Summary
The 4-quadrant forceps biopsy (FB) protocol for identifying Barrett's esophagus (BE) and esophageal dysplasia (ED) suffers from poor sensitivity due to significant sampling error. We investigated the benefit of wide-area transepithelial sampling with 3-dimensional computer-assisted analysis (WATS) used adjunctively to the combination of random and targeted FB in the detection of ED, and as a secondary outcome, BE. In this multicenter prospective trial, community endoscopists at 21 sites utilized WATS as an adjunct to both targeted and random FB in patients undergoing BE screening and surveillance. Investigators alternated taking FB and WATS samples first. WATS specimens were analyzed at CDx Diagnostics (Suffern, NY) while FB samples were analyzed by each site's regular pathologists. Data were de-identified and then aggregated for analysis. Of 12,899 patients enrolled, FB identified 88 cases of ED, and WATS detected an additional 213 cases missed by FB. These 213 cases represented an absolute increase of 1.65%, raising the yield from 0.68% to 2.33%. Adding WATS to FB increased the overall detection of ED by 242% (95% CI: 191%–315%). Fewer than 61 patients needed to be tested with WATS to identify an additional case of ED. The combination of random and targeted FB identified 1,684 cases of BE, and WATS detected an additional 2,570 BE cases. The absolute incremental yield of adding WATS to FB is 19.9%, increasing the rate of detection from 13.1% to 33%. Adding WATS to FB increased the overall detection of BE by 153% (95% CI: 144–162%). The number needed to test with WATS in order to detect an additional case of BE was 5. Whether FB or WATS was done first did not impact the results. In this study, comprised of the largest series of patients evaluated with WATS, adjunctive use of the technique with targeted and random FB markedly improved the detection of both ED and BE. These results underscore the shortcomings of FB in detecting BE-associated neoplasia, which can potentially impact the management and clinical outcomes of these patients.
Keywords: Barrett's esophagus, dysplasia, screening, surveillance
INTRODUCTION
Barrett's esophagus (BE) is a known premalignant condition characterized by the replacement of normal stratified squamous epithelium with columnar epithelium.1 It is associated with esophageal adenocarcinoma (EAC), which itself has seen a six-fold increase in incidence in the last three decades2 with a poor 5-year survival of only 15–20%.3 While the risk for progression to EAC with nondysplastic BE remains low, dysplastic changes have been found to increase the risk for progression to EAC.4
Therefore screening and surveillance protocols have been designed to detect BE and BE-associated dysplasia at its earliest stage.
Screening and surveillance guidelines for BE and esophageal dysplasia (ED) require targeted biopsy of any visible mucosal abnormality found on high resolution endoscopy followed by random 4-quadrant forceps biopsies (FB) obtained at 1–2 cm intervals (Seattle protocol). This recommended protocol is time-consuming and labor intensive, as evidenced by the fact that fewer than half of community-based gastroenterologists adhere to surveillance biopsy guidelines.5 Moreover, it is subject to considerable sampling error. Since intestinal metaplasia and dysplasia are often focally distributed within the columnar-lined mucosa, random 4-quadrant biopsies can easily miss these abnormalities resulting in low sensitivity for the detection of these lesions.6 This limitation cannot be overcome, even with extensive sampling.7 Wide-area transepithelial sampling with computer-aided three dimensional tissue analysis (WATS) is an adjunct to targeted and random 4-quadrant FB of the esophagus (CDx Diagnostics, Suffern, NY). It consists of an abrasive brush that is used to sample a large circumferential surface area of the esophagus obtaining a full-thickness tissue sample of the epithelium, including the lamina propria. Unlike standard exfoliative cytology, the WATS brush obtains microbiopsies or entire tissue fragments in addition to individual cells.
When deposited on the slide, the WATS sample consists of a disaggregated tissue specimen, up to 150 μm in thickness. These thick specimens cannot be effectively visualized by a standard manual microscope with a 3–4 μm depth of field.
Analysis of these specimens is therefore aided by a specialized computer imaging system using neural networks specifically optimized for evaluation of esophageal mucosa. The WATS computer captures up to fifty 3 μm ‘optical slices’ and integrates them together to creates a synthesized three dimensional image of the gland that is displayed to the pathologist including the uncut, in vivo appearance of the glandular surface not typically visible on histologic specimens. The computer-assisted microscope scans this synthesized three dimensional image and identifies and locates goblet cells and dysplasia within it for display to the pathologist. In addition, the exact coordinates of all computer-selected cells on the microscopic slide are shown on the monitor so that the pathologist can locate and confirm any abnormality on the slide. Images identified by the computer are reviewed by pathologists in conjunction with manual microscopy and are reported utilizing standard morphologic criteria for the diagnosis of both BE and ED.
Prior studies with WATS, which used a smaller sampling brush and a much more limited 30 μm three dimensional computer analysis system, have shown a significantly increased rate of BE and ED detection when used adjunctively to the combination of both targeted and random 4-quadrant FB.8,9 A recent multicenter study using a larger sampling brush and a 150 μm three dimensional computer analysis system demonstrated that in a BE surveillance population enriched with high risk patients with a history of ED, WATS was four times more effective at detecting high-grade dysplasia and EAC than the Seattle FB protocol.10 Another published study examining the interobserver agreement among pathologists using WATS found substantial agreement for low-grade dysplasia, high-grade dysplasia, and no dysplasia with an overall kappa value 0.86 (95% CI 0.75–0.97), a significant improvement over current histopathology assessment.11
Due to the inherent risks of missing BE and ED with the random 4-quadrant FB protocol, WATS represents a potentially valuable tool to improve disease detection, patient management, and outcomes. In this largest series of patients to date on this topic, we aim to evaluate the benefits of WATS in the detection of ED as a primary outcome and BE as a secondary goal, using the now standard, enhanced three dimensional computer analysis system and the larger sampling brush, as an adjunct to the combination of targeted and random FB in patients undergoing routine screening and surveillance in community practice settings.
METHODS
Men and women ages 18 years and older undergoing screening for suspected BE as well as those with known BE undergoing surveillance for dysplasia were enrolled by 58 community endoscopists at 21 sites. All cases were performed between June 2013 and July 2015. Patients with a suspicious lesion concerning for invasive cancer on endoscopy and requiring endoscopic resection were excluded from the study. Investigators were instructed to use both WATS and FB to sample suspected BE only in patients displaying salmon-colored mucosa in the tubular esophagus. The study was approved by an Institutional Review Board and informed consent was obtained from all patients.
WATS samples were obtained using a standardized 2-brush technique. In each kit, the following were included: two brush-biopsy catheters, two bar-coded glass slides, an alcohol/carbowax fixative pouch for sample preservation, 5 mL of alcohol, and a preaddressed packet for submitting the contents. One kit (i.e. 2 brushes) was used for every 5 cm of BE length to collect specimens. Proper WATS technique was reviewed with all participants before the study in person or by videoconference and written instructions were also provided. Investigators were instructed to alternate taking FB and WATS samples first to prevent potential sampling order bias.
The WATS brush was passed through a standard endoscope biopsy channel and brush biopsy was performed repeatedly in a craniocaudal direction against the surface of the mucosa, moving in a circumferential pattern to sample the entire BE segment. Minimal mucosal bleeding at the brush biopsy site indicated evidence of proper technique. The tissue specimen collected by the brush was placed on a glass slide and fixative was immediately applied. The bristle head of the brush was cutoff and placed into a container with 5 mL of alcohol. Using a second brush, an additional tissue sample was obtained and the bristle head of this brush was also placed into the same vial containing the bristle portion of the first brush.
FB was obtained with 4-quadrant biopsies every 1–2 cm per the discretion of each investigator. In addition to random 4-quadrant sampling, FB was utilized to sample any endoscopically visible mucosal abnormality (raised lesions, ulcerations etc), while investigators were instructed to use WATS specifically to test additional areas of the BE segment that would not necessarily have been sampled by FB. As this was a clinical registry study incorporating WATS in a community-based setting replicating its use in clinical practice, the requirements to sample salmon-colored mucosa in the tubular esophagus and to adhere to the Seattle biopsy protocol were unmonitored.
Three dimensional computer-synthesized images and associated slides from the WATS samples were analyzed at CDx Diagnostics by pathologists who were aided by the computer image analysis system previously described. To control for potential interobserver variability, two independent pathologists who did not participate in the study and who were blinded to the clinical and histologic data confirmed all cases reported as ED. FB specimens were analyzed by each investigator's pathologist using standard techniques. All pathologists were blinded to the results of the other technique.
Demographic, endoscopic, and pathology data were aggregated and de-identified prior to analysis.
The increase in yield using WATS as an adjunctive technique was calculated using the ratio of the number of cases that were positive with WATS but negative with FB, to the number of cases that were positive with FB. This was done separately for biopsies showing intestinal metaplasia and dysplasia. An independent statistician analyzed all of the data.
Confidence intervals for the added yield ratio were estimated using Fieller's theorem. Results were calculated with Mathematica software, version 9.
RESULTS
There were 12,899 patients included in the study and their demographic features are summarized in Table 1. There was a female predominance (61%), with a mean age of 56 years (18–93). Fourteen percent of all patients had a history of known BE.
Table 1.
Cohort demographics
| Total number of patients | 12,899 |
|---|---|
| Male:female | 39%:61% |
| Mean age (range) | 56 years (18–93) |
| Mean/median length of suspected Barrett's Segment (range) | 1.4 cm/1.1 cm (0.1–6.3 cm) |
| History of Barrett's esophagus | 14% |
Detection rates of ED and BE are shown in Table 2. Among the 12,899 patients, FB identified 88 cases (0.68% of the total population) of ED or neoplasia. An additional 213 cases were detected using WATS (75 low-grade dysplasia (LGD); 128 indefinite for dysplasia (IND); 10 high-grade dysplasia (HGD)/EAC) but missed on FB. These 213 cases represented an absolute increase of 1.65%, raising the yield from 0.68% to 2.33%. Thus, adding WATS to the random 4-quadrant FB protocol increased the overall detection of dysplasia by 242% (95% confidence interval 191%–315%). Fewer than 61 patients needed to be tested with WATS to identify an additional case of ED missed with FB.
Table 2.
All patients: increased detection of Barrett's esophagus or dysplasia
| Forceps biopsy results | |||||
|---|---|---|---|---|---|
| WATS results | HGD/EAC | IND/LGD | NDBE | No BE | Total |
| HGD/EAC | 8 | 1 | 5 | 5 | 19 |
| IND/LGD | 3 | 17 | 110 | 93 | 223 |
| NDBE | 1 | 40 | 928 | 2,570 | 3,539 |
| Negative | 1 | 17 | 641 | 8,459 | 9,118 |
| Total | 13 | 75 | 1,684 | 11,127 | 12,899 |
| Increased detection with WATS | |||||
| Relative increase vs. forceps | Absolute increase vs. forceps | Number needed to test | |||
| All Barrett's esophagus | 153% (95% CI: 144%–162%) | 19.9% | 5.0 | ||
| Dysplastic Barrett's | 242% (95% CI: 191%–315%) | 1.7% | 60.6 | ||
BE, Barrett's esophagus EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; IND, indefinite for dysplasia; LGD, low-grade dysplasia.
FB found 1,684 cases of BE (13.1% of total cases), and WATS detected 2,570 additional cases of BE that were missed by FB. The absolute incremental yield of adding WATS to FB is 19.9%, increasing the rate of detection from 13.1% to 33% when the two techniques are used adjunctively. Thus, adding WATS to the random 4-quadrant FB protocol increased the overall detection of BE by 153% (95% confidence interval 144– 162%). Only 5 patients needed to be tested with WATS to identify an additional case of BE missed with FB.
Tables 3 and 4 illustrate the increased yield by WATS in patients screened with no prior history of BE or ED (10,412 patients) and for those patients under surveillance with a prior history of BE or ED (2,487 patients), respectively. Among the screening patients without prior history of BE or ED, the addition of WATS to FB increased the overall detection of ED by 274% (95% CI: 194%-414%). In 12 screening patients identified with HGD/EAC utilizing WATS, 5 were called NDBE or no BE with FB, and in 7 patients under surveillance identified with HGD/EAC utilizing WATS, 5 were called NDBE or no BE with FB.
Table 3.
Barrett's screening patients: increased detection of Barrett's esophagus or dysplasia
| Forceps biopsy results | |||||
|---|---|---|---|---|---|
| WATS results | HGD/EAC | IND/LGD | NDBE | No BE | Total |
| HGD/EAC | 7 | 0 | 2 | 3 | 12 |
| IND/LGD | 2 | 3 | 41 | 61 | 107 |
| NDBE | 0 | 19 | 479 | 2,046 | 2,544 |
| Negative | 1 | 7 | 440 | 7,301 | 7,749 |
| Total | 10 | 29 | 962 | 9,411 | 10,412 |
| Increased detection with WATS | |||||
| Relative increase vs. forceps | Absolute increase vs. forceps | Number needed to test | |||
| All Barrett's esophagus | 213% (95% CI: 197%–230%) | 19.7% | 5.1 | ||
| Dysplastic Barrett's | 274% (95% CI: 194%–414%) | 1.0% | 97.3 | ||
BE, Barrett's esophagus; EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; IND, indefinite for dysplasia; LGD, low-grade dysplasia.
Table 4.
Barrett's surveillance patients: increased detection of Barrett's esophagus or dysplasia
| Forceps biopsy results | |||||
|---|---|---|---|---|---|
| WATS results | HGD/EAC | IND/LGD | NDBE | No BE | Total |
| HGD/EAC | 1 | 1 | 3 | 2 | 7 |
| IND/LGD | 1 | 14 | 69 | 32 | 116 |
| NDBE | 1 | 21 | 449 | 524 | 995 |
| Negative | 0 | 10 | 201 | 1,158 | 1,369 |
| Total | 3 | 46 | 722 | 1,716 | 2,487 |
| Increased detection with WATS | |||||
| Relative increase vs. forceps | Absolute increase vs. forceps | Number needed to test | |||
| All Barrett's esophagus | 73% (95% CI: 65%–81%) | 21.1% | 4.7 | ||
| Dysplastic Barrett's | 216% (95% CI: 156%–313%) | 4.3% | 23.5 | ||
BE, Barrett's esophagus; EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; IND, indefinite for dysplasia; LGD, low-grade dysplasia.
Whether FB or WATS was performed first in a particular session did not have an impact on the detection of patients with BE. Of the 4,550 cases where WATS was performed first, 1,375 cases of BE were detected (31.1% of cases), while in the 3,823 cases where FB was performed first, 1,268 cases of BE were detected (33.2% of cases). This difference was not statistically significant (P > 0.05).
No complications from WATS use were reported in any patient.
DISCUSSION
Since survival in EAC is strongly correlated with stage at diagnosis,12 screening to detect patients with BE, its precursor lesion, and surveillance to identify dysplasia and early stage neoplasia are recommended to decrease the morbidity and mortality related to this disease. This strategy, however, is not without controversy as results from several studies have not demonstrated any survival advantage in BE patients undergoing surveillance.13,14 For example, in the study by Corley et al., surveillance was not associated with a reduced risk of EAC-related mortality in 38 patients with a prior diagnosis of BE compared with 101 matched BE controls who did not die from EAC.13 Furthermore, several studies have also shown that relatively few patients with EAC are diagnosed with BE prior to their cancer diagnosis.15,16 These studies underscore the urgent need for an improved strategy that includes enhanced sampling methods that properly identify BE, earlier stage dysplasia and potentially curable early stage EAC.
We present analysis of a large multicenter cohort of screening and surveillance patients demonstrating that the adjunctive use of WATS to random 4-quadrant FB significantly increases detection of both ED and BE. For the primary goal of the study, the use of WATS led to the diagnosis of an additional 213 cases of ED including 10 with HGD/EAC that were missed with FB. Although the overwhelming majority of patients in the study were being screened and had no history of BE or neoplasia, our data suggest that WATS when used in a community-based setting is a promising tool for the diagnosis of BE-associated neoplasia. As a secondary goal, WATS was able to find an additional 2,570 cases of BE that were missed by FB, allowing for detection of 153% more BE (absolute increase of 19.9%). Our results showed a greater increased yield with WATS than what has been reported in prior studies with much smaller sample sizes. For example, Johansen et al. demonstrated a 39.8% increase in detection of BE with the addition of WATS to FB.8 Anandasabapathy et al. reported a 42% increase in detection of ED with the addition of WATS.9 Our improved results can be explained by the fact that our investigators utilized a larger brush than used in previous studies and that the computer microscope used in the pathology analysis was significantly improved. These studies demonstrate that sampling error resulting from random 4-quadrant FB can be greatly improved with WATS with the potential to overcome some of the inherent limitations associated with current standard screening and surveillance techniques.
Our study has several strengths including the use of a large population-based cohort of patients studied prospectively in multicenter community-based practices nationwide. Furthermore, our study accurately replicates the utilization of the WATS diagnostic test in a ‘real world’ community practice based clinical setting. Our results however, cannot be not generalizable to centers of excellence or academic centers where endoscopists rigorously adhere to performing the Seattle biopsy protocol.
There are also some limitations to our study. Although routine screening for BE in women is not recommended, women accounted for 61% of patients in our study. Data regarding females in our study who would be potential candidates for BE screening by exhibiting multiple risk factors for BE or EAC (age >50 years of age, Caucasian race, chronic or frequent GERD, central obesity, waist circumference >88 cm, waist to hip ratio >0.8, current or past history of smoking, a confirmed family history of BE or EAC) was not collected.
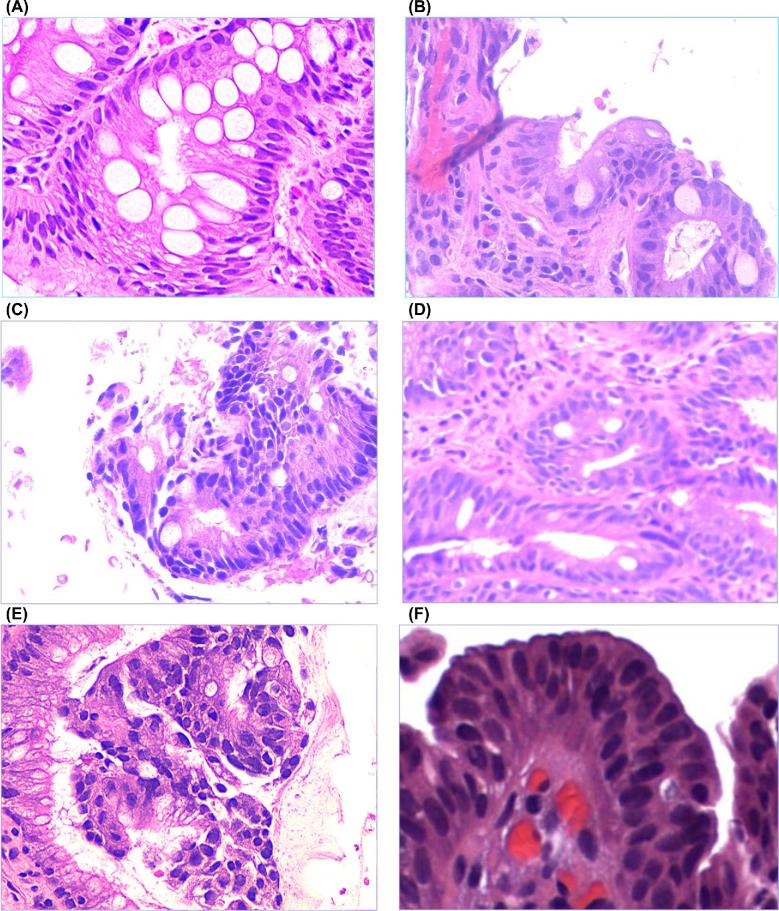
It may be argued that the biological behavior of WATS-detected BE or ED may differ from disease detected by FB. While the WATS computer assists the pathologist in the location of potentially abnormal cells within the thick WATS specimens, once identified, they are ultimately interpreted and reported based upon standard pathologic diagnostic criteria that are considered pathognomonic for the disease. This is illustrated in Figure 1 which depicts side by side pathology images from WATS and FB respectively in patients with BE, LGD, and HGD. Furthermore, blinded independent cytopathologists confirmed all cases of ED that were detected with WATS. Representative images from WATS positive/FB negative cases are illustrated in Figures 2 and 3.
Fig. 1.
Comparison of pathology results for: (a) nondysplastic Barrett's esophagus with WATS (b) nondysplastic Barrett's esophagus with forceps biopsy (c) low grade dysplasia with WATS (d) low grade dysplasia with forceps biopsy (e) high grade dysplasia with WATS and (f) high grade dysplasia with forceps biopsy.
Fig. 2.

WATS detected high grade dysplasia in a patient whose forceps biopsy results were reported as nondysplastic Barrett's esophagus. Computer-synthesized WATS 3-dimensional image stained with modified Papanicolaou. Note marked nuclear enlargement, hyperchromasia, pleomorphism, cell crowding, and complete effacement of the normal honeycomb pattern characteristic of the en face view of the nondysplastic intestinal gland.
Fig. 3.

In a second patient, WATS detected high grade dysplasia in a patient reported with nondysplastic Barrett's esophagus on forceps biopsy. WATS cell block stained with H&E. Note marked nuclear enlargement, hyperchromasia, pleomorphism, and cell crowding.
The progression rate of a FB negative/WATS positive LGD to HGD/EAC would provide additional confirmation that the biological behavior of a WATS-detected versus FB-detected dysplastic cell is the same. The data from a separate registry study demonstrating that the progression rates of WATS-detected BE and of WATS-detected LGD to HGD/EAC are comparable to the progression rates of FB-detected BE and of FB-detected LGD to HGD/EAC is currently being prepared for publication by the authors.
Although investigators were instructed to sample visible tongues of columnar mucosa in the tubular esophagus, investigators were not monitored, and the number and site of biopsies taken at each endoscopy were left to the discretion of the endoscopist. It is likely however, that in our registry study, adherence to endoscopic biopsy guidelines were followed by the great majority of endoscopists as they were verbally instructed to follow them prior to starting the study and the overwhelming majority of patients in our study did not exhibit long segment BE, a characteristic that is most associated with nonadherence to biopsy guidelines. Furthermore, the purpose of this study is to determine the benefits of adding WATS to the FB protocol actually utilized in practice by community-based endoscopists nationwide and not to the Seattle biopsy protocol. While clinicians were instructed to only sample the tubular esophagus, in patients with short segments of esophageal columnar epithelium, locating the gastroesophageal junction, identified as the most proximal extent of the gastric folds, may be difficult as its position is affected by respiration and gut motor activity. Therefore, it is likely that some patients in our study diagnosed with BE by WATS had in fact, cardia intestinal metaplasia (CIM). Sampling cases of BE with less than a 1 cm of metaplasia is problematic as there is significant interobserver and intraobserver variation in the estimation of BE length by probably as much as 1.0 cm.17
Finally, our study, which investigated the use of WATS as an adjunct to the combination of targeted and random FB was also not designed to address the question of whether WATS alone can substitute for, or is more effective than random FB in the detection of BE and BE-associated dysplasia. FB detected 641 cases of BE and 18 cases of ED including 5 patients with HGD/EAC not identified by WATS. Since FB was utilized not only for random 4-quadrant sampling but was the sole technique used to target visible mucosal abnormality, FB, not surprisingly, identified some patients with BE, ED and HGD/EAC that were not detected by WATS. By contrast, WATS was used to test large areas of the esophagus, which would have remained untested by both targeted and random FB. Therefore, increased adjunctive yield for BE and ED/neoplasia is the metric that is most meaningful. We clearly demonstrate that the use of WATS as an adjunct to both targeted and random FB does significantly improve the detection of ED as well as BE, in agreement with previously published studies.
In conclusion, our multicenter community-based experience, the largest collection of patients studied with the WATS diagnostic test to date, confirms the benefit of adding WATS to FB with significant added yields of both ED and BE. Our study suggests that by testing a larger portion of the esophagus with WATS which would not ordinarily be sampled by FB, high risk patients undergoing endoscopic surveillance programs for the diagnosis of BE neoplasia can be more readily identified, thereby potentially improving their management and eventual outcome.
Acknowledgments
The authors wish to thank Dr. Klaus Schreiber of CDx Diagnostics for providing images of WATS samples.
Notes
US Collaborative WATS Study Group: Hong JJ, Eastman TW, Harpole TJ, Kao J and Lim RG, United Surgery Center, Murrieta, CA; Seela S, Ramesh S and Sheela H, Endo-Surgical Center of Florida, Orlando, FL; McLaughlin WD, Rutland TJ, Tarwater SJ, Jackson DF, Crittenden JJ and Albares RP, Dothan Surgery Center, Dothan, AL; Feuer KR and Dumois RA, Citrus Surgical Center, Orlando, FL; Tran TT, Texoma Medical Center, Denison, TX; Reiss G, Metropolitan Gastroenterology Associates, Marrero, LA; Santoro JJ, Kaufman BP, Spaar JL and Rosman GA, Access Surgery Center, Egg Harbor Township, NJ; Hixon JS, Regional Medical Center, Anniston, AL; Beary DA, West Bank Surgery Center, Harvey, LA; Hellstern PA, Chandrupatla S and Mathur S, Citrus Endoscopy Center, Crystal River, FL; McCullough RW and Taormina MK, Midwest Physicians Surgery Center, Lees Summit, MO; Abshire SG and Noel JF, Lafayette General Endoscopy Center, Lafayette, LA; Dugan V, Gastroenterology Group AMC, Slidell, LA; Lee PS, Specialty Surgery Center, Irvine, CA; Block SC, Miller TD, Jabor MA, Kensing KP, Fenton BS, Ganga UM and Phipps TL, Lubbock Digestive Disease Associates, Lubbock, TX; Murray CJ, Rabito FG Jr. and Jenkins LP, Gastroenterology Group AMC, Covington, LA; Hamat HB, Chalasani R, Reddy GT and Thurman DR, North Houston Endoscopy and Surgery, Houston, TX; Berookim PP, La Peer Surgery Center, Beverly Hills, CA; Awan A, North Texas Gastroenterology Consultants, Denton, TX; Masters PA, Garza M, Pruitt A, De Melo S, Chumley DL, Singson Z, Dwivedi SK and Espinoza A, Gastroenterology Consultants, San Antonio, TX; Yu V, Specialty Surgery Center, Irvine, CA; Shinde T, Suncoast Endoscopy Center, Inverness, FL.
Specific author contributions:
Data interpretation: Michael S. Smith, Erkanda Ikonomi, Rajiv Bhuta, Natalya Iorio, Rahul D. Kataria, Vivek Kaul, Seth A. Gross; Drafting/review of manuscript: Michael S. Smith, Erkanda Ikonomi, Rajiv Bhuta, Natalya Iorio, Rahul D. Kataria, Vivek Kaul, Seth A. Gross; Approval of final draft submitted: Michael S. Smith, Erkanda Ikonomi, Rajiv Bhuta, Natalya Iorio, Rahul D. Kataria, Vivek Kaul, Seth A. Gross; Guarantor of the article: Michael S. Smith.
Financial support: The study was funded by CDx Diagnostics, which constructed the protocol and collected the data. De-identified data were then aggregated and sent to the authors for analysis and interpretation, after which the manuscript was written. Authors were not compensated by the sponsor for any of these efforts.
Potential competing interests: Drs. Smith, Kaul, and Gross have served as consultants for the sponsor and have received research support for other studies. Drs. Ikonomi, Bhuta, Kataria and Iorio have no potential interests to disclose.
Clinical trial number: NCT03008980.
Contributor Information
US Collaborative WATS Study Group:
J J Hong, T W Eastman, T J Harpole, J Kao, R G Lim, S Seela, S Ramesh, H Sheela, W D McLaughlin, T J Rutland, S J Tarwater, D F Jackson, J J Crittenden, R P Albares, K R Feuer, R A Dumois, T T Tran, G Reiss, J J Santoro, B P Kaufman, J L Spaar, G A Rosman, J S Hixon, D A Beary, P A Hellstern, S Chandrupatla, S Mathur, R W McCullough, M K Taormina, S G Abshire, J F Noel, V Dugan, P S Lee, S C Block, T D Miller, M A Jabor, K P Kensing, B S Fenton, U M Ganga, T L Phipps, C J Murray, Jr F G Rabito, L P Jenkins, H B Hamat, R Chalasani, G T Reddy, D R Thurman, P P Berookim, A Awan, P A Masters, M Garza, A Pruitt, S De Melo, D L Chumley, Z Singson, S K Dwivedi, A Espinoza, V Yu, and T Shinde
References
- 1. Reid B J, Weinstein W M. Barrett's esophagus and adenocarcinoma. Annu Rev Med 1987; 38: 477–92. [DOI] [PubMed] [Google Scholar]
- 2. Pohl H, Welch H G. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97: 142–6. [DOI] [PubMed] [Google Scholar]
- 3. Napier K J, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hvid-Jensen F, Pedersen L, Drewes A M, Sorensen H T, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011; 365: 1375–83. [DOI] [PubMed] [Google Scholar]
- 5. Abrams J A, Kapel R C, Lindberg G M et al.. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009; 7: 736–42; quiz 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma P. Review article: emerging techniques for screening and surveillance in Barrett's oesophagus. Aliment Pharmacol Ther 2004; 20: 63–70; discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 7. Spechler S J. Clinical practice. Barrett's esophagus. N Engl J Med 2002; 346: 836–42. [DOI] [PubMed] [Google Scholar]
- 8. Johanson J F, Frakes J, Eisen D. Computer-assisted analysis of abrasive transepithelial brush biopsies increases the effectiveness of esophageal screening: a multicenter prospective clinical trial by the EndoCDx Collaborative Group. Dig Dis Sci 2011; 56: 767–72. [DOI] [PubMed] [Google Scholar]
- 9. Anandasabapathy S, Sontag S, Graham D Y et al.. Computer-assisted brush-biopsy analysis for the detection of dysplasia in a high-risk Barrett's esophagus surveillance population. Dig Dis Sci 2011; 56: 761–6. [DOI] [PubMed] [Google Scholar]
- 10. Vennalaganti P R, Kaul V, Wang K K et al.. Increased detection of Barrett's esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest Endosc 2018; 87: 348–55. [DOI] [PubMed] [Google Scholar]
- 11. Vennalaganti P R, Naag Kanakadandi V, Gross S A et al.. Interobserver agreement among pathologists using wide-area transepithelial sampling with computer-assisted analysis in patients with Barrett's esophagus. Am J Gastroenterol 2015; 110: 1257–60. [DOI] [PubMed] [Google Scholar]
- 12. Menke-Pluymers M B, Schoute N W, Mulder A H, Hop W C, van Blankenstein M, Tilanus H W. Outcome of surgical treatment of adenocarcinoma in Barrett's oesophagus. Gut 1992; 33: 1454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corley D A, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss N S. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013; 145: 312–9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubenstein J H, Sonnenberg A, Davis J, McMahon L, Inadomi J M. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc 2008; 68: 849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fountoulakis A, Zafirellis K D, Dolan K, Dexter S P, Martin I G, Sue-Ling H M. Effect of surveillance of Barrett's oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004; 91: 997–1003. [DOI] [PubMed] [Google Scholar]
- 16. Bhardwaj A, McGarrity T J, Stairs D B, Mani H. Barrett's esophagus: emerging knowledge and management strategies. Pathol Res Int 2012; 2012: 814146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dekel R, Wakelin D E, Wendel C et al.. Progression or regression of Barrett's esophagus—is it all in the eye of the beholder? Am J Gastroenterol 2003; 98: 2612–5. [DOI] [PubMed] [Google Scholar]