Abstract
In the present study, phosphate solubilizing rhizobacterial isolate STJP from the rhizosphere of Stevia rebaudiana was identified as a Bacillus sp. on the basis of phenotypic, biochemical, and 16S rRNA gene sequencing. In addition to phosphate solubilization ability, isolate Bacillus sp. STJP produced a significant quantity of siderophore (16.06 µg/ml) and indole 3-acetic acid (30.59 µg/ml). In the greenhouse experiment, treatment with STJP along with tricalcium phosphate (TCP200) showed significant increase in the plant growth parameters, oil yield and P uptake in M. arvensis as compared to the control plants. Amongst all the treatments, highest oil yield and menthol content were observed when treated with Bacillus sp. STJP + TCP200. Hence, an integrated approach of using Bacillus sp. STJP along with TCP can be used to increase the production of menthol and oil yield of M. arvensis. This approach of using fertilizer along with phosphate solubilizing Bacillus sp. worked very well and was more effective in comparison with individual treatment of fertilizer or plant growth promoting rhizobacteria. A combined use of efficient phosphate solubilising bacteria loaded with plant growth promoting characters along with TCP can thus be far effective way for enhancing the yield of crops in a sustainable manner.
Keywords: Bacillus, Mentha arvensis, Menthol, Plant growth promoting rhizobacteria, Sustainable agriculture
Introduction
Medicinal and aromatic plants (MAPs) have proved to be the major sources of medicines in the world. They are extensively used for the treatment of several diseases and boost the economy as well as the living standard of farmers in several countries including India (WHO 2013). But nowadays, assurance of the efficacy, quality, and supply of MAPs and their products has become a major issue around the globe. Day by day, we are losing the natural reserves of MAPs mainly due to degradation of habitats (Stutte 2016). Hence, the need has arisen to cultivate the MAPs in an eco-friendly manner so as to maintain their continuous supply to get the useful products. For the cultivation of MAPs, the use of biological methods, so as to enhance the productivity is better and this can reduce the load of chemicals in the agro-ecosystems (Singh and Arora 2016). The use of plant growth promoting rhizobacteria (PGPR) can thus be more useful and good for the environment.
Mentha arvensis (Japanese mint or menthol mint) of family Lamiaceae, is widely used for the treatment of several diseases such as abdominal pain, cough, diarrhoea, fever, headache, nausea, sunburn, skin irritation and vomiting (Alankar 2009). The leaves of M. arvensis contain high levels of flavonoids and antioxidants. In addition, M. arvensis also contains an important secondary metabolite known as menthol which has potential applications in several industries including pharmaceutical, flavoring, food, agrochemicals and cosmetics. The worldwide production of menthol is estimated as 20,000 metric tons every year (Srivastava et al. 2002). India is the chief menthol producer (more than 80% of the total produce in the world) and its leading exporter as well (Bakry et al. 2016). Approximately, 0.145 million hectares (ha) of agricultural land is being used for growing M. arvensis in northern India’s Indo-Gangetic plains (Kumar et al. 2011). Day by day the demand for M. arvensis and its essential oil is increasing; hence it is important to increase the yields without affecting the quality and purity of agro-ecosystems.
Nutrient deficiency in soils is one of the major issue for agricultural production around the globe affecting the quantity and quality of crops (Tewari and Arora 2016). For the improvement of crop yields, farmers have commonly used chemical fertilizers for agricultural production causing an adverse effect on autochthonous organisms and deteriorating the quality of agro-ecosystems and aquatic resources (Arora et al. 2016). Most of the phosphate fertilizers remain unused and accumulate in the soil as precipitates. Apart from this, the accumulated phosphates (in soil) may also reach water bodies causing eutrophication. Therefore, the use of phosphate solubilising microbes along with the applied phosphate can provide a solution to the menace of accumulation of phosphates in the fields and water bodies. Bacillus sp. are well-known rhizobacteria which facilitate the plant growth either by solubilization of minerals like phosphorus or production of metabolites such as siderophores and phytohormones, and are excellent colonizers of the roots (Ahmad et al. 2018). In addition, Bacillus spp. have added advantage as their communities are found in the rhizosphere of wide varieties of crop plants and can survive stress condition very well (Abd-Allah et al. 2018). Use of Bacillus sp. has become a very important practice for eco-friendly and sustainable agriculture for enhancing crop production and control phytopathogens (Alori et al. 2017). An integrating approach of utilizing Bacillus spp. having multiple capabilities (including phosphate solubilization) along with phosphate fertilizers can enhance the growth of plants and maintain soil fitness even in presence of biotic and abiotic stresses (Mishra et al. 2016; Tewari and Arora 2018; Gouda et al. 2018).
Phosphate solubilizing bacteria (PSB) are emerging as important biofertilizers with the ability to utilize precipitated phosphorus (P) in the soil, leading to eco-friendly agriculture and maintaining the quality of agro-ecosystems (Pereira et al. 2015; Alori et al. 2017). PSB based biofertilizers are cheaper and can be of particular interest in nutrient (phosphorus) deficient soils (Shen et al. 2011). Although, in last few years, the role of PSB as attractive biofertilizers is on the rise, still, there are negligible reports on their utilization as biofertilizers with commercial use for enhancing the growth of M. arvensis and improving its oil content. With this aim, in the present study, PSB were isolated and checked for their plant growth promoting (PGP) characters followed by evaluation of the ability to enhance the growth, oil yield, and quantity of menthol in M. arvensis.
Materials and methods
Isolation and characterization of phosphate solubilizing rhizobacteria
PSB were isolated from the rhizosphere soil of S. rebaudiana, a medicinal plant growing in farmer’s field at Lucknow (26.7679°N, 80.9263°E), Uttar Pradesh, India. For isolation, 1 g of sample was dissolved in 9 ml of sterile water (pH 7.1). From fifth dilution (10−5), 0.1 ml of soil sample was spread on Pikovskaya’s (PVK) agar (Pikovskaya 1948) containing 0.5% tri-calcium phosphate and incubated at 28 °C for 72–120 h for checking phosphate solubilization. Isolates were also grown at 30 °C for 120 h in National Botanical Research Institute Phosphate (NBRIP) broth medium to check the amount of phosphate solubilization. The soluble P content (µg/ml) was determined by spectrophotometer at 600 nm (Nautiyal 1999). Selected isolate STJP (on the basis of phosphate solubilization ability) was identified by phenotypic (flagellated or non-flagellated, shape and size) and biochemical features such as oxidase, urease, catalase, gelatinase, indole, H2S, citrate, and nitrate production as per Bergey’s Manual of Systematic Bacteriology (Garrity 2005). The cell shape of the bacterium was confirmed by scanning electron microscopy (SEM) performed according to the method of Golding et al. (2016). Isolate STJP was further identified by 16S rRNA sequencing and sequence analysis (Maidak et al. 2000). The evolutionary distance of isolate STJP and the closely related taxa was calculated using MEGA (version 6.00) software.
Phosphatase enzyme assay
Phosphatase enzyme assay was performed as per the method of Tabatabai and Bremner (1969). Briefly, 100 µl bacterial culture was inoculated in NBRIP broth (50 ml) and incubated at 28 °C for 7 days. After incubation (24 h), the broth culture was centrifuged (speed: 10, 000 rpm, time: 10 min, temp: 4 °C) and supernatant was collected. One ml of supernatant was mixed with modified universal buffer (4 ml; pH 6.8) and subsequently, disodium p-nitrophenol phosphate (1 ml; 0.025 mM) was added in supernatant for quick analysis of phosphate enzyme activity and incubated at 28 °C for 60 min. After incubation, 1 drop of toluene was added and further incubated (time: 1 h; temp: 37 °C) and finally 0.5 M CaCl2 and 0.5 M NaOH (ratio 1:4) was mixed for inhibition of reaction. The obtained sample was filtered via the Whatman filter paper no 42 and the quantity of enzyme were examined in 1 µmol of p-nitrophenol/ml/min through spectrophotometer (420 nm). For standard, p-nitrophenol was used for the quantification of phosphatase.
Investigation of plant growth promoting characteristics
Bacterial isolate STJP was inoculated in tryptic soy broth (TSB) comprising (g/l); tryptone:15, NaCl: 8, soy peptone: 5, tryptophan: 1 (HiMedia, Mumbai), 1000 ml sterilized water; pH 7.0 in 50 ml Erlenmeyer flask (EF) and incubated at 30 °C for 72 h (120 rpm) for IAA production. After incubation, 5 ml culture broth was centrifuged (speed: 5000 rpm, time: 10 min, temp: 4 °C). The obtained supernatant was mixed with Salkowski reagent in the ratio of 3:2 and was left in the dark (20 min). Afterwards, sample was analyzed spectrophotometrically at 530 nm for quantitative estimation of IAA (µg/ml) (Brick et al. 1991). For quantifying of siderophore, isolate (STJP) was inoculated in nutrient broth (NB) and incubated for 72 h at 28 °C (130 rpm). After incubation, the culture was centrifuged to obtain supernatant (speed: 5000 rpm, time: 15 min, temp: 4 °C) and the collected sample was taken to examine the siderophore production (Reeves et al. 1983). The siderophore quantity was expressed in µmol benzoic acid/ml. The ability to fix nitrogen was checked as per Line and Loutit (1971) and production of hydrogen cyanide (HCN) was evaluated according to Millar and Higgins (1970).
Effect of isolate STJP on the growth of M. arvensis
The pot experiment was performed in University Greenhouse Horticulture Research Farm (HRF) for 90 days. Selected isolate STJP was grown in NB in a shaker (speed: 120 rpm, time; 48 h, temp: 28 °C). Afterwards, growth culture was centrifuged (speed: 5000 rpm, time: 10 min, temp: 4 °C) for collecting the pellet. Further, sterilized deionized water was mixed in the pellet to obtain population density of 108 CFU/ml (Maheshwari et al. 2012).
Sandy-loam soil [pH: 7.69, EC: 0.48 dS/m, N: 108 (kg/ha), K: 89 (kg/ha), and P: 35.26 (kg/ha)] was collected from HRF and 6 kg was filled in clay pots (20 cm × 20 cm × 20 cm). Rootlets of seedling of M. arvensis (var. CIMAP SARYU) were disinfected by ethanol (70%, time: 2 min) followed by sodium hypochlorite (2%, time: 5 min) and subsequently washed with deionized water for 10 min (Arora et al. 2001). The seedling was then dipped in inoculum suspension (108 CFU/ml: time: 20 min) and allowed to dry (time period: 30 min). Subsequently, the seedlings (five per pot) were sown as per following sets: T-1 control (M. arvensis); T-2 STJP; T-3 TCP100 (100 mg/kg in soil); T-4 STJP + TCP100; T-5 TCP200 (200 mg/kg in soil); T-6 STJP + TCP200 as per Mamta et al. (2010). The work was done with five replications for each set in a completely randomized design (CRD). Irrigation was done using tap water (in equal amount) for each pot as per requirement. The plants were uprooted 90 days after sowing (DAS) and checked for the shoot and root length, number of leaves, fresh and dry weight.
Examination of oil yield from M. arvensis
The dry M. arvensis plants were used for extraction of oil by Clevenger’s apparatus in hexane solvent. Before extraction, the fresh shoots (leaves and stem) were harvested and separately dried (temp: 45 °C, time: 96 h). Completely dry shoots were crushed, samples were dissolved in n-hexane (50 ml) for 10 h and oil was extracted as per method described by Langenau (1948). The obtained oil was filtered via Whatman filter paper no. 42. The filtered oil was evaporated using rotatory evaporator (temp: 40 °C, speed: 50 rev/min, vacuum pressure: 80 mbar) to obtain pure oil. The oil yield was calculated (g/pot) as per the method of Singh et al. (2013).
Effect of isolate STJP on menthol content
To determine menthol content, 5 ml of the methanol and water in a ratio of 75:25 v/v were properly mixed with mentha oil obtained from plants. Further, the samples were filtered through 0.45 M Millipore filter membrane. The filtered sample was injected in the high-pressure liquid chromatography (HPLC) (Model No. WATER—2489) with C-18 column for determination of menthol content. Menthol: water (HPLC grade) (75:25) with 2 × 10−5 M n-heptyl-p-aminobenzoate was used as mobile phase and pressure and flow rate was set 800 psi, and 1.5 ml/min (Perkin 1984). The wavelength was monitored at 290 nm and compared with standard menthol (Sigma-Aldrich, USA).
Examination of P uptake in M. arvensis
For examination of P uptake in M. arvensis, roots, stem, and leaves were harvested (90 DAS) from each pot and dried separately. Further, dried sample was digested and analyzed using atomic absorption spectroscopy (Model: AA240FS Fast Sequential AAS, USA) for quantification of P as described by Koenig and Johnson (1942).
Statistical analysis
Pot experiments were organized in a complete randomized design (CRD) method. One-way analysis of variance (ANOVA) was chosen for data analysis. Duncan’s multiple range test (DMRT) was used for comparison of means (Gomez and Gomez 1984) using the software of Statistical Package for Social Sciences (SPSS) version 15.
Results
Characterization of phosphate solubilizing rhizobacteria
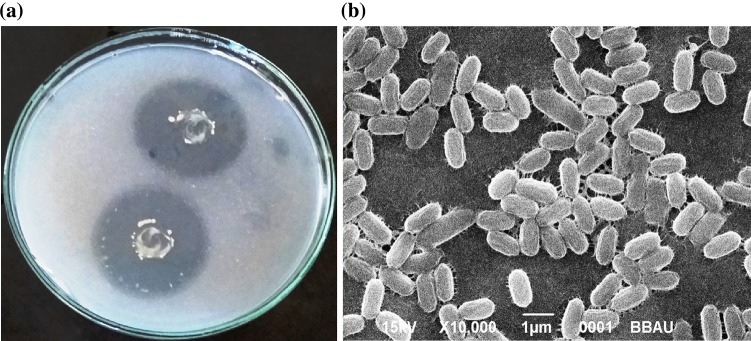
Eleven PSB were isolated from rhizospheric soil of S. rebaudiana. Among them, six isolates showed greater than 6 mm zone on PKV agar plates (Fig. 1a). Isolates were further screened for the amount of phosphate solubilization in liquid NBRIP medium. All isolates were able to carry out phosphate solubilization ranging from 8 to 610.33 µg/ml (Table 1). Among them, isolate STJP showed maximum P-solubilizing activity (610.33 µg/ml) in NBRIP medium and hence was selected for further study. Isolate STJP was found to be flagellated bacterium with peritrichous arrangement and cell size of 1.0–1.28 × 0.5–0.7 µm (Fig. 1b). Isolate STJP was found positive for oxidase, urease, catalase, and gelatinase, but negative for indole, H2S, citrate, and nitrate production. On the basis of phenotypic and biochemical traits, isolate STJP was found to be a member of genus Bacillus sp. on the basis of Bergey’s Manual of Systematic Bacteriology (Garrity 2005). The comparison of the whole 16S rRNA gene sequence of a neighbour isolate of STJP showed 99% similarities with Bacillus safensis strain NBRC 100820. The gene sequence data is submitted in NCBI database with Accession number KX372540 and can be said to belong to B. safensis clade. However, further studies are required to confirm the species of the strain.
Fig. 1.
a Phosphate solubilization by isolate STJP on Pikovskaya agar. b Cell shape of the bacterium STJP
Table 1.
Quantitative examination of phosphate solubilization by different isolates (µg/ml) (1–5 days)
| PSB isolates | 1st day | 2nd days | 3rd day | 4th day | 5th day |
|---|---|---|---|---|---|
| B1TNP | 48.33 ± 2.35 | 70.66 ± 2.49 | 122.33 ± 2.05 | 189.33 ± 2.49 | 188.33 ± 2.35 |
| B2NPT | 70.00 ± 4.08 | 80.66 ± 2.49 | 88.33 ± 2.35 | 234.33 ± 3.29 | 161.66 ± 2.35 |
| B1PPoNP | 10.00 ± 4.08 | 27.66 ± 2.05 | 68.33 ± 4.64 | 138.00 ± 1.63 | 134.66 ± 3.39 |
| NPSP | 89.33 ± 2.49 | 102.33 ± 6.12 | 31.00 ± 2.94 | 21.33 ± 1.88 | 8.00 ± 1.63 |
| NPPsP2 | 83.33 ± 2.35 | 113.33 ± 4.71 | 159.33 ± 0.94 | 192.00 ± 1.63 | 185.66 ± 3.29 |
| STJP | 139.00 ± 2.94 | 188.33 ± 6.23 | 347.66 ± 2.05 | 610.33 ± 2.05 | 604.00 ± 4.10 |
| STJP-12 | 052 ± 2.12 | 75.66 ± 3.29 | 101.66 ± 2.35 | 162.00 ± 1.63 | 235.00 ± 4.08 |
| STJP-18 | 31.33 ± 1.88 | 53.33 ± 3.39 | 91.66 ± 1.24 | 161.33 ± 1.88 | 132.66 ± 1.88 |
| STJP-20 | 89.66 ± 3.68 | 135.00 ± 4.08 | 237.66 ± 2.05 | 241.66 ± 2.35 | 208.33 ± 2.35 |
| STJP-22 | 036 ± 561 | 092.00 ± 2.16 | 38.00 ± 1.63 | 132.66 ± 2.49 | 114.33 ± 3.29 |
| STJP-30 | 37.66 ± 2.05 | 52.50 ± 1.87 | 88.33 ± 2.35 | 181.66 ± 2.35 | 132.33 ± 2.05 |
Bold values are for the selected isolate STJP which showed maximum phosphate solubilization on all the days of incubation
Data are mean of three replicates ± standard error of means
Phosphatase assay
The production of phosphatase by isolate STJP was checked every 24 h up to 7 days in NBRIP medium. Isolate STJP produced maximum phosphatase (81.45 U/ml) after 96 h which reduced with further incubation (data not shown).
Plant growth promoting characteristics
The intensity of pink color production within 30 min is the first visible confirmation of IAA production. Isolate STJP showed intense pink color and recorded a significant amount of IAA (30.59 µg/ml). Further, isolate STJP showed a good amount of siderophore production (16.06 µg/ml) and observed a high affinity for iron chelation. The color change of the filter paper from yellow to brown is a key for detection of HCN. STJP did not show HCN production as confirmed by the absence of any color change of the filter paper laden with picric acid. Isolate STJP was unable to grow on nitrogen-free medium when tried repeatedly confirming its inability to fix nitrogen.
Effect of STJP on growth promotion of M. arvensis
Bacterial isolate STJP positively affected the plant growth parameters as observed after 90 days. STJP showed stimulatory effect, in term of growth parameters as compared to the control (Table 2). Among all treatments, STJP + TCP200 recorded maximum enhancement in vegetative parameters as compared to the control.
Table 2.
Effect of isolate STJP on growth promotion of M. arvensis
| Treatments | Plant shoot length (cm) | Plant root length (cm) | Number of leaves | Total fresh weight (g) | Total dry weight (g) |
|---|---|---|---|---|---|
| Control | 18.00 ± 3.20c | 09.03 ± 1.66c | 42.66 ± 1.88c | 28.83 ± 1.24d | 13.03 ± 1.23d |
| STJP | 27.20 ± 2.08b | 14.73 ± 0.55ab | 59.66 ± 1.69b | 42.17 ± 2.10b | 19.49 ± 1.52bc |
| TCP100A | 18.93 ± 1.23c | 09.63 ± 0.91c | 44.00 ± 1.63c | 35.61 ± 2.15c | 16.87 ± 1.22c |
| STJP + TCP100 | 29.53 ± 0.80ab | 17.60 ± 1.62a | 61.66 ± 2.62ab | 42.28 ± 1.18b | 22.20 ± 0.98ab |
| TCP200B | 19.93 ± 0.66c | 10.13 ± 0.66bc | 44.33 ± 1.69c | 36.01 ± 2.12c | 17.22 ± 1.15c |
| STJP + TCP200 | 32.20 ± 2.01a | 18.23 ± 1.97a | 66.66 ± 3.39a | 49.05 ± 2.28a | 23.73 ± 1.81a |
Data are mean of three replicates ± standard error of means. Means, followed by the same letter in a column are not significantly different (P = 0.05) by Duncan’s multivariate test (DMRT)
ATCP100 (100 mg tricalcium phosphate in per kg of sterilized soil)
BTCP200 (200 mg tricalcium phosphate in per kg of sterilized soil)
STJP treatment of M. arvensis enhanced fresh and dry weight by 46.27% and 49.57% respectively, in comparison with the control plant. The combined application of TCP100 and STJP corresponded to 46.65% and 70.37% rise in fresh and dry weight respectively, in comparison with un-inoculated plants. Moreover, STJP + TCP200 treatment resulted in maximum fresh and dry weight enhancement (by 70.13% and 82.11%, respectively) as compared to the control plants (Table 2). The plant length of M. arvensis treated with STJP, STJP + TCP100, STJP + TCP200 recorded an increase of 55.12%, 74.36%, and 86.57%, respectively, as compared with the control plants. However, treatment with 100 mg TCP and 200 mg TCP only, did not affect significantly in the length of the plant. Although, M. arvensis treated with STJP showed significant increase in the leaf number (39.84%) as compared to the control plants but STJP + TCP200 application showed maximum increment in leaf number as compared to all other treatments. The number of leaves did not increase significantly after treatment with 100 mg and 200 mg TCP applications in comparison with the control plants.
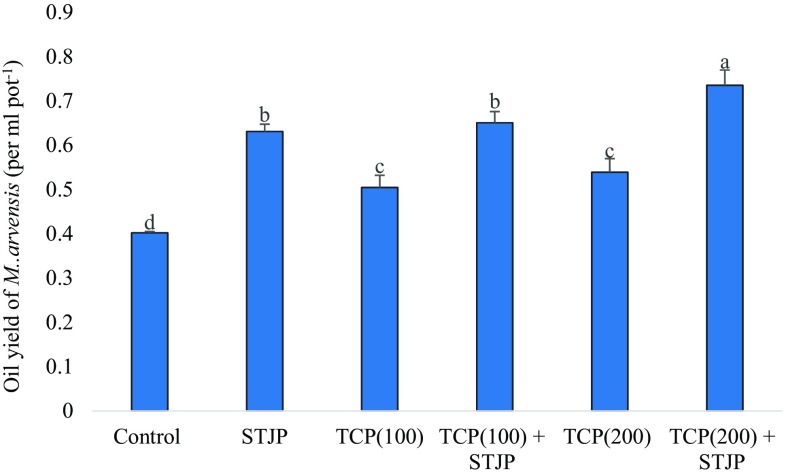
Effect of isolate STJP on oil yield and menthol content in M. arvensis
M. arvensis grown in soil amended with TCP showed higher oil yield as compared to without TCP amended soil (Fig. 2). A significant rise in oil yield of M. arvensis was observed after inoculation of STJP (56.71%) as compared to the control (without any treatment). There was 107.68% and 79.85% enhancement in oil yield by the treatment with STJP + TCP200 and TCP100 + STJP, respectively, in comparison with the control plant. Beside oil yield enhancement, the menthol content also increased significantly by the combined treatment of TCP and STJP (figure not shown). However, as in case of vegetative parameters, greatest enhancement in menthol content was shown when M. arvensis was treated with STJP + TCP200, as this treatment showed a maximum spike in peak (as compared to the control) when checked by HPLC.
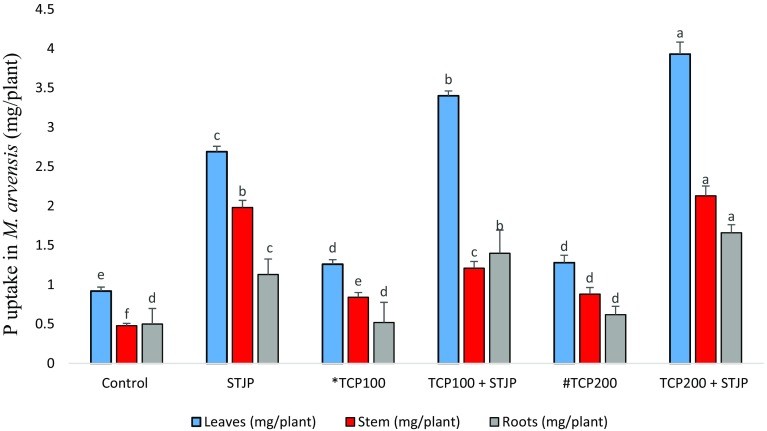
Fig. 2.
Effect of isolate STJP on P uptake in M. arvensis [data are mean of three replicates ± standard error of means. Means, followed by the same letter in a column are not significantly different (P = 0.05) by Duncan’s multivariate test (DMRT) (P ≤ 0.05)]
Effect of STJP on P uptake in M. arvensis
Total P uptake in M arvensis was studied after harvesting. The increase in P levels in roots, stem, and leaves were found to be 232%, 343.75%, and 327% respectively, by the STJP + TCP200 treatment which was maximum in comparison to any other treatment when compared with the control plant (Fig. 3).
Fig. 3.
Effect of isolate STJP on the yield of mentha oil in M. arvensis [data are mean of three replicates ± standard error of means. Means, followed by the same letter in a column are not significantly different (P = 0.05) by Duncan’s multivariate test (DMRT)]
Discussion
Menthol is one of the key plant metabolites with a wide range of applications in medicine and industries. According to an industrial report, the estimated production of menthol was 36,000 metric tonnes in 2017, which is much lower than the current global demand of approximately 45,000 metric tonnes (http://www.aosproduct.com/MARKET-REPORT/Menthol-Crystals-and-Mentha-oil). Hence, there is an urgent requirement to raise the menthol production which can be achieved in a better and sustainable manner if done through biological approaches. PGPR can thus play a key role in the enhancement of productivity of menthol producing plants. Among PGPR, Bacillus spp. are omnipresent in nature, and are widely used for enhancement of growth, development, and yield of plants by producing bioactive metabolites and nutrients (Radhakrishnan et al. 2017). These bacteria have the ability to solubilize phosphate in the soil and also the ability to produce a number of metabolites such as IAA and siderophores which are directly involved in nutrients uptake and plant growth (Ribeiro et al. 2018).
P is the second most important macronutrient which is also a limiting factor for plant growth and development apart from nitrogen. Most of the P in soil is found in an insoluble form such as aluminium phosphate (AlPO4) or ferric phosphate (FePO4) which cannot be taken up by crops (Chen et al. 2017). The ability of PSB to solubilize insoluble P and make it available to the plants by one of the several mechanisms such as producing phosphatase enzyme, releasing organic acids (gluconic, oxalic and succinic acids), ion exchange and chelation (Wei et al. 2018) are well known. Bacillus spp. and in particular, B. subtilis are widely reported as PSB (Radhakrishnan et al. 2017; Ahmad et al. 2017). In the current study, phosphate solubilizing isolate STJP from the rhizosphere of a medicinal plant and identified to be Bacillus sp. was tested as a potent PGPR for M. arvensis. Mukhtar et al. (2017) reported B. safensis and Bacillus megaterium as phosphate solubilizers, however in their study, Bacillus megaterium was found to be better phosphate solubilizing agent as compared to B. safensis. In the present study B. safensis isolate STJP was found to be effective phosphate solubilizer. The ability to solubilise phosphate by STJP was found to be better than reported earlier for B. safensis (Mukhtar et al. 2017).
Mechanisms of phosphate solubilization by PSB are very well known (Sharma et al. 2013; Behera et al. 2017). Phosphatase enzyme has been commonly reported for solubilization of inorganic phosphate (Wei et al. 2018). In our study, isolate STJP produced 81.25 U/ml phosphatase enzyme (after 96 h). There are many reports available on the production of phosphatase by Bacillus spp. (Parhamfar et al. 2016; Behera et al. 2017). The phosphatase enzyme cleaves the insoluble phosphate into phosphate ions and thus helps in uptake of ions by the plants. Maximum growth enhancement of M. arvensis was observed in the presence of STJP in combination with TCP200 treatment. This might be associated with the presence of a phosphatase enzyme produced by Bacillus sp. STJP. Isolate STJP produced 8.64 U/ml phosphatase after 24 h which increased with incubation period up to 96 h (81.45 U/ml). Similarly, Swain et al. (2012) reported that B. subtilis produced 30.2 U/ml phosphatase at 12 h incubation period but further increase in incubation period resulted in higher production of the enzyme.
P content was also increased to the maximum level in M. arvensis when treated with STJP + TCP200 as compared to the addition of TCP only. Treatment of STJP + TCP200 also resulted in maximum enhancement of plant growth parameters. Pereira et al. (2015) examined that Arthrobacter nicotinovorans EAPAA inoculated in TCP amended soil enhanced biomass and uptake of P in Zea mays plants.
In addition to phosphate solubilization, isolate STJP also showed a significant amount of IAA and siderophore production. Phytohormone IAA stimulates the lateral root formation, cell elongation, and also helps in cell division and differentiation in plant cells (Goswami et al. 2016). Siderophore plays a significant role in chelation of micronutrients such as iron even under limiting conditions (Arora and Verma 2017). Earlier studies have reported Bacillus spp. to be good producers of IAA and siderophores (Walpola and Yoon 2013). Researchers have also reported B. safensis as effective producer of IAA (Mukhtar et al. 2017) and siderophore (Chakraborty et al. 2013). Production of siderophore in the rhizosphere also helps in phosphate solubilisation and availability of phosphate ions to the plant through chelation of iron from precipitated forms (FePO4) (Sharma et al. 2013). B. safensis is a well-reported PGPR which has the ability to enhance the growth and productivity under biotic and abiotic conditions (Lateef et al. 2015). However, in the present work Bacillus sp. STJP capable of production of IAA, siderophore and phosphate solubilisation, significantly enhanced the growth of M. arvensis.
To the best of our knowledge, this is the first report on a PSB strain (B. safensis STJP) which has been employed successfully to significantly enhance the menthol content of M. arvensis simultaneously with plant growth and P content. Treatment with STJP + TCP200 (in pot trials) resulted in a maximum increment of oil content and it was approximately 37% higher than treatment with TCP200 alone. It has been informed earlier that the application of IAA increased the yield of menthol in Mentha piperita (Khan et al. 2015). Similarly, Hazzoumi et al. (2014) studied that essential oil content of Basil increased on the application of IAA. Isolate STJP is also a prolific IAA producer and this study also suggests the role of this growth hormone in enhancing the oil content of the plants might be associated with better growth of roots and shoots. P plays an important role in the enhancement of total biomass, and yields of bioactive components in the medicinal plants (Sharma et al. 2013). The deficiency of P in soils/plants causes a reduction in the net photosynthesis rate (PN) resulting in a decrease of biomass and oil content in crops (Xu et al. 2007). P also induces the synthesis of secondary metabolites and highly modulates the oil synthesis in medicinal plants via a nutrient-plant growth enhancement mechanism (Malusà et al. 2006; Nell et al. 2009) and menthol is a type of metabolite which is produced by M. arvensis. Also, IAA production results in improved root hair formation. Simultaneously release of IAA and phosphate solubilization activities by STJP most probably resulted in enhanced P uptake by the plant. This may justify the maximum increase in root weight in case of plants treated with PGPR and TCP200. It has been reported earlier that PSB having the capability to produce other metabolites such as IAA and siderophore are far more efficient in enhancing the growth and yields of crop plants (Koo and Cho 2009). In fact, Gupta et al. (2012) also reported the role of IAA and siderophore produced by PSB strains along with TCP in enhancing the growth and Aloin-A content of Aloe barbadensis. Hence, using B. safensis as a biofertilizer in an integrated approach with TCP could be an effective tactic for improvement of soil health, growth enhancement and oil yield in M. arvensis. When applied alone most of the TCP gets precipitated in the soil and causes stresses such as salinity and eutrophication as runoff to the water bodies (Tewari and Arora 2018). By the way of integrated approach of utilizing an efficient PGPR with multiple growth traits including efficient phosphate solubilizing abilities we can reduce the precipitation of phosphates in the soil and simultaneously diminish the eutrophication of water bodies like ponds and lakes which are integrated for water supply in agro-ecosystems (Arora et al. 2016; Gouda et al. 2018). PSB possessing PGP characters such as siderophore and IAA production can thus not only enhance the growth and metabolite content of medicinal plants but also protect from unnecessary pollution due to the addition of phosphate fertilizers.
Conclusion
The present study reports B. safensis, an efficient PGPR with phosphate solubilizing abilities, for growth promotion and increasing the oil content of M. arvensis. The experiments clearly show the efficiency of an integrated approach wherein fertilizer TCP and the PSB are co-inoculated to enhance the productivity of a very important medicinal and commercial plant. Bacillus sp. STJP is also an efficient producer of IAA and siderophore. The cocktail of PGP characters helped in enhancing the yield and oil content of M. arvensis. This is the first report suggesting the use of phosphate solubilizing Bacillus in enhancing the menthol content of M. arvensis. Menthol is a key plant metabolite having wide applications in various industries. Enhancement of oil yields that too through eco-friendly biological approach can be a major breakthrough for menthol production and development of the country’s economy.
Acknowledgements
The authors are thankful to Vice Chancellor, Babasaheb Bhimrao Ambedkar University, Lucknow, India for his support.
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest in this original article.
References
- Abd_Allah EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi F, Malik JA, Alharbi RI, Egamberdieva D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Interact. 2018;13(1):37–44. doi: 10.1080/17429145.2017.1414321. [DOI] [Google Scholar]
- Ahmad Z, Wu J, Chen L, Dong W. Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR. Sci rep. 2017;7(1):1777. doi: 10.1038/s41598-017-01940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Ahmad I, Hilger TH, Nadeem SM, Akhtar MF, Jamil M, Hussain A, Zahir ZA. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. Peer J. 2018;6:e5122. doi: 10.7717/peerj.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alankar S. A review on peppermint oil. Asian J Pharm Clin Res. 2009;2:4–6. [Google Scholar]
- Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora NK, Verma M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. 2017;7(6):381. doi: 10.1007/s13205-017-1008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora NK, Kang SC, Maheshwari DK. Isolation of siderophore producing strain of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci. 2001;25:674–677. [Google Scholar]
- Arora NK, Verma M, Prakash J, Mishra J. Bioformulations for sustainable agriculture. New Delhi: Springer; 2016. Regulation of biopesticides: global concerns and policies; pp. 283–299. [Google Scholar]
- Bakry AM, Fang Z, Ni Y, Cheng H, Chen YQ, Liang L. Stability of tuna oil and tuna oil/peppermint oil blend microencapsulated using whey protein isolate in combination with carboxymethyl cellulose or pollutant. Food Hydrocoll. 2016;60:559–571. doi: 10.1016/j.foodhyd.2016.04.026. [DOI] [Google Scholar]
- Behera BC, Yadav H, Singh SK, Mishra RR, Sethi BK, Dutta SK, Thatoi HN. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J Genet Eng Biotechnol. 2017;15:169–178. doi: 10.1016/j.jgeb.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick JM, Bostock RM, Silverstone SE. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U, Chakraborty BN, Chakraborty AP, Dey PL. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J Microbiol Biotechnol. 2013;29(5):789–803. doi: 10.1007/s11274-012-1234-8. [DOI] [PubMed] [Google Scholar]
- Chen M, Li Z, Huang P, Li X, Qu J, Wenyi Y, Qiwu Z. Mechanochemical transformation of apatite to phosphoric slow-release fertilizer and soluble phosphate. Proc Saf Environ Prot. 2017;114:91–96. doi: 10.1016/j.psep.2017.12.008. [DOI] [Google Scholar]
- Garrity G. Bergey’s manual of systematic bacteriology. New York: Springer; 2005. The proteobacteria, Part B the gammaproteobacteria; pp. 323–379. [Google Scholar]
- Golding CG, Lamboo LL, Beniac DR, Booth TF. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci Rep. 2016;6:26–516. doi: 10.1038/srep26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York: Wiley; 1984. [Google Scholar]
- Goswami D, Thakker JN, Dhandhukia PC. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. 2016;2:1127500. [Google Scholar]
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Gupta M, Kiran S, Gulati A, Singh B, Tewari R. Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol Res. 2012;167:358–363. doi: 10.1016/j.micres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Hazzoumi Z, Moustakime Y, Joutei KA. Effect of gibberellic acid (GA), indole acetic acid (IAA) and benzylaminopurine (BAP) on the synthesis of essential oils and the isomerization of methyl chavicol and trans-anethole in Ocimum gratissimum L. SpringerPlus. 2014;3(1):321. doi: 10.1186/2193-1801-3-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AF, Mujeeb FARINA, Aha FAROOQI, Farooqui ALVINA. Effect of plant growth regulators on growth and essential oil content in palmarosa (Cymbopogon martinii) Asian J Pharm Clin Res. 2015;8(2):373–376. [Google Scholar]
- Koenig RA, Johnson CR. Colorimetric determination of phosphorus in biological materials. Ind Eng Chem Anal. 1942;14:155–156. doi: 10.1021/i560102a026. [DOI] [Google Scholar]
- Koo SY, Cho KS. Isolation and characterization of a plant growth-promoting rhizobacterium, Serratia sp. SY5. J Microbiol Biotechnol. 2009;19:1431–1438. [PubMed] [Google Scholar]
- Kumar S, Suresh R, Singh V, Singh AK. Economic analysis of menthol mint cultivation in Uttar Pradesh: a case study of Barabanki district. Agric Econ Res Review. 2011;24:345–350. [Google Scholar]
- Langenau EE. The examination and analysis of essential oils, synthesis and isolated. In: Guenther E, editor. The essential oils. Princeton: Van Nostrand; 1948. pp. 229–367. [Google Scholar]
- Lateef A, Adelere IA, Gueguim-Kana EB. The biology and potential biotechnological applications of Bacillus safensis. Biologia. 2015;4:411–419. doi: 10.1080/13102818.2014.986360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line MA, Loutit MW. Non-symbiotic nitrogen-fixing organisms from some New Zealand tussock-grassland soils. Microbiology. 1971;66(3):309–318. [Google Scholar]
- Maheshwari DK, Dubey RC, Aeron A, Kumar B, Kumar S, Tewari S, Arora NK. Integrated approach for disease management and growth enhancement of Sesamum indicum L. utilizing Azotobacter chroococcum TRA2 and chemical fertilizer. World J Microbiol Biotechnol. 2012;28(10):3015–3024. doi: 10.1007/s11274-012-1112-4. [DOI] [PubMed] [Google Scholar]
- Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Stredwick JM, Garrity GM, Li B, Olsen GJ, Pramanik S, Schmidt TM, Tiedje JM. The RDP (ribosomal database project) continues. Nucleic Acids Res. 2000;28:173–174. doi: 10.1093/nar/28.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malusà EM, Russo A, Mozzetti C, Belligno A. Modification of secondary metabolism and flavonoid biosynthesis under phosphate deficiency in bean roots. J Plant Nutr. 2006;29:245–258. doi: 10.1080/01904160500474090. [DOI] [Google Scholar]
- Mamta P, Rahi V, Pathania A, Gulati B, Singh RK, Bhanwra, et al. Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside-A contents of Stevia rebaudiana Bertoni. Appl Soil Ecol. 2010;46:222–229. doi: 10.1016/j.apsoil.2010.08.008. [DOI] [Google Scholar]
- Millar RL, Higgins VJ. Association of cyanide with infection of birdsfoot trefoil by Stemphylium loti. Phytopathol. 1970;60(1):104–110. doi: 10.1094/Phyto-60-104. [DOI] [Google Scholar]
- Mishra J, Prakash J, Arora NK. Role of beneficial soil microbes in sustainable agriculture and environmental management. Clim Change Environ Sustain. 2016;4:137–149. doi: 10.5958/2320-642X.2016.00015.6. [DOI] [Google Scholar]
- Mukhtar S, Shahid I, Mehnaz S, Malik KA. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.) Microbiol Res. 2017;205:107–117. doi: 10.1016/j.micres.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Nell M, Vötsch M, Vierheilig H, Steinhellner S, Zitterl-Eglseer K, Franz C, Novak J. Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L) J Sci Food Agric. 2009;80:1090–1096. doi: 10.1002/jsfa.3561. [DOI] [Google Scholar]
- Parhamfar M, Badoei-Dalfard A, Parhamfar M, Fahimi Rad S. Purification and characterization of an extracellular phosphatase enzyme from Bacillus spp. J Cell Mol Res. 2016;8(2):90–97. [Google Scholar]
- Pereira SIA, Barbosa L, Castro PML. Rhizobacteria isolated from a metal-polluted area enhance plant growth in zinc and cadmium-contaminated soil. Int J Environ Sci Technol. 2015;12:2127–2142. doi: 10.1007/s13762-014-0614-z. [DOI] [Google Scholar]
- Perkin JE. High-performance liquid chromatographic assay of menthol using indirect photometric detection. J Chromatogr. 1984;303:430–439. [Google Scholar]
- Pikovskaya RI. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologiya. 1948;17:362–370. [Google Scholar]
- Radhakrishnan R, Hashem A, Abd Allah EF. Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MW, Pine L, Neilands JB, Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983;154:324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro VP, Marriel IE, de Sousa SM, de Paula Lana UG, Mattos BB, de Oliveira CA, Gomes EA. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz J Microbiol. 2018 doi: 10.1016/j.bjm.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:587. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F. Phosphorus dynamics: from soil to plant. Plant Physiol. 2011;156:997–1005. doi: 10.1104/pp.111.175232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Arora NK. Growth enhancement of medicinal plant Withania somnifera using phosphate solubilizing endophytic bacteria Pseudomonas sp. as bioinoculant. Inter J Sci Technol Soci. 2016;2:13–18. [Google Scholar]
- Singh R, Soni SK, Patel RP, Kalra A. Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crops Prod. 2013;45:335–342. doi: 10.1016/j.indcrop.2013.01.003. [DOI] [Google Scholar]
- Srivastava RK, Singh AK, Kalra A, Tomar VKS, Bansal RP, Patra DD, Chand S, Naqvi AA, Sharma S, Kumar S. Characteristics of menthol mint Mentha arvensis cultivated on industrial scale in the Indo-Gangetic plains. Ind Crops Prod. 2002;15(3):189–198. doi: 10.1016/S0926-6690(01)00113-3. [DOI] [Google Scholar]
- Stutte Gary W. ACS Symposium Series. Washington, DC: American Chemical Society; 2016. Controlled Environment Production of Medicinal and Aromatic Plants; pp. 49–63. [Google Scholar]
- Swain MR, Laxminarayana K, Ray RC. Phosphorus solubilization by thermotolerant Bacillus subtilis isolated from cow dung microflora. Agric Res. 2012;1(3):273–279. doi: 10.1007/s40003-012-0022-x. [DOI] [Google Scholar]
- Tabatabai MA, Bremner JM. Use of p-nitrophenol phosphate in assay of soil phosphatase activity. Soil Biol Biochem. 1969;1:301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- Tewari S, Arora NK. Fluorescent Pseudomonas sp. PF17 as an efficient plant growth regulator and biocontrol agent for sunflower crop under saline conditions. Symbiosis. 2016;68:99–108. doi: 10.1007/s13199-016-0389-8. [DOI] [Google Scholar]
- Tewari S, Arora NK. Role of salicylic acid from Pseudomonas aeruginosa PF23EPS + in growth promotion of sunfower in saline soils infested with phytopathogen Macrophomina phaseolina. Environ Sustain. 2018;1:49–59. doi: 10.1007/s42398-018-0002-6. [DOI] [Google Scholar]
- Walpola BC, Yoon MH. Isolation and characterization of phosphate solubilizing bacteria and their co-inoculation efficiency on tomato plant growth and phosphorous uptake. Afr J Microbiol Res. 2013;7:266–275. [Google Scholar]
- Wei Y, Zhao Y, Shi M, Cao Z, Lu Q, Yang T, Fan Y, Wei Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol. 2018;247:190–199. doi: 10.1016/j.biortech.2017.09.092. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO traditional medicine strategy 2014–2023. Geneva: World Health Organization; 2013. [Google Scholar]
- Xu HX, Weng XY, Yang Y. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ J Plant Physiol. 2007;54:741–748. doi: 10.1134/S1021443707060040. [DOI] [Google Scholar]