Abstract
Aims
Catheter ablation is considered the treatment of choice for many tachyarrhythmias, but convincing ‘real-world’ data on efficacy and safety are lacking. Using Swedish national registry data, the ablation spectrum, procedural characteristics, as well as ablation efficacy and reported adverse events are reported.
Methods and Results
Consecutive patients (≥18 years of age) undergoing catheter ablation in Sweden between 01 January 2006 and 31 December 2015 were included in the study. Follow-up (repeat ablation and vital status) was collected through 31 December 2016. A total of 26 642 patients (57 ± 15 years, 62% men), undergoing a total of 34 428 ablation procedures were included in the study. In total, 4034 accessory pathway/Wolff–Parkinson–White syndrome (12%), 7358 AV-nodal re-entrant tachycardia (21%), 1813 atrial tachycardia (5.2%), 5481 typical atrial flutter (16%), 11 916 atrial fibrillation (AF, 35%), 2415 AV-nodal (7.0%), 581 premature ventricular contraction (PVC, 1.7%), and 964 ventricular tachycardia (VT) ablations (2.8%) were performed. Median follow-up time was 4.7 years (interquartile range 2.7–7.0). The spectrum of treated arrhythmias changed over time, with a gradual increase in AF, VT, and PVC ablation (P < 0.001). Decreasing procedural times and utilization of fluoroscopy with time, were seen for all arrhythmia types. The rates of repeat ablation differed between ablation types, with the highest repeat ablation seen in AF (41% within 3 years). The rate of reported adverse events was low (n = 595, 1.7%). Death in the immediate period following ablation was rare (n = 116, 0.34%).
Conclusion
Catheter ablations have shifted towards more complex procedures over the past decade. Fluoroscopy time has markedly decreased and the efficacy of catheter ablation seems to improve for AF.
Keywords: Catheter ablation, Adverse events, Outcome
Introduction
Transvenous catheter ablation of cardiac arrhythmias was first described in the 1980s.1,2 In 1998, Haïssaguerre et al.3 introduced catheter ablation as a treatment option for atrial fibrillation (AF), which today is the dominating procedure in the majority of institutions. Over the past decades, the procedural techniques have gradually evolved and catheter ablation is currently considered the treatment of choice for many tachyarrhythmias. Technical developments, allowing precise visualization of the catheters and accurate tracking of ablation lesions and catheter movement, have reduced the need for fluoroscopy during the procedures.4–6 A wide range of diagnostic and ablations catheters designed for specific purposes, as well as different modalities of energy source, are available today. The continuous effort to improve the efficacy and safety is key in this development. Efficacy and safety of various catheter ablation treatments have primarily been in the setting of controlled trials or single-centre experiences from high-volume centres,7–10 while there is limited ‘real-world’ data about the safety and outcome of such procedure.11–15
Using national population-based register data with virtually complete coverage, this study aims at describing the evolution of catheter ablation over the past decade with respect to types of arrhythmias treated, procedural characteristics, and the efficacy and safety of ablation in Sweden.
Methods
Study population
Consecutive patients (≥18 years old at the time of the ablation) undergoing catheter ablation at one of the 11 ablation centres in Sweden (seven university institutions, three community hospitals, and one private institution) between 01 January 2006 and 31 December 2015 were included in the study. Follow-up data (repeat ablation and vital status) was collected through 31 December 2016.
Data collection
The Swedish Catheter Ablation Registry collects data on ablations performed in Sweden prospectively since 2004.16 Since 2006, all centres performing catheter ablation of cardiac arrhythmias in Sweden report to the register. Baseline characteristics are reported together with procedural characteristics, as well as data on in-hospital adverse events (see Supplementary material online, Table S1 for definitions). Patient consent was obtained by information and offer of an opt-out alternative. The completeness of key variables [personal identification number, age, gender, date of ablation, type of ablation, procedural time, fluoroscopy time, radiation dose, utilization of radio frequency or cryo energy, acute success (see Supplementary material online, Table S2 for definitions), repeat ablation, and vital status by 31 December 2016] is high. Coverage and register and data completeness are all exceeding 94% throughout the study period (see Supplementary material online, Table S3 for details). Acute success was not reported until 2008 for cavotricuspid isthmus ablation (CTI) and not until 2009 for ablation of AF, ventricular tachycardia (VT), and premature ventricular contraction (PVC) ablation. For ancillary variables, only variables with at least 60% data completeness are reported in this study. Patients having more than one type of ablation performed at a single occasion contributed with data to all ablation types (with the exception of AF ablation with concomitant CTI, in which case only the AF ablation was reported). Likewise, patients undergoing more than one ablation during the course of the study, contributed with data to all relevant ablation types (including multiple entries to the same ablation type in the case of repeat ablations).
Vital status was collected from the Swedish cause of death register, which is a high-quality, virtually complete register of all deaths in Sweden since 1952.17 The study was approved by the ethics committee of Umeå University and complied with the Declaration of Helsinki.
Statistical analysis
Data are presented as mean ± standard deviation (continuous variables) or percentage (categorical variables). When normal distribution could not be assumed, median and interquartile range (IQR) are used for continuous variables. For comparison between different years of ablation, independent sample Kruskal–Wallis test (continuous variables) or Mantel–Haenzsel test for trend (categorical variables) was used. When analysing the risk of subsequent repeat ablation, only patients undergoing a de novo ablation (for that particular arrhythmia) with acutely successful ablation, were included. Cumulative incidence function was used to analyse time to endpoint events.18 All tests were two-sided and a P < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics (IBM SPSS Statistics for Mac, Version 25.0. Armonk, NY, USA) or MATLAB R2016b (MathWorks Inc., Natick, MA, USA) running on Linux.
Results
A total of 26 642 patients (mean age 57 ± 15 years, 62% men), undergoing 34 428 ablation procedures, during which 34 562 different arrhythmic substrates were treated, were included in the study. In total, 4034 accessory pathways/Wolff–Parkinson–White syndrome (AP/WPW) (12%), 7358 AV-nodal re-entrant tachycardias (AVNRT) (21%), 1813 atrial tachycardias (AT) (5.2%), 5481 CTI (16%), 11 916 AF (35%), 2415 AV node ablations (AVN, 7.0%), 581 PVC (1.7%), and 964 VT ablations (2.8%) were performed. Median follow-up time was 4.7 years (IQR 2.7–7.0 years).
The age and gender distribution by type of ablation is presented in Table 1. In summary, a female predominance was seen among patients undergoing AVNRT ablation (60%), AVN ablation (53%), and ablation for PVC (56%), whereas the largest male predominance was seen among patients undergoing AF ablation (73%), CTI ablation (80%), or VT ablation (73%). Patients treated for AP/WPW were the youngest (41 ± 16 years), while those undergoing AVN ablation were substantially older (73 ± 9 years). The rate of reported concomitant heart disease among patients undergoing ablation of supraventricular tachycardia was low (Table 1).
Table 1.
Clinical and procedural characteristics
| AP/WPW (n = 4034) | AVNRT (n = 7358) | AT (n = 1813) | CTI (n = 5481) | AF (n = 11 916) | AVN (n = 2415) | PVC (n = 581) | VT (n = 964) | |
|---|---|---|---|---|---|---|---|---|
| Age | 41 ± 16 | 52 ± 16 | 55 ± 16 | 62 ± 12 | 59 ± 10 | 73 ± 9 | 49 ± 15 | 58 ± 15 |
| Male gender | 62% | 40% | 50% | 80% | 73% | 47% | 44% | 73% |
| Heart disease | ||||||||
| Ischaemic heart disease | 4.0% | 8.1% | 11% | 19% | 8.2% | |||
| Dilated cardiomyopathy | 1.6% | 1.5% | 5.8% | 11% | 3.3% | |||
| Hypertrophic cardiomyopathy | 0.5% | 0.4% | 1.3% | 1.4% | 1.5% | |||
| ARVC | 0% | 0% | 0% | 0.1% | 0% | |||
| Procedure time (min) | 120 (90–160) | 100 (80–129) | 175 (130–220) | 105 (80–140) | 180 (140–220) | 60 (44–75) | 152 (120–185) | 177 (138–225) |
| Fluoroscopy time (min) | 14 (8–24) | 8 (5–14) | 16 (10–7) | 14 (8–4) | 21 (13–34) | 5 (3–10) | 11 (6–20) | 17 (10–29) |
| Radiation dose (cGycm2) | 981 (400–2200) | 465 (200–1000) | 984 (409–2279) | 1045 (471–2200) | 1880 (969–3690) | 308 (121–800) | 568 (200–1480) | 1700 (607–3900) |
| RF-ablation | 94% | 66% | 89% | 76% | 88% | 99% | 97% | 99% |
| RF time (s) | 155 (79–317) | 135 (77–257) | 486 (240–992) | 725 (430–1220) | 2358 (1556–3408) | 150 (80–338) | 444 (251–804) | 804 (422–1655) |
| RF energy (kJ) | 5.2 (2.8–10) | 4.1 (2.4–7.5) | 15 (7.5–30) | 24 (14–40) | 65 (44–93) | 3.6 (2.0–8.3) | 12 (6.5–28) | 41 (17–71) |
| Cryo-ablation | 9% | 36% | 16% | 27% | 13% | 1% | 4% | 1% |
| Cryo time (s) | 480 (252–782) | 720 (480–1130) | 960 (524–1714) | 2295 (1654–3332) | 2148 (1669–3011) | 1814 (788–2077) | 730 (251–1578) | 937 (571–1809) |
| Acute success | 91% | 97% | 80% | 95% | 97% | 97% | 83% | 86% |
Fields with less than 60% completeness of data are not reported.
AF, ablation of atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; AT, ablation of atrial tachycardia; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
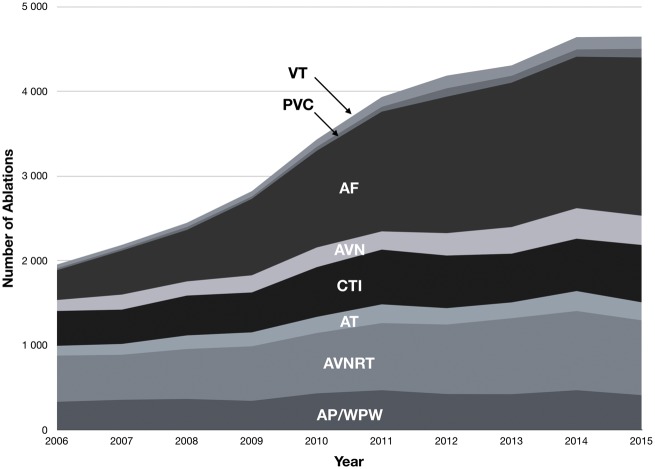
The types of ablations performed in Sweden changed over the studied decade, with the most marked difference seen in the proportion of ablations of AF (Figure 1). In 2006, 352 of the 1953 ablations performed were AF ablations (18%), while the corresponding number was 1869 of 4648 (40%) in 2015, translating to a 430% increase in the number of ablations of AF performed in Sweden during the study period. Similar increases, albeit with substantially lower absolute numbers, were seen for VT and PVC ablation (240% and 350% increase, respectively).
Figure 1.
Types of catheter ablations in Sweden between 2006 and 2015. P < 0.0001 (Pearson χ2). AF, ablation of atrial fibrillation; AT, ablation of atrial tachycardia; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
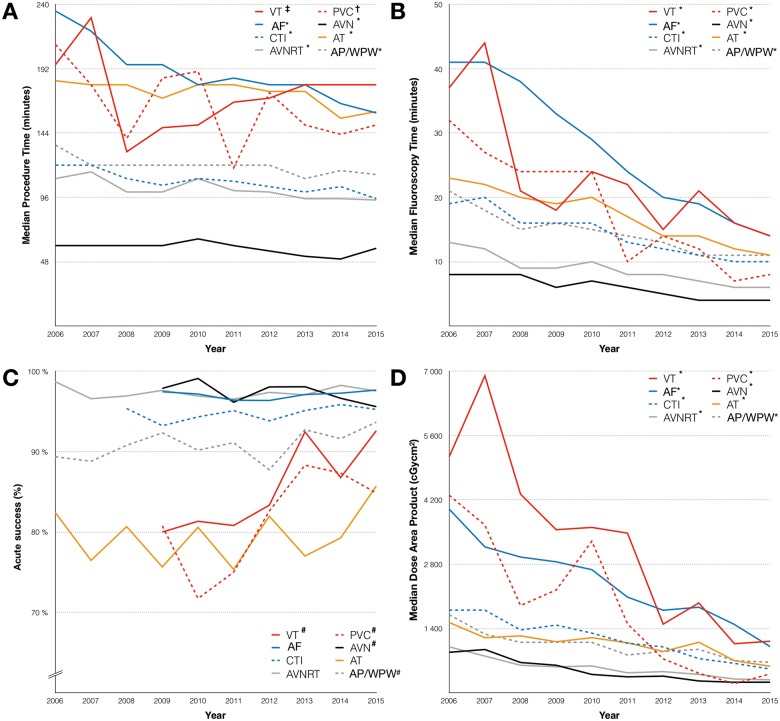
The procedural characteristics by ablation type are shown in Table 1. The acute success rate, as ascertained by the operator, was very high for all procedures with the exception of AT (80%), PVC (83%), and VT (86%). The vast majority of the ablations were performed using radiofrequency energy. The largest share of procedures performed using cryo energy were seen in AVNRT (36%) and CTI ablation (36%). Only 13% of the ablations of AF performed in Sweden between 2006 and 2015 were done using cryo-balloon. Considerable reduction in procedural time, fluoroscopy time, and radiation dose were seen over time across all ablation types, with the most pronounced differences in VT, PVC, and AF (Figure 2). Changes were observed in the acute success rate for AP/WPW, VT, and PVC ablation, with a higher success rate in more recently performed ablations (P < 0.05 for all trends). In contrast, a subtle decrease in success rate was seen for AVN ablation (P < 0.05, Figure 2).
Figure 2.
(A) Median procedure time, by ablation type and treatment year. (B) Median fluoroscopy time, by ablation type and treatment year. (C) Acute success, by ablation type and treatment year. (D) Median radiation dose, by ablation type and treatment year. *P < 0.001 (independent sample Kruskal–Wallis test); †P = 0.005 (independent sample Kruskal–Wallis test); ‡P = 0.001 (independent sample Kruskal–Wallis test); #P = 0.023 (Mantel–Haenzsel test for trend). AF, ablation of atrial fibrillation; AT, ablation of atrial tachycardia; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
The likelihood of a repeat ablation for the same arrhythmia as the index procedure, varied markedly depending on ablation type (Table 2). The lowest rates of repeat ablation were seen in patients undergoing AVN ablation (2.4% and 2.6% at 1 and 3 years, respectively). In contrast, the corresponding numbers for AF ablation were 20% and 41% (at 1 and 3 years). Moreover, the timing of repeat ablation differed depending on ablation type, while some types had most repeat ablations done within the first year (e.g. AVN ablation and AP/WPW), other had repeat ablations performed later to a larger extent (e.g. AF and AT) (Figure 3).
Table 2.
Long-term outcome (repeat ablation within 1 and 3 years)
| AVRT |
AVNRT |
AT |
CTI |
AF |
AVN |
PVC |
VT |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | Count | Percentage | 95% CI | |
| Redo procedure within 1 year | 192/3036 | 6.3 | 5.5–7.2 | 180/6384 | 2.8 | (2.4–3.2) | 121/1050 | 12 | 9.6–13 | 214/3651 | 5.9 | 5.1–6.6 | 1263/6460 | 20 | 19–21 | 42/1764 | 2.4 | 1.7–3.1 | 41/321 | 13 | 9.1–16 | 81/446 | 18 | 15–22 |
| Redo procedure within 3 years | 183/2372 | 7.7 | 6.6–8.8 | 225/4802 | 4.7 | (4.1–5.3) | 143/777 | 18 | 16–21 | 233/2591 | 9.0 | 7.9–10 | 1670/4113 | 41 | 39–42 | 29/1116 | 2.6 | 1.6–3.5 | 38/186 | 20 | 15–26 | 59/270 | 22 | 17–27 |
The risk of repeat ablation for the same arrhythmia as the original procedure. Only de novo procedures, deemed acutely successful are included. The numbers for redo procedures within 3 years, include only patients treated 2006 through 2013.
AF, ablation of atrial fibrillation; AT, ablation of atrial tachycardia; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CI, confidence interval; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
Figure 3.
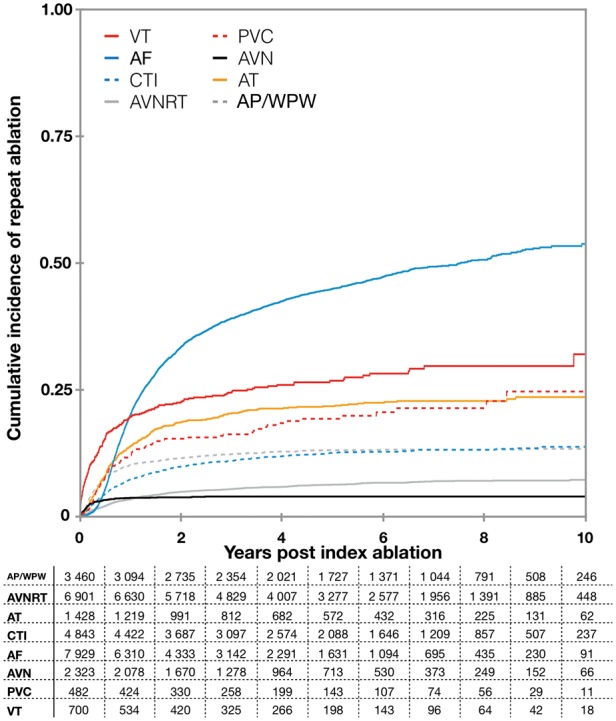
Cumulative incidence function plot illustrating the probability of repeat ablation (same arrhythmia) after a de novo ablation. Numbers at risk are illustrated in the accompanying table. AF, ablation of atrial fibrillation; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
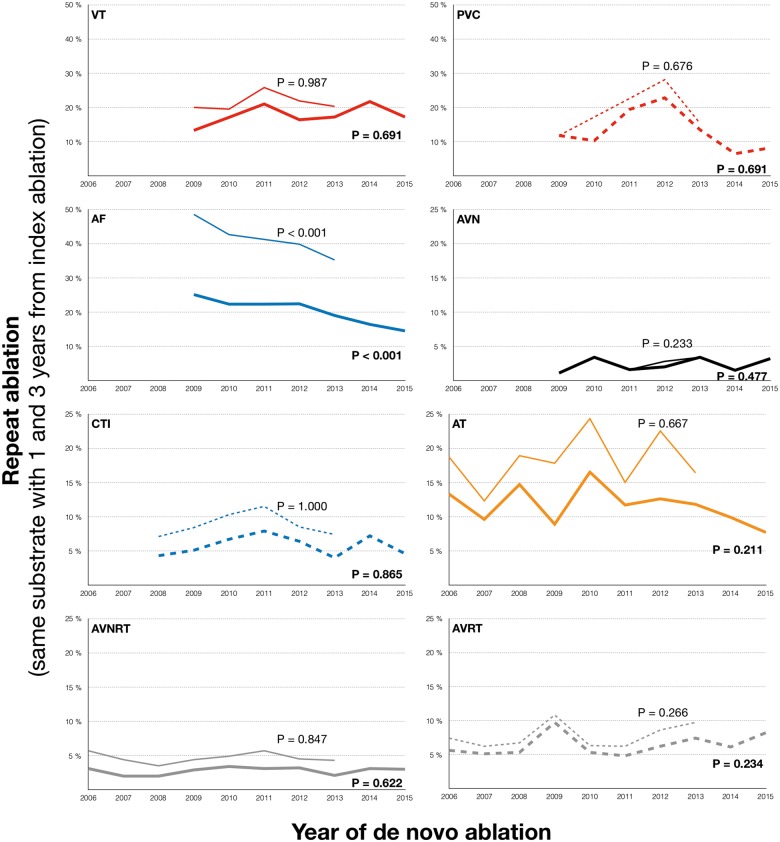
Over time, the risk of a repeat ablation for the same arrhythmia following a de novo procedure changed depending on arrhythmia type (Figure 4). For AF, a gradual decrease in the rates of the repeat ablations were seen over time (e.g. 25% repeat ablation within 1 year in 2009, compared with 15% in 2016, P < 0.0001, Mantel–Haenzsel test for trend). The repeat ablation rates for the remaining types of ablation were stable over time, without significant changes.
Figure 4.
Rate of repeat ablation (for the same substrate) in de novo ablations with acutely successful result (as judged by the operator by the end of the procedure), by arrhythmia substrate and year of index procedure. The thicker line represents the rate of repeat ablation within 1 year of the index procedure, whereas the thinner lines represent the rate of repeat ablation within 3 years of the index procedure. Analyses for trend was performed using Mantel–Haenzsel test for trend. AF, ablation of atrial fibrillation; AT, ablation of atrial tachycardia; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
The reported adverse events are summarized in Table 3. Overall, the event rate was low {595 events reported in 34 562 procedures [1.7%; 95% confidence interval (CI) 1.6–1.9%]}. The highest rates of adverse events were seen for AF ablation [329 events in 11 916 procedures (2.8%; 95% CI 2.5–3.1%)], for PVC [20 events in 581 procedures (3.4%; 95% CI 2.0–4.9%)], and for VT ablation [43 events in 964 procedures (4.5%; 95% CI 3.2–5.8%)]. The most frequently reported type of adverse event was pericardial effusion and/or tamponade [228 events in 34 562 procedures (0.66%)]. Within the first 3 months following ablation, a total of 116 patients (0.34%) died, 51 of whom died within the first month post-ablation. More than half of these patients (n = 72, 62%) had undergone VT (n = 24) or AV nodal ablation (n = 48). Eight patients who had undergone an ablation to treat AF died within 3 months of the ablation, five of these within a month of the procedure. The causes of death are listed in Table 4. Overall, the most common causes of death were chronic ischaemic heart disease (n = 31, 27%), malignant neoplasms (n = 14, 12%), and heart failure (n = 11, 9.5%).
Table 3.
Safety (adverse events per treatment group)
| AVRT (n = 4034) | AVNRT (n = 7358) | AT (n = 1813) | CTI (n = 5481) | AF (n = 11 916) | AVN (n = 2415) | PVC (n = 581) | VT (n = 964) | Total (n = 34 562) | |
|---|---|---|---|---|---|---|---|---|---|
| Cerebral haemorrhage | 5 (0.12%) | 2 (0.03%) | 3 (0.17%) | 8 (0.15%) | 30 (0.25%) | 4 (0.17%) | 4 (0.69%) | 9 (0.93%) | 65 (0.19%) |
| Cerebrovascular accident including TIA | 0 | 3 (0.04%) | 4 (0.22%) | 4 (0.07%) | 38 (0.32%) | 0 | 1 (0.17%) | 3 (0.31%) | 53 (0.15%) |
| Pericardial effusion | 17a (0.42%) | 12 (0.16%) | 4 (0.22%) | 8 (0.15%) | 158 (1.3%) | 7 (0.29%) | 6 (1.0%) | 16 (1.7%) | 228 (0.66%) |
| Major bleeding or haematoma | 10 (0.25%) | 3 (0.04%) | 4 (0.22%) | 12 (0.22%) | 50 (0.42%) | 3 (0.12%) | 3 (0.52%) | 4 (0.41%) | 89 (0.26%) |
| AV-block requiring treatment | 3 (0.07%) | 5 (0.07%) | 0 | 1 (0.02%) | 5 (0.04%) | 0 | 0 | 1 (0.10%) | 15 (0.04%) |
| Pulmonary emboli | 1 (0.02%) | 5 (0.07%) | 0 | 1 (0.02%) | 0 | 0 | 0 | 0 | 7 (0.02%) |
| Deep vein thrombosis | 0 | 1 (0.01%) | 0 | 0 | 1 (0.01%) | 1 (0.04%) | 0 | 0 | 3 (0.01%) |
| Peripheral emboli | 4 (0.10%) | 3 (0.04%) | 0 | 1 (0.02%) | 1 (0.01%) | 0 | 1 (0.17%) | 0 | 10 (0.03%) |
| Pulmonary vein stenosis | 0 | 0 | 0 | 0 | 9 (0.08%) | 0 | 0 | 0 | 9 (0.03%) |
| Persistent phrenic nerve injury | 0 | 0 | 0 | 0 | 2 (0.02%) | 0 | 0 | 0 | 2 (0.01%) |
| Unspecified adverse event | 19 (0.47%) | 21 (0.29%) | 7 (0.39%) | 10 (0.18%) | 35 (0.29%) | 6 (0.25%) | 5 (0.86%) | 10 (1.0%) | 113 (0.33%) |
| Any reported adverse event | 59 (1.5%) | 55 (0.75%) | 22 (1.2%) | 45 (0.82%) | 329 (2.8%) | 21 (0.87%) | 20 (3.4%) | 43 (4.5%) | 595 (1.7%) |
| 95% confidence interval | (1.0–1.8%) | (0.55–0.94%) | (0.71–1.7%) | (0.58–1.1%) | (2.5–3.1%) | (0.50–1.2%) | (2.0–4.9%) | (3.2–5.8%) | (1.6–1.9%) |
| Death within 30 days of ablation | 3 (0.07%) | 2 (0.03%) | 2 (0.11%) | 8 (0.15%) | 5 (0.04%) | 16 (0.66%) | 0 | 15 (1.6%) | 51 (0.15%) |
| Death 31–90 days after ablation | 0 | 7 (0.10%) | 2 (0.11%) | 11 (0.20%) | 3 (0.03%) | 32 (1.33%) | 1 (0.17%) | 9 (0.93%) | 65 (0.19%) |
AT, ablation of atrial tachycardia; AF, ablation of atrial fibrillation; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, Cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
Information on vital status was collected from the Swedish Cause of Death Register.
Table 4.
Causes of death within three months of ablation
| Cause of death | ICD-10 | AP/WPW (n = 4034) | AVNRT (n = 7358) | AT (n = 1813) | CTI (n = 5481) | AF (n = 11 916) | AVN (n = 2415) | PVC (n = 581) | VT (n = 964) | Total (n = 34 562) |
|---|---|---|---|---|---|---|---|---|---|---|
| Malignant neoplasms | C00–C97 | 1 | 2 | 5 | 4 | 1 | 1 | 14 (12%) | ||
| Diabetes mellitus | E10–E14 | 2 | 2 | 4 (3.4%) | ||||||
| Major cardiovascular diseases | ||||||||||
| Diseases of the heart | ||||||||||
| Acute myocardial infarction | I21–I22 | 2 | 1 | 2 | 2 | 3 | 10 (8.6%) | |||
| All other forms of chronic ischaemic heart disease | I20, I25 | 3 | 1 | 14 | 13 | 31 (27%) | ||||
| Pulmonary heart disease/pulmonary circulation | I26–I28 | 1 | 1 | 1 | 3 (2.6%) | |||||
| Non-rheumatic aortic valve disorders | I34 | 2 | 2 (1.7%) | |||||||
| Cardiomyopathy | I42 | 1 | 1 | 4 | 2 | 8 (6.9%) | ||||
| Conduction disorders and cardiac dysrhythmias | I44–I49 | 1 | 1 | 1 | 3 (2.6%) | |||||
| Heart failure | I50 | 4 | 6 | 1 | 11 (9.5%) | |||||
| Cerebrovascular diseases | ||||||||||
| Intracerebral and other intracranial haemorrhage | I61–I62 | 2 | 1 | 3 (2.6%) | ||||||
| Cerebral infarction | I63 | 1 | 1 | 2 | 1 | 5 (4.3%) | ||||
| Diseases of arteries, arterioles and capillaries | I70–I78 | 1 | 1 | 2 (1.7%) | ||||||
| Influenza and pneumonia | J10–J18 | 1 | 1 | 1 | 3 (2.6%) | |||||
| Chronic lower respiratory diseases | J40–J47 | 2 | 2 (1.7%) | |||||||
| Diseases of the digestive system | ||||||||||
| Vascular disorders of intestine | K55 | 1 | 1 | 2 (1.7%) | ||||||
| Nephritis, nephrotic syndrome and nephrosis | N19 | 1 | 1 (0.9%) | |||||||
| Motor vehicle accidents | V28 | 1 | 1 (0.9%) | |||||||
| Intentional self-harm | X60–X84 | 1 | 1 | 1 | 3 (2.6%) | |||||
| Other diseases | 1 | 5 | 2 | 8 (6.9%) | ||||||
| Number of deaths within 3 months of ablation | 3 | 9 | 4 | 19 | 8 | 48 | 1 | 24 | 116 (100%) |
AT, ablation of atrial tachycardia; AF, ablation of atrial fibrillation; AVN, AV-nodal ablation; AVNRT, AV-nodal re-entry tachycardia ablation; AP/WPW, accessory pathway/Wolff–Parkinson–White syndrome; CTI, cavotricuspid isthmus ablation; PVC, ablation of premature ventricular contraction; VT, ablation of ventricular tachycardia.
Discussion
Using a complete, population-based registry in the setting of a universal, single-payer healthcare system, this study demonstrates a doubling of the number of ablations over a 10-year period. Ablation of AF is the main driver behind this increase and accounts for 40% of all ablations performed in Sweden 2015. The rates of repeat ablation of AF is lower in recent years, in spite of the overall increase in ablation volumes. The use of fluoroscopy is steadily declining, with the largest decline seen for the more complex procedures. Overall, catheter ablation seems to be a safe procedure, with low reported adverse events rates, including mortality rates.
All catheter ablations performed in Sweden are prospectively reported to the Swedish Catheter Ablation Registry since 2006. In addition, the completeness of data has consistently been very high, when compared with the National Patient Register,19 with coverage percentage in the high 90s. Thus, the presented estimates of the number of ablations and repeat ablations are likely to be highly accurate. The mean ages and gender distributions observed in this study are largely as expected and are well in keeping with previously published reports using register data.11,15 Moreover, the presence of concomitant heart disease among patients undergoing ablation of supraventricular tachycardia is low, again in keeping with previously published data.11 Taken together, this implies that although the background information is limited, the treated population is likely to be representative of a typical ablation population in the setting of a universal, single-payer healthcare system.
Over the course of the study, the number of ablations for all ablation types increased. However, while the rate of increase between 2006 and 2015 was modest for some ablations, such as AP/WPW, AVNRT, and CTI (about a 30% increase for each of them), the increase was pronounced for ablation of AF (more than four times as many), VT and PVC (at least 2.5 times as many). Given the high prevalence of AF, AF ablation is the main driver between the increase in total ablation volumes. This trend is well in keeping with the strong recommendation for catheter ablation in patients with symptomatic AF on antiarrhythmic drug therapy in recent European Society of Cardiology (ESC) guidelines for the management of AF.20 Granted that the absolute numbers of VT and PVC ablations are still low, the impact on the annual ablation volumes is modest. However, in selected patient populations, VT ablation has a Class I recommendation in the current ESC Guidelines for the management of patients with ventricular arrhythmias,21 and the number of VT ablations is therefore likely to keep increasing in years ahead. Similar trends can be seen in other European countries, when looking at annually reported ablation volumes.22 However, the accuracy of those numbers is likely to vary substantially between countries, due to different abilities to accurately track the actual ablation numbers.
At some point after 2012 the relative proportion of AVN ablations seem to increase. Given the sparsity of data, the exact mechanism for this increase cannot be determined in this study, but one may speculate that it is an increase in the number of AVN ablations for rate control in cardiac resynchronization therapy driving this change.
In spite of a shift towards more complex procedures, a dramatic decrease in fluoroscopy time, as well as in radiation dose, is seen in this study. For AF ablation, the median fluoroscopy time and radiation dose was only a third in 2015 compared with that a decade earlier. Previously, smaller series have reported similar temporal trends.23 Importantly, although fluoroscopy has been widely used since the beginning of interventional electrophysiology, the procedure is de facto not dependent on fluoroscopic imaging. Consequently, non-fluoroscopic three-dimensional systems can be used to navigate electrophysiology catheters with no or minimal use of fluoroscopy. The introduction and refinement of three-dimensional mapping systems is likely to be a key component in the observed reduction in fluoroscopy time and radiation dose.4–6,24 In addition, the decreasing radiation doses may be attributed to more experienced operators, leading to the use of lower frame rates, pulsed fluoroscopy, the avoidance of magnification, and optimal adjustments of the fluoroscopy exposure rates.
Data on definite arrhythmia recurrence in this study is lacking, reliable data on repeat procedures are available. With some obvious limitations (e.g. patients may well have arrhythmia relapse without undergoing a repeat ablation), analyses of repeat ablation offer a reasonable surrogate for arrhythmia relapse. Furthermore, it is important to keep in mind that acute success is not a reliable marker of long-term success. An apparent discordance between acute and long-term success is illustrated in AF ablation, where the acute success rate was 97%, and the repeat ablation rate within 3 years exceeded 40%. Keeping all of the above in mind, a notable progressive decrease in the rates of repeat AF ablation was observed. This is well in keeping with the recent findings in the Danish ablation registry.15 As expected, the cumulative rate of AF relapse was higher in the study by Pallisgaard et al.15 (where relapse was studied rather than repeat ablation), but the decreasing trends are similar. Given the limited data on background information in this study, the exact underlying mechanism for this observation cannot be determined. However, the increased operator experience and the availability of improved ablation catheters and techniques are possibly important factors. This needs to be confirmed in subsequent studies.
Not surprisingly, the patterns of if and when the risk of a repeat ablation plateaus differed substantially between the different types of ablation. A high risk of early repeat ablation, sustained over time at a lower level, was seen for VT, PVC, and AT, whereas following a risk of early repeat ablation, the risk largely plateaued for AVN ablation, AVNRT, AP/WPW, and CTI. The highest risk of repeat ablation, by far, was seen for AF ablation. Although the likelihood of a repeat ablation decreased over time, it remains substantial in the longer term. This is in keeping with results reported from high-volume, single-centres.8,10
In this study, we demonstrate a very low rate of adverse events, with a reported adverse event rate of less than one in 50 cases. As expected, the risk of an adverse event was highly dependent on the type of ablation being performed, with a higher risk seen in ablation of VT, PVC, and AF. However, the reported risk of an adverse event in an AF ablation was 2.8%, which is substantially lower than that reported in the most recent worldwide survey on catheter ablation for AF.12 The rate of death following AF ablation in this study is considerably lower than in the worldwide survey. Of note, the most recent worldwide survey only reports on procedures performed between 2003 and 2006, and a recently published study indicate that the rate of adverse events is declining in more contemporary settings.23
Limitations of the study
Detailed background information apart from age and gender is largely lacking. This makes it more challenging to interpret some the reported data, in particular regarding VT ablation, where the patient population is likely to be heterogeneous. In this study uniform data on follow-up is lacking and repeat ablation was used as a surrogate for relapse of arrhythmia. This measure has inherent limitations and true effectiveness of the ablation procedures cannot be reliably determined without structured rhythm follow-up. This is particularly true for arrhythmias in which a relapse is not necessarily equivalent to a repeat ablation (e.g. AF and VT). Thus, repeat ablation should be viewed as the lower limit of the true relapse rate. Lastly, under-reporting of adverse events in this study is highly likely and the clinical routines for capturing adverse events differ between centres and a routine for auditing centres was lacking. Thus, the reported events rates should be considered as the lower limits of the true adverse event rate, which is bound to be higher than the reported. By collecting information on vital status from the Swedish cause of death register, this limitation was circumvented for mortality. Information on cause of death, beyond the cause stated in the Swedish cause of death register, was not available for the current analyses.
Conclusions
In a nation-wide, population-based study, in the setting of a universal, single-payer healthcare system, we demonstrate that the number of ablations more than doubled during the last decade. Ablation of AF is the main driver behind this increase and AF ablation accounted for 40% of all ablations in Sweden 2015. The rate of repeat AF ablation is lower in recent years, in spite of the overall increase in ablation volumes. The utilization of fluoroscopy is on a dramatically declining, with a more than 50% reduction overall. Furthermore, in this study catheter ablation procedures are associated with low rates of reported adverse events and death.
Funding
The Swedish Catheter Ablation Registry is funded by annual grants from the Swedish Association of Local Authorities and Regions. F.H. was funded by the Crafoord Foundation, Eva and Carl-Eric Larsson Foundation, Bundy Academy and Skåne University Hospital Research Foundation. J.C. was supported by grants from the Swedish Heart-Lung Foundation.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Gallagher JJ, Svenson RH, Kasell JH, German LD, Bardy GH, Broughton A, Critelli G.. Catheter technique for closed-chest ablation of the atrioventricular conduction system. N Engl J Med 1982;306:194–200. [DOI] [PubMed] [Google Scholar]
- 2. Borggrefe M, Budde T, Podczeck A, Breithardt G.. High frequency alternating current ablation of an accessory pathway in humans. J Am Coll Cardiol 1987;10:576–582. [DOI] [PubMed] [Google Scholar]
- 3. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J.. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 4. Stevenson WG, Delacretaz E, Friedman PL, Ellison KE.. Identification and ablation of macroreentrant ventricular tachycardia with the CARTO electroanatomical mapping system. Pacing Clin Electrophysiol 1998;21:1448–1456. [DOI] [PubMed] [Google Scholar]
- 5. Novak PG, Macle L, Thibault B, Guerra PG.. Enhanced left atrial mapping using digitally synchronized NavX three-dimensional nonfluoroscopic mapping and high-resolution computed tomographic imaging for catheter ablation of atrial fibrillation. Heart Rhythm 2004;1:521–522. [DOI] [PubMed] [Google Scholar]
- 6. Anter E, Tschabrunn CM, Contreras-Valdes FM, Li J, Josephson ME.. Pulmonary vein isolation using the Rhythmia mapping system: verification of intracardiac signals using the Orion mini-basket catheter. Heart Rhythm 2015;12:1927–1934. [DOI] [PubMed] [Google Scholar]
- 7. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C; FIRE and ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–2245. [DOI] [PubMed] [Google Scholar]
- 8. Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Kokturk B, Konstantinidou M, Metzner A, Fuernkranz A, Kuck KH.. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368–2377. [DOI] [PubMed] [Google Scholar]
- 9. Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S.. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation 2000;102:2619–2628. [DOI] [PubMed] [Google Scholar]
- 10. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O’Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haissaguerre M, Jais P.. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 11. Brachmann J, Lewalter T, Kuck KH, Andresen D, Willems S, Spitzer SG, Straube F, Schumacher B, Eckardt L, Danilovic D, Thomas D, Hochadel M, Senges J.. Long-term symptom improvement and patient satisfaction following catheter ablation of supraventricular tachycardia: insights from the German ablation registry. Eur Heart J 2017;38:1317–1326. [DOI] [PubMed] [Google Scholar]
- 12. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E.. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 13. Inama G, Pedrinazzi C, Adragao P, Alvarez M, Arribas F, Bonhorst D, Elvas L, Landolina M, Merino JL, Rodriguez E, De Sousa J, Gulizia M.. Five years of catheter ablation procedures in South-Western Europe: meta-analysis of National Registries. Pacing Clin Electrophysiol 2009;32:506–515. [DOI] [PubMed] [Google Scholar]
- 14. Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai K, Miyauchi Y, Yamane T, Morita N, Okumura K; Japanese Heart Rhythm Society Members. Clinical and procedural predictors of early complications of ablation for atrial fibrillation: analysis of the national registry data. Heart Rhythm 2014;11:2247–2253. [DOI] [PubMed] [Google Scholar]
- 15. Pallisgaard JL, Gislason GH, Hansen J, Johannessen A, Torp-Pedersen C, Rasmussen PV, Hansen ML.. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J 2018;39:442–449. [DOI] [PubMed] [Google Scholar]
- 16. Kesek M. Ablation procedures in Sweden during 2007: results from the Swedish Catheter Ablation Registry. Europace 2009;11:152–154. [DOI] [PubMed] [Google Scholar]
- 17. Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R.. The Swedish cause of death register. Eur J Epidemiol 2017;32:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putter H, Fiocco M, Geskus RB.. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–2430. [DOI] [PubMed] [Google Scholar]
- 19.The National Patient Register. https://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish (26 October 2018).
- 20. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K; Group ESCSD. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 21. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; Group ESCSD. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 22. Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H, Camm J.. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 report from the European Heart Rhythm Association. Europace 2017;19:ii1–ii90. [DOI] [PubMed] [Google Scholar]
- 23. Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S, Zei PC, Epstein LM, Tedrow UB, Michaud GF, Stevenson WG, Koplan BA.. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:854–860. [DOI] [PubMed] [Google Scholar]
- 24. De Ponti R. Reduction of radiation exposure in catheter ablation of atrial fibrillation: lesson learned. World J Cardiol 2015;7:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.