Abstract
Development of a suitable vaccine against visceral leishmaniasis (VL), a fatal parasitic disease, is considered to be vital for maintaining the success of kala-azar control programs. The fact that Leishmania-infected individuals generate life-long immunity offers a viable proposition in this direction. Our prior studies demonstrated that T-helper1 (Th1) type of cellular response was generated by six potential recombinant proteins viz. elongation factor-2 (elF-2), enolase, aldolase, triose phosphate isomerase (TPI), protein disulfide isomerase (PDI) and p45, derived from a soluble antigenic fraction (89.9–97.1 kDa) of Leishmania (Leishmania) donovani promastigote, in treated Leishmania patients and golden hamsters and showed significant prophylactic potential against experimental VL. Moreover, since, it is well-known that our immune system, in general, triggers production of specific protective immunity in response to a small number of amino acids (peptide), this led to the identification of antigenic epitopes of the above-stated proteins utilizing immunoinformatics. Out of thirty-six, three peptides-P-10 (enolase), P-14, and P-15 (TPI) elicited common significant lymphoproliferative as well as Th1-biased cytokine responses both in golden hamsters and human subjects. Further, immunization with these peptides plus BCG offered 75% prophylactic efficacy with boosted cellular immune response in golden hamsters against Leishmania challenge which is indicative of their candidature as potential vaccine candidates.
Keywords: visceral leishmaniasis, Th1 stimulatory proteins, immunoinformatics, T-cell epitopes, peptides, human PBMCs, hamsters, protective response
Introduction
Visceral leishmaniasis (VL or kala-azar), caused by Leishmania (Leishmania) donovani and Leishmania (Leishmania) infantum is the most severe form of leishmaniasis wherein the immune system of the affected individuals is severely impaired making them susceptible to secondary infections (1). Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan accounts for more than 90% of the recent VL cases (2). Though there is a drastic reduction in a number of active VL patients due to the available chemotherapeutics, the presence of potential reservoirs (asymptomatic and post kala-azar dermal leishmanoids patients) enforce the need of an effective VL vaccine in order to persist the success of current VL elimination program (3). The fact, that there is a development of protective immunity in VL patients in endemic areas, either naturally or after treatment, indicates toward the feasibility of a VL vaccine (4). Although canine VL vaccines are commercially available, no licensed vaccine has been developed against human VL till date (5).
Nowadays, vaccine designing becomes more refined with the improvement in the knowledge of targets of immune responses (6). Peptide epitope, the minimal immunogenic regions of a protein antigen, are the most precise vaccine components that guide the direction of immune responses, hence, forms the basis of peptide-based synthetic vaccines (7). Generation of optimal immune response with these vaccines requires better interaction between T- and B- cell epitopes of pathogen proteins and major histocompatibility complex (MHC) molecule of the host (8). Recent immunoinformatics approaches exploit various algorithms which can predict epitopes with the highest probability of inducing protective immune responses (9). A number of such vaccines have been evaluated against various parasitic diseases such as malaria, toxoplasmosis, and trypanosomiasis (10–13). These vaccines offer several merits over conventional ones particularly in terms of safety and regulatory issues and ease of production (7).
Development of Th1 type immune response, produced by CD4+ T cells, plays a vital role in mediating protection against intracellular pathogens including Leishmania (14), hence, T-cell stimulatory antigens have been explored as suitable vaccine candidates (15). Previously, in our laboratory, proteomic analysis of F2 fraction (ranging from 89.9 to 97.1 kDa) of soluble L. (L.) donovani (SLD) antigen revealed eighteen Th1 stimulatory proteins (16) out of which fifteen were developed as recombinant ones. Comparative immunogenicity of these fifteen recombinant proteins helped in the identification of six viz. aldolase, elongation factor-2 (elF-2), enolase, protein disulfide isomerase (PDI), p45 and triose phosphate isomerase (TPI) that elicited an optimum cellular response (17). In this study, we further undertook identification of potential T-cell epitopes from the above-stated six potential recombinant Th1 stimulatory proteins using immunoinformatics and evaluated the cellular responses in lymphocytes/PBMCs of treated Leishmania-infected hamsters/patients as well as the prophylactic efficacies of these peptides in golden hamster against L. (L.) donovani challenge. These peptides if found potential may further lead to the development of a chimeric vaccine for a specific and optimum immunity against VL.
Materials and Methods
Expression and Purification of Recombinant Proteins
Six recombinant Th1 stimulatory proteins namely aldolase, enolase, elF-2, p45, PDI, and TPI (Accession numbers: GQ220750.1, EU723850.1, EU929069.1, EU723851.1, EU723849.1, and EU867389.1, respectively), were expressed and purified using previously optimized protocols (18–22) and were used herein to compare the results obtained with the peptides identified in this study. The LPS content of the purified recombinant proteins was measured by Limulus amoebocyte lysate test (Pierce LAL Chromogenic Endotoxin Quantitation Kit, Thermo Scientific) and was found to be below 10 endotoxin units (EU)/mg of the recombinant protein.
In silico Prediction and Chemical Synthesis of Peptides
MHC class II binding epitopes were predicted from the above-stated six Th1 stimulatory proteins using Immuno Epitope Database (IEDB) and SYFPEITHI and were synthesized at 0.1 mmol scales using Fmoc-Chemistry by standard solid phase peptide synthesis chemistry.
Animal and Infection
Syrian golden hamsters served as a suitable animal model and were infected by amastigotes purified using percoll (Sigma-Aldrich, USA) density gradient centrifugation method, described by Chang (23). Briefly, the spleens from Leishmania-infected hamsters (45–60 day old) were removed aseptically and homogenized in sterile phosphate buffer saline (PBS) at 4°C. The suspension was centrifuged at 900Xg for 10 min at 4°C, to settle the tissue debris and the supernatant was again centrifuged at 3000Xg for 10 min at 4°C. The sediment containing amastigotes, after RBC lysis treatment, was resuspended in 45% percoll solution in PBS-EDTA (2 mM) which was overlaid in 95% percoll solution and centrifuged at 3,500Xg for 1 h at 4°C. A middle creamy or yellowish layer of amastigotes was aspirated, washed twice and then finally suspended in PBS for counting using an automated cell counter. These purified amastigotes were further used for infecting hamsters.
A batch of 30 hamsters was used for in vitro study. 0.1 ml of inoculum per animal containing 2 × 106 amastigotes/ml was inoculated in 20 hamsters intracardially (i.c.) whereas 10 hamsters were kept as uninfected control. One month later, the magnitude of infection in infected animals was assessed by rk39 dipstick (InBios International Inc., USA). Miltefosine (Synphabase, Switzerland), an oral anti-leishmanial drug, was administered in 10 out of 20 infected hamsters @ 40 mg/kg bodyweight daily for 5 days and these animals were referred as treated Leishmania-infected hamsters.
In vitro Evaluation of Peptides
In vitro cellular proliferation assay was performed both in mononuclear cells isolated from mesenteric lymph nodes of infected, treated and uninfected hamsters, as well as in peripheral blood mononuclear cells (PBMCs) from human subjects (Supplementary Table I) using the Ficoll Hypaque density gradient centrifugation (Histopaque 1077, Sigma-Aldrich, USA) as described by Garg et al. (24). Briefly, 0.1 ml of cell suspension in complete RPMI-1640 (Sigma-Aldrich, USA) containing 1 × 106 cells/ml was seeded in 96-well flat-bottom tissue culture plates (Nunc, Denmark) and stimulated with each of the 6 recombinant proteinsand their total 36 peptides @ 10 ng/ml in triplicates. Phytohaemagglutinin (PHA, 10 ng/ml Sigma-Aldrich, USA) for Patient's PBMCs and concanavalin A (ConA, 10 ng/ml, Sigma-Aldrich, USA) for hamster's mononuclear cells were used as standard mitogens to ascertain the procedural sensitivity. Cells were maintained in a CO2 incubator (37°C with 5% CO2) for 3 days. After the incubation, 100 μl of supernatant was taken out from each well and 50 μl of XTT (Biological Industries, Israel) was added to the remaining media containing cells for 4 h before the termination of the experiment. The absorbance of the samples, at 480 nm with 650 nm as a reference wavelength, was measured in a SPECTRAmax PLUS 384 microplate reader (Molecular Devices, USA). Wells without stimulants served as blank and their values were subtracted from the corresponding stimulated one.
Cytokine production was also estimated in cell culture supernatants of 3 potential peptide-stimulated cells by ELISA [(MyBioSource, USA (for hamster) and BD Pharmingen, USA (for human)] according to the manufacturer's protocol. The amount of cytokine production noticed in cell culture supernatants from unexposed healthy hamsters and humans was deducted from the infected one.
In vivo Evaluation of Peptides
A total of 60 hamsters was divided into 6 groups (n = 10/group) for efficacy study of 3 potential peptides based on the results of in vitro study. Of the six groups, hamsters belonging to group I to III were immunized intradermally (i.d.) on the footpad with each of the three peptides (P-10, P-14, and P-15) at a dose of 50 μg/50 μl/animal emulsified with an equal volume of BCG, whereas group IV received BCG alone. Fourteen days later, a booster with half of the first dose of the respective peptide plus BCG was given by intradermal route (i.d.) to all hamsters of Group I to III and Group IV received BCG alone. Hamsters of groups I to V were challenged intracardially (i.c.) 14 days after the last booster dose with 2 × 105 L. (L.) donovani purified amastigotes per animal. The amastigotes were isolated from the spleen of heavily infected (50–60 days old) hamsters as described previously (section Animal and infection). Hamsters in Group V and VI were kept as infected and uninfected controls, respectively. Five hamsters from each group were sacrificed on days 60 and 90 post-challenge (p.c.) for the assessment of parasite load and other immunological parameters. The parasite load was assessed as the number of amastigotes/100 cell nuclei in the Giemsa stained splenic dab-smears and expressed in percentage inhibition (PI) using the following formula (18).
Delayed-type hypersensitivity (DTH) assay was performed on days 60 and 90 p.c. as per the protocol described by Kumari et al. (25) on the contralateral footpad and the response was measured 24 h later in all the experimental groups. Evaluation of lymphoproliferative response was carried out in mesenteric lymph node-derived mononuclear cells from all the experimental groups on days 60 and 90 p.c. as per method described previously (24). The levels of Th1 and Th2 cytokines were measured in the hamster's serum by ELISA (MyBioSource, USA). In addition, the transcript of these cytokines were also estimated in their splenic tissues through quantitative Real-time RT-PCR (iQ5 LightCycler, BioRad) using the following reaction conditions: initial denaturation at 95°C for 2 min followed by 40 cycles, each consisting of denaturation at 95°C for 20 s, annealing at 60°C for 20 s and extension at 72°C for 16 s per cycle employing various sets of primers (Table 1). The mRNA expression of target genes was normalized to RPL18 (housekeeping gene) using the 2−ΔΔCt method (26) wherein the PCR signal of the target transcript in a treatment group is related to that of untreated control.
Table 1.
Sequences of forward and reverse primers of hamster cytokines used for quantitative real time RT-PCR.
| S. No. | Genes | Direction | Primer sequence | Product length |
|---|---|---|---|---|
| 1 | RPL 18 | Forward Reverse |
5′ GATAGATCCACTCCCATAACTG 3′ 5′ TACCTTCAACAATCAAGACATTC 3′ |
96 |
| 2 | TNF-α | Forward Reverse |
5′ TTCTCCTTCCTGCTTGTG 3′ 5′ CTGAGTGTGAGTGTCTGG 3′ |
131 |
| 3 | IFN-γ | Forward Reverse |
5′ GCTTAGATGTCGTGAATGG 3′ 5′ GCTGCTGTTGAAGAAGTTAG 3′ |
200 |
| 4 | IL-12 | Forward Reverse |
5′ TATGTTGTAGAGGTGGACTG 3′ 5′ TTGTGGCAGGTGTATTGG 3′ |
81 |
| 5 | TGF-β | Forward Reverse |
5′ ACGGAGAAGAACTGCTGTG 3′ 5′ GGTTGTGTTGGTTGTAGAGG 3′ |
178 |
| 6 | IL-4 | Forward Reverse |
5′ GCCATCCTGCTCTGCCTTC 3′ 5′ TCCGTGGAGTTCTTCCTTGC 3′ |
75 |
| 7 | IL-10 | Forward Reverse |
5′ TGCCAAACCTTATCAGAAATG 3′ 5′ AGTTATCCTTCACCTGTTCC 3′ |
102 |
Statistical Analysis
All the experiments were performed in two replicates. Statistical analysis was performed using GraphPad Prism 6.01 (GraphPad Software, San Diego, CA, USA). Results were expressed as mean ± SEM. Paired t-test was used to calculate statistical significant differences between mean values of treated groups stimulated with synthetic peptides and their respective recombinant proteins in Figure 1. Unpaired t-test was used to calculate the levels of significance between infected and treated groups in Figures 2, 5. In case of Figures 3, 4, one-way ANOVA was used to calculate the statistical significance between the vaccinated and infected group. A p value of < 0.05 was considered significant.
Figure 1.
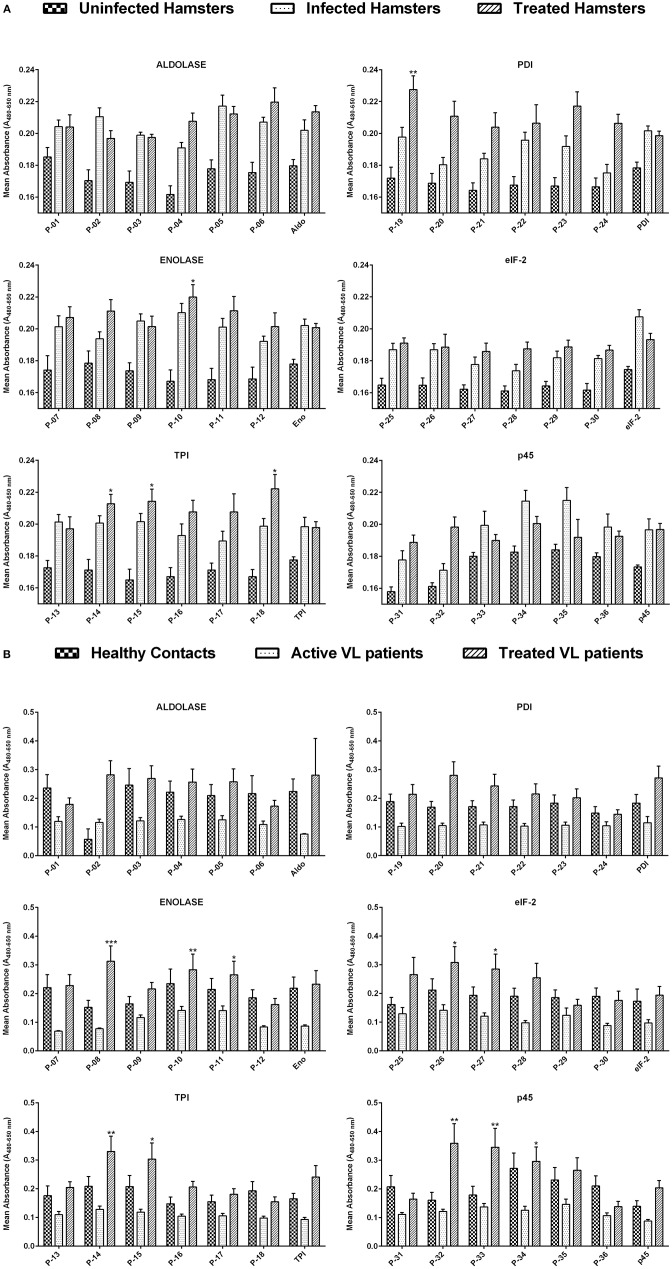
(A) Lymphoproliferative response in lymphocytes derived from the mesenteric lymph node of uninfected, infected as well as treated Leishmania-infected hamsters against 36 Th1 stimulatory peptides in comparison to their parent recombinant proteins. The concentration of peptides, as well as recombinant proteins, was adjusted to 1 ng/ml of cells. Each bar represents the pooled data of hamsters (Mean ± SE). Statistical significant differences were assessed using a paired t-test amid mean values of treated groups stimulated with synthetic peptides and their respective recombinant proteins. (B) Lymphoproliferative response in PBMCs of healthy contacts, active VL patients as well as treated VL patients against 36 Th1 stimulatory peptides and their parent recombinant proteins. The concentration of peptides, as well as recombinant proteins, was adjusted to 1 ng/ml of cells. Each bar represents the pooled data (Mean ± SE) and the levels of statistical significance were assessed between the mean values of treated groups, stimulated with synthetic peptides and their respective recombinant proteins by paired t-test. *p < 0.05; **p < 0.01; and ***p < 0.001.
Figure 2.
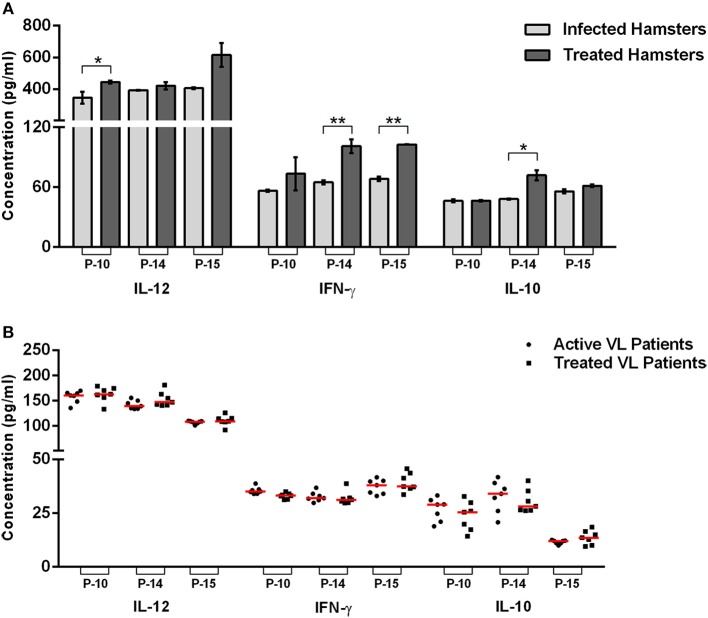
Cytokines level in the peptide-stimulated culture supernatant of lymphocyte derived from uninfected, infected, and treated Leishmania-infected hamsters (A) as well as in PBMCs derived from healthy contacts, active VL as well as treated VL patients (B). The levels of significance were calculated using unpaired t-test between infected and treated groups (*p < 0.05 and **p < 0.01).
Figure 3.
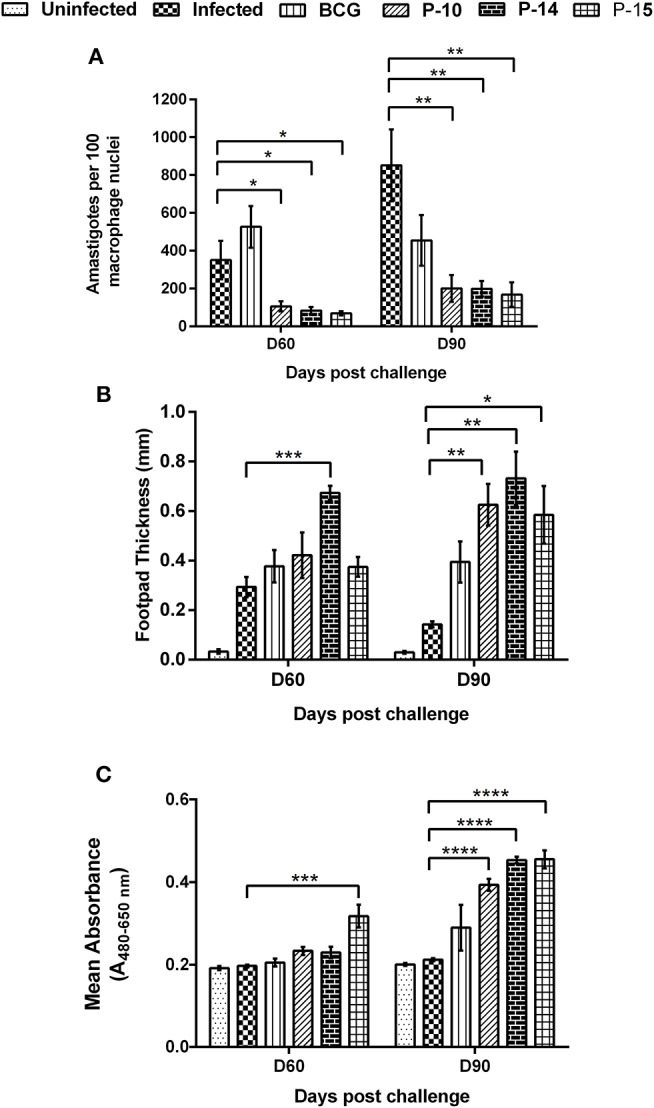
Parasite load in splenic dab smear of vaccinated hamsters (A), DTH response to SLD as footpad swelling (B) and lymphoproliferative response in vaccinated hamsters against SLD (C) in comparison to the unimmunized infected hamsters on days 60 and 90 p.c. One-way ANOVA was used to calculate the statistical significance between the vaccinated and infected group (*p < 0.05; **p < 0.01; ***p < 0.001, and ****p < 0.0001).
Figure 4.
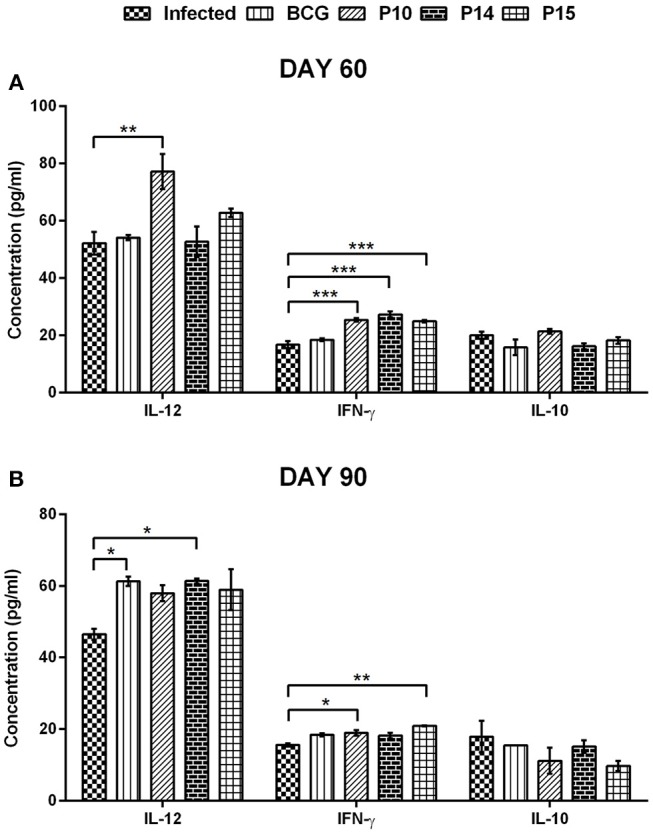
Cytokines level in serum samples of vaccinated hamsters at day 60 (A) and day 90 (B) p.c. Significance values indicate the difference in cytokine concentration between infected and treated hamsters (*p < 0.05; **p < 0.01; and ***p < 0.001) and was calculated using One-way ANOVA.
Results
In silico Prediction of Promiscuous T-Cell Epitopes
IEDB and SYFPEITHI servers were used to predict immunogenic peptides from the full-length protein sequences of six potential Th1 stimulatory proteins (LdAld, LdEno, LdTPI, LdPDI, LdelF2, and Ldp45). Both servers yielded several peptides (>100) out of which six top scoring peptides were selected against the HLA alleles which occurs most frequently in global population as well as against those recently reported to be involved in susceptibility to Leishmania infection in Indian population (27). Also, top scoring peptides were subjected to BlastP search for the identification of identical or highly similar sequences into the human genome. Peptides exhibiting higher identity with human proteins (>85%) in BLAST search were not considered. Finally, a total of 36 15-mer MHC-II binding peptides (six peptides of each protein) were chosen for the experimental evaluation as listed in Table 2.
Table 2.
Notation and sequence of peptides predicted against the alleles reported to be involved in susceptibility to Leishmania infection in Indian population.
| Proteins | S. No. | AA seq | Peptides | Proteins | S. No. | AA seq | Peptides |
|---|---|---|---|---|---|---|---|
| Aldo | P-01 | 179–193 | TLARYAILSQISGLV | PDI | P-19 | 85–99 | KYQIKGFPTLYIFRN |
| P-02 | 180–194 | LARYAILSQISGLVP | P-20 | 311–325 | HHVMETYTPVTAESV | ||
| P-03 | 137–151 | LDGYVKRASAYYKKG | P-21 | 212–236 | AESVKRFLATAVLDY | ||
| P-04 | 1–15 | MSRVTIFQSQLPACN | P-22 | 174–188 | NFVFVTDAAISPNDA | ||
| P-05 | 261–275 | YTVMTLARTMPAMLP | P-23 | 166–180 | ADSLRTQMNFVFVTD | ||
| P-06 | 263–277 | VMTLARTMPAMLPGV | P-24 | 371–385 | QNVMLLFYAPWCGHC | ||
| Eno | P-07 | 1–15 | MPIRKVYAREVLDSR | elF-2 | P-25 | 15–29 | AFSLTRFANMYAAKF |
| P-08 | 160–174 | VLPFQEFMIAPTKAT | P-26 | 566–580 | IYNVRAYLPVAESFG | ||
| P-09 | 188–202 | HALKVIIKSKYGQDA | P-27 | 189–203 | RGRFFAFGRIFSGKV | ||
| P-10 | 128–142 | LYRYIAGLAGTKDIR | P-28 | 2–16 | KGTVAIGSGLQAWAF | ||
| P-11 | 184–198 | SEVYHALKVIIKSKY | P-29 | 75–89 | DPIYQIFDAVMNEKK | ||
| P-12 | 336–350 | ACNSLLLKINQIGTI | P-30 | 117–131 | KTVMMKFLPAAETLL | ||
| TPI | P-13 | 204–218 | AARLRILYGGSVSAG | p45 | P-31 | 107–221 | GIHVDGYCAVVAHTI |
| P-14 | 201–215 | ADVAARLRILYGGSV | P-32 | 150–164 | LRQMRPGATIYQVTD | ||
| P-15 | 45–59 | TFVHIPLVQAKLRNP | P-33 | 172–186 | HYKVTPVDGVLSHMM | ||
| P-16 | 138–152 | NQTAKVVLSQTSAIA | P-34 | 306–320 | GEVVAHFKITVLISN | ||
| P-17 | 22–36 | IEKLVQVLNEHNISH | P-35 | 320–334 | NKKIEPITGLKPQKA | ||
| P-18 | 43–57 | APTFVHIPLVQAKLR | P-36 | 214–228 | KAQVWTLDIVMTSGK |
In vitro Evaluation of Peptides
The cellular response generated by mesenteric lymph node-derived mononuclear cells isolated from treated Leishmania-infected hamsters as well as PBMCs of treated Leishmania patients against thirty-six peptides was compared with their respective proteins by XTT, as shown in Figures 1A,B. In the case of hamsters, five peptides viz. P-10 of Enolase, P-14, P-15, and P-18 of TPI and P-19 of PDI induced significant cellular proliferation (mean OD ± SE 0.2199 ± 0.008, 0.2127 ± 0.006, 0.2143 ± 0.008, 0.2221 ± 0.009, and 0.2275 ± 0.009, respectively). However, in human PBMCs, ten peptides namely P-08, P-10, and P-11 of Enolase, P-14 and P-15 of TPI, P-26 and P-27 of elF-2, P-32, P-33, and P-34 of p45 induced significant lymphoproliferative response (0.3127 ± 0.053, 0.2831 ± 0.054, 0.2654 ± 0.047, 0.3301 ± 0.053, 0.3035 ± 0.056, 0.3075 ± 0.056, 0.2848 ± 0.052, 0.3587 ± 0.068, 0.3452 ± 0.066, and 0.2959 ± 0.050). Thus, among all the 5 and 10 peptides that were identified eliciting optimum cellular responses in treated groups of hamster and human, respectively, three peptides i.e., P-10, P-14, and P-15 were observed to be common in both. A significant proliferation was observed in patient's PBMCs as well as in hamster's mononuclear cells stimulated by standard mitogens (data not shown) indicating the experimental sensitivity.
Furthermore, cytokine production was estimated in cell culture supernatants of P-10, P-14, and P-15 stimulated cells. A significant production of Th1 cytokines (IL-12 and IFN-γ) in the supernatant of all the three peptides stimulated mono-nuclear cells derived from treated Leishmania-infected hamsters were noticed in comparison to the infected ones (Figure 2A). Amongst the three peptides, P-14, and P-15 induced significantly higher production of IFN-γ (101 ± 6.832 pg/mL and 102.8 ± 0.3368 pg/mL, respectively), in the treated group in comparison to infected control. However, in the case of P-10, there was an apparent increase in IFN-γ level but was not statistically significant. Likewise, the level of IL-12 was also found to be increased in treated hamsters stimulated with all the three peptides, wherein the values weresignificant in the case of P-10 (444.7 ± 9.533 pg/mL) only. Conversely, there was slight or no difference in the IL-10 level, a Th2 cytokine, between the infected and treated groups stimulated by P-10, P-14, and P-15. However, when assessed in PBMCs of treated VL patients, though, these peptides generated a slight increase in IL-12 level with decreased production of IL-10 (except P-15 stimulated cells), the values were statistically non-significant (Figure 2B). The level of IFN-γ was, however, found to be comparable to infected patients.
In vivo Evaluation of Three Potential Peptides
Three potential peptides (P-10, P-14, and P-15), based on the results of in vitro study, were further subjected for their prophylactic efficacy alongwith BCG in Syrian golden hamsters.
Determination of Parasite Load, DTH, and Lymphoproliferative Responses
A significant inhibition of parasitic multiplication was noticed in the spleen of P-10+BCG and P-15+BCG (75% inhibition) and P-10+BCG (65% inhibition) vaccinated animals as compared to infected controls on day 60 p.c. (Figure 3A). By day 90 p.c., 75% parasite inhibition was noticed in all the three peptides+BCG vaccinated groups as compared to infected ones. In contrast, higher parasite load was observed in hamsters immunized with BCG alone in comparison to the three peptide+BCG vaccinated hamsters both on days 60 and 90 p.c.
DTH and Leishmania-specific lymphoproliferative responses, the indices of cell-mediated immunity, were measured in animals of each experimental group. Though, there was an apparent increase in DTH response in BCG and all the three peptides+BCG vaccinated animals in comparison to infected control on day 60 p.c.; it was significant in hamsters immunized with P-14+BCG only (Figure 3B). By the day 90 p.c., however, the response became significant in all the three peptides+BCG vaccinated groups (not in BCG alone group) when compared with infected controls. Nevertheless, a substantial increase in lymphoproliferative response, in comparison to infected control, was evident only in the lymph node cells of hamsters vaccinated with P-15+BCG (0.3175 ± 0.027) on day 60 p.c., whereas by day 90 p.c., this response was found to be highly significant in all the three peptides+BCG vaccinated groups i.e., P-10+BCG (0.3931 ± 0.014), P-14+BCG (0.4533 ± 0.008), and P-15+BCG (0.4551 ± 0.022) which was in accordance with the DTH response (Figure 3C). Lesser non-significant proliferation was observed in animals vaccinated with BCG alone (0.2048 ± 0.010 and 0.2895 ± 0.056 on days 60 and 90 p.c., respectively).
Measurement of Cytokine Response in Serum Samples of Vaccinated Hamsters
The skewness in the cytokine milieu, if any, was assessed in the serum samples of hamsters following immunization and challenge. There was significant production of IFN-γ in all the three peptides+BCG vaccinated groups—P-10+BCG (25.32 ± 0.608 pg/mL), P-14+BCG (27.26 ± 0.992 pg/mL), and P-15+BCG (24.88 ± 0.431 pg/mL) on day 60 p.c. in comparison to unvaccinated infected control group (16.73 ± 1.238 pg/mL) (Figure 4A). However, on day 90 p.c. the IFN-γ level was almost similar and observed to be significantly higher in P-10+BCG (18.96 ± 0.740 pg/mL) and P-15+BCG (20.91 ± 0.153 pg/mL) vaccinated groups only (Figure 4B). The IFN-γ level in only BCG vaccinated animals remains almost similar to infected control at both the observation points. In addition, P-10+BCG vaccinated hamsters also significantly induced IL-12 (77.12 ± 6.117 pg/mL) with a perceptible increase in P-15+BCG (62.73 ± 1.473 pg/mL) vaccinated group on day 60 p.c. whereas, its level was noticed to be significant in BCG alone (6.26 ± 1.26 pg/mL) and P-14+BCG (61.33 ± 0.700 pg/mL) vaccinated hamsters on day 90 p.c. as compared to unvaccinated infected control group. Conversely, the level of IL-10, a Th2 cytokine, in BCG alone and all the peptide+BCG vaccinated groups was more or less comparable to the infected control groups on both days 60 and 90 p.c.
Analysis of Cytokine Profiles in the Splenic Tissue of Vaccinated Hamsters
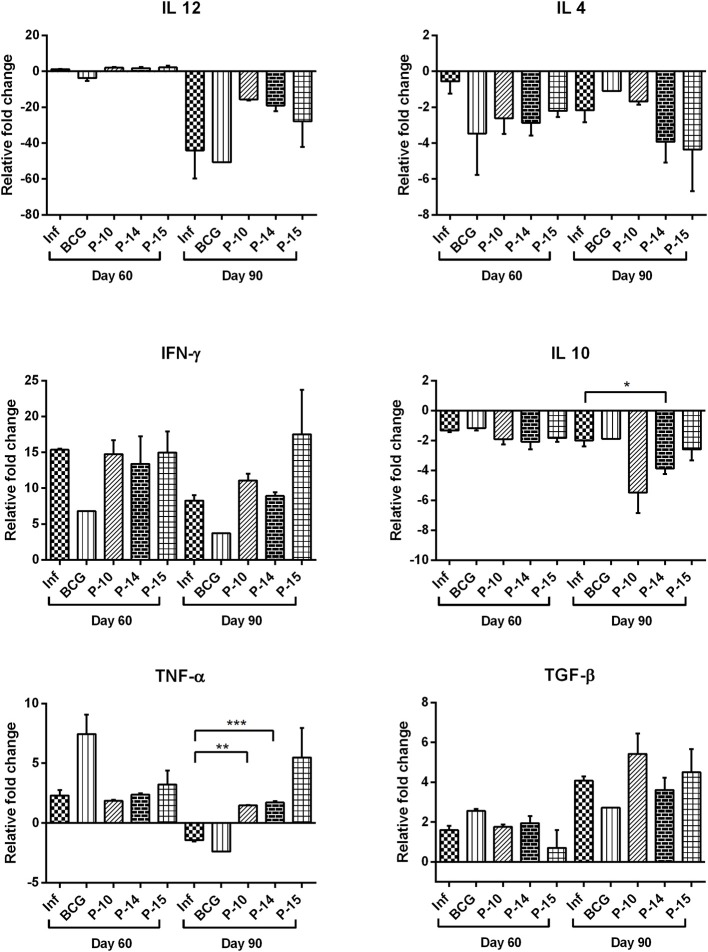
Cytokine production in all the experimental groups was further assessed at transcript levels by quantifying their expression levels in splenic tissue by qRT-PCR using RPL18 as an internal reference gene. Amongst Th1 cytokines, the mRNA expression of IFN-γ was observed to be similar in all the three peptides+BCG vaccinated groups as compared to that of infected control on day 60 p.c. which increased progressively by day 90 p.c especially in P-15+BCG vaccinated group (almost 2 fold). However, there was decreased mRNA expression of IFN-γ in BCG alone vaccinated group as compared to infected control at both observation points. Similarly, increased mRNA expression of IL-12 was noticed in all the three peptides+BCG vaccinated groups on day 60 p.c. but surprisingly by day 90 p.c. its expression was down-regulated in all the vaccinated groups. The expression of TNF-α, however, was found to be increased in all the three peptides+BCG vaccinated hamsters on day 90 p.c. which was significant only in P-10+BCG and P-14+BCG vaccinated groups in comparison to infected control group. On the contrary, the expression of Th2 cytokines, IL-4 and IL-10, in general, was down-regulated in all the vaccinated groups on both day 60 and 90 p.c., as compared to infected control whereas a slight increase in mRNA expression of TGF-β was observed in P-10+BCG and P-15+BCG by day 90 p.c (Figure 5).
Figure 5.
Cytokine mRNA expression profile of spleen of normal, infected, and vaccinated hamsters on days 60 and 90 p.c. by quantitative real-time-PCR. The levels of significance were calculated using unpaired t-test between infected and treated groups (*p < 0.05; **p < 0.01; and ***p < 0.001).
Discussion
Peptide-based synthetic vaccines offer a safer alternative to traditional vaccines as it utilizes shorter peptide fragments for eliciting an enhanced targeted immune response thus avoiding allergenic responses (28). The identification and proper selection of peptide antigens are, however, crucial for the development of an effective peptide-based synthetic vaccine (29). A large number of such vaccines have recently reached clinical trials (30). In case of leishmaniasis, several proteins such as Gp63, KMP-11, A2-protein, LPG, cysteine protease, etc. were explored for determining the potential T-cell activating peptides as reviewed by Joshi et al. and De Brito et al. (31, 32). Since the generation of Th1 biased cellular immunity is critical for mediating the protective response against intracellular pathogens including Leishmania (14), Th1 stimulatory peptides were, therefore considered to be suitable vaccine candidates. In our earlier study, we have identified six Th1 stimulatory proteins, which were found to elicit optimum cellular responses (17). Herein, we have further subjected these six proteins to bioinformatics analysis for the identification of potential T-cell epitopes.
Due to the existence of immense diversity among human leukocyte antigen (HLA) genes, they offer a selective advantage to the immune system against diverse microbial antigens (33). There are enormous evidence indicating a genetic association between HLA and numerous infectious diseases (34). Thus, knowledge of epitope recognition among the targeted human population is a critical factor in the designing of such vaccines (35). Thirty-six MHC II binding peptides were selected using IEDB and SYFPEITHI, keeping in view the fact that HLA-DRB1-HLA-DQA1 HLA class II region contributes to VL susceptibility in India (27). Also, MHC-II loaded antigen triggers CD4+ T-helper cells which further activate cellular as well as humoral immunity (36). Out of thirty-six peptides, three viz P-10, P-14, and P-15 were found to elicit a significant lymphoproliferative response in treated Leishmania exposed hamsters as well as human subjects as compared to their respective proteins. Although lymphoproliferative assay is a measure of T-cell function, heterogeneity of CD4+ T-cells modulates cytokine response either into protective or deleterious one (37) and therefore it was measured. A Th1 biased cytokine (IL-12 and IFN-γ) responses were observed in lymph node-derived mono-nuclear cells supernatant, incubated with these three peptides, from treated Leishmania-infected hamsters in comparison to infected one. However, in the case of PBMCs of treated VL patients, all the three peptides resulted in the increased generation of IL-12 with decrease production of IL-10 as compared to active VL patients, though statistically non-significant. The IFN-γ level was, however, found to be comparable with that of infected patients. A similar observation was made by Singh et al. (38) wherein no difference was observed in the IFN-γ level in SLA stimulated whole-blood cellsbetween active and cured VL patients in Indian Subcontinent although it was higher as compared to healthy subjects.
Encouraged with these observations, the prophylactic efficacies of these peptides were further evaluated in hamsters against Leishmania challenge. It is well-known that peptides are often weakly immunogenic when applied alone and thus require particulate carriers or adjuvants for enhancing their efficacy (29). In this study, BCG-an immunostimulatory adjuvant has been employed since it has been reported to be associated with induction of Th1 immune response via augmenting cell-mediated immune (CMI) response to the associated antigens (39) and is the most acceptable Th1 inducing adjuvant presently available for human use (40). The peptide vaccinated hamsters, in combination with BCG, offered a considerable protective response with 75% reduction in splenic parasitic load by day 90 p.c.
Since, chronic infections are usually characterized by T-cell exhaustion including loss of cellular proliferation and increase in pathogen burden, the development of strong CMI responses is believed to be contributing to healing Leishmania infection. In this study, the findings revealed that all the groups of peptide+BCG vaccinated hamsters have exhibited a significant lymphoproliferative response by day 90 p.c. which on the contrary, was severely impeded in the infected hamsters. Moreover, since, it has been postulated that the Th1 cell is the “inducer” of a DTH response (41) therefore, the hamsters of all the experimental groups were subjected to this hypersensitivity test. It was noticed that all the vaccinated hamsters elicited stronger DTH reaction as compared to infected control animals which showed the low level of parasite-specific DTH responses and this can be correlated with the disease progression.
Th1-type immunity, characterized by the production of IL-12 and IFN-γ, helps in the development of protective response against Leishmania infection (42). However, IL-10, an immunosuppressive cytokine, reduces the production of Th1 cytokines and their neutralization augments IFN-γ production and promotes amastigotes clearance (42). In our study, IFN-γ was found to be elevated in the splenic tissue as well as in the serum of all the peptide+BCG vaccinated groups by day 90 p.c. However, at the transcript level, IFN-γ was also found to be elevated in infected animals which were in accordance with the observation made by Melby et al. (43). IL-12, another Th1 cytokine, was found to be elevated in serum samples of vaccinated hamsters at both time points but at the transcript level, though it was initially found to be up-regulated on day 60 p.c., it eventually decreased by day 90 p.c. Amongst Th2 cytokines, IL-10 was found to be more or less similar in the serum samples of vaccinated and infected animals at both time points of observation. However, it was found to be down-regulated, at the transcript level, on day 60 and 90 p.c. which was in accordance to the observation made in treated VL patients (44). These differences in mRNA transcripts and serum cytokine levels might be due to various post-transcriptional, translational, and protein degradation regulatory mechanisms (45).
Furthermore, TNF-α reported to be associated with control of Leishmania infection through macrophage activation and tissue granuloma formation (46), was observed to be up-regulated in the splenic tissue of all the vaccinated hamsters indicating toward the generation of the Th1 type of immune response. On the other hand, among the Th2 cytokines, IL-4 was found to be down-regulated, at the transcript level, on day 60 and 90 p.c. However, the expression of TGF-β, which has been implicated in the susceptibility to VL due to its suppressor effects on macrophages during VL infection (47), on the contrary, was found to be almost similar or increased in peptide+BCG vaccinated groups at day 60 (P-10 and P-14) and day 90 (P-10 and P-15) as compared to infected control. This observation was in accordance with the findings reported by Araujo-Santos et al. (48).
Thus, the present study demonstrates that these three peptides (P-10, P-14, and P-15) with BCG offer noticeable immunoprotective responses in hamsters against experimental Leishmania challenge and thereby indicate toward their being the potential vaccine candidates. It is well-known that pathogen displays numerous copies of multiple antigens to the immune system and thus generate a holistic immune response. Therefore, it would be advisable to emphasize on the inclusion of multiple epitopes into a vaccine as it allows better coverage of antigen diversity of the pathogen and genetic polymorphism of the mammalian immune system. Keeping this in view, it would be worthwhile to develop a multi peptide-based synthetic vaccine conjugated with an immunostimulatory peptide so as to augment the protective immune response, thereby fortifying the host immunity in an effective way.
Ethics Statement
All the animal-based experimental procedures using Syrian golden hamsters (Mesocricetus auratus, 6–8-weeks-old), including their numbers, were approved by institutional animal ethical committee (IAEC, Approval No IAEC/2013/79/dated 31/07/2013) regulating according to the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA). Similarly, the protocols and study on human subjects, belonging to hyper-endemic areas of Bihar, were approved by Human Ethics Committee of the Kala-Azar Medical Research Center (KMRC), Muzaffarpur (Approval No EC-KAMRC/Vaccine/VL/2007-1). A written informed consent was taken from all the human subjects before their enrolment in this study.
Author Contributions
SJ, NY, and KR performed the human and animal experiments. VK, MS, and SJ conducted the in-silico studies. RA synthesized peptides under the supervision of WH. SS provides human samples. SJ and AD analyzed the data and wrote the manuscript. AD, AS, and SJ designed the study. AD supervised the entire project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our sincere gratitude to the Director, CSIR-CDRI for providing the necessary facilities for conducting this work. This work was supported by the Department of Biotechnology (DBT) [BT/PR7299/MED/29/678/2012]. Council of Scientific and Industrial Research (CSIR), University Grant Commission (UGC), and Indian Council of Medical Research (ICMR) provide financial assistance in terms of Junior and Senior Research Fellowships to SJ, NY, KR, VK, and RA. Department of Science and Technology (DST) provide financial assistance in term of Sir J. C. Bose Fellowship to AD.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00288/full#supplementary-material
References
- 1.Hailu A, Menon JN, Berhe N, Gedamu L, Hassard TH, Kager PA, et al. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J Infect Dis. (2001) 184:112–5. 10.1086/320994 [DOI] [PubMed] [Google Scholar]
- 2.WHO Leishmaniasis. Available online at: http://wwwwhoint/mediacentre/factsheets/fs375/en/ (2017).
- 3.Engwerda CR, Matlashewski G. Development of Leishmania vaccines in the era of visceral leishmaniasis elimination. Trans R Soc Trop Med Hyg. (2015) 109:423–4. 10.1093/trstmh/trv039 [DOI] [PubMed] [Google Scholar]
- 4.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. (2007) 5:873–82. 10.1038/nrmicro1748 [DOI] [PubMed] [Google Scholar]
- 5.Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. (2016) 34:2992–5. 10.1016/j.vaccine.2015.12.071 [DOI] [PubMed] [Google Scholar]
- 6.Furman D, Davis MM. New approaches to understanding the immune response to vaccination and infection. Vaccine. (2015) 33:5271–81. 10.1016/j.vaccine.2015.06.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. (2007) 6:404–14. 10.1038/nrd2224 [DOI] [PubMed] [Google Scholar]
- 8.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. (2001) 1:209–19. 10.1038/35105075 [DOI] [PubMed] [Google Scholar]
- 9.De Groot AS, Sbai H, Aubin CS, McMurry J, Martin W. Immuno-informatics: mining genomes for vaccine components. Immunol Cell Biol. (2002) 80:255–69. 10.1046/j.1440-1711.2002.01092.x [DOI] [PubMed] [Google Scholar]
- 10.Mahajan B, Berzofsky JA, Boykins RA, Majam V, Zheng H, Chattopadhyay R, et al. Multiple antigen peptide vaccines against Plasmodium falciparum malaria. Infect Immun. (2010) 78:4613–24. 10.1128/IAI.00533-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardin E. The past decade in malaria synthetic peptide vaccine clinical trials. Hum Vacc. (2010) 6:27–38. 10.4161/hv.6.1.9601 [DOI] [PubMed] [Google Scholar]
- 12.Serna C, Lara JA, Rodrigues SP, Marques AF, Almeida IC, Maldonado RA. A synthetic peptide from Trypanosomacruzi mucin-like associated surface protein as candidate for a vaccine against Chagas disease. Vaccine. (2014) 32:3525–32. 10.1016/j.vaccine.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Wang G, Zhang D, Yin H, Wang M. Screening and identification of novel B cell epitopes of Toxoplasma gondii SAG1. Parasit Vect. (2013) 6:125. 10.1186/1756-3305-6-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. (2000) 406:793–8. 10.1038/35021239 [DOI] [PubMed] [Google Scholar]
- 15.Kamhawi S, Oliveira F, Valenzuela JG. Using humans to make a human leishmaniasis vaccine. Sci Transl Med. (2014) 6:234fs18. 10.1126/scitranslmed.3009118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari S, Samant M, Misra P, Khare P, Sisodia B, Shasany AK, et al. Th1-stimulatory polyproteins of soluble Leishmaniadonovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine. (2008) 26:5700–11. 10.1016/j.vaccine.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 17.Joshi S, Yadav NK, Rawat K, Tripathi CD, Jaiswal AK, Khare P, et al. Comparative analysis of cellular immune responses in treated Leishmania patients and hamsters against recombinant th1 stimulatory proteins of Leishmaniadonovani. Front Microbiol. (2016) 7:312. 10.3389/fmicb.2016.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R, Kumar V, Kushawaha PK, Tripathi CP, Joshi S, Sahasrabuddhe AA, et al. Characterization of glycolytic enzymes-rAldolase and rEnolase of Leishmaniadonovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS ONE. (2014) 9:e86073 10.1371/journal.pone.0086073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushawaha PK, Gupta R, Sundar S, Sahasrabuddhe AA, Dube A. Elongation factor-2, a Th1 stimulatory protein of Leishmaniadonovani, generates strong IFN-gamma and IL-12 response in cured Leishmania-infected patients/hamsters and protects hamsters against Leishmania challenge. J Immunol. (2011) 187:6417–27. 10.4049/jimmunol.1102081 [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Kushawaha PK, Tripathi CD, Sundar S, Dube A. A novel recombinant Leishmaniadonovani p45, a partial coding region of methionine aminopeptidase, generates protective immunity by inducing a Th1 stimulatory response against experimental visceral leishmaniasis. Int J Parasitol. (2012) 42:429–35. 10.1016/j.ijpara.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 21.Kushawaha PK, Gupta R, Tripathi CD, Sundar S, Dube A. Evaluation of Leishmaniadonovani protein disulfide isomerase as a potential immunogenic protein/vaccine candidate against visceral Leishmaniasis. PLoS ONE. (2012) 7:e35670 10.1371/journal.pone.0035670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushawaha PK, Gupta R, Tripathi CD, Khare P, Jaiswal AK, Sundar S, et al. Leishmaniadonovani triose phosphate isomerase: a potential vaccine target against visceral leishmaniasis. PLoS ONE. (2012) 7:e45766. 10.1371/journal.pone.0045766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang KP. Human cutaneous leishmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. (1980) 209:1240–42. [DOI] [PubMed] [Google Scholar]
- 24.Garg R, Gupta Sk, Tripathi P, Naik S, Sundar S, Dube A. Immunostimulatory cellular responses0020of cured Leishmania-infected patients and hamsters against the integral membrane proteins and non-membranous soluble proteins of a recent clinical isolate of Leishmania donovani. Clin Exp Immunol. (2005) 40:149–56. 10.1111/j.1365-2249.2005.02745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumari S, Samant M, Khare P, Misra P, Dutta S, Kolli BK, et al. Photodynamic vaccination of hamsters with inducible suicidal mutants of Leishmaniaamazonensis elicits immunity against visceral leishmaniasis. Eur J Immunol. (2009) 39:178–91. 10.1002/eji.200838389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods. (2001) 25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 27.Fakiola M, Strange A, Cordell HJ, Miller EN, Pirinen M, Su Z, et al. Common variants in the HLA-DRB1-HLA-DQA1 HLA class II region are associated with susceptibility to visceral leishmaniasis. Nat Genet. (2013) 45:208–13. 10.1038/ng.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Exp Rev Vacc. (2007) 6:591–603. 10.1586/14760584.6.4.591 [DOI] [PubMed] [Google Scholar]
- 29.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. (2016) 7:842–54. 10.1039/C5SC03892H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK. Peptide vaccine: progress and challenges. Vaccines. (2014) 2:515–36. 10.3390/vaccines2030515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi S, Rawat K, Yadav NK, Kumar V, Siddiqi MI, Dube A. Visceral leishmaniasis: advancements in vaccine development via classical and molecular approaches. Front Immunol. (2014) 5:380. 10.3389/fimmu.2014.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Brito RCF, Cardoso JMO, Reis LES, Vieira JF, Mathias FAS, Roatt BM, et al. Peptide vaccines for leishmaniasis. Front Immunol. (2018) 9:1043. 10.3389/fimmu.2018.01043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Ann Rev Genom Human Genet. (2013) 14:301–23. 10.1146/annurev-genom-091212-153455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crux NB, Elahi S. Human leukocyte antigen (HLA) and immune regulation: how do classical and non-classical hla alleles modulate immune response to human immunodeficiency virus and hepatitis c virus infections? Front Immunol. (2017) 8:832. 10.3389/fimmu.2017.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern LJ, Calvo-Calle JM. HLA-DR: molecular insights and vaccine design. Curr Pharm Design. (2009) 15:3249–61. 10.2174/138161209789105171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciabattini A, Pettini E, Medaglini D. CD4(+) T cell priming as biomarker to study immune response to preventive vaccines. Front Immunol. (2013) 4:421. 10.3389/fimmu.2013.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta R, Kushawaha P, Tripathi CDP, Sundar S, Dube A. A novel recombinant Leishmaniadonovanip45, a partial coding region of methionine aminopeptidase, generates protective immunity by inducing a Th1 stimulatory response against experimental visceral leishmaniasis. Int J Parasitol. (2012) 42:429–35. 10.1016/j.ijpara.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 38.Singh OP, Gidwani K, Kumar R, Nylen S, Jones SL, Boelaert M, et al. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin Vacc Immunol. (2012) 6:961–6. 10.1128/CVI.00143-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng YQ, Naguib YW, Dong Y, Shi YC, Bou S, Cui Z. Applications of bacillus Calmette-Guerin and recombinant bacillus Calmette-Guerin in vaccine development and tumor immunotherapy. Exp Rev Vacc. (2015) 14:1255–75. 10.1586/14760584.2015.1068124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshua M, Mutiso JM, Macharia JC, Gicheru MM. A review of adjuvants for Leishmania vaccine candidates. J Biomed Res. (2010) 24:16–25. 10.1016/S1674-8301(10)60004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. (1999) 5:7. [PubMed] [Google Scholar]
- 42.Rodrigues V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasit Vect. (2016) 9:118. 10.1186/s13071-016-1412-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. (2001) 166:1912–20. 10.4049/jimmunol.166.3.1912 [DOI] [PubMed] [Google Scholar]
- 44.Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, et al. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. (2011) 186:3977–85. 10.4049/jimmunol.1003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. (2012) 13:227–32. 10.1038/nrg3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumang MC, Keogh C, Moldawer LL, Helfgott DC, Teitelbaum R, Hariprashad J, et al. Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol. (1994) 153:768–75. [PubMed] [Google Scholar]
- 47.Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, et al. Activation of TGF-beta by Leishmaniachagasi: importance for parasite survival in macrophages. J Immunol. (2003) 170:2613–20. 10.4049/jimmunol.170.5.2613 [DOI] [PubMed] [Google Scholar]
- 48.Araújo-Santos T, Andrade BB, Gil-Santana L, Luz NF, Dos Santos PL, de Oliveira FA, et al. Anti-parasite therapy drives changes in human visceral leishmaniasis-associated inflammatory balance. Sci Rep. (2017) 7:4334. 10.1038/s41598-017-04595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.