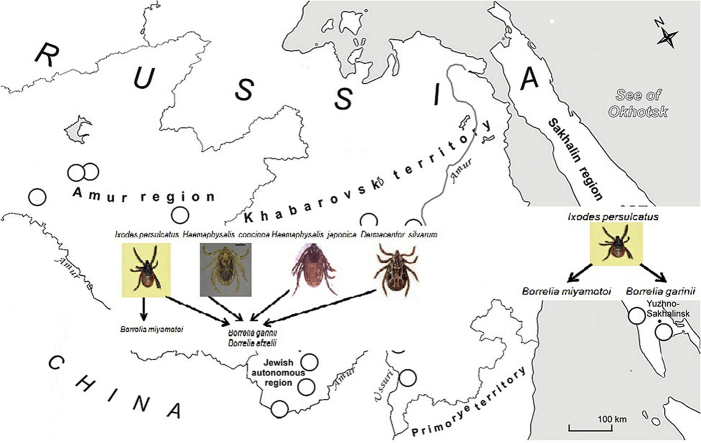
Abstract
Borrelia burgdorferi sensu lato (s.l.) DNA was detected by PCR in Ixodes persulcatus Schulze, 1930, Haemaphysalis concinna Koch, 1844, Haemaphysalis japonica douglasi Nuttall et Warburton, 1915 and Dermacentor silvarum Olenev, 1932 ticks collected in the Amur region, the Jewish Autonomous region, the Sakhalin region and on the Khabarovsk territory. Infection rate of I. persulcatus with B. burgdorferi s.l. 10–69% exceeded the corresponding values of three other tick species in all examined regions during 1999–2014 despite different tick abundance and dominance structure. Bacterial loads estimated on the base of quantitative real time PCR varied from 102 to 109 genome-equivalents per a tick with maximal values for I. persulcatus and H. japonica. Phylogenetic analysis of 16S rRNA gene and 5S—23S rRNA intergenic spacer nucleotide sequences revealed two species: 1) Borrelia garinii of Asian type NT29 with several isolates of European type 20047; 2) Borrelia afzelii with identical sequences of the majority of studied isolates and VS461 reference strain in all regions except the Sakhalin Island where B. afzelii was not found. Borrelia miyamotoi of the relapsing fever group was detected as monoinfection or in combination with B. burgdorferi s.l. in 4.0 ± 0.9% and 4.8 ± 0.9% I. persulcatus ticks, respectively. Multiple locus sequence analysis of three fragments of 16S rRNA, glpQ and p66 genes proved that all the Far Eastern B. miyamotoi isolates belonged to the Asian type identical to FR64b strain (GenBank CP004217) from Japan. Wide distribution of Borrelia DNA in ticks, relative genetic homogeneity with similar sequences of the coding regions and the intergenic spacer of Borrelia wild isolates and temporal stability with high homology levels of the Far Eastern isolates of B. garinii, B. afzelii and B. miyamotoi with previously described spirochetes from the surrounding regions of Russia, China and Japan allowed us to suggest multiple ecological niches as the stability factor of the parasitic system.
Keywords: Ixodid ticks, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Quantitative real-time PCR, Bacterial loads, Multiple locus sequence analysis (MLSA)
Graphical abstract
Highlights
-
•
Borrelia DNA was detected in four species of ixodid ticks by PCR.
-
•
Bacterial loads were up to 109 Borrelia genome-equivalents per a tick.
-
•
Infection rate and bacterial loads were highest for Ixodes persulcatus.
-
•
Phylogenetic analysis showed Borrelia garinii, B. afzelii and Borrelia miyamotoi.
-
•
Genetic homogeneity of Borrelia spp. suggests multiple ecological niches.
1. Introduction
Lyme borreliosis is one of the most common vector-borne zoonotic bacterial diseases in the world. Tens of thousands of cases are reported annually in temperate regions of North America, Eurasia and Australia (Rosa et al., 2005). Lyme disease has both acute (with a transient inflammatory skin rash known as erythema migrans, arthritis, carditis, neuropathies) and persistent clinical manifestations with chronic arthritis, neuroborreliosis, small-point rash, erythema migrans and other skin problems. The prevalence of particular Lyme disease symptoms varies between North America and Europe, with arthritis more common in the United States and neurological and skin disorders more common in Europe. These distinct clinical manifestations of Lyme disease might reflect the geographical distribution of Borrelia genospecies. The bacteria that cause human Lyme disease belong to a clade of 16 named species called Borrelia burgdorferi sensu lato. Among these species, Borrelia burgdorferi sensu stricto (s.s.) Borrelia garinii, Borrelia afzelii and “Borrelia bavariensis” sp. nov. are well-known causes of Lyme disease in North America, Europe (Casjens et al., 2011) and Russia (Rar et al., 2017). Borrelia finlandensis isolated from an Ixodes ricinus tick in Finland is closely related to B. burgdorferi s.s. and is suggested as candidate for new-species status (Casjens et al., 2011). Infection by B. burgdorferi s.s. is frequently associated with arthritis, B. garinii with neurological disease and B. afzelii with chronic skin disorders, although the correlation is not absolute.
Besides B. burgdorferi s.l. another genetically distant Borrelia spp. are associated with hard ticks including Borrelia miyamotoi and Borrelia sp. HM, Borrelia sp. HF, Borrelia sp. HK, Borrelia sp. HL in Haemaphysalis spp. ticks (Furuno et al., 2017; GenBank accession numbers LC170019-LC170035). B. miyamotoi phylogenetically clusters with the relapsing fever Borrelia species (Krause et al., 2015). The distinctive feature of B. miyamotoi and other relapsing fever group species includes expression of a glycerophosphodiester phosphodiesterase (GlpQ) gene (Krause et al., 2015). Transovarial transmission is another biological feature that distinguishes relapsing fever Borrelia species including B. miyamotoi from B. burgdorferi (Krause et al., 2015). Human cases of B. miyamotoi infection were first reported in 2011 in Russia (Platonov et al., 2011) and subsequently in the United States, Europe, and Japan. The most common clinical manifestations of B. miyamotoi infection are fever, fatigue, headache, chills, myalgia, arthralgia, and nausea with possible severe sequellae, including meningoencephalitis (Krause et al., 2015).
Spirochetes represent a phylum of bacteria phylogenetically distinct from other main bacterial groups (Rosa et al., 2005). B. burgdorferi (B31 strain) was the third microbial genome ever sequenced (Fraser et al., 1997). Overall, B. burgdorferi's genome consists of one megabase linear chromosome and a variety of circular and linear plasmids ranging in size from 9 to 62 kb. The chromosome, unlike many other eubacteria, has no relation to either the bacteria's virulence or to the host-parasite interaction (Fraser et al., 1997). Some of the plasmids are necessary for the B. burgdorferi life cycle in wild nature but not for propagation of the bacteria in culture. The genetic variations of Borrelia burgdorferi contribute to varying degrees of infection and dissemination (Theisen et al., 1995; Kurtenbach et al., 2006).
Mechanisms controlling the Borrelia selection are multiple-niche polymorphism and negative frequency-dependent selection (Kurtenbach et al., 2006; Samuels and Radolf, 2010). Multiple-niche polymorphism is maintained within a population due to the varying amount possible ecological niches such as various biotops, reservoir hosts and vectors. Both genetic diversity, various gene expression patterns and protein polymophrism are required for B. burgdorferi adaptation to phylogenetically divergent arthropods and vertebrate hosts. In negative frequency-dependent selection, B. burgdorferi rare low-frequency variants may have a selective advantage because of lack of an immunological response of arthropod hosts.
Despite high prevalence of Lyme borreliosis in the Far East of Russia (3.3–5.45 confirmed cases per 100,000 population) little is currently known about Borrelia biodiversity (Sato et al., 1996; Mediannikov et al., 2005; Liu et al., 2012). High prevalence of B. burgdorferi s.l. (Korenberg et al., 1993), predominance of B. garinii over B. afzelii (Li et al., 1998; Mediannikov et al., 2005) and absence of Borrelia burgdorferi sensu stricto or Borrelia japonica isolates (Li et al., 1998; Morozov and Morozova, 2012 and references therein) were thoroughly examined. Meanwhile, genetic diversity, geographic distribution, natural hosts and vectors of B. miyamotoi are at the beginning of study (Mukhacheva et al., 2015). Our aim was the comparative analysis of Borrelia species in ixodid ticks collected from various biotopes in the Far East of Russia.
2. Materials and methods
2.1. Ticks
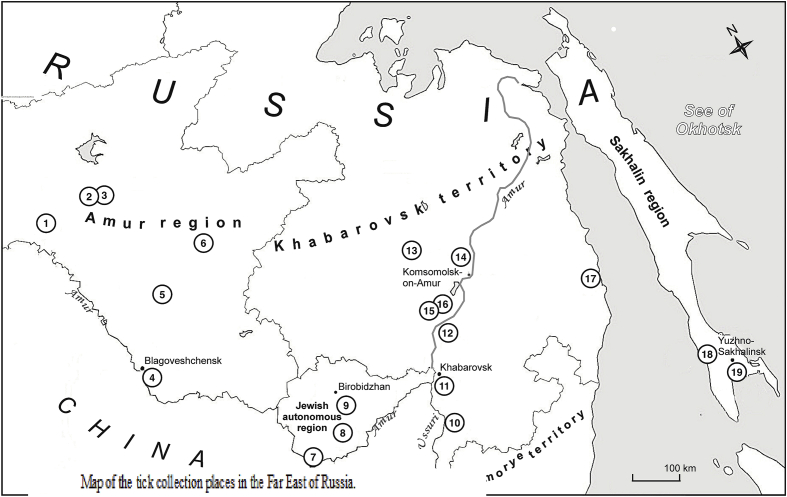
Were collected from vegetation by using 60 × 100 cm cotton cloths (flag), ticks were removed from the “flag” and placed in cotton wet bandage and cotton bag. Average number of ixodid ticks was calculated as “ticks per one flag - hour” is the score of relative abundance of ticks that is calculated as the number of unfed adult ticks averaged per 1 h, . Ticks species were determined on the base of their morphological traits according to (Filippova; 1985). Adult questing ticks were flagged from vegetation during May and June (when ticks are the most active in the region) 1999–2014 in the Far East (Amur region, Jewish Autonomous region, Khabarovsk territory and Sakhalin region) of Russia in sampling sites 1–19 differing in their geographic location, climatic conditions, vegetation and anthropogenic pressure (Fig. 1 and Table 2). Sites 1–6 are located in the Amur region; sites 7–9 - in the Jewish Autonomous region, 10–17 on the Khabarovsk territory; and sites 18 and 19 - in the Sakhalin region. A few premature ticks can also be collected from vegetation but we focused on adult ixodid ticks due to their epidemiological importance.
Fig. 1.
Map of the tick collection places in the Far East of Russia.
Table 2.
Geographic locations of collection places and ratio of the ixodid tick species.
| Site № | District | Сollection place (or exact location) | Biotop | Geographic coordinates of the site |
Year | Average number (ticks per 1 flag-hour) | Tick species ratio (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | E | Ixodes persulcatus P. Schulze, 1930 | Haemaphysalis concinna Koch, 1844 | Haemaphysalis japonica douglasii Nuttall et Warburton, 1915 | Dermacentor silvarum Olenev, 1932 | ||||||
| Amur region | |||||||||||
| 1 | Magdagachinsky | Ductui | larch-birch forest | 53°22′ | 126°08′ | 2011 | 85.3 | 33.6 | 65.6 * | 0 | 0.8 |
| 2 | Zeisky | Zeya | pine –birch forest | 53°44′ | 127°15′ | 2010 | N/A** | ||||
| 3 | Shimanovsky | Belovezh | larch-birch forest | 52°19′ | 127°24′ | 2011 | 122.0 | 4.9 | 95.1 | 0 | 0 |
| 4 | Blagoveshchensky | Raduga camp | deciduous forest | 50°40′ | 127°42′ | 2007 | 41.2 | 15.5 | 84.5 | 0 | 0 |
| Blagoveshchensky | Raduga camp | deciduous forest | 50°40′ | 127°42′ | 2008 | 13.8 | 41.4 | 58.6 | 0 | 0 | |
| 5 | Svobodnensky | Kosmodrom | broadleaved forest | 51°53′ | 128°20′ | 2011 | 30.0 | 8.9 | 88.9 | 0 | 2.2 |
| 6 | Selemdzhinsky | Norsk | mixed deciduous forest | 52°20′ | 129°53′ | 2010 | 16.0 | 0 | 100 | 0 | 0 |
| Jewish Autonomous region | |||||||||||
| 7 | Oktyabrsky | Stolbovoye | broadleaved forest | 47°55ʹ | 131°03ʹ | 2013 | 19.3 | 6.9 | 51.7 | 24.1 | 17.3 |
| 8 | Leninsky | Churki | coniferous–broadleaved forest | 48°04ʹ | 132°39ʹ | 2013 | 14.3 | 38.0 | 44.0 | 18.0 | 0 |
| 9 | Birobidzhansky | Birshosse, 17th km | coniferous–broadleaved forest | 48°41ʹ | 132°48ʹ | 2013 | 71.4 | 54.0 | 35.2 | 10.8 | 0 |
| Khabarovsk territory | |||||||||||
| 10 | Lazo | Kiinsk | deciduous forest | 47°59ʹ | 134°49ʹ | 2014 | 38.3 | 2.6 | 93.0 | 4.4 | 0 |
| 11 | Khabarovsky | Khekhtzir | coniferous–broadleaved forest | 48°15′ | 135°00′ | 1999–2014 | 182.9 | 88.9 | 2.5 | 5.4 | 3.2 |
| 12 | Nanaisky | Troitzkoe | mixed broadleaved forest | 49°22′ | 136°36′ | 2014 | 13.8 | 40.6 | 52.2 | 7.2 | 0 |
| 13 | Solnechny | Gorny | coniferous–broadleaved forest | 50°45′ | 136°27′ | 2009 | 62.0 | 100 | 0 | 0 | 0 |
| 14 | Solnechny | Solnechny | mixed coniferous–small-leaved forest | 50°26′ | 136°58′ | 2014 | 103.5 | 99.0 | 1.0 | 0 | 0 |
| 15 | Komsomolsky | Khurba | secondary fine-leaved forest | 50°24′ | 136°52′ | 2009 | 25.0 | 18.0 | 72.0 | 0 | 0 |
| 16 | Komsomolsky | Taezhny | coniferous–broadleaved forest | 50°35′ | 136°54′ | 2014 | 4.0 | 90.0 | 10.0 | 0 | 0 |
| 17 | Vaninsky | Toki | larch-small-leaved forest | 49°07′ | 140°18′ | 2011 | 57.0 | 100 | 0 | 0 | 0 |
| Sakhalin region | |||||||||||
| 18 | Kholmsky | Pionery | mixed coniferous–small-leaved forest | 47°16′ | 142°02′ | 2011 | 39.0 | 100 | 0 | 0 | 0 |
| 19 | Yuzhno-Sakhalinsky | Isvestkovy | mixed coniferous–small-leaved forest | 46°50′ | 142°56′ | 2011 | 60.9 | 100 | 0 | 0 | 0 |
Notes: ∗ –the dominant ixodid species percents are marked in bold; **- average numbers of ixodid ticks were not calculated.
2.2. Borrelia DNA detection
Arthropod suspensions were prepared from individual ticks in disposable tubes with pestles in liquid nitrogen. Since 2013 «TissueLyser LT» (Qiagen, Germany) kit was used.
Total nucleic acids were isolated from individual tick suspensions using phenol-chloroform deproteinization with subsequent alcohol precipitation (“Vector Best”, Russia) during 1999–2009 and later in 2010–2014 it was replaced with adsorption of nucleic acids on silica by using “Ribosorb” (“InterLabService”, Russia).
PCR with primers SL (Demaerschalck et al., 1995) and mastermix (“Syntol”, Russia) with subsequent electrophoresis was performed in 1999–2006; test-system for Borrelia burgdorferi s.l. detection (“IzoGen”, Russia) was used during 2007–2009. Real time PCR detection and quantitation were began to use in 2010 initially with the kit “AmpliSens B. burgdorferi sensu lato - FL” and later in 2011–2014 – with “AmplySens TBE, B. burgdorferi, A. phagocytophila, E. muris/E. chaffensis - FL” (“InterLabService”, Russia). Borrelia miyamotoi DNA was detected by PCR with subsequent electrophoresis using primers specific to p66 gene (Fomenko et al., 2010) in 2011–2013 and by real time PCR in 2014 with the kit “Vecto Borrelia miyamotoi - FL” (Vector Best, Russia) according to the manufacturer's instructions.
2.3. Bacteirial loads
Bacteirial loads were estimated using quantitative real time PCR as described above with calibration curve of dependence between quantities of Borrelia burgdorferi s.s. strain B31 DNA with the known concentrations and threshold cycles (Ct) of fluorescence as previously published (Morozova et al., 2011).
2.4. Nucleotide sequences
Nucleotide sequences of PCR products were determined using primers (Fomenko et al., 2008, 2010), BigDye 3.1 Terminator Cycle Sequencing Kit and DNA analyzer ABI 3500 (Applied Biosystems, USA). GenBank (http://www.ncbi.nlm.nih.gov) accession numbers of the Borrelia spp. determined in our study are shown in Table 1. Phylogenetic analysis was performed using MEGA 6.06 (5 alternative algorithms, 1,000 replications) (Tamura et al., 2013). Reference nucleotide sequences were the following: B. afzelii VS461 (NR164748), B. afzelii VS461 (L30135), B. afzelii HLJ01 (CP003882), Borrelia sp. Tokachi-J-IP21f (EF160140); B. garinii CZ (CP007564), B. garinii BqVir (CP003151), B. garinii 20047 (NR043413), B. garinii N337 (AB035398), B. garinii N341 (AB035395), B. garinii F632 (AB035403), B. garinii NT29 (L30130), B. garinii 20047 (L30119), B. garinii SZ 8-1 (JX570876), B. garinii Itn-4150 (AM748054), B. bavariensis PBi (CP000013), B. burgdorferi B31 (CP009656), B. finlandensis SV1 (NZ_ABJZ02000005), B. valasiana Tom4006 (CP009117); B. miyamotoi FR64b (CP004217), B. miyamotoi Izh-5 (CP024205), B. miyamotoi Nsk57 (EF488992), B. miyamotoi 14T114 (KU749376), B. miyamotoi Lipetzk-09(1)-IR (JF951382), B. miyamotoi tik485 (KJ412189), B. miyamotoi CT14D4 (CP010308), B. miyamotoi LB-2001 (CP006647), B. miyamotoi Khabarovsk-1 (KU845210), B. miyamotoi 57Nsk (FJ940729), Borrelia sp. Seq3-tick (AB824855), B. miyamotoi M24FT2 (KX885477), B. miyamotoi 57Nsk (EU645995), B. miyamotoi tik508 (KJ425365), B. miyamotoi An80 NX1 (KF054069); Borrelia sp. HM (LC170034), Borrelia sp. HM (LC170035), B. recurentus A1 (CP000993). Coincidence of topologies of 5 phylogenetic trees constructed by means of 5 alternative algorithms (Tamura et al., 2013) for each locus of Borrelia genomes and reasonable bootstrap support indexes of cladistic groups above 70 were considered as significant.
Table 1.
Accession numbers of nucleotide sequences of Borrelia isolates determined in our study.
| Borrelia species | sequence |
|||
|---|---|---|---|---|
| 16S rRNA | 5S—23S IGS | p66 | glpQ | |
| B. garinii | KY312010 - KY312015, KY312118, KY346888 - KY346892, KY346970 - KY346973, KY348800, | KY924779, KY937676 - KY937682, KY963154 - KY963161 | ||
| B. afzelii | KX622580, KX622581, KX622852, KX688604 | KX685726 - KX685729 | ||
| B. miyamotoi | KX769848 - KX769851 | KX812709 - KX812712 | КХ898133 | |
2.5. Statistical comparisons
Were carried out using percentages with the Standard Error of the Percentage (SEP) (Sheskin, 2011). Continuous variables were compared using Student's t-test. P values < 0.05 were assumed to be significant.
3. Results
3.1. Distribution of ixodidae ticks in the Far East of Russia
Ixodid ticks were collected in the Far East of Russia both in the mainland part (the Amur, the Jewish Autonomous regions and the Khabarovsk territory) and on Sakhalin Island during 1999–2014 (Fig. 1, Table 2). Totally, 2,158 individual ixodid ticks were collected, identified and examined by means of PCR for Borrelia burgdorferi s.l. detection. In 2011–2014 part of them (519 I. persulcatus) was also assayed to detect B. miyamotoi DNA. The ixodid tick populations in the continental collection places included four prevalent tick species: Ixodes persulcatus P. Schulze, 1930; Haemaphysalis concinna Koch, 1844; Haemaphysalis japonica douglasi Nuttall et Warburton, 1915 and Dermacentor silvarum Olenev, 1932 in different proportions, while on the Sakhalin Island only I. persulcatus ticks were found (Fig. 1, Table 2). In populous southern and central parts of the Amur region and on the Khabarovsk territory, where agrolandscapes replaced the original forests, H. concinna was predominant, and average tick density in deciduous and broadleaved forests (sites 4–6, 10, 12, 15) was essentially less than in larch-birch and pine-birch forests (site 1, 3) or in the coniferous forest landscapes with I. persulcatus prevalence on the Khekhtzir mountains (Bolshekhekhtzirsky wildlife reserve, site 11) and in Solnechny district (site 14). In the Jewish Autonomous region in coniferous–broadleaved forest (site 9) predominant I. persulcatus ticks reached the maximum population density (Table 2). Structure of tick populations of the Sakhalin Island differed from mainland, I. persulcatus was the only detected tick species (Table 2). Humidity of soil seems to be a limited factor for ixodid ticks development and is especially crucial parameter for I. persulcatus (Filippova; 1985). Therefore, the species remains abundant and predominant in coniferous–broadleaved forests far away from towns and villages. Other tick species including Haemaphysalis spp. can survive in less humid conditions and gradually replace the former prevailing I. persulcatus.
3.2. PCR detection of Borrelia DNA
PCR detection of B. burgdorferi s.l. in I. persulcatus ticks was successful in all explored biotops and areas. Borrelia DNA detection rate reached 69.0 ± 4.6% in larch-small-leaved forest (with I. persulcatus domination) on the Khabarovsk territory (site 17) and 68.8 ± 6.8 in larch-birch forest (with H. concinna domination) in the Amur region (site 1) with low anthropogenic pressure and relatively high tick population density. Borrelia DNA detection rate 0–28.6% for other tick species was significantly below the corresponding values for I. persulacatus collected from the same regions (P < 0.01) (Table 3). For comparison, totally in the Far East of Russia the average infection rate of I. persulcatus during 1999–2014 was 36.4 ± 1.2%, whereas H. japonica - 14.3 ± 5.1%, D. silvarum - 6.9 ± 4.8% and H. concinna - 2.9 ± 0.8%.
Table 3.
PCR detection of the Borrelia burgdorferi sensu lato DNA in the individual ixodid ticks collected from vegetation.
| Site No | Year | District | The Borrelia burgdorferi sensu lato infection rate in ixodid ticks |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ixodes persulcatus Schulze, 1930 |
Haemaphysalis concinna Koch,1844 |
Haemaphysalis japonica douglasi Nuttall et Warburton,1915 |
Dermacentor silvarum Olenev,1932 |
|||||||||||
| Number of studied ticks | Ticks with the Borrelia DNA | Rate (%) | Number of studied ticks | Ticks with the Borrelia DNA | Rate (%) | Number of studied ticks | Ticks with the Borrelia DNA | Rate (%) | Number of studied ticks | Ticks with the Borrelia DNA | Rate (%) | |||
| Amur region | ||||||||||||||
| 1 | 20111 | Magdagachinsky | 48 | 33 | 68.8 ± 6.8 | 51 | 2 | 3.9 ± 2.7 | 0 | 1 | 0 | |||
| 2 | 20111 | Shimanovsky | 9 | 2 | 22.2 ± 14.7 | 89 | 4 | 4.5 ± 2.2 | 0 | 2 | 0 | |||
| 3 | 20111 | Svobodnensky | 12 | 2 | 16.7 ± 11.2 | 85 | 3 | 3.5 ± 2.0 | 0 | 3 | 0 | |||
| 4 | 20101 | Selemdzhinsky | 0 | 56 | 0 | 0 | 0 | 0 | ||||||
| 5 | 20101 | Zeisky | 82 | 34 | 41.5 ± 5.5 | 0 | 0 | 17 | 2 | 11.8 ± 8.1 | ||||
| 6 | 20072 | Blagoveshchenky | 30 | 8 | 26.7 ± 8.2 | 70 | 0 | 0 | 0 | 0 | ||||
| 6 | 20082 | Blagoveshchenky | 27 | 12 | 44.4 ± 9.7 | 73 | 0 | 0 | 0 | 0 | ||||
| 1–6 | 2007 -20111,2 | 208 | 91 | 43.8 ± 3.4 | 424 | 9 | 2.1 ± 0.7 | 0 | 23 | 2 | 8.7 ± 6.0 | |||
| Jewish Autonomous region | ||||||||||||||
| 7 | 20131 | Oktyabrsky | 20 | 2 | 10.0 ± 6.9 | 2 | 0 | 0 | 0 | – | – | 5 | 0 | 0 |
| 8 | 20131 | Leninsky | 23 | 6 | 26.1 ± 9.4 | 0 | – | – | 12 | 0 | 0 | 0 | ||
| 9 | 20131 | Birobidzhansky | 50 | 27 | 54.0 ± 7.1 | 13 | 0 | 0 | 0 | 0 | 0 | |||
| 7–9 | 20131 | 93 | 35 | 37.6 ± 5.1 | 15 | 0 | 0 | 12 | 0 | 5 | 0 | 0 | ||
| Khabarovsk territory | ||||||||||||||
| 10 | 20141 | Lazo | 15 | 3 | 20.0 ± 10.7 | 21 | 1 | 4.8 ± 4.8 | 13 | 0 | 1 | 0 | ||
| 11 | 1999–20092 | Khabarovsky (Khekhtzir) | 530 | 152 | 28.7 ± 2.0 | 0 | 0 | 0 | ||||||
| 11 | 2010–20141 | Khabarovsky (Khekhtzir) | 250 | 136 | 54.4 ± 3.2 | 0 | 0 | 0 | ||||||
| 12 | 20141 | Nanaisky | 26 | 9 | 34.6 ± 9.5 | 21 | 4 | 19.0 ± 8.8 | 3 | 1 | 33.3 ± 33.3 | 0 | ||
| 13 | 20092 | Solnechny | 40 | 10 | 25.0 ± 6.9 | 0 | 0 | 0 | ||||||
| 14 | 20141 | Solnechny | 66 | 17 | 25.8 ± 5.4 | 0 | 0 | 0 | ||||||
| 15 | 20092 | Komsomolsky | 50 | 10 | 20.0 ± 5.7 | 0 | 0 | 0 | ||||||
| 16 | 20141 | Komsomolsky | 21 | 7 | 33.3 ± 10.5 | 0 | 21 | 6 | 28.6 ± 10.1 | 0 | ||||
| 17 | 20111 | Vaninsky | 100 | 69 | 69.0 ± 4.6 | 0 | 0 | 0 | ||||||
| 10–17 | 1999 -20141,2 | 1098 | 413 | 37.6 ± 1.5 | 42 | 5 | 11.9 ± 5.1 | 37 | 7 | 18.9 ± 6.5 | 1 | 0 | ||
| Sakhalin region | ||||||||||||||
| 18 | 20111 | Kholmsky | 100 | 19 | 19.0 ± 3.9 | 0 | 0 | 0 | ||||||
| 19 | 20111 | Yuzhno-Sakhalinsky | 100 | 24 | 24.0 ± 4.3 | 0 | 0 | 0 | ||||||
| 18, 19 | 20111 | 200 | 43 | 21.5 ± 2.9 | ||||||||||
| Far East | ||||||||||||||
| 1–19 | 1999 -20141,2 | 1599 | 582 | 36.4 ± 1.2 | 481 | 14 | 2.9 ± 0.8 | 49 | 7 | 14.3 ± 5.1 | 29 | 2 | 6.9 ± 4.8 | |
Notes:1 – results of real time PCR, 2 – data of PCR with subsequent electrophoresis.
B. miyamotoi DNA was detected in 2.0–33.3% I. persulcatus ticks collected from various biotopes of the Jewish Autonomous region, the Sakhalin region and the Khabarovsk territory (Table 4). B. miyamotoi was not found in the Amur region (Table 4) despite availability of I. persulcatus on the examined site 1 (Table 2). On an average, B. miyamotoi was detected in 8.9 ± 1.3% I. persulcatus ticks (as monoinfection in 4.0 ± 0.9% or as mixed infection with B. burgdorferi s.l. in 4.8 ± 0.9%) with reduced rate in comparison with B. burgdorferi s.l. (Table 3, Table 4).
Table 4.
PCR detection of the Borrelia miyamotoi DNA in individual Ixodes persulcatus ticks collected from vegetation.
| Site No | Year | District | The Borrelia miyamotoi DNA detection in I. persulcatus ticks |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studied ticks |
B. miyamotoi monoinfection |
B. miyamotoi + B. burgdorferi s. l. |
B. miyamotoi in total |
||||||
| Ticks with the Borrelia DNA | Rate (%) | Ticks with the Borrelia DNA | Rate (%) | Ticks with the Borrelia DNA | Rate (%) | ||||
| Amur region | |||||||||
| 1 | 20111 | Magdagachinsky | 48 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jewish Autonomous region | |||||||||
| 7 | 20132 | Oktyabrsky | 20 | 0 | 0 | 2 | 10.0 ± 6.9 | 2 | 10.0 ± 6.9 |
| 8 | 20132 | Leninsky | 23 | 0 | 0 | 1 | 4.3 ± 4.2 | 1 | 4.3 ± 4.2 |
| 9 | 20132 | Birobidzhansky | 50 | 0 | 0 | 5 | 10.0 ± 4.3 | 5 | 10.0 ± 4.3 |
| 7–9 | 20132 | 93 | 0 | 0 | 8 | 8.6 ± 2.9 | 8 | 8.6 ± 2.9 | |
| Khabarovsk territory | |||||||||
| 11 | 20132 | Khabarovsky | 50 | 0 | 0 | 1 | 2.0 ± 2.0 | 1 | 2.0 ± 2.0 |
| 11 | 20142 | Khabarovsky | 50 | 5 | 10.0 ± 4.3 | 4 | 8.0 ± 3.9 | 9 | 18.0 ± 5.5 |
| 10 | 20142 | Lazo | 15 | 4 | 26.7 ± 11.8 | 1 | 6.7 ± 6.7 | 5 | 33.3 ± 12.6 |
| 12 | 20142 | Nanaisky | 26 | 2 | 7.7 ± 5.3 | 1 | 3.8 ± 3.8 | 3 | 11.5 ± 6.4 |
| 14 | 20142 | Solnechny | 66 | 3 | 4.5 ± 2.6 | 3 | 4.5 ± 2.6 | 6 | 9.1 ± 3.6 |
| 16 | 20142 | Komsomolsky | 21 | 1 | 4.8 ± 4.8 | 2 | 9.5 ± 6.6 | 3 | 14.3 ± 7.8 |
| 17 | 20111 | Vaninsky | 50 | 0 | 0 | 1 | 2.0 ± 2.0 | 1 | 2.0 ± 2.0 |
| 10-12, 14, 16, 17 | 2013–20141,2 | 278 | 15 | 5.4 ± 1.4 | 13 | 4.7 ± 1.3 | 28 | 10.1 ± 1.8 | |
| Sakhalin region | |||||||||
| 18 | 20111 | Kholmsky | 50 | 3 | 6.0 ± 3.4 | 2 | 4.0 ± 2.8 | 5 | 10.0 ± 4.3 |
| 19 | 20111 | Yuzhno-Sakhalinsky | 50 | 3 | 6.0 ± 3.4 | 2 | 4.0 ± 2.8 | 5 | 10.0 ± 4.3 |
| 18, 19 | 20111 | 100 | 6 | 6.0 ± 2.4 | 4 | 4.0 ± 2.0 | 10 | 10.0 ± 3.0 | |
| Far East | |||||||||
| 1–19 | 2011–20141,2 | 519 | 21 | 4.0 ± 0.9 | 25 | 4.8 ± 0.9 | 46 | 8.9 ± 1.3 | |
Notes: 1 – data of PCR with electrophoresis detection; 2 – results of real-time PCR.
3.3. Quantitative estimations of Borrelia DNA in ixodid ticks
Average threshold cycles (Ct) of real time PCR with the analyzed B. burgdorferi s.l. DNA significantly differed among four examined tick species with the maximal deduced bacterial loads for I. persulcatus and H. japonica whereas those for H. concinna and D. silvarum were essentially lower (Table 5). Bacterial loads estimated on the base of quantitative real time PCR varied in a wide range from 102 to 109 genome-equivalents per a tick (Table 5). Based on the calibration curve of Ct from quantities of B. burgdroferi B31 genome-equivalents (Morozova et al., 2011) and the Lukyanov-Matz equation, one might estimate the average number of genome-equivalents per a tick 5.63 × 107 for I. persulcatus, 5.55 × 107 for H. japonica, 1.13 × 104 for H. concinna and 2.61 × 103 for D. silvarum (Table 5). The highest bacterial loads for I. persulcatus along with infection rate with B. burgdorferi s.l. exceeded the corresponding values of other tick species in all the examined regions during the whole period of observations (1999–2014) despite different tick abundance and dominance structure revealed the leading role of the taiga ticks in transmission of Borrelia. Similar quantitations for B. miyamotoi in I. persulcatus revealed the average bacterial load near 104 genome-equivalents per a tick in mono- and mixed infections (Table 6) that were in 10–1000 times less compared to amounts of B. burgdroferi s.l. (p < 0.001).
Table 5.
Threshold cycles (Ct) of quantitative real time PCR and deduced estimations of Borrrelia burgdorferi sensu lato DNA amounts in ixodid ticks in the Far East of Russia.
| Region/territory | No of tick collection site | Ixodidae tick species | Number of samples with the Borrelia DNA | Ct range (min-max) | Average Ct | Average quantity of genome-equivalents per a tick |
|---|---|---|---|---|---|---|
| Amur | 1 | I. persulcatus | 33 | 11.47–31.28 | 22.84 ± 7.05 | 1.46 × 10(5) |
| Amur | 2 | I. persulcatus | 2 | 15.05–17.35 | 16.20 ± 1.63 | 1.46 × 10(7) |
| Amur | 3 | I. persulcatus | 2 | 19.43–19.73 | 19.58 ± 0.21 | 1.40 × 10(6) |
| Amur | 5 | I. persulcatus | 34 | 18.93–30.32 | 24.73 ± 2.70 | 5.93 × 10(5) |
| Jewish Autonomous | 7 | I. persulcatus | 2 | 12.44–14.74 | 13.59 ± 1.63 | 5.35 × 10(9) |
| Jewish Autonomous | 8 | I. persulcatus | 6 | 11.24–15.15 | 12.84 ± 1.41 | 8.75 × 10(9) |
| Jewish Autonomous | 9 | I. persulcatus | 27 | 10.68–17.44 | 13.41 ± 1.85 | 6.06 × 10(9) |
| Khabarovsk | 10 | I. persulcatus | 3 | 14.65–30.04 | 19.90 ± 8.79 | 6.72 × 10(7) |
| Khabarovsk | 11 | I. persulcatus | 136 | 10.62–31.84 | 20.03 ± 6.42 | 6,16 × 10(7) |
| Khabarovsk | 12 | I. persulcatus | 9 | 10.42–23.73 | 18.48 ± 6.33 | 1.81 × 10(8) |
| Khabarovsk | 14 | I. persulcatus | 17 | 10.79–25.97 | 17.97 ± 5.59 | 2.57 × 10(8) |
| Khabarovsk | 16 | I. persulcatus | 7 | 11.01–22.53 | 14.58 ± 4.01 | 2.69 × 10(9) |
| Khabarovsk | 17 | I. persulcatus | 69 | 9.33–33.91 | 22.56 ± 7.03 | 1.07 × 10(7) |
| Sakhalin | 18 | I. persulcatus | 19 | 11.71–31.64 | 19.28 ± 7.57 | 1.04 × 10(8) |
| Sakhalin | 19 | I. persulcatus | 24 | 11.27–31.05 | 20.07 ± 7.20 | 5.99 × 10(7) |
| In total | I. persulcatus | 390 | 9.33–33.91 | 20.16 ± 0.18 | 5.63 × 10(7) | |
| Amur | 1 | H. concinna | 2 | 29.49–34.20 | 31.85 ± 3.33 | 2.84 × 10(2) |
| Amur | 2 | H. concinna | 4 | 29.98–33.21 | 32.02 ± 1.41 | 2.52 × 10(2) |
| Amur | 3 | H. concinna | 3 | 30.30–33.01 | 31.29 ± 1.49 | 4.18 × 10(2) |
| Khabarovsk | 10 | H. concinna | 1 | 30.69 | 30.69 | 3.81 × 10(4) |
| Khabarovsk | 12 | H. concinna | 4 | 24.42–26.48 | 24.95 ± 0.04 | 2.88 × 10(6) |
| In total | H. concinna | 14 | 24.42–34.20 | 26.53 ± 3.60 | 1.13x10(4) | |
| Khabarovsk | 12 | H. japonica | 1 | 13.96 | 13.96 | 4.14 × 10(9) |
| Khabarovsk | 16 | H. japonica | 6 | 20.47–21.86 | 21.22 ± 0.48 | 4.50 × 10(5) |
| In total | H. japonica | 7 | 13.96–21.86 | 20.18 ± 2.78 | 5.55х10(7) | |
| Amur | 5 | D. silvarum | 2 | 28.35–28.94 | 28.65 ± 0.42 | 2.61x10(3) |
Table 6.
Quantitative comparative analysis of Borrelia miyamotoi and Borrrelia burgdorferi sensu lato Ct and bacterial loads in Ixodes persulcatus ticks collected in the Far East of Russia.
| District | Number of tick collection site |
Borrelia miyamotoi monoinfection |
Mixed infection with Borrelia miyamotoi and B. burgdorferi s. l. |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of samples with the Borrelia DNA | Ct range (min-max) | Average Ct | Average quantity of genome-equivalents per a tick | Number of samples with the Borrelia DNA | Ct range (min-max) B. miyamotoi/B. burgdorferi s. l. | Average Ct B. miyamotoi/B. burgdorferi s. l. | Average quantity of genome-equivalents per a tick B. miyamotoi/B. burgdorferi s. l. | ||
| Jewish Autonomous region | |||||||||
| Oktyabrsky | 7 | 0 | 2 | 32.89–33.79/12.44–14.74 | 33.34 ± 0.64/13.59 ± 1.63 | 3,03 × 10(3)/5.35 × 10(9) | |||
| Leninsky | 8 | 0 | 1 | 35.25/12.87 | 35.25/12.87 | 8.07 × 10(2)/8.81 × 10(9) | |||
| Birobidzhansky | 9 | 0 | 5 | 33.66–36.02/10.84–13.84 | 34.92 ± 1.17/12.20 ± 1.16 | 1,01 × 10(3)/1.40 × 10(10) | |||
| Khabarovsk territory | |||||||||
| Lazo | 10 | 4 | 33.94–37.24 | 35.55 ± 1.54 | 6.56 × 10(2) | 1 | 37.31/30.04 | 37.31/30.04 | 1.94 × 10(2)/5.98 × 10(4) |
| Khabarovsky | 11 | 5 | 27.74–31.55 | 30.27 ± 1.73 | 2.55 × 10(4) | 5 | 17.53–30.80/11.92–28.85 | 25.37 ± 6.51/21.62 ± 7.60 | 7.61 × 10(5)/2.05 × 10(7) |
| Nanaisky | 12 | 2 | 35.34–38.58 | 36.96 ± 2.29 | 2.47 × 10(2) | 1 | 33.80/11.17 | 33.80/11.17 | 2.21 × 10(3)/2.86 × 10(10) |
| Solnechny | 14 | 3 | 22.78–33.25 | 29.75 ± 6.03 | 3.65 × 10(4) | 3 | 32.54–35.00/12.46–25.97 | 33.92 ± 1,26/17.57 ± 7.33 | 2,03 × 10(3)/3.39 × 10(8) |
| Komsomolsky | 16 | 1 | 18.72 | 7.64 × 10(7) | 2 | 21.62–36.19/11.01–12.68 | 28,91 ± 10.30/11,85 ± 1.18 | 6.54 × 10(4)/1.79 × 10(10) | |
| In total | 7–12, 14, 16 | 15 | 18.72–38.25 | 31.69 ± 5.36 | 9.52 × 10(3) | 20 | 21.62–36.19/10.84–30.04 | 31.70 ± 5.72/16.37 ± 6.34 | 9.56 × 10(3)/7.95 × 10(8) |
3.4. Phylogenetic analysis of Borrelia species diversity in the Far East of Russia
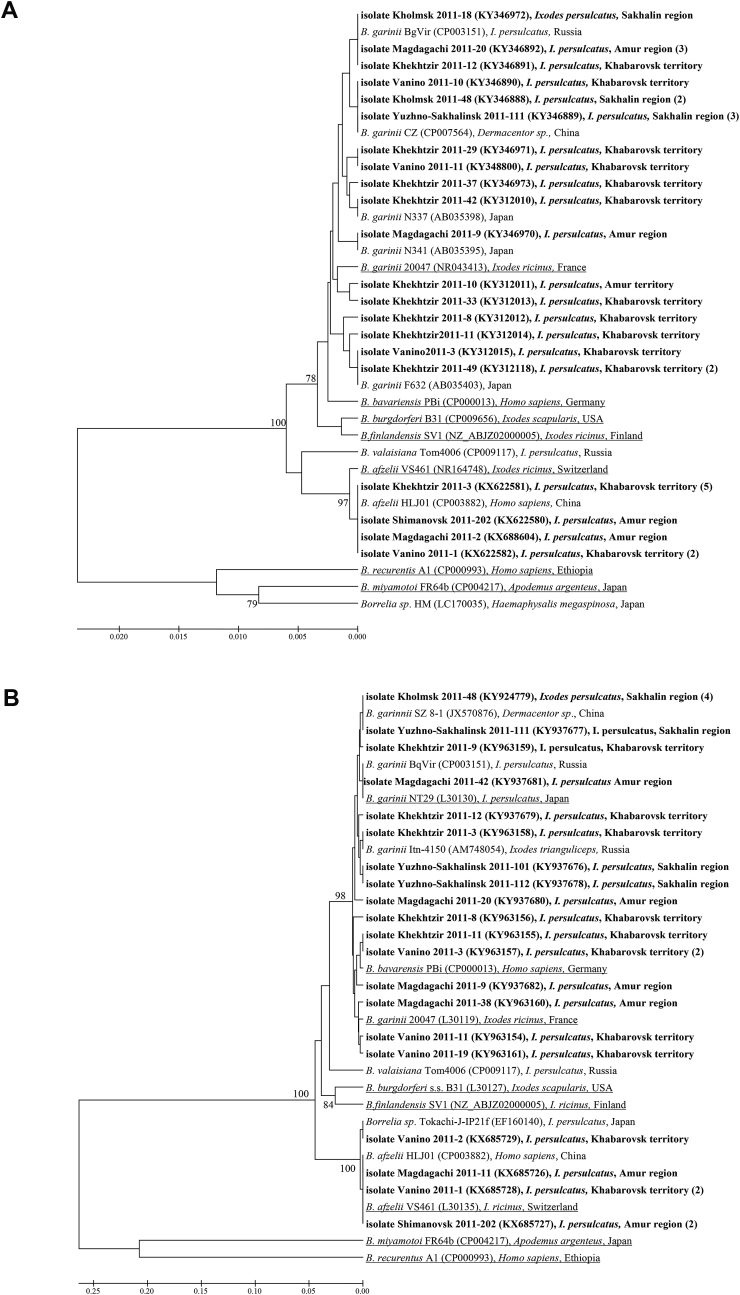
Genetic diversity of Borrelia in the Far East of Russia (Fig. 2) was similar to surrounding area of Eurasia (Kurtenbach et al., 2006) including sequence from a tick collected from migrating bird in Japan (AB015911). Sequences of 16S rRNA gene and 5S—23S rRNA intergeniс spacer (ITS) of B. garinii corresponding to Asian type with prototype NT29 and European type with reference strain 20047 were found in all the examined regions of the Far East. The majority of B. afzelii isolates from ixodid ticks was identical to B. afzelii HLJ01 strain (CP003882) from China and clustered with strain VS461 (GenBank accession number NR164748) from Switzerland (Fig. 2) with the single nucleotide polymorphism (SNP) in 5S—23S ITS of Borrelia isolate Vanino 2011-2 (KX685729) similar to Borrelia sp. isolate Tokachi-J-IP21f (EF160140) from Japan. One should note that the only B. garinii (9 isolates) were found on the Sakhalin Island despite the simultaneous circulation of both B. garinii (23 isolates) and B. afzelii (12 isolates) in the continental part of the Far East of Russia. Observed fluctuations of ratios between B garinii and B. afzelii in two areas of the Khabarovsk territory with prevalence of B. garinii over B. afzelii (18:8, respectively) and in two collection places of the Amur region with the corresponding ratio species between B. garinii and B. afzelii near 1 (5:4) were not significant due to small sampling sizes (Fig. 2). Phylogenetic analysis of nucleotide sequences of both 16S rRNA gene and 5S—23S rRNA ITS does not permit to distinguish between B. garinii and Borrelia bavarensis (Fig. 2). Both species belong to the same clade with good bootstrap indexes (Fig. 2).
Fig. 2.
Phylogenetic analysis of the nucleotide sequences of Borrelia 16S rRNA gene fragment of 733 bp long (A) and 5S—23S ITS of 211–218 bp long (B) using Mega 6.06 software, UPGMA algorithm and 1,000 replications. Phylogenetic trees constructed by means of 5 alternative algorithms (Maximum likelihood, Neighbor-Joining, Minimum-Evolution, UPGMA and Maximum Parsimony) show similar topologies and reasonable bootstrap support. Nucleotide sequences of Borrelia isolates determined in our study are shown in bold. The number of identical sequences of Borrelia isolates determined in these ixodid ticks is shown in parentheses. Branches corresponding to the reference strains are underlined.
3.5. MLSA analysis of B. miyamotoi
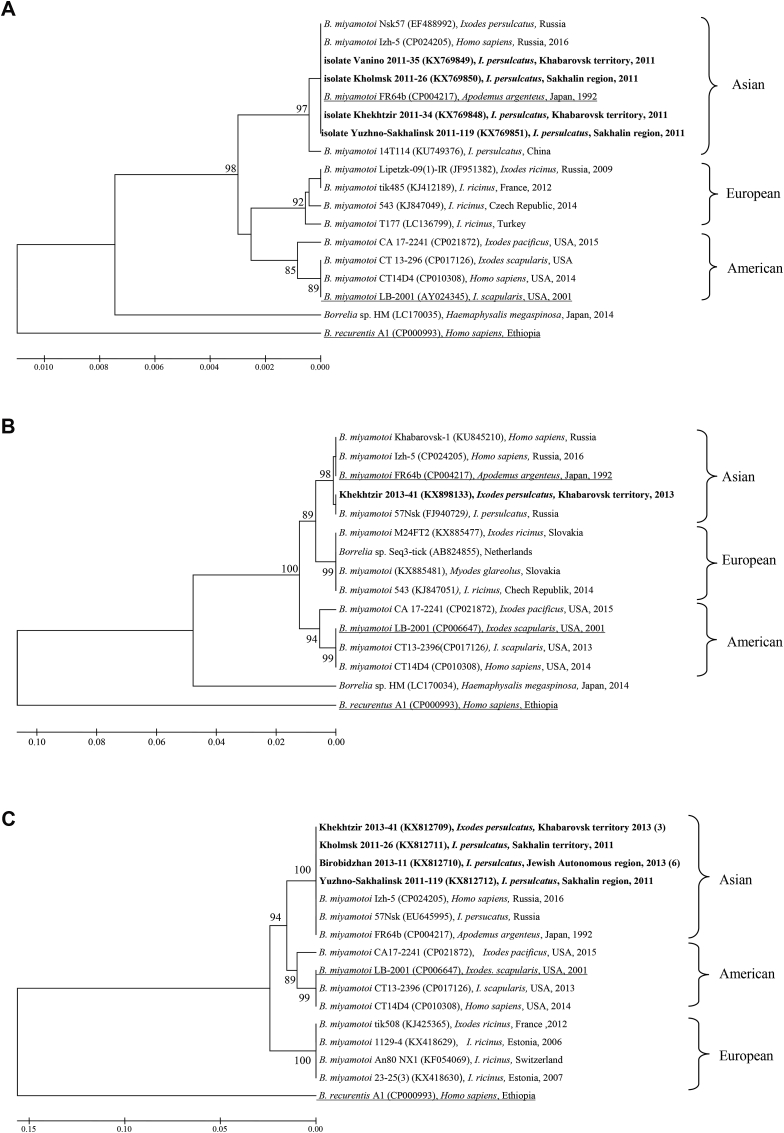
Multiple locus sequence analysis (MLSA) of B. miyamotoi nucleotide sequences of three fragments of 16S rRNA, glpQ and p66 genes showed similar patterns (Fig. 3). Isolates of B. miyamotoi from the Far East of Russia belong to the Asian group previously found in Japan, China, Siberia and Ural (Fig. 3). All our nucleotide sequences of 16S rRNA and p66 genes were identical to each other and to the strain FR64b (CP004217) from Japan, the only glpQ gene sequence includes the SNP identical to Siberian strain 57Nsk (FJ940729).
Fig. 3.
Multiple locus sequence analysis (MLSA) of Borrelia miyamotoi 16S rRNA gene fragment of 1248 bp (A), glpQ gene fragment 536 bp (B) and p66 gene fragment 526 bp long (C) using Mega 6.06 software, UPGMA algorithm and 1,000 replications. Phylogenetic trees constructed by means of 5 alternative algorithms (Maximum likelihood, Neighbor-Joining, Minimum-Evolution, UPGMA and Maximum Parsimony) show similar topologies and reasonable bootstrap support. Nucleotide sequences of Borrelia isolates determined in our study are shown in bold. The number of identical sequences of Borrelia isolates determined in these ixodid ticks is shown in parentheses. Branches corresponding to the reference strains are underlined.
4. Discussion
In the Far East of Russia, 22 hard tick species of the family Ixodidae have been identified, among them 11 species: Ixodes maslovi Emelyanova and Kozlovskaya, 1967; Ixodes uriae White, 1852; Ixodes lividus Koch, 1844; Ixodes signatus Birula, 1895; Ixodes angustus Neumann, 1899; Ixodes pavlovskyi Pomerantsev, 1946; Ixodes persulcatus P. Schulze, 1930; Haemaphysalis phasianna Saito and Wassef, 1974; Haemaphysalis concinna Koch, 1844; Haemaphysalis japonica Nuttall et Warburton, 1915 and Dermacentor silvarum Olenev, 1932 were found on the Khabarovsk territory. The four tick species including I. persulcatus, H. concinna, H. japonica, D. silvarum predominate and have epidemiological importance (Volkov, 2005). During our period of observation 1999–2014 the four prevailing tick species - I. persulcatus, H. concinna, H. japonica, D. silvarum were found in all examined regions. Minor tick species were not observed, therefore, they were not analyzed. Monodominant populations of ixodids with the only species I. persulcatus remain on the Sakhalin Island and on Kamchatka Peninsula (Pukhovskaya et al., 1991, 2010). Tick numbers and dominance structure depend on anthropogenic influence. In afforestation biotopes both total number of ixodid ticks and proportion of taiga ticks dissipate (Volkov, 2005). Wide distribution of Borrelia DNA in the ixodid ticks of different species collected from all the studied areas of the Far East of Russia including Kamchatka Peninsula (Pukhovskaya et al., 2010) was shown (Table 2, Table 3, Table 4). Nevertheless, maximal values of Borrelia DNA detection rate and bacterial loads were observed for I. persulcatus that together with its wide distribution throughout forest zone of Eurasia suggest its leading role as vector of B. burgdorferi s.l. Our comparative analysis confirmed the previous observation of principal role of I. persulcatus to cause Lyme disease in China and at the border with Russia (Liu et al., 2012).
Phylogenetic analysis of 16S rRNA gene and 5S—23S rRNA intergenic spacer nucleotide sequences revealed two species of B. burgdorferi s.l. complex, Borrelia garinii of prevailing Asian type NT29 with a few isolates of European type 20047 and Borrelia afzelii close toVS461 reference strain in ixodid ticks collected from all the examined regions besides the Sakhalin Island with the B. garinii only. One should note that currently according to BLAST homology search among GenBank deposited nucleotide sequences Borrelia DNA genetic variability in I. persulcatus ticks remains low: 1) for 16S rRNA gene - 0% for B. afzelii, 0–1% for B. garinii, 0–2% for B. miyamotoi; 2) for 5S—23S ITS - 0–2% for B. garinii and 1–2% for B. afzelii; 3) for glpQ – 0–4% for B. miyamotoi; 4) for p66 – 0–5% for B. miyamotoi. One should note that variability of B. miyamotoi glpQ and p66 gene fragments slightly exceeded nucleotide change levels of 16S rRNA gene and 5S—23S ITS. The remarkable genetic stability of Borrelia might suggest epigenetic regulation for adaptation to evolutionary divergent invertebrate vectors and vertebrate.
Molecular typing of Borrelia on the base of a single fragment of coding region or intergenic spacer as well as numerous attempts to use random set of several genetic loci were not always successful (Baranton and Postic, 2006). It may be caused by orthologous and paralogous genes localized on numerous plasmids with varying copy numbers in wild populations and possible loss during laboratory passaging. Therefore, description of natural diversity of Borrelia by analysis of laboratory strains or single locus (Sato et al., 1996) and especially fragment of the plasmid gene encoding outer surface protein A (Mediannikov et al., 2005) can lead to misrepresentations and wrong interpretations. MLSA of wild isolates (without a laboratory passage) with similar topologies of phylogenetic trees with reasonable bootstrep support (Fig. 2, Fig. 3) provided the convincing evidences of spatial homogeneity and temporal stability of species.
Differences exist between Borrelia isolates according to tick vector and geographic region, but little genetic difference has been found between isolates within a given geographic area or with the same tick vector association (Fig. 2, Fig. 3) (Morozov and Morozova, 2012 and references therein). Despite detection of Borrelia valaisiana in China (Hao et al., 2011) they were not detected in the Far East of Russia (Fig. 2). Low-frequency Borrelia variants were not found in ixodid ticks surviving without immune system at ambient temperatures (Fig, 2, 3). Genetic similarity was observed for Borrelia isolates from invertebrate vectors and vertebrate reservoir hosts as well as from patients (Fig. 2, Fig. 3). The available data support concept of multiple ecological niches (such as various vectors, reservoir hosts and biotops) with minimal genetic polymorphism. But further study of Borrelia adaptation to divergent arthropods and vertebrate hosts is required. Rich ixodofauna of the Far East of Russia along with multiple vertebrate reservoir hosts (Volkov, 2005) provide multiple ecological niches not only for tick-borne borrelia with high spacial and temporal genetic stability but even for relative stable wild populations of RNA-containing tick-borne encephalitis virus (Pukhovskaya et al., 2018).
However, recent study revealed that in Siberia main part of borrelioses was caused by B. miyamotoi but not B. burgdorferi s.l. as previously believed (Titkov et al., 2018). The recently described infection with B. miyamotoi that is transmitted by the same vector - I. persulcatus ticks and proceeds as fever with unspecific symptoms without erythema and relatively rare serious complications (Krause et al., 2015). Despite lower bacterial loads and decreased infection rate of ticks with B. miyamotoi compared to B. burgdorferi s.l. the epidemiological significance is emerging. High abundance of ixodid ticks in the Far East of Russia, high infection rate of different tick species with Borrelia spp. with enormous bacterial loads and absence of vaccines may be reasons of stable and high Lyme disease rate.
5. Conclusion
Comparative analysis of both infection rate and bacterial loads of four prevailing species of ixodid ticks with Borrelia burgdorferi s.l. revealed the leading role of Ixodes persulcatus in the Far East of Russia. Genetic homogeneity and temporal stability of B. garinii, B. afzelii and B. miyamotoi suggest multiple ecological niches as mechanism providing their stability.
Conflicts of interest
There are no conflicts to declare.
References
- Baranton G., Postic D. Multi Locus Sequence Analysis (MLSA) as alternative to Whole DNA/DNA hybridization (WDDH) in Borrelia burgdorferi sensu lato taxonomy. In: Cabello F.C., Hulinska D., Hodfrey H.P., editors. Molecular Biology of Spirochetes, NATO Science Series: Life and Behavioral Sciences. 2006. pp. 135–145. [Google Scholar]
- Casjens S.R., Fraser-Liggett C.M., Mongodin E.F., Qiu W.-G., Dunn J.J., Luft B.J., Schutzer S.E. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J. Bacteriol. 2011;193:1489–1490. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaerschalck I., Ben Messaoud A., De Kesel M., Hoyois B., Lovet Y., Hoet Ph, Bigaignon G., Bollen A., Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 1995;33(3):602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippova N.A. Nauka; Leningrad: 1985. Taiga Tick Ixodes Persulcatus Schulze (Acarina, Ixodidae) p. 416. (in Russain) [Google Scholar]
- Fomenko N.V., Livanova N.N., Chernousova N.Y. Diversity of Borrelia burgdorferi sensu lato in natural foci of Novosibirsk region. Int. J. Med. Microbiol. 2008;298(Supplemental 1):139–148. [Google Scholar]
- Fomenko N.V., Livanova N.N., Bogoyarkov V.Yu, Kozlova I.V., Shulaykina I.V., Pukhovskaya N.M., Tokarevich K.N., Livanov S.G., Doroschenko E.K., Ivanov L.I. Detection of Borrelia miyamotoi in ticks Ixodes persulcatus from Russia. Parasitology. 2010;44:201–211. (in Russian) [PubMed] [Google Scholar]
- Fraser C.M., Casjens S., Huang W.M., Sutton G.G., Clayton R., Lathigra R., White O., Ketchum K.A., Dodson R., Hickey E.K., Gwinn M., Dougherty B., Tomb J.F., Fleischmann R.D., Richardson D., Peterson J., Kerlavage A.R., Quackenbush J., Salzberg S., Hanson M., van Vugt R., Palmer N., Adams M.D., Gocayne J., Weidman J., Utterback T., Watthey L., McDonald L., Artiach P., Bowman C., Garland S., Fuji C., Cotton M.D., Hors K., Roberts K., Hatch B., Smith H.O., Venter J.C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Furuno K., Lee K., Itoh Y., Suzuki K., Yonemitsu K., Kuwata R., Shimoda H., Watarai M., Maeda K., Takano A. Epidemiological study of relapsing fever borreliae detected in Haemaphysalis ticks and wild animals in the western part of Japan. PLoS One. 2017;12:e0174727. doi: 10.1371/journal.pone.0174727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Hou X., Geng Zh, Wan K. Distribution of Borrelia burgdorferi sensu lato in China. J. Clin. Microbiol. 2011;49:647–650. doi: 10.1128/JCM.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg E.I., Kryuchechnikov V.N., Kovalevsky Y.V. Advances in investigations of Lyme borreliosis in the territory of the former USSR. Eur. J. Epidemiol. 1993;9:86–91. doi: 10.1007/BF00463095. [DOI] [PubMed] [Google Scholar]
- Krause P., Fish D., Narasimhan S., Barbour A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K., Hanincová K., Tsao J.I., Margos G., Fish D., Ogden N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- Li M., Masuzawa T., Takada N., Ishiguro F., Fujita H., Iwaki A., Wang H., Wang J., Kawabata M., Yanagihara Y. Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia and Japan. Appl. Environ. Microbiol. 1998;64:2705–2709. doi: 10.1128/aem.64.7.2705-2709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yuan C., Cui Y.F., Li B.X., Wu L.J., Liu Y. Investigation of Borrelia spp. in ticks (Acari: Ixodidae) at the border crossings between China and Russia in heilongjiang province, China. Asian Pac. J. Trop. Med. 2012;5:459–464. doi: 10.1016/S1995-7645(12)60078-9. [DOI] [PubMed] [Google Scholar]
- Mediannikov O.Y., Ivanov L., Zdanovskaya N., Vorobyova R., Sidelnikov Y., Fournier P.E., Tarasevich I., Raoult D. Diversity of Borrelia burgdorferi sensu lato in Russian Far East. Microbiol. Immunol. 2005;49:191–197. doi: 10.1111/j.1348-0421.2005.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Morozov I., Morozova O. Lambert Academic Publishing (LAP); Saarbrucken, Germany: 2012. p. 151. (From Borrelia Genome Structure to Gene Functions). [Google Scholar]
- Morozova O.V., Grishechkin A.E., Konkova-Reidman A.B. Quantitative estimations of Borrelia and Bartonella DNA and tick-borne encephalitis virus RNA in ticks Ixodes persulcatus collected in Chelyabinsck region. Mol. Gen. Mikrobiol. Virusol. 2011;1:35–38. (in Russian) [Google Scholar]
- Mukhacheva T.A., Salikhova I.I., Kovalev S.Y. Multilocus spacer analysis revealed highly homogeneous genetic background of Asian type of Borrelia miyamotoi. Infect. Genet. Evol. 2015;31:257–262. doi: 10.1016/j.meegid.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Platonov A.E., Karan L.S., Kolyasnikova N.M., Makhneva N.A., Toporkova M.G., Maleev V.V., Fish D., Krause P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukhovskaya N.M., Dolgikh A.M., Vereta L.A., Pletnev A.G. Natural foci of tick-borne encephalitis on the south-western coast of Sakhalin. Med. Parasitol. Parasitarnie Bolezni. 1991;2:48–50. (in Russian) [PubMed] [Google Scholar]
- Pukhovskaya N.M., Rar V.A., Ivanov L.I., Vysochina N.P., Igolkina YaP., Fomenkova N.V., Zaraichenkova N.V., Plashkova V.V., Oreshkina S.G., Maltseva I.P., Chistov V.A., Chechikova V.A. PCR detection of the causative agents of feral herd infections transmitted by ticks on the Kamchatka Peninsula. Med. Parasitol. Parasitarnie Bolezni. 2010;4:36–39. (in Russian) [PubMed] [Google Scholar]
- Pukhovskaya N.M., Morozova O.V., Vysochina N.P., Belozerova N.B., Bakhmetyeva S.V., Zdanovskaya N.I., Seligman S.J., Ivanov L.I. Tick-borne encephalitis virus in arthropod vectors in the Far East of Russia. Ticks Tick Borne Dis. 2018;9:824–833. doi: 10.1016/j.ttbdis.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Rar V., Livanova N., Tkachev S., Kaverina G., Tikunov A., Sabitova Y., Igolkina Y., Panov V., Livanov S., Fomenko N., Babkin I., Tikunova N. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasites Vectors. 2017;10:258. doi: 10.1186/s13071-017-2186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P.A., Tilly K., Stewart P.E. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 2005;3:129–143. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- Samuels D.S., Radolf J.D. Horizon Scientific Press; 2010. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. [Google Scholar]
- Sato Yu, Miyamoto K., Iwaki A., Masuzava T., Yanagihara Ya, Korenberg E.I., Gorelova N.B., Volkov V.I., Ivanov L.I., Liberova R.N. Prevalence of Lyme disease spirochetes in Ixodes persulcatus and wild rodents in far eastern Russia. Appl. Environ. Microbiol. 1996;62:3887–3889. doi: 10.1128/aem.62.10.3887-3889.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin D.J. fifth ed. Chapman & Hall/CRC; NY: 2011. Handbook of Parametric and Nonparametric Statistical Procedures. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Borre M., Mathiesen M.J., Mikkelsen B., Lebech A.M., Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J. Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titkov A.V., Platonov A.E., Stukolova O.A. Epidemiological features of ixodes tick-borne borrelioses in the Krasnoyarsk territory in the context of searching for the cases of infection caused by Borrelia miyamotoi. J. Microbiol. Epidemiol. Immunobiol. 2018;3:10–18. (in Russian) [Google Scholar]
- Volkov V.I. 2005. Medical Ecological Atlas of the Khabarovsk Territory and the Jewish Autonomous Region. Khabarovsk (in Russian) [Google Scholar]