Abstract
Multiple sclerosis (MS) is the most prevalent immune-mediated disease affecting the central nervous system. A treatment strategy with multiple therapies is a frequent clinical scenario. Unmonitored multi-drug use can lead to adverse outcomes, higher health care costs and medication non-adherence. The primary aim of this study was to evaluate the frequency of polypharmacy and related clinicodemographic factors in a single-center MS patient cohort. Furthermore, medication aspects of therapy management were examined. After the patients agreed to participate in the study, data were collected through patient interviews, patient records and clinical investigations. Subsequently, a statistical data analysis regarding various medication subgroups and polypharmacy (use of at least five drugs) was performed. Polypharmacy was observed in 56.5% of the patients (N = 306). High degrees of disability (odds ratio [OR] = 1.385), comorbidities (OR = 4.879) and inpatient treatment (OR = 5.146) were associated with a significantly higher risk of polypharmacy (p ≤ 0.001). Among patients with polypharmacy, disease-modifying drugs, antihypertensives, gastrointestinal drugs, thrombosis prophylactics, osteoporosis medications and sedatives were frequently used. In summary, polypharmacy plays a large role in MS patients, especially in those with higher degrees of disability, those with comorbidities and those treated in an inpatient setting.
Introduction
With 2.3 million people affected globally1, multiple sclerosis (MS) is the most frequent immune-mediated disease of the central nervous system2. It causes pathological loss of synapses, demyelination and axonal damage, which can lead to various combinations of symptoms3. Both genetic and environmental factors play a role in the manifestation of the disease4–6. There is a risk of developing MS in any age group, while most patients are diagnosed between the ages of 20 and 49 years7.
The introduction of interferon-beta-1b preparations in 1993 as the first disease-modifying drugs (DMDs) heralded the development of a range of new immunomodulatory drugs for MS8–11. Meanwhile, 16 different DMDs have been approved12. Considering the course of disease, quite diverse symptoms can arise, such as sensory disturbances13, pareses, spasticity14, fatigue15, pain16, emotional disturbances17 and coordination disturbances18. Therefore, in addition to the DMDs, targeted symptomatic therapeutic approaches, treatment of comorbidities besides MS and individual use of complementary medicines play a substantial role for the patients’ quality of life19. Given such complex therapeutic scenarios, it is essential to take polypharmacy into account. In our study, polypharmacy is defined as the use of five or more medications, which constitutes the most common definition20–25.
In the USA, 10% of the general population and 30% of the older generation is taking five or more medications simultaneously26–28. Similar rates of polypharmacy have been observed in other countries, such as the United Kingdom29, China30 and India31. Older people are often affected by polypharmacy as they suffer more frequently from comorbidities and therefore take more medications. For instance, previous research has shown a correlation between polypharmacy and gut microbiota composition in aging, leading to a detrimental clinical outcome32. Possible consequences of an inappropriate monitoring of polypharmacy in affected patients include rising socioeconomic costs and side effects33, serious drug interactions34, lack of adherence due to medication complexity35 and rehospitalizations36. Until now, the number of studies addressing partial aspects of polypharmacy in MS patients is limited37–40.
For this reason, the primary aim of our study was to examine the frequency of polypharmacy in a single-center MS patient cohort. Furthermore, we analyzed the sociodemographic and clinical-neurological factors associated with polypharmacy as well as medication aspects of therapy management. Finally, we examined differences in medication between younger and older MS patients, between outpatients and inpatients and between patients with and without polypharmacy (PwP and Pw/oP).
Results
Study population
The sociodemographic data of the study population, comprising 306 patients with a diagnosis of MS (N = 300) or clinically isolated syndrome (CIS) (N = 6), are presented in Table 1. The average age was 48.7 years and 71.2% of the patients were women. Over half of the patients were retired due to their health situation or due to age, while 37.6% were still in employment. Approximately three quarters (72.5%) of the patients were in a partnership and almost the same amount (73.5%) had at least one child. Nearly half of the patients (48.0%) had one sibling, while 38.9% had two or more.
Table 1.
Sociodemographic data of the examined MS patients (N = 306).
| N (%) | Range | M (SD)a or medianb | |
|---|---|---|---|
| Gender | |||
| Female | 218 (71.2) | ||
| Male | 88 (28.8) | ||
| Age (Years) | 19–86 | 48.7 (13.1)a | |
| ≤29 | 23 (7.5) | ||
| 30–39 | 64 (20.9) | ||
| 40–49 | 64 (20.9) | ||
| 50–59 | 96 (31.4) | ||
| ≥60 | 59 (19.3) | ||
| School years | 6–16 | 10b | |
| Educational level | |||
| No training | 6 (2.0) | ||
| Skilled worker | 206 (67.3) | ||
| Technical college | 19 (6.2) | ||
| University | 75 (24.5) | ||
| Employment status | |||
| In training | 6 (2.0) | ||
| Employed | 115 (37.6) | ||
| Unemployed | 10 (3.3) | ||
| Retiree | 168 (54.9) | ||
| Others | 7 (2.3) | ||
| Partnership | |||
| Yes | 222 (72.5) | ||
| No | 84 (27.5) | ||
| Place of residence | |||
| Rural community | 85 (27.8) | ||
| Provincial town | 57 (18.6) | ||
| Medium-sized town | 43 (14.1) | ||
| City | 121 (39.5) | ||
| Number of children | 0–4 | 1b | |
| 0 | 81 (26.5) | ||
| 1 | 87 (28.4) | ||
| ≥2 | 138 (45.1) | ||
| Number of siblings | 0–13 | 1b | |
| 0 | 40 (13.1) | ||
| 1 | 147 (48.0) | ||
| ≥2 | 119 (38.9) |
M, mean value; MS, multiple sclerosis; N, number of patients; SD, standard deviation. aMean value (standard deviation); bmedian.
The clinical-neurological data of the patient cohort are presented in Table 2. The median EDSS score was 3.5, with individual scores ranging between 1.0 and 9.0. The majority of the patients (62.7%) had CIS/relapsing-remitting MS (RRMS), followed by secondary progressive MS (SPMS) (26.1%) and primary progressive MS (PPMS) (11.1%). The disease duration varied between 6 weeks and 50 years, with a median of 11 years. Almost two thirds of the patients (64.4%) suffered from comorbidities. The proportion of inpatients (52.3%) was roughly equal to the proportion of outpatients (47.7%).
Table 2.
Clinical data of the examined MS patients (N = 306).
| N (%) | Range | Median | |
|---|---|---|---|
| EDSS | 1.0–9.0 | 3.5 | |
| 1.0–3.5 | 170 (55.5) | ||
| 4.0–5.5 | 47 (15.4) | ||
| ≥6.0 | 89 (29.1) | ||
| Disease course | |||
| CIS/RRMS | 192 (62.7) | ||
| SPMS | 80 (26.1) | ||
| PPMS | 34 (11.1) | ||
| Disease duration (Years) | 0–50 | 11 | |
| 0*−5 | 88 (28.8) | ||
| 6–10 | 57 (18.6) | ||
| 11–15 | 54 (17.6) | ||
| 16–20 | 52 (17.0) | ||
| ≥21 | 55 (18.0) | ||
| Comorbidities | |||
| Pw/oSI | 109 (35.6) | ||
| PwSI | 197 (64.4) | ||
| Patient Care | |||
| Outpatients | 146 (47.7) | ||
| Inpatients | 160 (52.3) |
CIS, clinically isolated syndrome; EDSS, expanded disability status scale; MS, multiple sclerosis; N, number of patients; PPMS, primary progressive MS; PwSI, patients with secondary illnesses; Pw/oSI, patients without secondary illnesses; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS.
*Six weeks as the lowest disease duration.
Comparison between patients with polypharmacy and patients without polypharmacy
In the analysis of the examined patients according to all medications taken, 56.5% were categorized as PwP. Conducting the analysis with regard to long-term medications only, the proportion of PwP amounted to 42.2%. In total, the average number of medications taken by the patients was 5.7, with a range from 1 to 19.
Studying polypharmacy according to all medications, PwP were, on average, significantly older than Pw/oP (53.0 vs. 43.0 years) and had significantly more children (1.4 vs. 1.2) (Table 3). Pw/oP had a significantly higher number of school years (10.9 vs. 10.4 years) and were more frequently employed (52.6% vs. 26.0%) than PwP. After adjusting the p-values by means of false discovery rate (FDR) correction, all of the aforementioned differences remained significant, except for the number of children. Defining polypharmacy by using long-term medications only, the same sociodemographic variables showed significant differences between PwP and Pw/oP and these differences also remained significant following FDR correction.
Table 3.
Comparison of sociodemographic data between patients with and without polypharmacy.
| N | Polypharmacy (all medications) | Polypharmacy (long-term medications only) | ||||
|---|---|---|---|---|---|---|
| Pw/oP | PwP | Statistics | Pw/oP | PwP | Statistics | |
| 133 | 173 | 177 | 129 | |||
| Genderc | p = 0.799Fi | p = 1.000Fi | ||||
| Female | 96 (72.2) | 122 (70.5) | 126 (71.2) | 92 (71.3) | ||
| Male | 37 (27.8) | 51 (29.5) | 51 (28.8) | 37 (28.7) | ||
| Age (Years)a | 43.0 (11.4) | 53.0 (12.7) | t(304) = −7.170, p < 0.001t | 43.2 (11.6) | 56.2 (11.2) | t(304) = −9.815, p < 0.001t |
| School yearsa | 10.9 (1.4) | 10.4 (1.3) | t(277.487) = 3.322, p = 0.001t* | 10.9 (1.4) | 10.2 (1.2) | t(289.722) = 4.599, p < 0.001t* |
| Educational levelc | X2(3) = 4.634, p = 0.201Chi | X2(3) = 3.301, p = 0.348Chi | ||||
| No training | 3 (2.3) | 3 (1.7) | 3 (1.7) | 3 (2.3) | ||
| Skilled worker | 81 (60.9) | 125 (72.3) | 113 (63.8) | 93 (72.1) | ||
| Technical college | 9 (6.8) | 10 (5.8) | 11 (6.2) | 8 (6.2) | ||
| University | 40 (30.1) | 35 (20.2) | 50 (28.2) | 25 (19.4) | ||
| Employment statusc | X2(5) = 38.139, p < 0.001Chi | X2(5) = 47.944, p < 0.001Chi | ||||
| In training | 5 (3.8) | 1 (0.6) | 6 (3.4) | 0 (0.0) | ||
| Employed | 70 (52.6) | 45 (26.0) | 89 (50.3) | 26 (20.2) | ||
| Unemployed | 6 (4.5) | 4 (2.3) | 9 (5.1) | 1 (0.8) | ||
| Retiree | 47 (35.3) | 121 (69.9) | 68 (38.4) | 100 (77.5) | ||
| Other | 5 (3.8) | 2 (1.2) | 5 (2.8) | 2 (1.6) | ||
| Partnershipc | p = 0.301Fi | p = 0.245Fi | ||||
| Yes | 101 (75.9) | 121 (69.9) | 133 (75.1) | 89 (69.0) | ||
| No | 32 (24.1) | 52 (30.1) | 44 (24.9) | 40 (31.0) | ||
| Place of residencec | X2(3) = 1.307, p = 0.728Chi | X2(3) = 3.081, p = 0.379Chi | ||||
| Rural community | 39 (29.3) | 46 (26.6) | 53 (29.9) | 32 (24.8) | ||
| Provincial town | 23 (17.3) | 34 (19.7) | 30 (16.9) | 27 (20.9) | ||
| Medium-sized town | 16 (12.0) | 27 (15.6) | 21 (11.9) | 22 (17.1) | ||
| City | 55 (41.4) | 66 (38.2) | 73 (41.2) | 48 (37.2) | ||
| Number of childrena | 1.2 (1.0) | 1.4 (1.0) | t(304) = −2.085, p = 0.038t | 1.2 (1.0) | 1.5 (1.1) | t(304) = −3.350, p = 0.001t |
| Number of siblingsa | 1.7 (1.7) | 1.9 (1.8) | t(304) = −0.921, p = 0.358t | 1.6 (1.7) | 2.0 (1.8) | t(304) = −1.661, p = 0.098t |
N, number of patients; PwP, patients with polypharmacy; Pw/oP, patients without polypharmacy.
aMean value (standard deviation); cnumber of patients (%); ChiChi-square test; FiFisher’s exact test; ttwo-sample two-tailed Student’s t-test; *not assuming equal variances (Levene’s test: p < 0.05).
The comparison of the clinical-neurological data between Pw/oP and PwP yielded significant differences for all investigated clinical factors (Table 4). As expected, PwP showed substantially higher disability levels than Pw/oP (Fig. 1) and had, on average, a longer disease duration. PwP were more frequently affected by SPMS, while Pw/oP predominantly had CIS/RRMS. All PPMS patients showed polypharmacy. It was apparent that approximately twice as many PwP had comorbidities compared to Pw/oP (Fisher’s exact test: p < 0.001). Furthermore, the proportion of inpatients with polypharmacy was two to three times higher than of those without polypharmacy (Fisher’s exact test: p < 0.001). These significant differences also remained following correction of the p-values for multiple testing (FDR < 0.05). Potential clinicodemographic factors predicting polypharmacy (considering all medications) were examined by using a multivariable logistic regression model with forward variable selection. This revealed that the presence of comorbidities (Wald(1) = 26.620; p < 0.001; odds ratio [OR] = 4.879) and inpatient care (Wald(1) = 25.253; p < 0.001; OR = 5.146) as well as higher EDSS scores (Wald(1) = 11.769; p = 0.001; OR = 1.385) were significantly associated with the risk of polypharmacy (Figs 2 and 3).
Table 4.
Clinical data comparison between patients with and without polypharmacy.
| N | Polypharmacy (all medications) | Polypharmacy (long-term medications only) | ||||
|---|---|---|---|---|---|---|
| Pw/oP | PwP | Statistics | Pw/oP | PwP | Statistics | |
| 133 | 173 | 177 | 129 | |||
| EDSSb | 2.5 | 4.5 | z = −7.991, p < 0.001U | 3.0 | 6.0 | z = −8.062, p < 0.001U |
| Disease coursec | X2(2) = 74.871, p < 0.001Chi | X2(2) = 78.310, p < 0.001Chi | ||||
| CIS/RRMS | 119 (89.5) | 73 (42.2) | 148 (83.6) | 44 (34.1) | ||
| SPMS | 14 (10.5) | 66 (38.2) | 21 (11.9) | 59 (45.7) | ||
| PPMS | 0 (0.0) | 34 (19.7) | 8 (4.5) | 26 (20.2) | ||
| Disease duration (Years)b | 10 | 13 | z = −2.234, p = 0.025U | 9 | 15 | z = −4.592, p < 0.001U |
| Comorbiditiesc | p < 0.001 Fi | p < 0.001 Fi | ||||
| Pw/oSI | 76 (57.1) | 33 (19.1) | 97 (54.8) | 12 (9.3) | ||
| PwSI | 57 (42.9) | 140 (80.9) | 80 (45.2) | 117 (90.7) | ||
| Patient carec | p < 0.001 Fi | p < 0.001 Fi | ||||
| Outpatients | 102 (76.7) | 44 (25.4) | 116 (65.5) | 30 (23.3) | ||
| Inpatients | 31 (23.3) | 129 (74.6) | 61 (34.5) | 99 (76.7) | ||
CIS, clinically isolated syndrome; MS, multiple sclerosis; N, number of patients; PwP, patients with polypharmacy; PwSI, patients with secondary illnesses; Pw/oP, patients without polypharmacy; Pw/oSI, patients without secondary illnesses; PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS.
bMedian; cnumber of patients (%) ChiChi-square test; FiFisher’s exact test; UMann-Whitney U test.
Figure 1.
Comparison of the patients’ EDSS score with regard to polypharmacy and patient care. Comparing Pw/oP (N = 133) and PwP (N = 173) (according to all medications) as well as outpatients (N = 146) and inpatients (N = 160), the boxplot shows the distribution of the patients’ degree of disability for each subgroup. The upper and lower quartiles of the EDSS ratings are marked by the boxes. The whiskers range to the minimum and maximum values, while the medians are indicated by horizontal lines. EDSS, expanded disability status scale; N, number of patients; p, p-value; PwP, patients with polypharmacy; Pw/oP, patients without polypharmacy; UMann-Whitney U test.
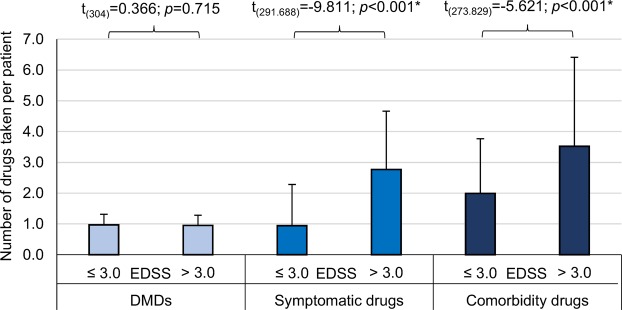
Figure 2.
Number of drugs taken with respect to the patients’ degree of disability and therapy goals. The medications were split into three groups according to the therapy goals: DMDs, symptomatic drugs and comorbidity drugs. The patients were divided into two subgroups with EDSS ≤ 3.0 (N = 143) and >3.0 (N = 163), respectively. The bars mark the average number of drugs taken and the error bars show the standard deviation. Considering the drug intake of MS patients, the numbers of symptomatic and comorbidity drugs taken were significantly higher for patients with higher degrees of disability. DMDs, disease-modifying drugs; EDSS, expanded disability status scale; MS, multiple sclerosis; N, number of patients; t, two-sample two-tailed Student’s t-test; *not assuming equal variances (Levene’s test: p < 0.05).
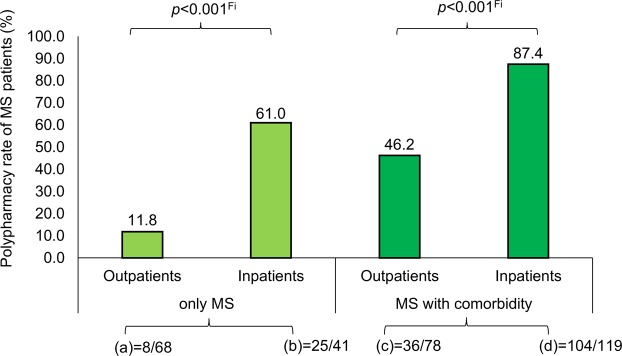
Figure 3.
Polypharmacy rates regarding type of patient care and comorbidities. We split the patients (N = 306) into four groups according to the presence of comorbidities and inpatient/outpatient status. (a) Outpatients with MS only. (b) Inpatients with MS only. (c) Outpatients with MS and comorbidities. (d) Inpatients with MS and comorbidities. Regarding the polypharmacy rates of the four patient groups, MS inpatients with comorbidities showed the highest rate of polypharmacy. FiFisher’s exact test; MS, multiple sclerosis.
Comparing the pharmacological data, the average number of medications taken was twice to three times as high in the PwP group than in the Pw/oP group (according to all medications: 8.1 vs. 2.6; according to long-term medications only: 8.8 vs. 3.4). Adding up the number of all medications taken by the 306 patients yielded a total of 1738 recorded medications (counted with repetitions). Despite the quite similar cohort sizes of PwP (N = 173) and Pw/oP (N = 133) in our study, 80.3% of the medications were taken by the PwP group.
The only medication categories without significant differences between Pw/oP and PwP were DMDs (z = −1.013; p = 0.311) when evaluating polypharmacy according to all medications and DMDs (z = −0.344; p = 0.731) and pro re nata (PRN) medications (z = −1.385; p = 0.166) when regarding long-term medications only (Table 5).
Table 5.
Pharmacological data comparison between patients with and without polypharmacy.
| N | Polypharmacy (all medications) | Polypharmacy (long-term medications only) | ||||
|---|---|---|---|---|---|---|
| Pw/oP | PwP | Statistics | Pw/oP | PwP | Statistics | |
| 133 | 173 | 177 | 129 | |||
| All medicationsa | 2.6 (1.0) | 8.1 (2.9) | z = −15.075, p < 0.001U | 3.4 (1.8) | 8.8 (2.9) | z = −13.655, p < 0.001U |
| Long-term medicationsa | 2.0 (1.0) | 6.4 (3.0) | z = −13.040, p < 0.001U | 2.2 (1.1) | 7.6 (2.5) | z = −15.050, p < 0.001U |
| PRN drugsa | 0.6 (0.8) | 1.7 (1.5) | z = −7.154, p < 0.001U | 1.2 (1.5) | 1.2 (1.2) | z = −1.385, p = 0.166U |
| Prescription-only drugsa | 2.0 (1.0) | 6.5 (2.9) | z = −13.855, p < 0.001U | 2.6 (1.6) | 7.1 (2.9) | z = −12.859, p < 0.001U |
| OTC drugsa | 0.6 (0.7) | 1.6 (1.5) | z = −7.280, p < 0.001U | 0.8 (0.9) | 1.7 (1.5) | z = −6.367, p < 0.001U |
| DMDa | 0.9 (0.3) | 1.0 (0.4) | z = −1.013, p = 0.311U | 1.0 (0.4) | 1.0 (0.3) | z = −0.344, p = 0.731U |
| Symptomatic drugsa | 0.5 (0.7) | 3.0 (1.8) | z = −11.990, p < 0.001U | 1.0 (1.3) | 3.2 (1.9) | z = −10.301, p < 0.001U |
| Comorbidity drugsa | 1.1 (0.9) | 4.1 (2.6) | z = −11.561, p < 0.001U | 1.5 (1.2) | 4.7 (2.7) | z = −11.365, p < 0.001U |
DMD, disease-modifying drugs; N, number of patients; OTC, over-the-counter; PRN, pro re nata; PwP, patients with polypharmacy; Pw/oP, patients without polypharmacy.
aMean value (standard deviation) of the number of drugs taken per patient; UMann-Whitney U test.
In terms of routes of drug administration, PwP took twice to over three times more intravenously, perorally and subcutaneously administered drugs than Pw/oP (Table 6). These significant differences remained after correcting the p-values for multiple testing (FDR < 0.05).
Table 6.
Comparison of routes of drug administration with respect to the presence of polypharmacy.
| Route of administration | Polypharmacy (all medications) | Polypharmacy (long-term medications only) | ||||
|---|---|---|---|---|---|---|
| Pw/oP | PwP | Statistics | Pw/oP | PwP | Statistics | |
| N | 133 | 173 | 177 | 129 | ||
| buccala | 0.0 (0.0) | 0.0 (0.2) | z = −1.762, p = 0.078U | 0.0 (0.2) | 0.0 (0.2) | z = −1.327, p = 0.185U |
| conjunktivala | 0.0 (0.0) | 0.1 (0.3) | z = −2.957, p = 0.003U | 0.0 (0.1) | 0.1 (0.3) | z = −3.333, p = 0.001U |
| cutaneousa | 0.0 (0.1) | 0.1 (0.3) | z = −2.340, p = 0.019U | 0.0 (0.1) | 0.1 (0.3) | z = −2.712, p = 0.007U |
| intramusculara | 0.1 (0.2) | 0.0 (0.2) | z = −0.757, p = 0.449U | 0.1 (0.3) | 0.0 (0.2) | z = −0.856, p = 0.392U |
| intravenousa | 0.4 (0.5) | 0.8 (0.7) | z = −5.141, p < 0.001U | 0.5 (0.6) | 0.8 (0.6) | z = −3.868, p < 0.001U |
| nasala | 0.0 (0.1) | 0.0 (0.1) | z = −0.187, p = 0.852U | 0.0 (0.1) | 0.0 (0.1) | z = −0.225, p = 0.822U |
| perorala | 1.7 (1.0) | 6.2 (2.6) | z = −14.381, p < 0.001U | 2.3 (1.5) | 6.9 (2.6) | z = −13.596, p < 0.001U |
| pulmonarya | 0.0 (0.1) | 0.1 (0.2) | z = −1.307, p = 0.191U | 0.0 (0.1) | 0.1 (0.3) | z = −2.197, p = 0.028U |
| rectala | 0.0 (0.0) | 0.0 (0.2) | z = −1.242, p = 0.214U | 0.0 (0.2) | 0.0 (0.1) | z = −0.220, p = 0.826U |
| subcutaneousa | 0.4 (0.6) | 0.8 (0.7) | z = −5.332, p < 0.001U | 0.5 (0.6) | 0.8 (0.7) | z = −4.865, p < 0.001U |
| sublinguala | 0.0 (0.1) | 0.0 (0.2) | z = −0.803, p = 0.422U | 0.0 (0.1) | 0.0 (0.2) | z = −0.038, p = 0.970U |
| vaginala | 0.0 (0.0) | 0.0 (0.2) | z = −1.762, p = 0.078U | 0.0 (0.1) | 0.0 (0.1) | z = −0.319, p = 0.750U |
N, number of patients; PwP, patients with polypharmacy; Pw/oP, patients without polypharmacy.
aMean value (standard deviation) of the number of drugs taken per patient; UMann-Whitney U test.
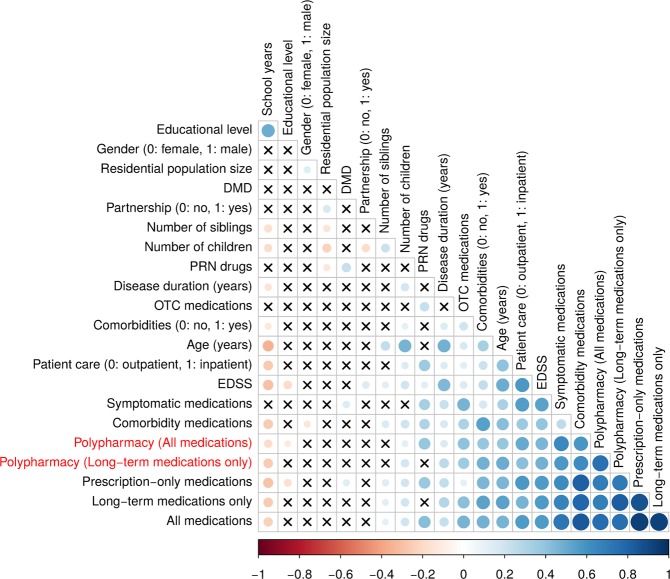
Pearson’s correlation coefficients demonstrated significant relations between several variables, as illustrated in the correlation matrix in Fig. 4. For instance, EDSS and age correlated significantly with each other.
Figure 4.
Correlation matrix visualization of the correlation between variables and polypharmacy status. Using the “corrplot” R package, the correlation matrix heatmap was built. The degree of pairwise correlation with respect to Pearson’s correlation coefficient is displayed by the colour gradient. The crosses represent absence of significance (p-values > 0.05). A significant correlation was seen, for instance, between age and comorbidities. DMD, disease-modifying drug; EDSS, expanded disability status scale; OTC, over-the-counter; PRN, pro re nata.
Drug spectrum
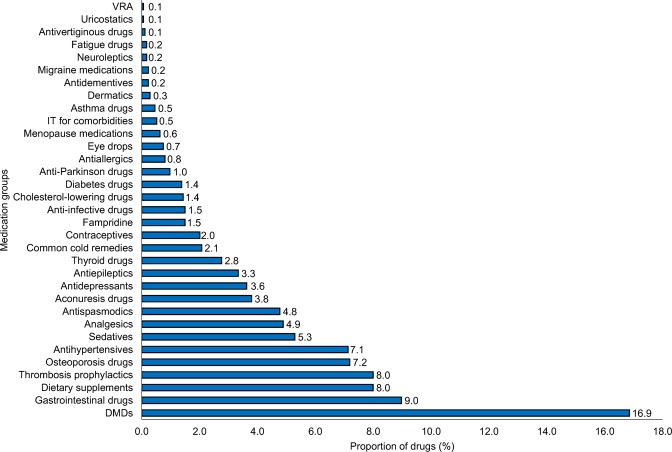
The most frequently used medication groups in our study were DMDs (16.9%), gastrointestinal drugs (9.0%), dietary supplements (8.0%), thrombosis prophylactics (8.0%), osteoporosis medications (7.2%) as well as antihypertensives (7.1%) (Fig. 5).
Figure 5.
Proportion of categories of medications used by MS patients. The proportions of the medication groups were calculated according to the total number of drugs taken by the 306 patients included in our study (N = 1738). The proportions ranged from 0.1% (VRA, uricostatics) to 16.9% (DMDs). DMDs, disease-modifying drugs; IT, immunotherapy; MS, multiple sclerosis; N, number of medications; VRA, vasopressin receptor antagonists.
Significant differences between PwP and Pw/oP in the frequency of drugs taken emerged for all of the aforementioned medication groups except for the DMDs (Table 7). On average, PwP, older patients and inpatients took drugs more frequently than Pw/oP, younger patients and outpatients. As an exception, contraceptives were taken more often by Pw/oP, outpatients and younger patients. There were no significant differences for antiallergics, anti-dementia drugs, dermatics, fatigue medications, menopause medications, migraine medications, neuroleptics, thyroid medications, uricostatics and vasopressin receptor antagonists (Fisher’s exact test: p > 0.05).
Table 7.
Comparison of medications regarding polypharmacy, age and patient care.
| N | Polypharmacy (all medications) | Polypharmacy (long-term medications only) | Age (Years) | Patient care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pw/oPa | PwPa | FDRFi | Pw/oPa | PwPa | FDRFi | <60a | ≥60a | FDRFi | OPa | IPa | FDRFi | |
| 133 | 173 | 177 | 129 | 247 | 59 | 146 | 160 | |||||
| Aconuresis drugs | 8.3 | 26.6 | <0.001 | 7.3 | 34.1 | <0.001 | 17.0 | 25.4 | 0.243 | 9.6 | 26.9 | <0.001 |
| Analgesics | 18.0 | 31.8 | 0.015 | 23.2 | 29.5 | 0.298 | 25.5 | 27.1 | 1.000 | 25.3 | 26.3 | 1.000 |
| Antiallergics | 3.8 | 4.6 | 0.956 | 4.5 | 3.9 | 1.000 | 5.3 | 0.0 | 0.167 | 5.5 | 3.1 | 0.730 |
| Antidementives | 0.0 | 2.3 | 0.194 | 0.0 | 3.1 | 0.051 | 0.8 | 3.4 | 0.266 | 0.7 | 1.9 | 0.907 |
| Anti-depressants | 8.3 | 26.6 | <0.001 | 9.0 | 31.8 | <0.001 | 15.8 | 30.5 | 0.055 | 12.3 | 24.4 | 0.024 |
| Antiepileptics | 3.0 | 27.7 | <0.001 | 4.0 | 34.9 | <0.001 | 13.0 | 33.9 | <0.001 | 7.5 | 25.6 | <0.001 |
| Antihyper-tensives | 6.0 | 39.9 | <0.001 | 8.5 | 48.1 | <0.001 | 17.8 | 55.9 | <0.001 | 11.6 | 37.5 | <0.001 |
| Anti-infective drugs | 0.8 | 12.7 | <0.001 | 1.7 | 15.5 | < 0.001 | 5.7 | 15.3 | 0.076 | 4.1 | 10.6 | 0.108 |
| Anti-Parkinson drugs | 0.8 | 7.5 | 0.010 | 1.7 | 8.5 | 0.019 | 3.6 | 8.5 | 0.257 | 2.1 | 6.9 | 0.113 |
| Antispas-modics | 3.8 | 35.8 | <0.001 | 9.0 | 39.5 | <0.001 | 19.4 | 32.2 | 0.094 | 6.8 | 35.6 | <0.001 |
| Antivertiginous drugs | 0.0 | 1.2 | 0.669 | 0.0 | 1.6 | 0.243 | 0.0 | 3.4 | 0.094 | 0.0 | 1.3 | 0.823 |
| Asthma drugs | 0.0 | 3.5 | 0.063 | 0.0 | 4.7 | 0.010 | 1.2 | 5.1 | 0.171 | 2.1 | 1.9 | 1.000 |
| Cholesterol-lowering drugs | 0.8 | 13.9 | <0.001 | 1.1 | 17.8 | <0.001 | 4.0 | 25.4 | <0.001 | 2.7 | 13.1 | 0.003 |
| Common cold remedies | 5.3 | 15.0 | 0.015 | 8.5 | 14.0 | 0.199 | 9.3 | 16.9 | 0.189 | 11.0 | 10.6 | 1.000 |
| Contraceptives | 15.0 | 8.7 | 0.162 | 16.4 | 4.7 | 0.004 | 14.2 | 0.0 | <0.001 | 19.9 | 3.8 | <0.001 |
| Dermatics | 1.5 | 1.7 | 1.000 | 1.1 | 2.3 | 0.716 | 1.6 | 1.7 | 1.000 | 1.4 | 1.9 | 1.000 |
| Diabetes drugs | 0.8 | 8.1 | 0.007 | 2.3 | 8.5 | 0.028 | 3.6 | 10.2 | 0.106 | 2.1 | 7.5 | 0.080 |
| Dietary supplements | 21.1 | 38.7 | 0.003 | 23.7 | 41.1 | 0.004 | 31.2 | 30.5 | 1.000 | 32.2 | 30.0 | 0.979 |
| DMDs | 92.5 | 91.9 | 1.000 | 91.5 | 93.0 | 0.716 | 92.3 | 91.5 | 0.967 | 95.9 | 88.8 | 0.079 |
| Eye drops | 0.0 | 6.4 | 0.007 | 0.6 | 7.8 | 0.003 | 2.4 | 8.5 | 0.097 | 2.7 | 4.4 | 0.858 |
| Fampridine | 0.8 | 14.5 | <0.001 | 5.1 | 13.2 | 0.036 | 7.7 | 11.9 | 0.455 | 4.1 | 12.5 | 0.033 |
| Fatigue drugs | 0.0 | 1.7 | 0.358 | 0.0 | 2.3 | 0.111 | 0.8 | 1.7 | 0.653 | 0.7 | 1.3 | 1.000 |
| Gastrointes-tinal drugs | 11.3 | 68.2 | <0.001 | 24.9 | 69.0 | <0.001 | 37.2 | 69.5 | <0.001 | 11.6 | 72.5 | <0.001 |
| IT for comorbidities | 0.0 | 4.0 | 0.035 | 0.0 | 5.4 | 0.004 | 1.2 | 6.8 | 0.084 | 1.4 | 3.1 | 0.783 |
| Menopause medications | 1.5 | 5.2 | 0.183 | 1.7 | 6.2 | 0.091 | 4.0 | 1.7 | 0.885 | 2.1 | 5.0 | 0.435 |
| Migraine medications | 0.8 | 1.2 | 1.000 | 1.1 | 0.8 | 1.000 | 1.2 | 0.0 | 1.000 | 0.7 | 1.3 | 1.000 |
| Neuroleptics | 0.8 | 1.2 | 1.000 | 0.6 | 1.6 | 0.654 | 0.8 | 1.7 | 0.653 | 0.7 | 1.3 | 1.000 |
| Osteoporosis drugs | 17.3 | 49.1 | <0.001 | 22.0 | 53.5 | <0.001 | 30.8 | 54.2 | 0.004 | 22.6 | 46.9 | <0.001 |
| Sedatives | 8.3 | 44.5 | <0.001 | 18.1 | 43.4 | <0.001 | 24.3 | 47.5 | 0.004 | 4.8 | 50.6 | <0.001 |
| Thrombosis prophylactics | 7.5 | 64.7 | <0.001 | 18.6 | 69.0 | <0.001 | 31.6 | 74.6 | <0.001 | 7.5 | 69.4 | <0.001 |
| Thyroid drugs | 13.5 | 15.6 | 0.800 | 12.4 | 17.8 | 0.257 | 14.2 | 16.9 | 0.777 | 15.8 | 13.8 | 0.907 |
| Uricostatics | 0.0 | 0.6 | 1.000 | 0.0 | 0.8 | 0.497 | 0.4 | 0.0 | 1.000 | 0.0 | 0.6 | 1.000 |
| VRA | 0.0 | 0.6 | 1.000 | 0.0 | 0.8 | 0.497 | 0.4 | 0.0 | 1.000 | 0.0 | 0.6 | 1.000 |
DMD, disease-modifying drug; FDR, adjusted p-value according to false discovery rate; IP, inpatients; IT, immunotherapy; OP, outpatients; PwP, patients with polypharmacy; Pw/oP, patients without polypharmacy; VRA, vasopressin receptor antagonists.
aFrequency of use of medication groups (%); FiFisher’s exact test.
Discussion
Our prospective single-center study aimed to examine the prevalence of polypharmacy in a cohort of MS patients as well as possible influencing factors associated with polypharmacy. To date, there are only few studies addressing polypharmacy in MS patients37–40. The available studies analyzed quality of life and relapse rate38, occurring symptoms such as fatigue and cognitive ability37, the use of antiepileptic drugs or antidepressants39 and the aspect of rehospitalization40. The focus of our study was on presenting a real-world snapshot of a German MS cohort with respect to the prevalence of polypharmacy, supplemented by an investigation of the associations of polypharmacy with sociodemographic and clinical-neurological factors. Moreover, we considered the whole range of medications, identifying the most frequent medication groups used by the examined MS patients and in particular by PwP.
The study population showed an average age of 48.7 years, resembling values found in other studies examining polypharmacy in MS37–39. A low employment rate (37.6% in our study) despite the relatively young average age can be explained by the limiting nature of the disease, e.g. due to fatigue and cognitive disturbances15,41. Even at lower levels of disability, such impairments can lead to an incapacity to work.
As expected, the patients’ degree of disability laid in the moderate range with a median EDSS score of 3.5. For the German MS registry (N = 48386 patients in the registry population), a median EDSS score of 3.0 was reported42. Similar findings were described in other studies37,42. The MS subtype proportions in our study (62.7% CIS/RRMS, 26.1% SPMS and 11.1% PPMS) were in accordance with the expected distribution43. With regard to hospitalization, there were approximately equal numbers of outpatients and inpatients in our study. The treatment of SPMS and PPMS with, for instance, glucocorticosteroid (GCS) pulses or triamcinolone acetonide took place in the inpatient setting44,45. For this reason, inpatients included more SPMS and PPMS patients, while CIS/RRMS patients more frequently received outpatient care.
There are various ways to define polypharmacy: The division into minor polypharmacy (two to four medications) and major polypharmacy (five or more medications)46, the prescription of two or more medications with the same therapeutic objective47,48 or two or more medications which belong to the same chemical substance class47. However, the most common definition of polypharmacy is exceeding a certain number of medications29. In our study, polypharmacy was defined as the use of five or more medications, as this definition is well-established and frequently used in the literature20–25.
The proportion of PwP in the present study was 56.5% when analyzing polypharmacy according to all medications. The second classification, in which pro re nata drugs (PRN) were excluded, yielded a polypharmacy rate of 42.2%. These polypharmacy rates of our MS cohort resemble those of other polypharmacy studies on MS patients, reporting rates of 14.9%38 to 59%39. The rate of 14.9% was relatively low because first- and second-generation DMDs, general immunosuppressants and GCS38 have not been considered for examining polypharmacy.
Distinguishing polypharmacy by including or excluding PRN drugs offers, on the one hand, the opportunity to take a general view on all medications and, on the other hand, the investigation of medications which are taken regularly and on a long-term basis. Comparing these two definitions, the analysis considering all medications may provide a broader assessment because many patients additionally take as-needed medications like OTC and herbal preparations38.
Regarding sociodemographic data, the relatively high average age and high retirement rate in the group of PwP can be attributed to the increasing likelihood of suffering from comorbidities with age. Earlier studies have already demonstrated that a higher age at the time of MS diagnosis is associated with comorbidities49,50. Accordingly, the number of medications taken also rises with age.
The association between higher EDSS scores and polypharmacy is paralleled by higher proportions of SPMS and PPMS patients among PwP51 and, consequently, a higher proportion of inpatients in the PwP group. Moreover, the significantly higher age of the PwP explains the significantly longer mean disease duration compared to Pw/oP37. A significant difference between Pw/oP and PwP also emerged in terms of comorbidities: Among the PwP, comorbidities were almost twice as prevalent as among the Pw/oP. There are two major reasons for this observation: First, the occurrence of comorbidities leads to an increasing number of medications taken. Secondly, certain MS drugs can cause secondary illnesses and side effects52, requiring further medical interventions12.
Generally, distinguishing between comorbidities as separate diagnoses and disease symptoms is a debated issue. For instance, is depression a comorbidity or a symptom of MS? In some studies, depression has been associated with more lesions at particular brain areas and so it could be a secondary manifestation of MS53. However, there is no consistent causality. Consequently, for implementing a more general definition of comorbidities, we followed the principles laid down by the “International Workshop on Comorbidities in MS”54–62.
The more detailed analysis of the pharmacological data revealed that PwP took, on average, much more drugs than Pw/oP (mean values: 8.1 vs. 2.6). The DMDs did not contribute to this quantitative medication difference between Pw/oP and PwP (Table 5), as immunotherapy in MS is generally maintained as a monotherapy63. Accordingly, a higher number of DMDs among PwP was not to be expected. Twenty-three (7.5%) of the 306 patients were not currently taking any DMD. Some of these patients were in the process of having their treatment adjusted or opted to stop the treatment due to side effects12. Nine patients (2.9%) had two DMDs in their medication plans, which have been recorded in the patient interview and by reviewing the patient records. In each case, one of these two medications was a GCS which was used to treat an acute relapse occurring at the time of the survey. All other 274 patients (89.5%) have taken precisely one DMD. Following the guidelines of the German Neurological Society, an early initiation of DMD treatment is recommended after diagnosis. Thus, few MS patients are not treated. Recent data of a German National MS Cohort showed that after a median time of 167 days, the majority of early-stage CIS/RRMS patients (762/1124) started DMD therapy64. In our study, the median disease duration was 11 years, so nearly all patients used DMDs.
Regarding the routes of drug administration, peroral medications constituted the largest share of routes of administration in this study, with 74.1%. The finding that the majority of the recorded medications were administered in this way, which is generally the most popular one65, can be explained by the fact that peroral administration is easy to understand, uncomplicated and well-manageable.
Examining the question of which sociodemographic and clinical-neurological factors in combination are substantially associated with polypharmacy, the following results emerged: On the one hand, polypharmacy was correlated with higher levels of disability (Wald(1) = 11.769; p = 0.001; OR = 1.385). Thus, with each 1.0 step on the EDSS, the risk of polypharmacy rises by 38.5%. This may be referred to the increase of medical therapies, which are used to compensate and to reduce symptoms like spasticity14, pain16 and gait disturbances66. Furthermore, the presence of secondary illnesses was associated with polypharmacy (Wald(1) = 26.620; p < 0.001; OR = 4.879). Thus, the risk of polypharmacy among patients with secondary illnesses (PwSI) was almost five times as high as in patients without secondary illnesses (Pw/oSI), which can be attributed to the additionally prescribed treatments due to the occurrence of comorbidities. There was also an association between the type of patient care and polypharmacy: Inpatients had a five times higher risk of polypharmacy than outpatients (Wald(1) = 25.253; p < 0.001; OR = 5.146). An explanation for this may be that the inpatients in our study were mostly SPMS or PPMS patients who generally show higher EDSS scores than RRMS patients51. More strongly disabled patients were therefore mostly treated in the inpatient setting. Moreover, inpatients are more likely taking prophylactic medications, e.g. thrombosis prophylactics, leading to a further rise in the number of medications taken.
However, the patient care differs among countries. For instance, Germany and France have a distinctive inpatient healthcare system, while patients in the United Kingdom and Canada are mostly treated in outpatient settings67,68. There are many global differences in the access and management of MS treatment69. In some countries, governments and health insurances do not fully compensate for the costs of DMD treatment1,70,71. Access to DMDs is strongly dependent on treatment costs. For a US MS patient, the costs of DMD treatment (at least US$ 50,000 per year) are two to three times higher than in Australia or Canada72. Despite these differences, the essential associations found in our study should be generalizable for other countries.
In the analysis of the spectrum of medications, DMDs, osteoporosis medications, antihypertensives, gastrointestinal drugs, thrombosis prophylactics and dietary supplements were identified as the most frequently used medication groups in MS in our study. DMDs form the basis for MS immunotherapy to prevent relapses and to alleviate the progression of the disease2. There is evidence that patients with cardiovascular diseases are at higher risk of developing a further one73 and so those patients are more likely to use more than one cardiovascular drug. This matter as well as higher age and therapeutic side effects are related to the observed more frequent use of antihypertensives74, gastrointestinal drugs75 and osteoporosis drugs76. The majority of thrombosis prophylactics and proton pump inhibitors in our study were administered during the inpatient hospital stay for GCS pulse treatment, following the German guidelines for the diagnosis and treatment of MS77. The use of dietary and herbal supplements in the general population has become a trend78,79 because they are available in any price range and prescriptions by physicians are not necessary. Dietary supplements may be useful to support MS treatment. However, further studies are necessary to evaluate the effect of supplements on relapse rate and progression of MS80,81.
The establishment of a well-thought-out medication management considering an adjustment of medications is essential to optimize treatment. The patients’ medication plans should be regularly checked by physicians and pharmacists for identifying unnecessary or missing prescriptions as well as drug interactions. Therefore, a well working network between the patients’ physicians and pharmacists has to be established. Furthermore, evidence-based, non-medicinal approaches such as physiotherapy82–84 or cognitive-behavioral talking therapy85 can offer alternatives to or complement medications. In addition, there is evidence that the mortality risk of older polypharmacy patients can be reduced by a healthy lifestyle86.
Limitations of this study that should be mentioned are the lack of controls and the absent explanatory power regarding causality. A longitudinal study with controls could reveal new insights on causal relationships and could point out further MS-specific factors underlying polypharmacy. Nonetheless, this study gave a current overview of the prevalence and the medication situation in a large representative single-center MS cohort. Our study may be the starting point for further studies addressing testable hypotheses.
In summary, our study showed that polypharmacy plays an important role for MS patients. Polypharmacy in MS is linked to higher degrees of disability, the presence of comorbidities and an inpatient treatment scenario. Future computational analyses of the medication plans of MS patients could be conducted to assess potential and clinically relevant medication interactions.
Materials and Methods
This cross-sectional study was conducted at the Department of Neurology and the Division of Neuroimmunology at the University Medicine Rostock. The data were collected between March 2017 and May 2018. In order to gather sociodemographic data, clinical-neurological data and current medication data, patients were examined by the following means: anamnesis, structured patient interview, patient records and clinical examination. The study was conducted in accordance with the EU General Data Protection Regulation. The inclusion criterion for this study was a confirmed diagnosis of MS or CIS according to the revised McDonald criteria from 201087. Overall, 309 patients attended the examination. Three of them declined to participate in the study for personal reasons. Thus, 306 patients were included and analyzed in this study. Prior informed consent was obtained from all individual participants included in the study. This study was approved by the University of Rostock’s ethics committee (permit number A 2014-0089) and carried out in line with the Declaration of Helsinki.
Data collection
The patients were examined with respect to sociodemographic, clinical-neurological and pharmacological factors.
Sociodemographic data: These included gender, age, number of school years (not including time spent in training or higher education), educational level, employment status and place of residence, with the latter subdivided into rural community (<5000 residents), small town (5000–20000 residents), medium-sized town (20000–100000) and city (>100000). Moreover, partnership status, number of children and number of siblings were recorded.
Clinical-neurological data: To categorize the degree of disability, Kurtzke’s Expanded Disability Status Scale (EDSS) was used88. MS subtypes were classified into RRMS, PPMS, SPMS and patients with CIS (N = 6)89. Furthermore, we recorded the disease duration since the time of the initial diagnosis of MS/CIS as well as data on the presence of comorbidities (Pw/oSI and PwSI) and the type of patient care (outpatient, inpatient).
Pharmacological data: The trade name, indication, active ingredients, dosage and route of administration were obtained for the various preparations from the patients’ medication plans.
To ensure the completeness of the collected data, for each patient, a structured patient interview and a review of the medical records were conducted. In the analysis, we only considered medications which were actually taken as stated by the patients.
Inpatient and outpatient scenario
At the Department of Neurology of the University of Rostock, there are wards for inpatients as well as for outpatients. At the outpatients’ ward, patients with a usually stable disease situation had a routine appointment. While waiting for the checkup there, the outpatients were asked to participate in this study. After patients’ agreement, the structured interview and the review of the medical records were performed. At the inpatients’ ward, there were patients with more severe disease courses, patients with an increase in disease activity and patients with adverse events during DMD therapy. Usually, inpatients staid there a couple of days. During this time, the patients were asked to take part in this study and after their agreement, the interview and the patient record review were conducted.
Medication analysis
A more precise analysis of the medications was undertaken by evaluating them according to three criteria.
Dosing schedule: A distinction was made between long-term and as-needed (pro re nata; PRN) medications. Long-term medications are those taken daily or at regular intervals, e.g. once a week or once a month. Such medications are used to treat long-term illnesses or complaints. In contrast, PRN medications are taken at irregular intervals to treat acute or sporadic complaints.
Prescription status: A distinction was made between prescription-only and OTC preparations.
Therapeutic objective: A distinction was made between DMDs90, specific symptomatic drugs for MS and medications to treat comorbidities. The symptomatic drugs aim at a targeted alleviation of specific MS symptoms, such as spasticity or pain. Medications to treat comorbidities comprise drugs for the therapy of secondary illnesses and for other reasons, e.g. contraception.
Definition of polypharmacy and comorbidities
The number of five medications was set as the threshold to compare Pw/oP and PwP. Accordingly, patients who took five or more medications were counted as PwP. This constitutes the most common definition of polypharmacy20–25. Polypharmacy was analyzed, on the one hand, according to the total number of medications (i.e. the sum of long-term and PRN medications) and, on the other hand, according to the number of long-term medications only.
For defining comorbidities, we followed the study by Laroni et al.49 and adhered to the recommendations for observational studies of comorbidity in MS by Marrie et al.55 (“International Workshop on Comorbidities in MS”)54,56–62. Accordingly, PwSI were defined based on the patient records and the physicians’ expert opinion. Patients without comorbidities were categorized as Pw/oSI, while those categorized as PwSI had to have at least one comorbidity.
Statistical analysis
Statistical analyses were conducted using PASW Statistics 18 (IBM). Patient data were anonymized before entry into the database. The collected data were tested for homogeneity of variances (Levene’s test). For the comparative analysis of Pw/oP and PwP, we used two-sample two-tailed Student’s t-tests, Fisher’s exact tests, Chi-square tests and Mann-Whitney U tests. To determine which associations between polypharmacy (defined by the total number of medications taken) as response variable and eight sociodemographic variables (gender, age, number of school years, educational level, partnership, place of residence, number of children and number of siblings) as well as four clinical-neurological variables (comorbidities, inpatient/outpatient care, disease duration and EDSS) as explanatory variables could be seen as statistically significant, we conducted a stepwise binary logistic regression with forward variable selection based on the likelihood ratio. The significance level was set at α = 0.05. To take into account alpha error inflation in the case of multiple testing, the p-values were corrected according to FDR91. The pairwise interdependencies between various variables were identified by the analysis of Pearson’s correlation coefficients. Using the “corrplot” R package, we obtained a correlation matrix.
Acknowledgements
The authors would like to express their special thanks to Antje Bombor, Ina Schröder, Angela Steffan, Sylvia Röhring and Nele Retzlaff of the Department of Neurology at the University of Rostock for providing help in the data acquisition and to Sarah L. Mannion for professional translation service. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author Contributions
U.K.Z. inspired and directed the study. N.F. gathered, analysed and interpreted the data, drafted the manuscript and prepared all tables and figures. M.H. helped with the data analysis. U.K.Z. and M.H. contributed to the manuscript design, helped in the interpretation of the results and corrected and improved the paper. All authors read and approved the final manuscript.
Data Availability
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
Competing Interests
M.H. received speaking fees and travel funds from Bayer HealthCare, Biogen, Novartis and Teva. U.K.Z. received research support as well as speaking fees and travel funds from Almirall, Bayer HealthCare, Biogen, Merck Serono, Novartis, Sanofi and Teva. N.F. declares no financial competing interests. M.H., U.K.Z. and N.F. declare no non-financial competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael Hecker and Uwe Klaus Zettl contributed equally.
References
- 1.Browne P, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawachi I, Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry. 2017;88:137–145. doi: 10.1136/jnnp-2016-313300. [DOI] [PubMed] [Google Scholar]
- 4.James T, et al. Impact of genetic risk loci for multiple sclerosis on expression of proximal genes in patients. Hum. Mol. Genet. 2018;27:912–928. doi: 10.1093/hmg/ddy001. [DOI] [PubMed] [Google Scholar]
- 5.Olafsson S, et al. Fourteen sequence variants that associate with multiple sclerosis discovered by meta-analysis informed by genetic correlations. NPJ Genom. Med. 2017;2:24. doi: 10.1038/s41525-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patsopoulos, N. et al. The Multiple Sclerosis Genomic Map. Role of peripheral immune cells and resident microglia in susceptibility, 10.1101/143933 (2017).
- 7.Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: Prevalence and impact. Health Rep. 2018;29:3–8. [PubMed] [Google Scholar]
- 8.Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 9.The IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 10.The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group Interferon beta-1b in the treatment of multiple sclerosis: Final outcome of the randomized controlled trial. Neurology. 1995;45:1277–1285. [PubMed] [Google Scholar]
- 11.Zettl UK, Hecker M, Aktas O, Wagner T, Rommer PS. Interferon β-1a and β-1b for patients with multiple sclerosis. Updates to current knowledge. Expert Rev. Clin. Immunol. 2018;14:137–153. doi: 10.1080/1744666X.2018.1426462. [DOI] [PubMed] [Google Scholar]
- 12.Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: Pharmacotherapy options for patients. Expert Opin. Pharmacother. 2018;19:483–498. doi: 10.1080/14656566.2018.1446944. [DOI] [PubMed] [Google Scholar]
- 13.Koutsis G, et al. A neurophysiological study of facial numbness in multiple sclerosis: Integration with clinical data and imaging findings. Mult. Scler. Relat. Disord. 2016;9:140–146. doi: 10.1016/j.msard.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Patejdl R, Zettl UK. Spasticity in multiple sclerosis. Contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun. Rev. 2017;16:925–936. doi: 10.1016/j.autrev.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: A review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun. Rev. 2016;15:210–220. doi: 10.1016/j.autrev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, K. L., Bethea, J. R. & Fischer, R. Neuropathic Pain in Multiple sclerosis—Current Therapeutic Intervention and Future Treatment Perspectives in Perspectives in treatment and pathogenesis (eds Zagon, I. S. & McLaughlin, P. J.) 53–69 (Codon Publications, Brisbane, Australia, 2017). [PubMed]
- 17.Solaro C, Gamberini G, Masuccio FG. Depression in Multiple Sclerosis: Epidemiology, Aetiology, Diagnosis and Treatment. CNS Drugs. 2018;32:117–133. doi: 10.1007/s40263-018-0489-5. [DOI] [PubMed] [Google Scholar]
- 18.Ayache SS, et al. Tremor in multiple sclerosis: The intriguing role of the cerebellum. J. Neurol. Sci. 2015;358:351–356. doi: 10.1016/j.jns.2015.09.360. [DOI] [PubMed] [Google Scholar]
- 19.Kochs L, Wegener S, Sühnel A, Voigt K, Zettl UK. The use of complementary and alternative medicine in patients with multiple sclerosis: A longitudinal study. Complement. Ther. Med. 2014;22:166–172. doi: 10.1016/j.ctim.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Jörgensen T, Johansson S, Kennerfalk A, Wallander MA, Svärdsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann. Pharmacother. 2001;35:1004–1009. doi: 10.1345/aph.10351. [DOI] [PubMed] [Google Scholar]
- 21.Linjakumpu T, et al. Use of medications and polypharmacy are increasing among the elderly. J. Clin. Epidemiol. 2002;55:809–817. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 22.Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J. Am. Acad. Nurse Pract. 2005;17:123–132. doi: 10.1111/j.1041-2972.2005.0020.x. [DOI] [PubMed] [Google Scholar]
- 23.Haider SI, Johnell K, Thorslund M, Fastbom J. Analysis of the association between polypharmacy and socioeconomic position among elderly aged > or = 77 years in Sweden. Clin. Ther. 2008;30:419–427. doi: 10.1016/j.clinthera.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Richardson K, Ananou A, Lafortune L, Brayne C, Matthews FE. Variation over time in the association between polypharmacy and mortality in the older population. Drugs Aging. 2011;28:547–560. doi: 10.2165/11592000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Onder G, et al. Polypharmacy in nursing home inEurope: results from the SHELTER study. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:698–704. doi: 10.1093/gerona/glr233. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland JJ, et al. Co-prescription trends in a large cohort of subjects predict substantial drug-drug interactions. PLoS ONE. 2015;10:e0118991. doi: 10.1371/journal.pone.0118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushardt RL, Massey EB, Simpson TW, Ariail JC, Simpson KN. Polypharmacy: Misleading, but manageable. Clin. Interv. Aging. 2008;3:383–389. doi: 10.2147/cia.s2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci. Data. 2017;4:170167. doi: 10.1038/sdata.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne RA. The epidemiology of polypharmacy. Clin. Med. (Lond.) 2016;16:465–469. doi: 10.7861/clinmedicine.16-5-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Yan H, Wang D. Polypharmacy and its correlates in village health clinics across 10 provinces of Western China. J. Epidemiol. Community Health. 2010;64:549–553. doi: 10.1136/jech.2008.085415. [DOI] [PubMed] [Google Scholar]
- 31.Rambhade S, Chakarborty A, Shrivastava A, Patil UK, Rambhade A. A survey on polypharmacy and use of inappropriate medications. Toxicol. Int. 2012;19:68–73. doi: 10.4103/0971-6580.94506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ticinesi A, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017;7:11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014;13:57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazargan M, et al. Non-adherence to medication regimens among older African-American adults. BMC Geriatr. 2017;17:163. doi: 10.1186/s12877-017-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sganga F, et al. Polypharmacy and health outcomes among older adults discharged from hospital: results from the CRIME study. Geriatr. Gerontol. Int. 2015;15:141–146. doi: 10.1111/ggi.12241. [DOI] [PubMed] [Google Scholar]
- 37.Thelen JM, Lynch SG, Bruce AS, Hancock LM, Bruce JM. Polypharmacy in multiple sclerosis: relationship with fatigue, perceived cognition, and objective cognitive performance. J. Psychosom. Res. 2014;76:400–404. doi: 10.1016/j.jpsychores.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Jelinek GA, et al. Medication use in a large international sample of people with multiple sclerosis: associations with quality of life, relapse rate and disability. Neurol. Res. 2015;37:662–673. doi: 10.1179/1743132815Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beiske GAG, Holmøy T, Beiske AG, Johannessen SI, Johannessen Landmark C. Antiepileptic and Antidepressive Polypharmacy in Patients withMultiple Sclerosis. Mult. Scler. Int. 2015;2015:317859. doi: 10.1155/2015/317859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans C, et al. Adherence to disease-modifying therapies for multiple sclerosis and subsequent hospitalizations. Pharmacoepidemiol. Drug Saf. 2017;26:702–711. doi: 10.1002/pds.4207. [DOI] [PubMed] [Google Scholar]
- 41.DeLuca GC, Yates RL, Beale H, Morrow SA. Cognitive impairment in multiple sclerosis: Clinical, radiologic and pathologic insights. Brain Pathol. 2015;25:79–98. doi: 10.1111/bpa.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiel S, et al. Neuroimmunological Registries in Germany. Neurology International Open. 2018;02:E25–E39. [Google Scholar]
- 43.Höer A, et al. Multiple sclerosis in Germany: Data analysis of administrative prevalence and healthcare delivery in the statutory health system. BMC Health Serv. Res. 2014;14:381. doi: 10.1186/1472-6963-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rommer PS, et al. Effects of repeated intrathecal triamcinolone-acetonide application on cerebrospinal fluid biomarkers of axonal damage and glial activity in multiple sclerosis patients. Mol. Diagn. Ther. 2014;18:631–637. doi: 10.1007/s40291-014-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu-Mugheisib M, Benecke R, Zettl UK. Management of spasticity in progressive multiple sclerosis: Efficacy of repeated intrathecal triamcinolone acetonide administration. Curr. Pharm. Des. 2012;18:4564–4569. doi: 10.2174/138161212802502251. [DOI] [PubMed] [Google Scholar]
- 46.Bjerrum L, Rosholm JU, Hallas J, Kragstrup J. Methods for estimating the occurrence of polypharmacy by means of a prescription database. Eur. J. Clin. Pharmacol. 1997;53:7–11. doi: 10.1007/s002280050329. [DOI] [PubMed] [Google Scholar]
- 47.Brager R, Sloand E. The spectrum of polypharmacy. Nurse Pract. 2005;30:44–50. doi: 10.1097/00006205-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laroni A, et al. Assessing association of comorbidities with treatment choice and persistence in MS: A real-life multicenter study. Neurology. 2017;89:2222–2229. doi: 10.1212/WNL.0000000000004686. [DOI] [PubMed] [Google Scholar]
- 50.Marrie RA, et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72:117–124. doi: 10.1212/01.wnl.0000333252.78173.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bøe Lunde HM, et al. Employment among patients with multiple sclerosis-a population study. PLoS ONE. 2014;9:e103317. doi: 10.1371/journal.pone.0103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan TB, Berkowitz AL, Samuels MA. Cardiovascular Dysfunction in Multiple Sclerosis. Neurologist. 2015;20:108–114. doi: 10.1097/NRL.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 53.Files DK, Jausurawong T, Katrajian R, Danoff R. Multiple sclerosis. Prim. Care. 2015;42:159–175. doi: 10.1016/j.pop.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Marrie RA, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Mult. Scler. 2015;21:263–281. doi: 10.1177/1352458514564491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marrie RA, et al. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology. 2016;86:1446–1453. doi: 10.1212/WNL.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marrie RA, et al. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult. Scler. 2015;21:282–293. doi: 10.1177/1352458514564490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marrie RA, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult. Scler. 2015;21:318–331. doi: 10.1177/1352458514564485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marrie RA, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult. Scler. 2015;21:294–304. doi: 10.1177/1352458514564489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marrie RA, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult. Scler. 2015;21:342–349. doi: 10.1177/1352458514564486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrie RA, et al. The incidence and prevalence of comorbid gastrointestinal, musculoskeletal, ocular, pulmonary, and renal disorders in multiple sclerosis: A systematic review. Mult. Scler. 2015;21:332–341. doi: 10.1177/1352458514564488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marrie RA, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult. Scler. 2015;21:305–317. doi: 10.1177/1352458514564487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Culpepper WJ. The incidence and prevalence of comorbidity in multiple sclerosis. Mult. Scler. 2015;21:261–262. doi: 10.1177/1352458515574151. [DOI] [PubMed] [Google Scholar]
- 63.Wingerchuk DM, Carter JL. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo. Clin. Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 64.von Bismarck O, et al. Treatment choices and neuropsychological symptoms of a large cohort of earlyMS. Neurol. Neuroimmunol. Neuroinflamm. 2018;5:e446. doi: 10.1212/NXI.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witticke D, Seidling HM, Klimm HD, Haefeli WE. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer. Adherence. 2012;6:679–684. doi: 10.2147/PPA.S35950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens V, Goodman K, Rough K, Kraft GH. Gait impairment and optimizing mobility in multiple sclerosis. Phys. Med. Rehabil. Clin. N. Am. 2013;24:573–592. doi: 10.1016/j.pmr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: Results from five European countries. Mult. Scler. 2012;18:7–15. doi: 10.1177/1352458512441566. [DOI] [PubMed] [Google Scholar]
- 68.Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: The costs and utilities of MS patients in Canada. J. Popul. Ther. Clin. Pharmacol. 2012;19:11–25. [PubMed] [Google Scholar]
- 69.Wilsdon, T., Barron, A., Mitchell-Heggs, A. & Ginoza, S. Access to medicines for multiple sclerosis: Challenges and opportunities. Available at http://www.crai.com/sites/default/files/publications/CRA-Biogen-Access-to-MS-Treatment-Final-Report.pdf (2013).
- 70.Edlin M, Sonnenreich P. Trends in managing multiple sclerosis. P T. 2008;33:611–614. [PMC free article] [PubMed] [Google Scholar]
- 71.Owens GM. Managed care aspects of managing multiple sclerosis. Am. J. Manag. Care. 2013;19:307–12. [PubMed] [Google Scholar]
- 72.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry. Too big to fail? Neurology. 2015;84:2185–2192. doi: 10.1212/WNL.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathur R, Hull SA, Badrick E, Robson J. Cardiovascular multimorbidity: The effect of ethnicity on prevalence and risk factor management. Br. J. Gen. Pract. 2011;61:262–70. doi: 10.3399/bjgp11X572454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang J, Gillespie C, Ayala C, Loustalot F. Prevalence of Self-Reported Hypertension and Antihypertensive Medication Use Among Adults Aged ≥18 Years - United States, 2011-2015. MMWR Morb. Mortal. Wkly. Rep. 2018;67:219–224. doi: 10.15585/mmwr.mm6707a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delcher A, et al. Multimorbidities and Overprescription of Proton Pump Inhibitors in Older Patients. PLoS ONE. 2015;10:e0141779. doi: 10.1371/journal.pone.0141779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold, R. Diagnose und Therapie der Multiplen Sklerose. German Neurological Society. Available at https://www.dgn.org/leitlinien/2333-ll-31-2012-diagnose-und-therapie-der-multiplen-sklerose (2012).
- 78.Lentjes, M. A. H. The balance between food and dietary supplements in the general population. Proc. Nutr. Soc. 78, 97–109 (2019). [DOI] [PMC free article] [PubMed]
- 79.O’Brien SK, Malacova E, Sherriff JL, Black LJ. The Prevalence and Predictors of Dietary Supplement Use in the Australian Population. Nutrients. 2017;9:1154. doi: 10.3390/nu9101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmitz K, et al. “Disease modifying nutricals” for multiple sclerosis. Pharmacol. Ther. 2015;148:85–113. doi: 10.1016/j.pharmthera.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 81.von Geldern G, Mowry EM. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat. Rev. Neurol. 2012;8:678–689. doi: 10.1038/nrneurol.2012.194. [DOI] [PubMed] [Google Scholar]
- 82.Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: A pilot study. Clin. Rehabil. 2007;21:771–781. doi: 10.1177/0269215507077602. [DOI] [PubMed] [Google Scholar]
- 83.Broekmans T, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult. Scler. 2011;17:468–477. doi: 10.1177/1352458510391339. [DOI] [PubMed] [Google Scholar]
- 84.Cakt BD, et al. Cycling progressive resistance training for people with multiple sclerosis: A randomized controlled study. Am. J. Phys. Med. Rehabil. 2010;89:446–457. doi: 10.1097/PHM.0b013e3181d3e71f. [DOI] [PubMed] [Google Scholar]
- 85.Dennison L, Moss-Morris R. Cognitive-behavioral therapy: what benefits can it offer people with multiple sclerosis? Expert Rev. Neurother. 2010;10:1383–1390. doi: 10.1586/ern.10.111. [DOI] [PubMed] [Google Scholar]
- 86.Martinez-Gomez D, et al. A healthy lifestyle attenuates the effect of polypharmacy on total and cardiovascular mortality: A national prospective cohort study. Sci. Rep. 2018;8:12615. doi: 10.1038/s41598-018-30840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 89.Lublin FD, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamout BI, Alroughani R. Multiple Sclerosis. Semin. Neurol. 2018;38:212–225. doi: 10.1055/s-0038-1649502. [DOI] [PubMed] [Google Scholar]
- 91.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.