wheat chromosomes. BAC genomic libraries were also screened to develop FISH chromosome markers (Zhang et al. 2004). Komuro et al. (2013) screened 2000 plasmid wheat clones in order to detect multiple tandem repeated sequences, using in situ hybridization, and selected 47 of them, which gave clear signals on wheat chromosomes. Apart from physical mapping of DNA sequences onto chromosomes, the major breakthrough in chromosome identification was the development of an in situ hybridization technique utilizing total genomic DNA as a probe (GISH). This variant of in situ hybridization appeared to be a powerful tool for characterization of alien introgressions in cereals. The first GISH was carried out on chromosomes of synthetic hybrids of Hordeumchilense Roemer & Schultes, 1817 × Secaleafricanum Stapf, 1901 (Schwarzacher et al. 1989) and Triticumaestivum (wheat) × S.cereale Linnaeus, 1753 (rye) hybrids (Le et al. 1989). This technique is based on the divergence of repetitive DNA (Belyayev and Raskina 1998; Belyayev et al. 2001a; b) and was effectively used for identification of alien chromosomes/chromosome segments in hybrids or translocation lines of cereals (Schwarzacher et al. 1989; 1992; Leitch et al. 1990). GISH in combination with FISH was also used to study the genome constitution of natural and artificial hybrids, or to identify the introgression of alien chromosomes or chromosome segments (Jiang and Gill 2006).
The structure and organization of chromosomes of species belonging to the genera Aegilops and Triticum are collinear, as chromosomes within each homoeologous group are related by descent from a chromosome of the ancestor of the Triticum-Aegilops complex (Akhunov et al. 2003). Hence, large numbers of cytogenetic markers have a similar localization in the same homoeologous group (McCouch 2001). Moreover, this genetic resemblance can hamper the use of GISH in some instances (Majka et al. 2017). The synteny between the homoeologous Aegilops and Triticum chromosomes may be disturbed because of chromosome rearrangements, which appeared during the evolution process (Devos et al. 1993; Zhang et al. 1998). Moreover, it is known that the level of chromosome synteny decreases the more distant a chromosome region is from the centromere. It is also decreased in regions with increased meiotic recombination rates, also known as hotspots of recombination on chromosome arms (Akhunov et al. 2003). Such changes result in distribution variability of chromosome markers. This review summarizes cyto-molecular techniques, which differentiate Aegilops and Triticum chromosomes, and are used most often for effective tracking of Aegilops chromosomes (or chromosome segments) in cultivated cereals.
Banding methods for identification of Aegilops chromatin introgression
Since the 1970s C-banding and N-banding techniques were used to distinguish the chromosomes of Aegilops-Triticum complex (Friebe et al. 1992; Gill and Kimber 1974; Landjeva and Ganeva 2000). C-banding has been employed to study genetic diversity and to create karyotypes of many Aegilops species. Giemsa C-banding was one of the first methods which allowed for identification of all 21 chromosome pairs of wheat (Endo 1986; Gill et al. 1991). This method was widely used to identify Aegilops-Triticum chromosome addition, substitution and translocation lines (Friebe et al. 1991; 1992; 1995; 1996a; 1996b; 1999; 2000; 2003). The results obtained by means of C-banding chromosome analysis of the majority of goatgrasses were reported in a series of articles describing the most important genomes of Aegilops (Badaeva et al. 1996a; 2002; 2004). C-banding analyses allowed the authors to discover that the S-genome of Ae.speltoides was most syntenic to B- and G-genomes of Triticum, but was different from other species of section Sitopsis (Badaeva et al. 1996a). Moreover, those authors observed minor polymorphisms in C-banding patterns of chromosomes of D-genome (Badaeva et al. 2002) and U-genome (Badaeva et al. 2004) belonging to different Aegilops species. All those results were later compared and confirmed by means of FISH studies (FISH methods are described in the third section of this review).
Polymorphisms in C-banding patterns were also utilised to distinguish Aegilops chromosomes in wheat genetic background. Ae.speltoides turned out to be one of the largest sources of valuable genes and was used to develop Aegilops-Triticum introgression lines. Friebe et al. (1991) used C-bands to establish the chromosome constitution of wheat streak mosaic virus (WSMV) and greenbug (Schizaphisgraminum Rondani, 1852) resistant lines, derived from wheat - Agropyronintermedium - Aegilopsspeltoides crosses. Three lines carried 7S(7A) chromosome substitution (derived from Ae.speltoides). The results indicated that the greenbug resistance gene Gb5 was located on chromosome 7S. This chromosome was also used to transfer leaf rust (caused by Pucciniatriticina Eriksson, 1899) resistance gene combined with greenbug resistance gene Gb5 into wheat genetic background (Dubcovsky et al. 1998). The authors induced a homologous recombination events using ph1b wheat mutant and developed Ti7AS-7S#1S-7AS.7AL translocation line conferring resistance to leaf rust and Ti7AS.7AL-7S#1L-7AL line conferring resistance to greenbug. The chromosome segments transferred from Ae.speltoides were characterized by means of C-banding and the fact of the translocation was supported by restriction fragment length polymorphisms (RFLP) analysis. Friebe et al. (1996a) applied C-banding analysis to identify T4AS.4AL-7S#2S chromosome translocations in wheat - Ae.speltoides lines with Lr28 leaf rust resistance gene. Moreover, a chromosome translocation (2B.2S) involved in the Lr35/Sr39 transfer derived from Ae.speltoides was identified using a C-banding method (Friebe et al. 1996a). C-banding technique was also used to determine the introgression carrying Yr8/Sr34 yellow rust and stem rust resistance genes from Ae.comosa Smith, 1806 into wheat. Miller (1988) detected 2AS-2ML.2MS and 2DS-2ML.2MS chromosome translocations. Friebe et al. (1992) adopted the C-banding method and identified complete set of chromosomes of Ae.caudata Linnaeus, 1753 in the amphiploid Triticumaestivum cv ‘Alcedo’ - Ae.caudata. Furthermore, the authors developed six chromosome addition lines in which the Ae.caudata chromosome pairs were called B, C, D, F, E and G. Friebe et al. (1995) established a karyotype of Ae.umbellulata Zhukovsky, 1928 using C-banding analysis of ten accessions collected in ten different geographic locations. This approach allowed for the identification of individual alien chromosomes in wheat-Ae.umbellulata chromosome monosomic and telosomic addition and wheat - Ae.umbellulata translocation lines (Friebe et al. 1995).
One of the most notable applications of the C-banding technique was the identification of radiation-induced translocation lines resistant to leaf rust (Lr9) and assignment of Lr9 loci to 6UL chromosome of Ae.umbellulata. The following chromosome translocations were identified by means of C-banding analysis: 6BL.6BS-6UL, T4BL.4BS-6UL, 2DS.2DL-6UL, T6BS.6BL-6UL and 7BL.7BS-6UL (Friebe et al. 1995). C-banding method was also used to identify 3BL.3BS-3S and 3DL.3DS-3S chromosome translocations conferring resistance to powdery mildew (Pm13 gene), which was transferred from Ae.longissima Schweinfurth & Muschler, 1912 into wheat (Ceoloni et al. 1992; Friebe et al. 1996a). Another powdery mildew gene (Pm32) was transferred from Ae.speltoides into wheat and T1BL-1SS chromosomal translocation was revealed by means of C-banding analysis (Hsam et al. 2003). However, in some cases the C-banding method was not sufficient to discriminate between Aegilops-Triticum translocations. For example, C-banding patterns of the translocated 7DL arms from Aegilopsventricosa Tausch, 1837, carrying Pch1 gene (responsible for resistance to eyespot) in cultivars Rendevous and Roazon was impossible to visualize as the patterns identified in 7DL chromosome of Chinese Spring wheat and 7DL of Ae.ventricosa were similar (Martin 1991). It was not until more sensitive C-banding protocol was applied that clear differences in the C-banding patterns between 7D of Chinese Spring and 7D of Ae.ventricosa were demonstrated by Badaeva et al. (2008). Another difficulty was reported by Apolinarska et al. (2010), who could not unambiguously identify the Aegilopsvariabilis Eig, 1929-rye chromosome translocations by means of C-banding.
The N-banding method was less often used to investigate Aegilops-Triticum introgression lines. Landjeva and Ganeva (1996; 2000) reported the N-banded karyotype of Aegilopsovata Linnaeus, 1753 (syn. Ae.geniculata Roth, 1787) and the chromosomal constitution of its partial amphiploid with bread wheat Triticumaestivum cv.‘Chinese Spring’. N-banding patterns made it possible to distinguish all Ae.ovata and wheat chromosomes. Ganeva et al. (2000) also used this technique, supported by gliadin electrophoresis, to reveal the structural changes in chromosomes 1A, 2A, 4B, 6B, 7B, 1D, and 2D of the Ae.umbellulata-wheat amphiploid (2n=6x=42, AABBUU), which showed leaf rust resistance conferred by Lr9 gene homolog. C- and N-banding methods are effective techniques to distinguish alien chromatin in a large number of introgression lines. However, the precise identification of translocation breakpoints requires additional supporting technique – in most cases genomic in situ hybridization (GISH) would suffice.
Fluorescence in situ hybridization methods for identification of Aegilops introgressions
A combination of molecular techniques and classical cytology became a breakthrough tool for science and crop breeding, especially for the development and characterization of Aegilops-Triticum introgression lines. First reports of adaptation of fluorescence in situ hybridization protocol for analyses of wheat chromosomes were published by Rayburn and Gill (1985) and Yamamoto and Mukai (1989). The ideal set of chromosome markers should cover the entire chromosome arms. This is a crucial requirement, which defines the usefulness of cytological landmarks for the identification of chromosome translocations. Hence, the most useful landmarks are DNA repetitive sequences that are richly represented in almost all chromosome regions, and can be used for evaluation of intra- and interspecific or intergeneric chromosome polymorphisms (Table 1).
Table 1.
Tandem repeats used as effective FISH markers for identification of Aegilops chromatin introgression.
| Tandem repeats | Clones/sequences | References |
|---|---|---|
| Satellite DNA sequences | pAs1, pSc119.2, pTa-71, pTa-86, pTa-465, pTa-535, pTa-566, pTa-713, pTa-794 | Badaeva et al. 1996a; b; 2015; Schneider et al. 2005; Zhao et al. 2016; Kwiatek et al. 2015; 2016a; 2016b; 2017a; 2017b; Goriewa-Duba et al. 2018 |
| Microsatellite DNA sequences (simple sequence repeats - SSR) | AAC, ACG, GAA | Molnar et al. 2005; 2011 |
To date the most popular probe used for identification of Aegilops-Triticum chromosomes is a D-genome specific repetitive DNA sequence called pAs1, derived from of Aegilopssquarrosa Linnaeus, 1753 (syn. Ae.tauschii Cosson, 1849; 2n = 14, genome DD) (Nagaki et al. 1995; Rayburn and Gill 1985). This sequence is AT rich (65.2%) and is widely distributed in many species of Aegilops-Triticum complex. It is included into Afa-family repeated sequences, because the recognition site of AfaI restriction enzyme was the most conserved sequence in this unit (Nagaki et al. 1995). Another much-used chromosome marker is a pSc119.2 repetitive sequence, derived from rye (Secalecereale) (Bedbrook et al. 1980). FISH landmarks of pSc119.2 and pAs1 are widely distributed in the chromosomes of Aegilops and Triticum species. A combination of those two probes was the first effective marker set used for chromosome identification of Triticum (Mukai et al. 1993) and Aegilops (Badaeva et al. 1996a; b; Schneider et al. 2005) species. However this set of markers was insufficient to describe some of Aegilops segments transferred into Triticum chromosomes. Hence, there was a need to develop more diversified and abundant chromosome landmarks.
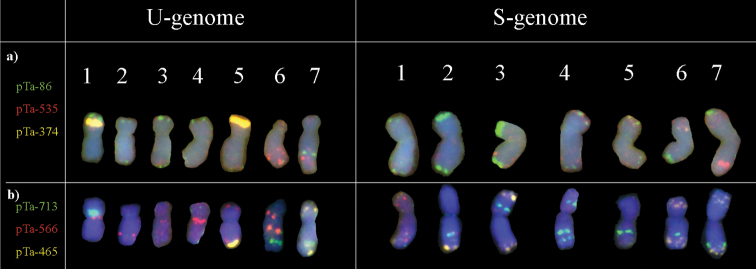
Vershinin et al. (1994) identified dpTa1 family of repetitive sequences that are present in subtelomeric and interstitial regions of chromosomes belonging to Aegilops-Triticum complex. Salina et al. (2004; 1998; 2009) isolated, characterized and designated repetitive sequence called Spelt-1, which is located in subtelomeric regions of Ae.speltoides. Another repetitive sequence, Spelt52, pGC1R-1 belongs to the family of tandem repeats pAesKB52, located at subtelomeric regions of chromosomes Ae.speltoides, Ae.longissima and Ae.sharonensis Eig, 1928 (Anamthawat-Jonsson and Heslop-Harrison 1993; Zhang et al. 2002; Salina et al. 2004). Additionally, Kishii and Tsujimoto (2002) characterized TaiI family of tandem repeats, which are localized to the centromeric regions. Moreover, there are some groups of repetitive sequences, originated from related genera such as Secale sp. (subtelomeric repeats represented by 350 family pSc200 and pSc250) (Vershinin et al. 1994) and Hordeumvulgare Linnaeus, 1753 (HvRT telomere-associated sequences) (Kilian and Kleinhofs 1992), which are also represented in chromosomes of cultivated wheat or triticale. Other repetitive sequences that effectively discriminate between Aegilops and Triticum chromosomes were derived from BAC libraries of species belonging to Triticeae tribe. Komuro et al. (2013) screened 2000 plasmid wheat clones for signal occurence using FISH. 47 clones showed distinct signals on wheat chromosomes, and clones pTa-86 and pTa-535 were related to pSc119.2 and pAs1, respectively (Komuro et al. 2013). Kwiatek et al. (2017a; 2017b) used pTa-86, pTa-103, pTa-k374, pTa-465, pTa-535, pTa-k566, and pTa-713 to discriminate between the chromosomes of Aegilopsbiuncialis de Visiani, 1851, Ae.ovata, respectively and Ae.kotschyi Boissier, 1846 (unpublished, Figure 1) which were transferred into a triticale genetic background. This set of chromosome markers allowed for the identification of 1BS-1BL.5ML, 5MS-5ML.1BL, 7US.6BS-6BL, 6BS.7US-7UL, 1BS-1BL.5ML and 5MS-5ML.6BL chromosome translocations (Kwiatek et al. 2017a). Zhao et al. (2016) combined pSc119.2, pTa71 and pTa-713 and identified each of the 14 pairs of Ae.variabilis chromosomes.
Figure 1.
Karyograms of Aegilopskotschyi 2n=4x=28 chromosomes; UUSS) showing U- and S-genome chromosomes after two rounds of FISH with: a pTa-86 (green; Atto-488 fluorochrome; Jena Bioscience), pTa-535 (red; Atto-550 fluorochrome; Jena Bioscience), pTa-374 (25S rDNA; yellow; Atto-647 fluorochrome; Jena Bioscience) and b pTa-713 (green; Atto-488 fluorochrome; Jena Bioscience), pTa-k566 (red; Atto-550 fluorochrome; Jena Bioscience) and pTa-465 (yellow; Atto-647 fluorochrome; Jena Bioscience) probes (Kwiatek, unpublished)
Apart from the use of long repetitive sequences, one of the most effective ways to saturate chromosome regions with markers as much as possible is to apply microsatellite sequences as cytomolecular probes. Such trinucleotide sequences (i.e. AAC, GAA, ACG) were used to distinguish between chromosomes of wheat (Cuadrado et al. 2000) and Aegilops (Molnar et al. 2011). Furthermore, GISH effectively complemented FISH analysis so as to locate and identify the Aegilops-Triticum chromosome translocation breakpoints (Friebe et al. 1992; Kwiatek et al. 2017a). A combination of banding techniques and FISH/GISH methods were used for precise Aegilops chromosome identification in a Triticum background during the development of introgression lines with valuable traits. Friebe et al. (1995) combined C-banding and GISH using total genomic DNA of Ae.umbellulata to identify the chromosome breakpoints in radiation-induced Triticum-Aegilops translocation lines resistant to leaf rust (Lr9), which involved 4B and 6B chromosomes of wheat and 4U chromosome of Ae.umbellulata. In addition, Friebe et al. (2003) used C-banding and FISH to identify Ae.sharonensis chromosomes carrying gametocidal genes in a wheat genetic background. A 4BS.4BL-4S chromosome translocation was identified using clone pGclR-1, which is a 258 bp fragment of a tandem repetitive element and hybridizes to telomeric and subtelomeric regions of Ae.speltoides, Ae.sharonensis, and Ae.longissima chromosomes (Friebe et al. 2000).
A combination of C-banding and GISH methods was also used for development of wheat introgression lines with resistance genes against one of the most virulent races of stem rust (Pucciniagraminisvar.tritici Persoon, 1794), namely Ug99. Liu et al. (2011a) used this combination of cytomolecular methods, supported by SSR marker analysis, to identify three Robertsonian translocations (T3AL·3SsS, T3BL·3SsS and T3DL·3SsS) and one recombinant (T3DS-3SsS·3SsL) line with stem rust resistance as a common feature of the analysed forms. Faris et al. (2008) examined a durum wheat-Aegilopsspeltoides chromosome translocation line (DAS15), which was resistant to Ug99 and six other races of stem rust. GISH methods made it possible to identify 2BL-2SL.2SS translocation, which harbours stem rust resistance. GISH was also used to identify the 5DL-5MgL·5MgS chromosome translocation, which introduced resistance to stem rust races RKQQC and TTKSK (Ug99) into wheat (Liu et al. 2011b). Chromosome 5Mg of Ae.geniculata is also a source of leaf and yellow rust resistance genes (Lr57 and Yr40, respectively). Kuraparthy et al. (2007) identified wheat-Ae.geniculata translocation lines (5DL·5DS-5MgS) using GISH. Molnar et al. (2005) combined GAA sequence probe with GISH to discriminate between the 1U, 2U, 4U and 5U chromosomes of Ae.biuncialis in wheat introgression lines, which showed limited tolerance to drought stress. Furthermore, Schneider et al. (2005) combined GISH and FISH using three repetitive DNA clones (pSc119.2, pAs1, and pTa71) to identify 2M, 3M, 7M, 3U, and 5U chromosome pairs in those lines. FISH/GISH methods, using pSc119.2, pAs1, 5S and 35S rDNA (from pTa71) sequence FISH probes together with GISH probes were also used to identify 2Dt and 3Dt chromosomes, carrying Lr39 and Lr32 genes, respectively in Ae.tauschii-triticale introgression lines (Kwiatek et al. 2015). The same set of FISH markers was used together with GISH to discriminate between 2S and 3S chromosomes of Ae.variabilis, which were transferred into triticale with intent to introduce the powdery mildew resistance gene Pm13 (Kwiatek et al. 2016a). Mirzaghaderi et al. (2014) observed FISH patterns of the Ut- and Ct -genome chromosomes of Ae.triuncialis Linnaeus, 1753 and Ae.cylindrica (Host, 1802) in wheat background. The following probes: pSc119.2-1, pTa535-1, pAs1-1, (CTT)10 and the 45S rDNA clone from wheat (pTa71), supported by GISH, were sufficient to discriminate between three different non-reciprocal homologous or heterologous translocations involving Cc and Dc chromosomes of Ae.cylindrica.
Modifications and changes of FISH protocols for identification of Aegilops introgressions
In order to screen large populations of Aegilops-Triticum introgression forms, the methods for cytomolecular marker analysis should be easy to handle and cost-efficient. FISH protocols require fluorescent DNA probes, heat treatment and are labour and time consuming. There are reports describing modifications and changes to the protocols used to conduct repetitive sequence preparation for FISH. One of such techniques, primed in situ labeling (PRINS), combines polymerase chain reaction (PCR) with FISH to visualize sequences on chromosomes (Koch et al. 1989). This technique is based on the annealing of short, sequence-specific unlabelled DNA to denatured chromosomes (Kubalakova et al. 2001). Tang et al. (2014) designed oligonucleotides to replace the repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for Aegilops-Triticum chromosome identification. Kwiatek et al. (2016b) and Goriewa-Duba et al. (2018) developed specific primers to amplify some of the repetitive sequences reported by Komuro et al. (2013) from wheat genomic DNA. This approach reduces the time and the costs of BAC library maintenance. The modifications of FISH protocols also facilitate the chromosome identification. Cuadrado and Jouve (2010) investigated telomeres of barley (Hordeumvulgare L.) using non-denaturing FISH (ND-FISH). This method was used to study chromosomes of Triticum (Fu et al. 2015). The analytical potential of this technique was demonstrated by Tang et al. (2018), who developed new oligo probes that make possible the identification of particular chromosomal segments, i.e.: the intercalary regions of 4AL and 2DL chromosome arms, and the pericentromeric regions of 3DL and 6DS arms of wheat chromosomes.
Another way to saturate the chromosome arms with markers is the use of cDNA probes. Danilova et al. (2014) carried out FISH experiment with more than 60 full length wheat cDNAs, which were selected using BLAST against mapped EST markers (expressed sequence tags). FISH analysis revealed 1U-6U chromosome translocation in Aegilopsumbellulata and showed synteny between chromosome A of Ae.caudata and group-1 wheat chromosomes. There are certain reports, showing technical modifications of FISH procedures, which reduce the time and costs of experiments. For example, Kwiatek et al. (2016b) used four different fluorescence labels (Atto488, Atto550, Atto647 and DAPI) that made possible the examination of three different probes at the same time. Of course, this approach requires investing in excitation wavelength specific filter cubes, which are cost-consuming. When there is a need to examine hundreds of plants resulting from genetic crosses, in some cases the time and labour consuming cytological methods could be substituted. For example, Rey and Prieto (2017) used dot-blot genomic hybridization experiments instead of microscopy to detect alien genetic introgressions to bread wheat.
Closing remarks: large scale selection of Aegilops-Triticum introgressions, perspectives for the future
Cytogenetic methods seem to be essential to verify genomic constitution in interspecific hybrids. The main problems are: limited sensitivity and spatial resolution, laborious and expensive protocols, which seriously limit the application of cytogenetic markers for large scale selection of Aegilops-Triticum introgressions. High-resolution and high-throughput methods are being progressively developed for identification of micro-introgressions, chromosome breakpoints and spatial localization of alien chromatin in donor nuclei. These require the use of new DNA markers, sequencing and new combinations of cytomolecular techniques. For example, three dimension FISH (3D-FISH) was applied to track the spatial organization of rye chromatin in wheat host genome (Burešová 2018). However, the main aim for development of Aegilops-Triticum introgressions is the transfer of desirable genes. Hence, there is a need to improve the cytogenetic methods for single gene physical mapping. Danilova et al. (2014) used single copy gene FISH with probes developed from cDNA of cytosolic acetyl-CoA carboxylase (ACCase) gene (Acc-2) and mapped them onto chromosomes of wheat. Another promising tool can be the combination of CRISPR (clustered regularly interspaced short palindromic repeats) with FISH. Deng et al. (2015) used a bacterial protein, CRISPR, combined with RNA sequences as probes to find the genes of interest. This method is comparably rapid and allows for keeping natural organization of the nucleus. What is more, CRISPR-FISH enables the analysis of spatial relationships between the genetic elements that are significant for gene expression. Apart from identification of Aegilops-Triticum introgressions, newly developed cytogenetic markers and methods could shed some light on the behaviour of chromatin, incorporated into the wheat genome, and show the results of the interaction between wheat genome and expression of introduced alien genes.
Acknowledgements
The authors would like to acknowledge and thank Dr. Harrold Bockelman at the USDA/ARS Small Grains Laboratory, Aberdeen (ID, USA) for providing the seeds of Aegilopskotschyi (PI 226615). This publication is being co-financed by the framework of Ministry of Science and Higher Education program as “Regional Initiative Excellence” in years 2019–2022, project no. 005/RID/2018/19. In addition, we would like to thank all of the reviewers and manuscript editor for their careful review of the manuscript and for their excellent suggestions for improving our initial work.
Citation
Kwiatek MT, Kurasiak-Popowska D, Mikołajczyk S, Niemann J, Tomkowiak A, Weigt D, Nawracała J (2019) Cytological markers used for identification and transfer of Aegilops spp. chromatin carrying valuable genes into cultivated forms of Triticum. Comparative Cytogenetics 13(1): 41–59. https://doi.org/10.3897/CompCytogen.v13i1.30673
References
- Akhunov ED, Akhunova AR, Linkiewicz AM, Dubcovsky J, Hummel D, Lazo G, Chao S, Anderson OD, David J, Qi L, Echalier B, Gill BS, Miftahudin J, Gustafson JP, La Rota M, Sorrells ME, Zhang D, Nguyen HT, Kalavacharla V, Hossain K, Kianian SF, Peng J, Lapitan NLV, Wennerlind EJ, Nduati V, Anderson JA, Sidhu D, Gill KS, McGuire PE, Qualset CO, Dvořák J. (2003) Synteny perturbations between wheat homoeologous chromosomes caused by locus duplications and deletions correlate with recombination rates. Proceedings of the National Academy of Sciences of the United States of America 100: 10836–10841. 10.1073/pnas.1934431100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anamthawat-Jonsson K, Heslop-Harrison JS. (1993) Isolation and characterization of genome-specific DNA sequences in Triticeae species. Molecular and General Genetics 240(2): 151–158. 10.1007/BF00277052 [DOI] [PubMed] [Google Scholar]
- Apolinarska B, Wiśeniewska H, Wojciechowska B. (2010) Aegilops-rye amphiploids and substitution rye used for introgression of genetic material into rye (Secalecereale L.). Journal of Applied Genetics 51: 413–420. 10.1007/bf03208871 [DOI] [PubMed] [Google Scholar]
- Badaeva ED, Amosova AV, Goncharov NP, Macas J, Ruban AS, Grechishnikova IV, Zoshchuk SA, Houben A. (2015) A set of cytogenetic markers allows the precise identification of all A-genome chromosomes in diploid and polyploid wheat. Cytogenetics Genome Research 146: 71–79. 10.1159/000433458 [DOI] [PubMed] [Google Scholar]
- Badaeva ED, Amosova AV, Muravenko OV, Samatadze TE, Chikida NN, Zelenin AV, Friebe B, Gill BS. (2002) Genome differentiation in Aegilops. 3. Evolution of the D-genome cluster. Plant Systematics and Evolution 231: 163–190. 10.1007/s006060200018 [DOI] [Google Scholar]
- Badaeva ED, Amosova AV, Samatadze TE, Zoshchuk SA, Shostak NG, Chikida NN, Zelenin AV, Raupp WJ, Friebe B, Gill BS. (2004) Genome differentiation in Aegilops. 4. Evolution of the U-genome cluster. Plant Systematics and Evolution 246: 45–76. 10.1007/s00606-003-0072-4 [DOI] [Google Scholar]
- Badaeva ED, Dedkova OS, Koenig J, Bernard S, Bernard M. (2008) Analysis of introgression of Aegilopsventricosa Tausch. genetic material in a common wheat background using C-banding. Theoretical and Applied Genetics 117: 803–811. 10.1007/s00122-008-0821-4 [DOI] [PubMed] [Google Scholar]
- Badaeva ED, Friebe B, Gill BS. (1996a) Genome differentiation in Aegilops. 1. Distribution of highly repetitive DNA sequences on chromosomes of diploid species. Genome 39: 293–306. 10.1139/g96-040 [DOI] [PubMed] [Google Scholar]
- Badaeva ED, Friebe B, Gill BS. (1996b) Genome differentiation in Aegilops. 2. Physical mapping of 5S and 18S-26S ribosomal RNA gene families in diploid species. Genome 39: 1150–1158. 10.1139/g96-145 [DOI] [PubMed] [Google Scholar]
- Bedbrook JR, Jones J, O’Dell M, Thompson RD, Flavell RB. (1980) A molecular description of telomeric heterochromatin in secale species. Cell 19: 545–560. 10.1016/00928674(80)90529-2 [DOI] [PubMed] [Google Scholar]
- Belyayev A, Raskina O. (1998) Heterochromatin discrimination in AegilopsSpeltoides by simultaneous genomic in situ hybridization. Chromosome Research 6: 559–566. 10.1023/a:1009292726034 [DOI] [PubMed] [Google Scholar]
- Belyayev A, Raskina O, Nevo E. (2001a) Detection of alien chromosomes from S-genome species in the addition/substitution lines of bread wheat and visualization of A-, B- and D-genomes by GISH. Hereditas 135: 119–122. 10.1111/j.1601-5223.2001.00119.x [DOI] [PubMed] [Google Scholar]
- Belyayev A, Raskina O, Nevo E. (2001b) Evolutionary dynamics and chromosomal distribution of repetitive sequences on chromosomes of Aegilopsspeltoides revealed by genomic in situ hybridization. Heredity (Edinburg) 86: 738–742. 10.1046/j.1365-2540.2001.00891.x [DOI] [PubMed] [Google Scholar]
- Burešová V. (2018) Spatial organization of rye chromatin in wheat host genome revealed by 3D-FISH. EMBO Workshop, Plant Genome Stability and Change. Gatersleben, Germany, 13 pp. [Google Scholar]
- Ceoloni C, Signore Gd, Ercoli L, Donini P. (1992) Locating the alien chromatin segment in common wheat-Aegilopslongissima mildew resistant transfers. Hereditas 116: 239–245. 10.1111/j.16015223.1992.tb00148.x [DOI] [Google Scholar]
- Cuadrado A, Cardoso M, Jouve N. (2008) Increasing the physical markers of wheat chromosomes using SSRs as FISH probes. Genome 51: 809–815. 10.1139/g08-065 [DOI] [PubMed] [Google Scholar]
- Cuadrado Á, Jouve N. (2010) Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH). Chromosoma 119: 495–503. 10.1007/s00412-010-0273-x [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Schwarzacher T, Jouve N. (2000) Identification of different chromatin classes in wheat using in situ hybridization with simple sequence repeat oligonucleotides. Theoretical and Applied Genetics 101: 711–717. 10.1007/s001220051535 [DOI] [Google Scholar]
- Danilova TV, Friebe B, Gill BS. (2014) Development of a wheat single gene FISH map for analyzing homoeologous relationship and chromosomal rearrangements within the Triticeae. Theoretical and Applied Genetics 127: 715–730. 10.1007/s00122-013-2253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Shi X, Tjian R, Lionnet T, Singer RH. (2015) CASFISH: CRISPR/Cas9-mediated in situ labelling of genomic loci in fixed cells. Proceedings of the National Academy of Sciences 112: 11870–11875. 10.1073/pnas.1515692112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos KM, Atkinson MD, Chinoy CN, Francis HA, Harcourt RL, Koebner RMD, Liu CJ, Masojć P, Xie DX, Gale MD. (1993) Chromosomal rearrangements in the rye genome relative to that of wheat. Theoretical and Applied Genetics 85: 673–680. 10.1007/bf00225004 [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Lukaszewski AJ, Echaide M, Antonelli EF, Porter DR. (1998) Molecular characterization of two Triticumspeltoides interstitial translocations carrying leaf rust and greenbug resistance genes. Crop Science 38: 1655–1660. 10.2135/cropsci1998.0011183X003800060040x [DOI] [Google Scholar]
- Dvořák J, Luo MC, Yang ZL, Zhang HB. (1998) The structure of Aegilopstauschii genepool and the evolution of hexaploid wheat. Theoretical and Applied Genetics 97(4): 657–670. 10.1007/s001220050942 [DOI] [Google Scholar]
- Dvořák J, Terlizzi P, Zhang HB, Resta P. (1993) The evolution of polyploid wheats: identification of the A genome donor species. Genome 36(1): 21–31. 10.1139/g93-004 [DOI] [PubMed] [Google Scholar]
- Endo TR. (1986) Complete identification of common wheat chromosomes by means of the C-banding technique. The Japanese Journal of Genetics 61: 89–93. 10.1266/jjg.61.89 [DOI] [Google Scholar]
- Endo T, Gill BS. (1984) Somatic karyotype, heterochromatin distribution, and nature of chromosome differentiation in common wheat, Triticumaestivum L. em Thell. Chromosoma 89: 361–369. 10.1007/BF00331253 [DOI] [Google Scholar]
- Faris JD, Xu SS, Cai X, Friesen TL, Jin Y. (2008) Molecular and cytogenetic characterization of a durum wheat-Aegilopsspeltoides chromosome translocation conferring resistance to stem rust. Chromosome Research 16: 1097–1105. 10.1007/s10577-008-1261-3 [DOI] [PubMed] [Google Scholar]
- Feldman M, Levy AA. (2012) Genome evolution due to allopolyploidization in wheat. Genetics 192: 763774. 10.1534/genetics.112.146316 [DOI] [PMC free article] [PubMed]
- Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS. (1996a) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91: 59–87. 10.1007/bf00035277 [DOI] [Google Scholar]
- Friebe B, Jiang J, Tuleen N, Gill BS. (1995) Standard karyotype of Triticumumbellulatum and the characterization of derived chromosome addition and translocation lines in common wheat. Theoretical and Applied Genetics 90: 150–156. 10.1007/bf00221010 [DOI] [PubMed] [Google Scholar]
- Friebe B, Kynast RG, Gill BS. (2000) Gametocidal factor-induced structural rearrangements in rye chromosomes added to common wheat. Chromosome Research 8: 501–511. 10.1023/a:1009219722418 [DOI] [PubMed] [Google Scholar]
- Friebe B, Mukai Y, Dhaliwal HS, Martin TJ, Gill BS. (1991) Identification of alien chromatin specifying resistance to wheat streak mosaic and greenbug in wheat germ plasm by C-banding and in situ hybridization. Theoretical and Applied Genetics 81: 381–389. 10.1007/bf00228680 [DOI] [PubMed] [Google Scholar]
- Friebe B, Tuleen NA, Badaeva ED, Gill BS. (1996b) Cytogenetic identification of Triticumperegrinum chromosomes added to common wheat. Genome 39: 272–276. 10.1139/g96-037 [DOI] [PubMed] [Google Scholar]
- Friebe B, Tuleen NA, Gill BS. (1999) Development and identification of a complete set of Triticumaestivum-Aegilopsgeniculata chromosome addition lines. Genome 42: 374–380. 10.1139/gen-42-3-374 [DOI] [Google Scholar]
- Friebe B, Schubert V, Blüthner WD, Hammer K. (1992) C-banding pattern and polymorphism of Aegilopscaudata and chromosomal constitutions of the amphiploid T.aestivum-Ae.caudata and six derived chromosome addition lines. Theoretical and Applied Genetics 83: 589–596. 10.1007/bf00226902 [DOI] [PubMed] [Google Scholar]
- Friebe B, Zhang P, Gill BS, Nasuda S. (2003) Characterization of a knock-out mutation at the Gc2 locus in wheat. Chromosoma 111: 509–517. 10.1007/s00412-003-0234-8 [DOI] [PubMed] [Google Scholar]
- Fu S, Chen L, Wang Y, Li M, Yang Z, Qiu L, Yan B, Ren Z, Tang Z. (2015) Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Scientific Reports 5: 10552. 10.1038/srep10552 [DOI] [PMC free article] [PubMed]
- Ganeva G, Georgieva V, Panaiotova M, Stoilova T, Balevska P. (2000) The transfer of genes for brown rust resistance from Aegilopsumbellulata Eig. to wheat (Triticumaestivum L.) genome. Genetika 36: 71–76. [PubMed] [Google Scholar]
- Gerlach WL, Bedbrook JR. (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Research 7: 1869–1885. 10.1093/nar/7.7.1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach WL, Dyer TA. (1980) Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Research 8: 4851–4865. 10.1093/nar/8.21.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill BS, Friebe B, Endo TR. (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticumaestivum). Genome 34: 830–839. 10.1139/g91-128 [DOI] [Google Scholar]
- Gill BS, Kimber G. (1974) Giemsa C-banding and the evolution of wheat. Proceedings of the National Academy of Sciences of the United States of America 71: 4086–4090. 10.1073/pnas.71.10.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnina KA, Kondratenko EY, Blinov AG, Goncharov NP. (2009) Phylogeny of the A genomes of wild and cultivated wheat species. Russian Journal of Genetics 45(11): 1360–1367. 10.1134/S1022795409110106 [DOI] [PubMed] [Google Scholar]
- Goncharov NP. (2011) Genus Triticum L. taxonomy: the present and the future. Plant Systematics and Evolution 295: 1–11. 10.1007/s00606-011-0480-9 [DOI] [Google Scholar]
- Goriewa-Duba K, Duba A, Kwiatek M, Wiśniewska H, Wachowska U, Wiwart M. (2018) Chromosomal distribution of pTa-535, pTa-86, pTa-713, 35S rDNA repetitive sequences in interspecific hexaploid hybrids of common wheat (Triticumaestivum L.) and spelt (Triticumspelta L.). PLoS One 13: e0192862. 10.1371/journal.pone.0192862 [DOI] [PMC free article] [PubMed]
- Hsam SLK, Lapochkina IF, Zeller FJ. (2003) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticumaestivum L. em Thell.). 8. Gene Pm32 in a wheat-Aegilopsspeltoides translocation line. Euphytica 133: 367–370. 10.1023/a:1025738513638 [DOI] [Google Scholar]
- Iordansky AB, Zurabishvili TB, Badaev NS. (1978) Linear differentiation of cereal chromosomes I. Common wheat and its supposed ancestors. Theoretical and Applied Genetics 51: 145–152. 10.1007/BF00273138 [DOI] [PubMed] [Google Scholar]
- Jiang J, Gill BS. (2006) Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49: 1057–1068. 10.1139/g06-076 [DOI] [PubMed] [Google Scholar]
- Kihara H. (1924) Cytologische und genetische studien bei wichtigen getreidearten mit besonderer rücksicht auf das verhalten der chromosomen und die sterilität in den bastarden. Memoirs of the College of Science, Kyoto Imperial University 1: 1–200. [Google Scholar]
- Kihara H. (1954) Considerations on the evolution and distribution of Aegilops species based on the analyser-method. Cytologia: 19 336–357. 10.1508/cytologia.19.336 [DOI]
- Kilian A, Kleinhofs A. (1992) Cloning and mapping of telomere-associated sequences from Hordeumvulgare L. Molecular and General Genetics 235: 153–156. 10.1007/bf00286193 [DOI] [PubMed] [Google Scholar]
- Kilian B, Mammen K, Millet E, Sharma R, Graner A, Salamini F, Hammer K, Özkan H. (2011) Aegilops. In: Kole C. (Ed.) Wild crop relatives: genomic and breeding resources: Cereals.Springer Berlin Heidelberg, Berlin, Heidelberg, 1–76. 10.1007/978-3-642-14228-4_1 [DOI]
- Kishii M, Tsujimoto H. (2002) Genus-specific localization of the TaiI family of tandem-repetitive sequences in either the centromeric or subtelomeric regions in Triticeae species (Poaceae) and its evolution in wheat. Genome 45: 946–955. 10.1139/g02-059 [DOI] [PubMed] [Google Scholar]
- Koch JE, Kølvraa S, Petersen KB, Gregersen N, Bolund L. (1989) Oligonucleotide-priming methods for the chromosome-specific labelling of alpha satellite DNA in situ. Chromosoma 98: 259–265. 10.1007/bf00327311 [DOI] [PubMed] [Google Scholar]
- Komuro S, Endo R, Shikata K, Kato A. (2013) Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 56: 131–137. 10.1139/gen-2013-0003 [DOI] [PubMed] [Google Scholar]
- Konarev A, Gavrilyuk I, Migushova E. (1974) Differentiation of diploid wheats as indicated by immunochemical analysis. Doklady Vsesoyuznoj Akademii Selskohozyajstvennih Nauk (Proceedings of All-Union Academy of Agricultural Sciences) (USSR) 6: 12. [In Russian]
- Kubalakova M, Vrana J, Cihalikova J, Lysak MA, Dolezel J. (2001) Localisation of DNA sequences on plant chromosomes using PRINS and C-PRINS. Methods in Cell Science 23: 71–82. 10.1023/A:1013193516001 [DOI] [PubMed] [Google Scholar]
- Kuraparthy V, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, Gill BS. (2007) Characterization and mapping of cryptic alien introgression from Aegilopsgeniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theoretical and Applied Genetics 114: 1379–1389. 10.1007/s00122-007-0524-2 [DOI] [PubMed] [Google Scholar]
- Kwiatek M, Belter J, Majka M, Wisniewska H. (2016a) Allocation of the S-genome chromosomes of Aegilopsvariabilis Eig. carrying powdery mildew resistance in triticale (x Triticosecale Wittmack). Protoplasma 253: 329–343. 10.1007/s00709-015-0813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek M, Majka M, Majka J, Belter J, Suchowilska E, Wachowska U, Wiwart M, Wiśniewska H. (2016b) Intraspecific polymorphisms of cytogenetic markers mapped on chromosomes of Triticumpolonicum L. PLoS One 11: e0158883. 10.1371/journal.pone.0158883 [DOI] [PMC free article] [PubMed]
- Kwiatek M, Majka M, Wisniewska H, Apolinarska B, Belter J. (2015) Effective transfer of chromosomes carrying leaf rust resistance genes from Aegilopstauschii Coss. into hexaploid triticale (X Triticosecale Witt.) using Ae.tauschii x Secalecereale amphiploid forms. Journal of Applied Genetics 56: 163168. 10.1007/s13353-014-0264-3 [DOI] [PMC free article] [PubMed]
- Kwiatek MT, Majka J, Majka M, Belter J, Wisniewska H. (2017a) Adaptation of the pivotal-differential genome pattern for the induction of intergenomic chromosome recombination in hybrids of synthetic amphidiploids within Triticeae Tribe. Frontiers in Plant Science 8. 10.3389/fpls.2017.01300 [DOI] [PMC free article] [PubMed]
- Kwiatek MT, Wiśniewska H, Ślusarkiewicz-Jarzina A, Majka J, Majka M, Belter J, Pudelska H. (2017b) Gametocidal factor transferred from Aegilopsgeniculata Roth can be adapted for large-scale chromosome manipulations in cereals. Frontiers in Plant Science 8. 10.3389/fpls.2017.00409 [DOI] [PMC free article] [PubMed]
- Landjeva S, Ganeva G. (1996) N-banded karyotype of Aegilopsovata and chromosomal constitution of its amphiploid with Triticumaestivum. Plant Breeding 115: 330–334. 10.1111/j.14390523.1996.tb00928.x [DOI] [Google Scholar]
- Landjeva SP, Ganeva GD. (2000) Chromosome N-banding polymorphism in Aegilopsgeniculata Roth. Genetic Resources and Crop Evolution 47: 35–41. 10.1023/a:1008723220664 [DOI] [Google Scholar]
- Le HT, Armstrong KC, Miki B. (1989) Detection of rye DNA in wheat-rye hybrids and wheat translocation stocks using total genomic DNA as a probe. Plant Molecular Biology Reporter 7: 150–158. 10.1007/bf02669631 [DOI] [Google Scholar]
- Leitch AR, Mosgoller W, Schwarzacher T, Bennett MD, Heslop-Harrison JS. (1990) Genomic in situ hybridization to sectioned nuclei shows chromosome domains in grass hybrids. Journal of Cell Science 95: 335–341 [DOI] [PubMed] [Google Scholar]
- Li L-F, Liu B, Olsen KM, Wendel JF. (2015) Multiple rounds of ancient and recent hybridizations have occurred within the Aegilops-Triticum complex. New Phytologist 208: 11–12. 10.1111/nph.13563 [DOI] [PubMed] [Google Scholar]
- Liu W, Jin Y, Rouse M, Friebe B, Gill B, Pumphrey MO. (2011a) Development and characterization of wheat-Ae.searsii Robertsonian translocations and a recombinant chromosome conferring resistance to stem rust. Theoretical and Applied Genetics 122: 1537–1545. 10.1007/s00122-011-1553-4 [DOI] [PubMed] [Google Scholar]
- Liu W, Rouse M, Friebe B, Jin Y, Gill B, Pumphrey MO. (2011b) Discovery and molecular mapping of a new gene conferring resistance to stem rust, Sr53, derived from Aegilopsgeniculata and characterization of spontaneous translocation stocks with reduced alien chromatin. Chromosome Research 19: 669–682. 10.1007/s10577-011-9226-3 [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ, Xu X. (1995) Screening large populations of wheat hybrids by C-banding. Cereal Research Communications 23: 9–13. [Google Scholar]
- Majka J, Majka M, Kwiatek M, Wiśniewska H. (2017) Similarities and differences in the nuclear genome organization within Pooideae species revealed by comparative genomic in situ hybridization (GISH). Journal of Applied Genetics 58: 151–161. 10.1007/s13353-016-0369-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. (1991) Untersuchungen zur charakterisierung und identifizierung von Aegilopsventricosa chromosomen und deren nutzung in der weizenzüchtung. Ph.D. Thesis: Technical University of Munich, Germany.
- McFadden FE, Sears ER. (1946) The origin of Triticumspelta and its free-threshing hexaploid relatives. Journal of Heredity 37: 81–107. 10.1093/oxfordjournals.jhered.a105590 [DOI] [PubMed] [Google Scholar]
- McCouch SR. (2001) Genomics and synteny. Plant Physiology 125: 152–155. 10.1104/pp.125.1.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TE. (1988) The introduction of a major gene for resistance to powdery mildew of wheat, Erysiphegraminisf.sp.tritici, from Ae.speltoides into wheat to integrated cereal production. In: EUCARPIA Cereal Section Meeting. Wageningen, The Netherlands, 179–183.
- Mirzaghaderi G, Houben A, Badaeva ED. (2014) Molecular-cytogenetic analysis of Aegilopstriuncialis and identification of its chromosomes in the background of wheat. Molecular Cytogenetics 7: 91. 10.1186/s13039-014-0091-6 [DOI] [PMC free article] [PubMed]
- Mirzaghaderi G, Mason AS. (2017) Revisiting pivotal-differential genome evolution in wheat. Trends in Plant Science 22: 674–684. 10.1016/j.tplants.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Molnar I, Cifuentes M, Schneider A, Benavente E, Molnar-Lang M. (2011) Association between simple sequence repeat-rich chromosome regions and intergenomic translocation breakpoints in natural populations of allopolyploid wild wheats. Annals of Botany 107: 65–76. 10.1093/aob/mcq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar I, Schneider A, Molnar-Lang M. (2005) Demonstration of Aegilopsbiuncialis chromosomes in a wheat background by genomic in situ hybridization (GISH) and identification of U chromosomes by FISH using GAA sequences. Cereal Research Communications 33: 673–680. 10.1556/CRC.33.2005.2-3.134 [DOI] [Google Scholar]
- Mukai Y, Nakahara Y, Yamamoto M. (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36: 489–494. 10.1139/g93-067 [DOI] [PubMed] [Google Scholar]
- Nagaki K, Tsujimoto H, Isono K, Sasakuma T. (1995) Molecular characterization of a tandem repeat, Afa family, and its distribution among Triticeae. Genome 38: 479–486. 10.1139/g95-063 [DOI] [PubMed] [Google Scholar]
- Ozkan H, Tuna M, Arumuganathan K. (2003) Nonadditive Changes in Genome Size During Allopolyploidization in the Wheat (Aegilops-Triticum) Group. Journal of Heredity 94: 260–264. 10.1093/jhered/esg053 [DOI] [PubMed] [Google Scholar]
- Petersen G, Seberg O, Yde M, Berthelsen K. (2006) Phylogenetic Relationships of Triticum and Aegilops and Evidence for the Origin of the A, B, and D Genomes of Common Wheat (Triticumaestivum). Molecular Phylogenetics and Evolution 39(1): 70–82. 10.1016/j.ympev.2006.01.023 [DOI] [PubMed] [Google Scholar]
- Rakszegi M, Molnár I, Lovegrove A, Darkó É, Farkas A, Láng L, Bedő Z, Doležel J, Molnár-Láng M, Shewry P. (2017) Addition of Aegilops U and M Chromosomes Affects Protein and Dietary Fiber Content of Wholemeal Wheat Flour. Frontiers in Plant Science 8. 10.3389/fpls.2017.01529 [DOI] [PMC free article] [PubMed]
- Rayburn AL, Gill BS. (1985) Use of biotin-labeled probes to map specific DNA sequences on wheat chromosomes. Journal of Heredity 76: 78–81. 10.1093/oxfordjournals.jhered.a110049 [DOI] [Google Scholar]
- Rey M-D, Prieto P. (2017) Detection of alien genetic introgressions in bread wheat using dot-blot genomic hybridisation. Molecular Breeding 37: 32. 10.1007/s11032-017-0629-5 [DOI] [PMC free article] [PubMed]
- Ruban A, Badaeva ED. (2018) Evolution of the S-Genomes in Triticum-Aegilops alliance: evidences from chromosome analysis. Frontiers in Plant Science 9:1756. 10.3389/fpls.2018.01756 [DOI] [PMC free article] [PubMed]
- Sarkar P, Stebbins GL. (1956) Morphological evidence concerning the origin of the B genome in wheat. American Journal of Botany 43: 297–304. 10.1002/j.1537-2197.1956.tb10494.x [DOI] [Google Scholar]
- Salina EA, Numerova OM, Ozkan H, Feldman M. (2004) Alterations in subtelomeric tandem repeats during early stages of allopolyploidy in wheat. Genome 47: 860–867. 10.1139/g04-044 [DOI] [PubMed] [Google Scholar]
- Salina EA, Pestsova EG, Adonina IG, Vershinin AV. (1998) Identification of a new family of tandem repeats in Triticeae genomes. Euphytica 100: 231–237. 10.1023/a:1018360324242 [DOI] [Google Scholar]
- Salina EA, Sergeeva EM, Adonina IG, Shcherban AB, Afonnikov DA, Belcram H, Huneau C, Chalhoub B. (2009) Isolation and sequence analysis of the wheat B genome subtelomeric DNA. BMC Genomics 10: 414. 10.1186/1471-2164-10-414 [DOI] [PMC free article] [PubMed]
- Salse J, Bolot S, Throude M, Jouffe V, Piegu B, Quraishi UM, Calcagno T, Cooke R, Delseny M, Feuillet C. (2008) Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell 20: 11–24. 10.1105/tpc.107.056309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Linc G, Molnar I, Molnar-Lang M. (2005) Molecular cytogenetic characterization of Aegilopsbiuncialis and its use for the identification of 5 derived wheat-Aegilopsbiuncialis disomic addition lines. Genome 48: 1070–1082. 10.1139/g05-062 [DOI] [PubMed] [Google Scholar]
- Schwarzacher T, Anamthawat-Jónsson K, Harrison GE, Islam AKMR, Jia JZ, King IP, Leitch AR, Miller TE, Reader SM, Rogers WJ, Shi M, Heslop-Harrison JS. (1992) Genomic in situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theoretical and Applied Genetics 84: 778–786. 10.1007/bf00227384 [DOI] [PubMed] [Google Scholar]
- Schwarzacher T, Leitch A, Bennett M, Heslop-Harrison J. (1989) In situ localization of parental genomes in a wide hybrid. Annals of Botany 64: 315–324. 10.1093/oxfordjournals.aob.a087847 [DOI] [Google Scholar]
- Sears ER. (1948) The cytology and genetics of the wheats and their relatives. Advances in Genetics 3b: 239–270. 10.1016/S0065-2660(08)60470-8 [DOI] [PubMed]
- Slageren van MW. (1994) Wild wheats: a monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae). Agriculture University Papers, Wageningen, 512 pp. [Google Scholar]
- Tang S, Tang Z, Qiu L, Yang Z, Li G, Lang T, Zhu W, Zhang J, Fu S. (2018) Developing new oligo probes to distinguish specific chromosomal segments and the A, B, D genomes of wheat (Triticumaestivum L.) using ND-FISH. Frontiers in Plant Science 9. 10.3389/fpls.2018.01104 [DOI] [PMC free article] [PubMed]
- Tang Z, Li M, Chen L, Wang Y, Ren Z, Fu S. (2014) New types of wheat chromosomal structural variations in derivatives of wheat-rye hybrids. PLoS One 9: e110282. 10.1371/journal.pone.0110282 [DOI] [PMC free article] [PubMed]
- Vershinin A, Svitashev S, Gummesson PO, Salomon B, von Bothmer R, Bryngelsson T. (1994) Characterization of a family of tandemly repeated DNA sequences in Triticeae. Theoretical Applied Genetics 89: 217–225. 10.1007/bf00225145 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Mukai Y. (1989) Application of fluorescence in situ hybridization to molecular cytogenetics of wheat. Wheat Information Service: 30–32.
- Zhang H, Jia J, Gale MD, Devos KM. (1998) Relationships between the chromosomes of Aegilopsumbellulata and wheat. Theoretical and Applied Genetics 96: 69–75. 10.1007/s001220050710 [DOI] [Google Scholar]
- Zhang W, Qu L, Gu H, Gao W, Liu M, Chen J, Chen Z. (2002) Studies on the origin and evolution of tetraploid wheats based on the internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. Theoretical and Applied Genetics 104: 1099–1106. 10.1007/s00122-002-0887-3 [DOI] [PubMed] [Google Scholar]
- Zhang P, Li W, Fellers J, Friebe B, Gill BS. (2004) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112: 288–299. 10.1007/s00412-004-0273-9 [DOI] [PubMed] [Google Scholar]
- Zhao L, Ning S, Yu J, Hao M, Zhang L, Yuan Z, Zheng Y, Liu D. (2016) Cytological identification of an Aegilopsvariabilis chromosome carrying stripe rust resistance in wheat. Breeding Science 66: 522529. 10.1270/jsbbs.16011 [DOI] [PMC free article] [PubMed]
- Zohary D, Feldman M. (1962) Hybridization between amphidiploids and the evolution of polyploids in the wheat (Aegilops-Triticum) group. Evolution 16: 44–61. 10.1111/j.1558-5646.1962.tb03197.x [DOI] [Google Scholar]