Summary
Acid exposure time commonly varies from day-to-day in prolonged wireless pH monitoring. Thus, diagnosis based on the number of days with abnormal acid burden may be misleading or inconclusive. We hypothesize that assessing longitudinal patterns of acid exposure may be diagnostically useful. Therefore, this study aims to describe acid exposure trajectories and evaluate agreement between identified trajectory patterns and conventional grouping. In this retrospective cohort study, we assessed patients with nonresponse to proton pump inhibitor therapy who underwent wireless pH monitoring (≥72 h) off therapy between August 2010 and September 2016. The primary outcome was esophageal acid exposure time. Subjects were grouped as 0, 1, 2, and 3+ days positive based on number of days with an acid exposure time >5.0%. Latent class group-based mixture model identified distinct longitudinal acid exposure trajectory groups. Of 212 subjects included 44%, 18%, 14%, and 24% had 0, 1, 2, 3+ days positive, respectively. Group-based modeling identified three significantly stable acid exposure trajectories: low (64%), middle (28%), and high (8%). Trajectory grouping and days positive grouping agreed substantially (weighted K 0.69; 95% CI: 0.63–0.76). Trajectory grouping identified 62% of subjects with conventionally inconclusive studies (one or two days positive) into the low trajectory. Agreement between trajectory groups when using three versus four days of monitoring was substantial (K 0.70; CI: 0.61–0.78). In summary, we found that patients with nonresponse to proton pump inhibitors follow three acid exposure trajectories over prolonged pH-monitoring periods: low, middle, and high. Compared to conventional day positive grouping, the trajectory modeling identified the majority of inconclusive days positive into the low trajectory group. Analyzing prolonged wireless pH data according to trajectories may be a complimentary method to conventional grouping, and may increase precision and accuracy in identifying acid burden.
Keywords: Bravo, gastroesophageal reflux disease, proton pump inhibitor nonresponder, reflux monitoring
ABBREVIATIONS
- AET
acid exposure time
- BIC
Bayesian information criteria
- GERD
gastroesophageal reflux disease
- PPI
proton pump inhibitor
INTRODUCTION
Esophageal pH monitoring provides a measurement of esophageal acid exposure and symptom–reflux association and is commonly used to assess nonresponse to acid suppression in patients with symptoms suggestive of gastroesophageal reflux disease (GERD). Presently, ambulatory esophageal pH monitoring is available in two forms: a catheter-based pH monitoring system and a wireless pH system. The wireless pH system is a catheter-free pH monitoring technology that works via a transorally positioned capsule attached to the distal esophagus, which transmits esophageal pH readings to an external receiver via telemetry.1,2 Compared to the conventional catheter-based system, the wireless pH system is better tolerated by patients, enables monitoring over prolonged periods (up to 96 hours), and increases the diagnostic yield of abnormal esophageal acid exposure.1–6,7
While prolonged wireless pH monitoring offers several advantages, the diagnostic interpretation of prolonged pH monitoring is fraught with several important issues. A common challenge encountered in clinical practice and reported in the literature surrounds the day-to-day variability in acid exposure time (AET).8,9 When severity of AET differs from one day to another during a study period, it is unclear whether to rely on average acid exposure time, the worst day, or a composite of days for diagnosis.2,10,11 Further confounding this issue is that cut-offs for abnormal esophageal AET over prolonged wireless pH monitoring periods (72 hours or longer) are based on normative data from studies that examined AETs during 24- and 48-hour wireless pH periods. In these studies, the 95th percentile of normal AET varied between 4.4 and 5.3%, and therefore an AET cut-off of 5.0% is generally considered pathologic.12–15 However, this extrapolation of AET cut-offs based on prior studies, which examined shorter durations of wireless pH monitoring may not be appropriate. Given the widespread use of prolonged wireless pH monitoring, clarification on interpretation is critically needed. Inconsistent and inaccurate diagnoses could make the difference between a recommendation for or against antireflux surgery.
To address these issues, we investigated a cohort of patients undergoing prolonged wireless pH monitoring with presupposed GERD and persistent symptoms despite proton pump inhibitor (PPI) therapy. In this cohort, we aimed to describe longitudinal acid exposure trajectories and evaluate agreement between identified trajectory patterns and conventional diagnostic metrics. We hypothesized that an assessment of longitudinal patterns in acid exposure would have diagnostic value compared to evaluations based on conventional and extrapolated cut-offs.
MATERIALS and METHODS
Study design and subjects
This retrospective cohort study assessed PPI nonresponders seen at a single academic center between August 2010 and September 2016. This study was approved by the Northwestern University Institutional Review Board. Patients were included if they were adults age 18 years or older who underwent prolonged wireless pH monitoring off antisecretory therapy for evaluation of typical (heartburn, regurgitation) or atypical (chest pain, cough, sore throat, throat clearing, voice hoarseness, and globus) GERD symptoms despite double-dose PPI therapy for a minimum of eight weeks. All patients had less than a 50% patient-reported symptom response to PPI therapy. Patients were excluded if they had erosive esophagitis, Barrett's esophagus, esophageal stricture, or an esophageal diverticulum. Additionally, patients were excluded if the wireless pH-metry was not analyzable over at least 16 hours per day and 72 hours total.
Wireless pH monitoring protocol
Following calibration of the wireless catheter in reference solutions, the delivery catheter system (Bravo; Medtronic, Mineappolis, MN) was introduced transorally and advanced to 6 cm proximal to the endoscopically identified squamocolumnar junction, corresponding to 5 cm above the proximal border of the lower esophageal sphincter. All wireless pH monitoring systems were placed during endoscopy under intravenous conscious sedation with the patient in the left lateral decubitus position. Once the system was in appropriate position, the external portable vacuum pump was switched on to apply suction to the well of the capsule and suck in adjacent esophageal mucosa. After 15 seconds, the plastic safety guard was removed and activation button depressed.
Following catheter placement patients received detailed instructions. The patients were instructed to wear the pager-sized receiver on their waist and remain within 3 to 5 feet of the receiver at all times. Patients were encouraged to continue their usual activities and consume their typical diet. Patients were instructed to log symptoms and meals in a written diary as well as via receiver. Wireless pH recordings were analyzed via manufacturer software (AccuView Reflux Software; Medtronic, Mineappolis, MN) for esophageal acid exposure and symptom association parameters.
Outcomes, definitions, and groups
The primary outcome measured was esophageal AET defined as the percent time esophageal acid exposure was below a pH of 4.0. A day was considered positive if AET was greater than 5.0%.12–14 In accordance with our conventional interpretation, the number of days a study was positive was calculated. Subjects were categorized into four groups based on the number of positive days during the study period as 0 days positive, 1 day positive, 2 days positive or ≥3 days positive (Table 1).
Table 1.
Groups based on number of days positive
| Conventional groups | N = 212 |
|---|---|
| 0 days positive | 93 (44%) |
| 1 day positive | 39 (18%) |
| 2 two days positive | 29 (14%) |
| 3+ (three or more) days positive | 51 (24%) |
Data analysis
Analyses utilized all available data from subjects meeting inclusion criteria. There were no formal power calculations for this study, and we did not impute missing data. The overall objective of the analysis was to perform a technical assessment of acid exposure data.
To address the primary aim (describe patterns of acid exposure trajectories over time), primary analyses employed a semiparametric, group-based mixture model (SAS PROC TRAJ) to separate distinct clusters of longitudinal acid exposure trajectories over the four days of observation; trajectory analyses were naïve to any other variables.16,17 This procedure identifies unobserved, or latent, subgroups within a population. In this study, the latent subgroups were determined according to latent growth, or longitudinal, curves of acid exposure. The optimal number of latent curves for the population was determined by mixture models. With a hypothesis that anywhere between three to six trajectory patterns of acid exposure exist among this population, we used the Bayesian information criteria (BIC) for model selection to identify the optimal number of trajectory patterns to fit the data. The goal of using BIC for model selection was to identify which parameters are similar and estimate what outcomes may be meaningful to identify data clusters representative of clinical phenotypes. We next assessed the agreement between trajectory groups and days positive groups via the kappa statistics and spearman sample correlation coefficients.
We planned an additional sensitivity analysis to ensure that the wireless pH monitoring results in this study were consistent with that observed in published literature. To assess for day-to-day variability in AET, we employed a series of linear mixed models with random patient effect and fixed day (‘time’) and days positive effects. In addition, we explored predictors (heartburn, regurgitation, etc.) of elevated AET (greater than 5%) using generalized estimating equations (GEE) and generalized linear models with logit link.
We performed two posthoc, exploratory analyses. First, we used generalized logistic regression modeling to examine predictors of trajectory group membership. Second, we repeated trajectory analysis (PROC TRAJ) using just three days of data instead of all four days to explore the sensitivity of trajectory analyses to the number of days of monitoring.
RESULTS
Baseline characteristics
Overall, 212 subjects met inclusion criteria over the study period. The mean age was 46.7 ± 15.1 years, and 64% (136) were female. The majority (69%, n = 146) were Caucasian. Thirty-nine subjects (18%) had a hiatal hernia, 13% measured at 1 to 2 cm in size, and 3% measured at larger than 2 cm in size. The most common symptom presentation was heartburn (50%, n = 106), followed by regurgitation (24%, n = 50), and chest pain (24%, n = 50). Five percent (n = 11) presented with cough alone, and 12% (n = 25) presented with laryngeal complaints including voice hoarseness, sore throat, and/or throat clearing alone (Table 2).
Table 2.
Baseline subject and study characteristics
| Baseline characteristics | N = 212 |
|---|---|
| Age, years | 46.7 ± 15.1 |
| Female gender | 136 (64%) |
| Race | |
| White | 146 (69%) |
| African-American | 11 (5%) |
| Asian | 5 (2%) |
| Other/Unknown | 50 (24%) |
| Hiatal hernia | 39 (18%) |
| 1–2 cm in size | 28 (13%) |
| >2 cm in size | 7 (3%) |
| Symptoms | |
| Regurgitation | 50 (24%) |
| Heartburn | 106 (50%) |
| Chest pain | 50 (24%) |
| Cough only | 11 (5%) |
| Laryngeal complaints only | 25 (12%) |
| Study protocol characteristics | |
| 4 days of monitoring | 165 (78%) |
| Monitoring overlapped with weekend | 115 (54%) |
| Time of capsule placement, hour | 10.9 ± 2.3 |
With regard to the wireless pH monitoring study protocol, the majority (78%, n = 165) had a full four days of monitoring; the 212 subjects contributed a total of 801 days of monitoring. Fifty-four percent (n = 115) of subjects’ monitoring periods occurred over the weekend (Friday to Sunday), and the mean time of day wireless pH capsule placement was 10.9 ± 2.3 hours (Table 2).
AET summary statistics
The median overall AET was 2.90% (IQR 0.90% to 6.90%). Based upon the threshold of AET of greater than 5%, we categorized patients into zero (0 days positive), one (1 day positive), two (2 days positive), and three or more (3+ days positive) days positive with 44% (n = 93), 18% (n = 39), 14% (n = 29), and 24% (n = 51) falling into each group, respectively (Table 1).
Primary analysis: group-based trajectory modeling
Group-based trajectory modeling identified that patients tended to follow one of three acid exposure trajectory patterns: low (Group 1, 64%), middle (Group 2, 28%), and high (Group 3, 8%) exposure patterns. Mean AET in the low, middle, and high groups were 2.1 ± 2.1, 7.5 ± 3.9, and 12.9 ± 4.6, respectively. Overall, the model was statistically significant (P < 0.001), suggesting significant stability within each trajectory group and significant differences between the groups (Fig. 1).
Fig. 1.
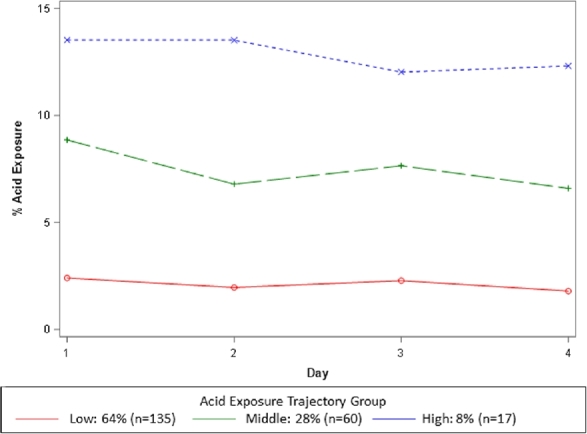
Group-based acid exposure trajectories.
Primary analysis: agreement between trajectory group and days positive group
The agreement between trajectory grouping and days positive group was substantial (weighted Kappa = 0.69 [95% CI: 0.63, 0.76], Spearman's sample correlation coefficient = 0.84 [0.80, 0.88]).18 The model classified all (100%, 93) of 0 days + subjects in the low acid exposure trajectory (Group 1), and 100% (17) of high acid exposure trajectory (Group 3) had three or more days positive. The model classified the majority (n = 33/39, 85%) of those subjects with one day positive into the low acid exposure trajectory, and the majority of those with two days positive (20/29, 69%) into the middle (Group 2) trajectory (Table 3).
Table 3.
Agreement between days positive and trajectory grouping
| Trajectory grouping | |||||
|---|---|---|---|---|---|
| Frequency row% Col% | Low AET | Mid AET | High AET | Total | |
| Number of days positive grouping | 0 | 93 | 0 | 0 | 93 |
| 100% | 0% | 0% | |||
| 69% | 0% | 0% | |||
| 1 | 33 | 6 | 0 | 39 | |
| 85% | 15% | 0% | |||
| 24% | 10% | 0% | |||
| 2 | 9 | 20 | 0 | 29 | |
| 31% | 69% | 0% | |||
| 7% | 33% | 0% | |||
| 3+ | 0 | 34 | 17 | 51 | |
| 0% | 67% | 33% | |||
| 0% | 57% | 100% | |||
| Total | 135 | 60 | 17 | 212 | |
A priori sensitivity analyses
Mixed modeling results revealed significant variability in AET day-to-day (P < 0.001) and across groups (P < 0.001). In particular, after adjustment for multiple comparisons, the mean AET was significantly greater on Day 1 compared to Day 2 (Tukey-adjusted P = 0.013) or Day 4 (Tukey-adjusted P < 0.001, Fig. 2). In generalized linear models using GEE, the following variables were significantly associated with AET >5.0%: symptoms of regurgitation (OR 1.9, 95% CI 1.2–3.2; P = 0.011), and presence of hernia (OR 2.2, 95% CI 1.2–4.0; P = 0.011). Although not significant, subjects presenting with cough only were less likely to present with AET > 5.0% (OR 0.3, 95% CI 0.1–1.1; P = 0.070) (Table 4).
Fig. 2.
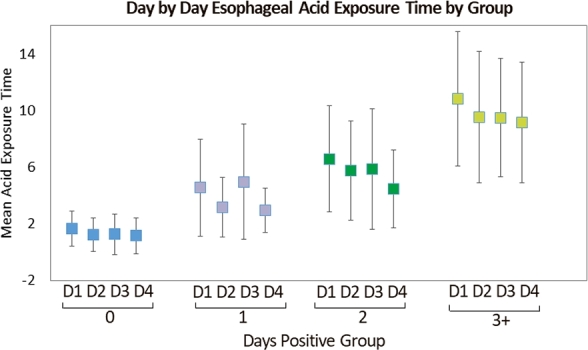
Day by day esophageal acid exposure time by days positive group.
Table 4.
Variables associated with an acid exposure time >5.0%
| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Hiatal hernia | 2.2 | 1.2–4.0 | 0.01 |
| Heartburn | 1.4 | 0.9–2.2 | 0.12 |
| Regurgitation | 1.9 | 1.2–3.2 | 0.01 |
| Laryngeal complaints only | 0.7 | 0.4–1.4 | 0.38 |
| Chest pain | 0.8 | 0.5–1.3 | 0.33 |
| Cough only | 0.3 | 0.1–1.1 | 0.07 |
| Time of capsule placement | 1.0 | 0.9–1.2 | 0.58 |
| No weekend overlap | 1.4 | 0.9–2.3 | 0.09 |
Post-hoc sensitivity analyses
In the generalized logit models examining predictors associated with trajectory group membership, presence of any hernia was significantly associated with higher acid exposure group membership (overall P = 0.002; OR for high acid exposure group vs. low acid exposure group = 6.9 [2.4, 20.0]), and regurgitation symptoms were marginally significantly associated (overall P = 0.069; OR for high acid exposure vs. low acid exposure = 3.4 [1.2, 9.6]).
When using three days of monitoring to determine trajectory patterns versus four days of monitoring, we found substantial agreement (Kappa = 0.70 with 95% confidence limits: 0.61–0.78; Spearman's r = 0.88).
DISCUSSION
Prolonged wireless pH monitoring is a well-tolerated high-yield diagnostic tool increasingly used to evaluate GERD, and particularly PPI non-response. An important advantage of prolonged monitoring is the ability to nonintrusively assess real-life patterns of esophageal physiology over an extended duration. However, the diagnostic approach to prolonged wireless pH monitoring in light of variable day to day acid exposure time is not clear. In this study of 212 wireless pH monitoring studies off of PPI therapy performed among adult PPI nonresponders, we explored the diagnostic value of grouping longitudinal patterns of acid exposure in comparison to conventional methods. Group-based trajectory modeling identified that three significantly stable and fairly flat trajectories of acid exposure (low, middle, and high) exist. These trajectories were centered around acid exposures of 2%, 7%, and 13%, respectively. Notably, the trajectory analysis was naïve to the typical threshold of 5% for AET. Specifically, trajectory modeling identified that 62% (42/68) of conventionally inconclusive studies (1 or 2 days positive) exhibited a low acid exposure trajectory, and that 67% (34/51) of conventionally abnormal studies (3 or more days positive) exhibited a middle acid exposure trajectory. Group-based trajectory modeling seemed to be a complimentary method to conventional diagnostic practices when studies were inconclusive or abnormal. Furthermore, trajectory groupings had significantly high agreement when modeled over three days or four days of pH data, suggesting that 72 hours may be a sufficient duration for prolonged monitoring.
Reflux monitoring guides important management decisions such as whether to continue PPI therapy, and moreover, whether to proceed with antireflux surgery.19–21 Hence, reliable diagnostic approaches for reflux monitoring are essential. At present, consensus on the optimal diagnostic approach to prolonged monitoring studies is lacking. Various diagnostic approaches have been described, each with its own limitation.11 In a retrospective study of 93 wireless pH studies by Scarpulla et al., the DeMeester score significantly varied across diagnoses based on the first day, overall average or worst day.8 Similarly, Sweis et al. found that among 38 prolonged wireless pH monitoring studies, average total esophageal acid exposure was pathologic in 37% of cases whereas the worst day analysis was pathologic in 47% of cases.10 Indeed, our study identified that the esophageal acid exposure significantly varies by day and highlighted the fact that assessment of number of days positive risks misdiagnosis and mismanagement.
Since prolonged wireless pH monitoring offers the ability to examine trends in esophageal acid burden over time, we sought to test a novel concept. We hypothesized that patients with PPI nonresponse exhibit distinct patterns (i.e. latent class trajectories) of acid exposure, and that grouping patients according to longitudinal trajectory patterns of acid exposure may be an alternative, and perhaps more informative, diagnostic approach. Latent class trajectory analyses have been used to understand longitudinal patterns across the medical field including diabetes, obesity, and cardiovascular diseases.22,23 Our trajectory model identified three groups of acid exposure trajectories, and overall the model significantly agreed with the conventional number of days positive diagnostic approach. As expected, there was 100% agreement for 0 days positive and a low acid exposure trajectory. This highlights the excellent negative predictive value of 0 days positive across a 96-hour prolonged wireless pH study, and in these scenarios an added longitudinal assessment of acid exposure trajectory is not useful. However, the group-based trajectory modeling was particularly valuable and provided unique management considerations for 64% (76/119) of studies that were conventionally grouped as 1, 2, and 3+ days positive. For instance, the clinical relevance of the 1 or 2 day positive study is currently unclear and variably interpreted from a normal study to objective refractory GERD. Results from this study suggest that patients with 1 or 2 day positive studies could be stratified on the basis of their acid exposure trajectory where patients with low acid exposure trajectories likely do not have GERD, and should not be managed with acid suppression (PPI) or antireflux interventions. An example of this from our study includes a patient with PPI nonresponse with the following AETs by day: D1 5.3%, D2 1.2%, D3 5.1%, and D4 2.4% off of PPI. Conventional grouping would classify this patient as having 2 days positive, but not provide additional value as to whether this patient meets criteria for GERD and should be continued on acid suppression versus whether this patient does not have GERD and PPI should be discontinued. In this case, trajectory modeling grouped the patient as following a low acid exposure trajectory. Therefore, based on the complimentary trajectory analysis, it would be reasonable to discontinue PPI and consider alternate etiologies of symptom generation.
Another unresolved issue in wireless pH monitoring is the optimal duration of monitoring. Multiple studies report on the increased diagnostic yield monitoring over 24 hours to 48 hours to 96 hours.10,12,24,25 However, depending on resource availability and volume, implementing a 96-hour protocol may pose substantial clinical challenges. To examine the diagnostic yield between three versus four days of monitoring, we performed a post-hoc analysis of pH data across three and four days. Three distinct acid exposure trajectory groups were identified when studied over three days or four days, and there was significantly high agreement between both models. These results suggest that 72 hours may be a sufficient prolonged monitoring period if 96 hours are not possible.
There are important limitations to this study. Our objective was to explore patterns of acid exposure using a sophisticated statistical model, and thus we did not assess symptom–reflux correlation or patient outcomes. Additionally, we examined group-based trajectory modeling within an internal patient cohort, and we have yet to validate the model in an external or normative group. Furthermore, it is our clinical practice to perform pH-impedance monitoring on PPI therapy for patients with a high pretest likelihood of GERD rather than wireless pH off of PPI, and thus, by the nature of selection bias in this retrospective study many patients that we see with erosive reflux disease and/or large hiatal hernia were not included in this cohort.26 Nonetheless, our a priori sensitivity analyses demonstrated that the elevated acid exposure time was associated with hiatal hernia and regurgitation, as would be expected, supporting generalizability and reproducibility of our cohort. Although group-based trajectory modeling to discern patterns of acid exposure would require additional software analytics that is currently unavailable in real-time, the notion of assessing longitudinal trajectories of acid exposure can be conceptually applied in real-time and may be particularly valuable for inconclusive studies.
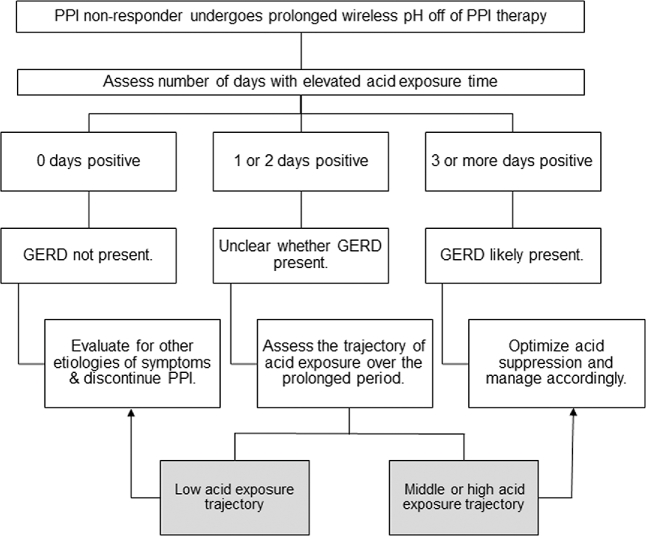
In summary, the group-based trajectory modeling of prolonged wireless pH studies identified three significantly distinct, but stable groups of acid exposure trajectories, despite heavy day-to-day variability in acid exposure. The results support the evaluation of esophageal acid trajectories as a complimentary diagnostic approach in prolonged pH monitoring, particularly for scenarios that are otherwise inconclusive. While esophageal acid exposure trajectory grouping is an exploratory model that requires validation and software integration, at present clinicians can look to patterns of acid exposure when faced with unclear diagnoses or day-to-day variability in acid exposure (Fig. 3).
Fig. 3.
Potential diagnostic value of acid exposure trajectory assessment in the setting of PPI nonresponse and prolonged wireless pH monitoring off PPI.
Conflicts of interest: Rena Yadlapati: Consultant for Ironwood, Medtronic, Diversatek; Sabine Roman: Consultant for Medtronic, research grant from Crospon and Sandhill, speaker for Mayoly Spindler, John E. Pandolfino: Consultant for Crospon, Ironwood, Torax, Astra Zeneca, Takeda, Impleo, Medtronic, Sandhill. Jody D. Ciolino: None to declare. Jenna Craft: None to declare.
Notes
Research Funding Support: Rena Yadlapati and John E. Pandolfino supported by NIH R01 DK092217 (John E. Pandolfino).
Specific author contributions: Study concept and design: Rena Yadlapati, Jody D. Ciolino, Jenna Craft, John E. Pandolfino; Acquisition of data: Rena Yadlapati, Jenna Craft, Sabine Roman, John E. Pandolfino; Analysis and interpretation of data: Rena Yadlapati, Jody D. Ciolino, Sabine Roman, John E. Pandolfino; Drafting of manuscript: Rena Yadlapati, Jody D. Ciolino, Jenna Craft, Sabine Roman, John E. Pandolfino; Critical revision of the manuscript for important intellectual content: Rena Yadlapati, Jody D. Ciolino, Jenna Craft, Sabine Roman, John E. Pandolfino; Study oversight: Rena Yadlapati, John E. Pandolfino.
Writing assistance: None.
References
- 1. Pandolfino J E, Kwiatek M A. Use and utility of the Bravo pH capsule. J Clin Gastroenterol 2008; 42: 571–8. [DOI] [PubMed] [Google Scholar]
- 2. Roman S, Mion F, Zerbib F, Benamouzig R, Letard J C, Bruley des Varannes S. Wireless pH capsule-yield in clinical practice. Endoscopy 2012; 44: 270–6. [DOI] [PubMed] [Google Scholar]
- 3. Capovilla G, Salvador R, Spadotto L et al.. Long-term wireless pH monitoring of the distal esophagus: prolonging the test beyond 48 hours is unnecessary and may be misleading. Dis Esophagus 2017; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Prakash C, Clouse R E. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3: 329–34. [DOI] [PubMed] [Google Scholar]
- 5. Hirano I, Zhang Q, Pandolfino J E, Kahrilas P J. Four-day Bravo pH capsule monitoring with and without proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2005; 3: 1083–8. [DOI] [PubMed] [Google Scholar]
- 6. Lacy B E, Dukowicz A C, Robertson D J, Weiss J E, Teixeira P, Kelley M L Jr. Clinical utility of the wireless pH capsule. J Clin Gastroenterol 2011; 45: 429–35. [DOI] [PubMed] [Google Scholar]
- 7. Grigolon A, Consonni D, Bravi I, Tenca A, Penagini R. Diagnostic yield of 96-h wireless pH monitoring and usefulness in patients' management. Scand J Gastroenterol 2011; 46: 522–30. [DOI] [PubMed] [Google Scholar]
- 8. Scarpulla G, Camilleri S, Galante P, Manganaro M, Fox M. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol 2007; 102: 2642–7. [DOI] [PubMed] [Google Scholar]
- 9. Ahlawat S K, Novak D J, Williams D C, Maher K A, Barton F, Benjamin S B. Day-to-day variability in acid reflux patterns using the BRAVO pH monitoring system. J Clin Gastroenterol 2006; 40: 20–24. [DOI] [PubMed] [Google Scholar]
- 10. Sweis R, Fox M, Anggiansah A, Wong T. Prolonged, wireless pH-studies have a high diagnostic yield in patients with reflux symptoms and negative 24-h catheter-based pH-studies. Neurogastroenterol Motil 2011; 23: 419–26. [DOI] [PubMed] [Google Scholar]
- 11. Grigolon A, Bravi I, Duca P, Pugliese D, Penagini R. Prolonged wireless pH monitoring: importance of how to analyse oesophageal acid exposure. Scand J Gastroenterol 2010; 45: 1133–4. [DOI] [PubMed] [Google Scholar]
- 12. Pandolfino J E, Richter J E, Ours T, Guardino J M, Chapman J, Kahrilas P J. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98: 740–9. [DOI] [PubMed] [Google Scholar]
- 13. Ayazi S, Lipham J C, Portale G et al.. Bravo catheter-free pH monitoring: normal values, concordance, optimal diagnostic thresholds, and accuracy. Clin Gastroenterol Hepatol 2009; 7: 60–67. [DOI] [PubMed] [Google Scholar]
- 14. Wenner J, Johnsson F, Johansson J, Oberg S. Wireless oesophageal pH monitoring: feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol 2005; 40: 768–74. [DOI] [PubMed] [Google Scholar]
- 15. Ang D, Xu Y, Ang T L et al.. Wireless oesophageal pH monitoring: establishing values in a multiracial cohort of asymptomatic Asian subjects. Dig Liver Dis 2013; 45: 371–6. [DOI] [PubMed] [Google Scholar]
- 16. Jones B L, Nagin D S. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007; 35: 542–71. [Google Scholar]
- 17. Jones B L, Nagin D S, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001; 29: 374–93. [Google Scholar]
- 18. Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 19. Katz P O, Gerson L B, Vela M F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108: 308–28; quiz 329. [DOI] [PubMed] [Google Scholar]
- 20. Jobe B A, Richter J E, Hoppo T et al.. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013; 217: 586–97. [DOI] [PubMed] [Google Scholar]
- 21. Kahrilas P J, Shaheen N J, Vaezi M F et al.. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135: 1383–1391.e5, 1391 e1381-1385. [DOI] [PubMed] [Google Scholar]
- 22. Walsh E I, Shaw J, Cherbuin N. Trajectories of BMI change impact glucose and insulin metabolism. Nutr Metab Cardiovasc Dis 2017. [DOI] [PubMed] [Google Scholar]
- 23. Albaum J M, Carsley S, Chen Y et al.. Persistent high non-high-density lipoprotein cholesterol in early childhood: A latent class growth model analysis. J Pediatr 2017; 191: 152–7. [DOI] [PubMed] [Google Scholar]
- 24. Chander B, Hanley-Williams N, Deng Y, Sheth A. 24 versus 48-hour bravo pH monitoring. J Clin Gastroenterol 2012; 46: 197–200. [DOI] [PubMed] [Google Scholar]
- 25. Domingues G R, Moraes-Filho J P, Domingues A G. Impact of prolonged 48-h wireless capsule esophageal pH monitoring on diagnosis of gastroesophageal reflux disease and evaluation of the relationship between symptoms and reflux episodes. Arq Gastroenterol 2011; 48: 24–29. [DOI] [PubMed] [Google Scholar]
- 26. Roman S, Gyawali C P, Savarino E et al.. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29: 1–15. [DOI] [PubMed] [Google Scholar]