Abstract
Curative effect and adverse reactions of oxaliplatin combined with endostar in the interventional treatment of primary hepatic carcinoma (PHC) were investigated. A total of 101 PHC patients from October 2012 to December 2014 in The First Affiliated Hospital of Xi'an Jiaotong University were retrospectively collected. Fifty patients in combined therapy group were treated with oxaliplatin combined with endostar, while the remaining 51 patients in oxaliplatin group were treated with oxaliplatin alone. The treatment lasted for a total of 4 cycles (20 days as 1 cycle). The ratios of cluster of differentiation 3 (CD3)+, CD4+ and CD8+ were detected via enzyme-linked immunosorbent assay (ELISA). The objective response rate in combined therapy group was 92.00%, which was significantly higher than that in oxaliplatin group (74.51%). The main adverse reactions showed no statistical difference between the two groups (P>0.05). The median progression-free survival (PFS) was 8.6 months in combined therapy group and 6.3 months in oxaliplatin group, while the median overall survival (OS) was 12.9 months in combined therapy group and 10.6 months in oxaliplatin group. After treatment, CD4+ and CD3+ levels in the peripheral blood in both groups were obviously lower than those before treatment, but the CD8+ level was obviously higher than that before treatment. At the same time, changes in the ratio of T lymphocyte subsets in combined therapy group were superior to those in oxaliplatin group, displaying statistically significant differences (P<0.05). Oxaliplatin combined with endostar has a good curative effect in the treatment of PHC with mild adverse reactions, which can prolong the survival time of patients, improve the levels of T lymphocyte subsets and increase the immunity of patients, so it is worthy of promotion and application in clinic.
Keywords: endostar, oxaliplatin, primary hepatic carcinoma, combined medication, immune cells
Introduction
Primary hepatic carcinoma (PHC) is one of the most common malignant tumors threatening human health, which is prone to metastasis and recurrence. According to data published by the American Cancer Society (1), the incidence rate of PHC in 2016 in China increased by 40% compared to that a decade ago. China is a country with high prevalence of hepatitis B and hepatic carcinoma, and hepatitis B virus (HBV) carriers account for 20% of the total population. Besides, about 356,000 people suffer from PHC every year with approximately 332,000 deaths, and both morbidity and mortality rates of PHC are among the top three in the world, second only to lung cancer (2).
Surgical operation is a major means in the treatment of PHC currently, and both radical excision and liver transplantation are still the most effective methods to improve the long-term survival rate. According to statistics of Chirikov et al (3), the 5-year survival rate can be 49–72% in patients with early-stage hepatic carcinoma after hepatectomy, liver transplantation or minimally-invasive therapy, but these methods are only applicable to early-stage patients without metastasis. Due to the hidden onset, no specificity and rapid development of PHC, patients have been mostly in the advanced stage with a poor prognosis when diagnosed. Local treatment methods, such as transcatheter arterial chemoembolization (TACE), percutaneous ethanol injection (PEI) and high-intensity focused ultrasound (HIFU), are often not effective due to the limitations of indications (4). In recent years, new-generation drugs, such as oxaliplatin and endostar, have emerged successively, achieving good effects in the treatment of tumors, and laying a solid foundation for the research and treatment of hepatic carcinoma. Worldwide studies have demonstrated that (5,6) oxaliplatin is a third-generation platinum drug, which can inhibit DNA synthesis, produce cytotoxicity and anti-tumor activity and induce apoptosis of hepatic carcinoma cells, thus effectively treating PHC. Moreover, it can alleviate symptoms, control the development of disease and prolong the survival time, so it has drawn attention. Endostar, a human endostatin, can block the nutrition supply of tumor cells, inhibit neovascularization and accelerate apoptosis of cancer cells (7), thus reducing the vascular endothelial growth factor (VEGF) in patients, improving the therapeutic efficiency and increasing the survival rate of PHC patients. Immune cells can reduce the toxic side effects in the drug therapy of patients with hepatic carcinoma, and stimulate and enhance the body's immune function, thereby reducing the recurrence rate of patients and prolonging the progression-free survival (PFS) and overall survival (OS) (8). Plenty of results have been achieved in the study on single application of oxaliplatin in other tumors. However, there are still few reports on the curative effect of oxaliplatin combined with endostar in the treatment of hepatic carcinoma and its influence on immune cells. In this experiment, the curative effect of oxaliplatin combined with endostar in the treatment of hepatic carcinoma and its immunological influence were investigated.
Materials and methods
Data of patients
A total of 101 patients with PHC admitted to The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China) from October 2012 to December 2014 were respectively collected, of which 50 cases were enrolled in combined therapy group and treated with oxaliplatin and endostar, including 28 males and 22 females with an average age of 53±10.2 years, and the remaining 51 cases were included in oxaliplatin group and treated only with oxaliplatin, including 32 males and 19 females with an average age of 54±9.4 years.
The study was approved by the Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong University. Signed informed consents were obtained from the patients or the guardians.
Inclusion and exclusion criteria
Inclusion criteria were: Patients who were clinically diagnosed with hepatic carcinoma, had complete clinical data and no distant metastasis, and had not received any systematic drug treatment or any chemo-radiotherapy. Exclusion criteria were: Patients with cardiovascular or cerebrovascular diseases, patients that could not receive radiotherapy, patients complicated with other malignant tumors, patients that suffered from unsoundness of feet, or patients in gestation or lactation period.
Treatment methods
Patients in combined therapy group were treated with oxaliplatin and endostar. First, 130 mg/m2 oxaliplatin (H20000337; Jiangsu Hengrui Medicine Co., Ltd.) was added into 500 ml 5% glucose solution, and the mixture was dripped intravenously for 6 h, 2 times a day, with 20 days as 1 cycle. Moreover, they were also given endostar (S20050088; Shandong Medgenn Bioengineering Co., Ltd.) from 1–14 days via intravenous drip of 250–500 ml normal saline added with 15 mg endostar for 6 h, and the drug was administered again after 7 days. Patients in oxaliplatin group were given slow intravenous infusion of 130 mg/m2 oxaliplatin added with 500 ml 5% glucose solution for 6 h, 2 times a day, with 20 days as 1 cycle. During the treatment process, patients that suffered from vomiting or fever could be given anti-vomiting or anti-pyretic treatment. Curative effects and toxic side effects of patients in the two groups were observed after 4 cycles of treatment and survival rate analysis was performed after 5 cycles.
Enzyme-linked immunosorbent assay (ELISA)
Before and after treatment, 3 ml fasting venous blood was collected from patients in the two groups and centrifuged at 2,300 × g for 20 min at 4°C to get the serum. The ratios of serum CD3+, CD4+ and CD8+ cells (cat. nos 17617-1-AP, 19068-1-AP, and 21256-1-AP; Wuhan Sanying Biotechnology, Wuhan, China) in the two groups before and after treatment, were compared. ELISA kits and all operations (Wuhan Sanying Biotechnology) strictly followed protocol of the kit.
Curative effect and toxicity assessment
The assessment of response rate was according to Revised Edition of Response Evaluation Criteria in Solid Tumors (9) and it is divided into complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD) and objective remission rate (CR+PR). The evaluation of toxic side effects was in accordance with National Cancer Institute Common Toxicity Criteria (10). Relevant adverse reactions after treatment were assessed, including fever, pain, nausea, emesis, anorexia, increased bilirubin, nephrotoxicity, neurotoxicity, hypoproteinemia, thrombocytopenia and anemia, which were taken as main assessment indexes for short-term efficacy.
Statistical analysis
The data in this experiment were analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The measured data are presented as mean ± standard deviation (SD). t-test was adopted for the comparison between two groups and F-test was used for the comparison among groups. Measurement data are expressed as ratio (%) and analyzed using χ2 test. Kaplan Meier method and log-rank test was used for the comparison of the survival curves. P<0.05 indicates that the difference was statistically significant.
Results
Comparison of data of patients
Only tumor-node-metastasis (TNM) staging, treatment range and number of tumors had differences between the two groups (P<0.05), while other factors had no significant differences (P>0.05) (Table I).
Table I.
Comparison of basic data between the two groups of patients [n (%)].
| Groups | ||||
|---|---|---|---|---|
| items | Combined therapy (n=50) | Oxaliplatin (n=51) | χ2 | P-value |
| Sex | ||||
| Male | 28 (56.00) | 32 (62.75) | 0.476 | 0.490 |
| Female | 22 (44.00) | 19 (37.25) | ||
| Age (years) | ||||
| >50 | 23 (46.00) | 28 (54.90) | 0.800 | 0.371 |
| ≤50 | 27 (54.00) | 23 (45.10) | ||
| History of disease | ||||
| Hepatitis B | 16 (32.00) | 20 (39.22) | 0.705 | 0.703 |
| Hepatitis C | 25 (50.00) | 24 (47.06) | ||
| Others | 9 (18.00) | 7 (13.73) | ||
| TNM stage | 0.240 | 0.624 | ||
| I–II | 31 (62.00) | 34 (66.67) | ||
| III–IV | 19 (38.00) | 17 (33.33) | ||
| Diameter of tumor | 0.170 | 0.680 | ||
| ≥2 cm | 39 (78.00) | 38 (74.51) | ||
| <2 cm | 11 (22.00) | 13 (25.49) | ||
| Treatment range | 1.208 | 0.227 | ||
| Sub-segment | 20 (40.00) | 13 (25.49) | ||
| Segment | 14 (28.00) | 19 (37.25) | ||
| Lobe | 13 (26.00) | 15 (29.41) | ||
| Whole liver | 3 (6.00) | 4 (7.84) | ||
| No. of tumors treated | 0.286 | 0.775 | ||
| 1 | 19 (38.00) | 17 (33.33) | ||
| 2 | 11 (22.00) | 13 (25.49) | ||
| 3 | 4 (8.00) | 6 (11.76) | ||
| 4 | 5 (10.00) | 3 (5.88) | ||
| ≥5 | 11 (22.00) | 12 (23.53) | ||
Comparison of curative effect between the two groups of patients
There was CR+PR in 46 patients (92.00%) in combined therapy and 38 patients (74.51%) in oxaliplatin group, and CR in 29 patients (58.00%) in combined therapy and 23 patients (45.11%) in oxaliplatin group. The objective response rate, CR and PR rates in combined therapy were obviously higher than those in oxaliplatin group, displaying statistically significant differences (P<0.05) (Table II).
Table II.
Comparison of curative effect between the two groups of patients [n (%)].
| Groups | ||||
|---|---|---|---|---|
| items | Combined therapy (n=50) | Oxaliplatin (n=51) | χ2 | P-value |
| CR | 29 (58.00) | 23 (45.11) | ||
| PR | 17 (34.00) | 15 (29.41) | 1.774 | 0.076 |
| SD | 2 (4.00) | 10 (19.61) | ||
| PD | 2 (4.00) | 3 (5.88) | ||
| CR+PR | 46 (92.00) | 38 (74.51) | 5.517 | 0.019 |
Comparison of adverse reactions between the two groups
The main adverse reactions included pain (34.00%), increased bilirubin (24.00%) and neurotoxicity (22.00%) in combined therapy group, and pain (29.42%), hypoproteinemia (21.57%) and neurotoxicity (15.69%) in oxaliplatin group, showing no statistically significant differences (P>0.05) (Table III).
Table III.
Comparison of adverse reactions between the two groups [n (%)].
| Groups | ||||
|---|---|---|---|---|
| items | Combined therapy (n=50) | Oxaliplatin (n=51) | χ2 | P-value |
| Fever | 3 (6.00) | 2 (3.91) | 0.232 | 0.630 |
| Pain | 17 (34.00) | 15 (29.42) | 0.246 | 0.620 |
| Nausea and vomiting | 5 (10.00) | 3 (5.82) | 0.586 | 0.444 |
| Anorexia | 3 (6.00) | 2 (3.91) | 0.232 | 0.630 |
| Increased bilirubin | 12 (24.00) | 7 (13.73) | 1.745 | 0.187 |
| Hypoproteinemia | 6 (12.00) | 11 (21.57) | 1.651 | 0.199 |
| Thrombocytopenia | 5 (10.00) | 4 (7.84) | 0.145 | 0.704 |
| Anemia | 5 (10.00) | 6 (11.76) | 0.081 | 0.776 |
| Neurotoxicity | 11 (22.00) | 8 (15.69) | 0.659 | 0.417 |
| Renal toxicity | 1 (2.00) | 0 (0.00) | 0.490 | |
The median PFS was 8.6 months in combined therapy group and 6.3 months in oxaliplatin group, while the median OS was 12.9 months in combined therapy group and 10.6 months in oxaliplatin group, showing statistically significant differences (P<0.05). TNM stage, treatment range and number of tumor were independent risk factors (Figs. 1 and 2).
Figure 1.
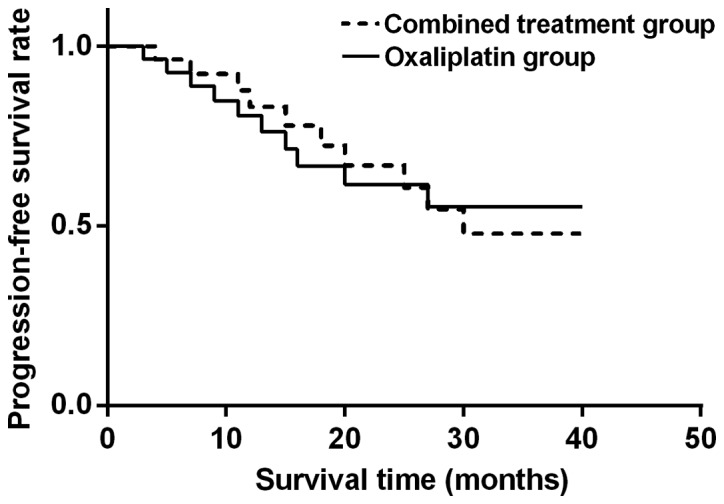
Analysis of PFS in combined therapy and oxaliplatin group. PFS in oxaliplatin group is lower than that in combined therapy group, and the difference is statistically significant (P<0.05).
Figure 2.
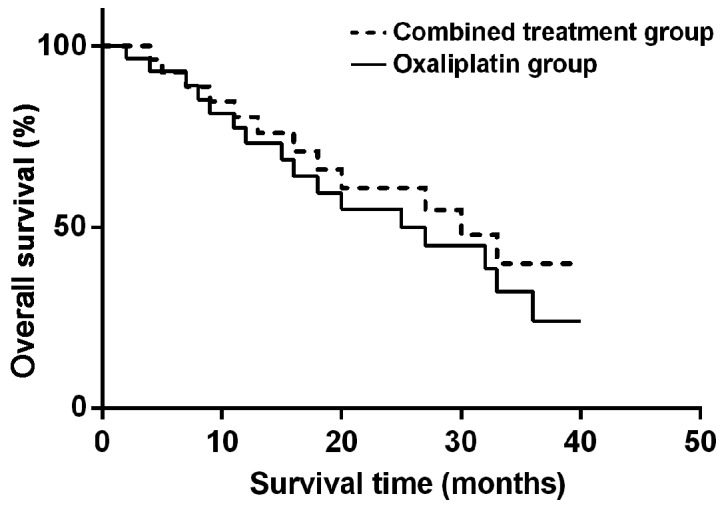
Analysis of OS in combined therapy and oxaliplatin group. OS in combined therapy is higher than that in oxaliplatin group, and the difference is statistically significant (P<0.05).
Comparison of immune cell levels between the two groups before and after treatment
Before treatment, there were no statistically significant differences in CD3+, CD4+ and CD8+ cells between the two groups (P>0.05). After treatment, CD4+ and CD3+ in the peripheral blood in both groups were obviously lower than those before treatment, but CD8+ was obviously higher than that before treatment. Moreover, changes in the ratio of T lymphocyte subsets in combined therapy group were superior to those in oxaliplatin group, displaying statistically significant differences (P<0.05) (Table IV).
Table IV.
Comparison of immune cell levels in the peripheral blood between the two groups before and after treatment.
| Groups | Time | CD3+ | CD4+ | CD8+ |
|---|---|---|---|---|
| Combined therapy | Before treatment | 47.96±6.32 | 45.54±6.23 | 28.64±6.38 |
| After treatment | 41.25±3.65a | 38.21±3.97b | 50.21±4.39c | |
| t | 7.393 | 6.420 | 22.211 | |
| P-value | <0.001 | <0.001 | <0.001 | |
| Oxaliplatin | Before treatment | 45.43±6.35 | 43.21±6.31 | 26.23±6.28 |
| After treatment | 20.12±3.21 | 27.39±2.96 | 36.28±3.52 | |
| t | 28.875 | 19.629 | 9.879 | |
| P-value | <0.001 | <0.001 | <0.001 |
P<0.05, the CD3+ level is higher than that in oxaliplatin group after treatment.
P<0.05, the CD4+ level is higher than that in oxaliplatin group.
P<0.05, the CD8+ level is higher than that in oxaliplatin group.
Discussion
Both mortality and morbidity rates of PHC in China show increasing trends. Most patients were already in the middle-advanced stage when definitely diagnosed with hepatic carcinoma, losing the optimal opportunity for treatment. To effectively control the progression of disease, systemic chemotherapy is often adopted. Systemic chemotherapy is the most commonly-used treatment means for the advanced PHC, it can kill PHC cells with small trauma and has wide-ranging indications, and significantly improve the quality of life and effectively prolong the survival time of patients. However, hepatic carcinoma cells are prone to drug resistance to chemotherapy drugs with significant toxic side effects (11), so the curative effect is always unsatisfactory.
Khan et al (12) proposed for the first time that angiogenesis in malignant tumors is the main cause of tumor proliferation and growth, and tumor tissues are able to induce neovascularization, so malignant tumors can be inhibited through inhibiting tumor neovascularization. Endostar, a kind of human endostatin, can block the nutrition supply of tumor cells, inhibit neovascularization and accelerate the apoptosis of cancer cells (13). Oxaliplatin is a third-generation platinum drug, which can inhibit DNA synthesis, produce cytotoxicity and antitumor activity (14), and induce apoptosis of PHC cells. There is a synergistic effect in the combined application of oxaliplatin and endostar, so the toxicity does not overlap, making the toxic side effects tolerable and reducing the risk of patients during treatment. The optimal dose and medication method can be adopted in the combined application, increasing the efficiency and prolonging the survival time. Besides, the combined application is characterized by convenient operation and moderate costs (15). Results of this study revealed that both CR and PR rates in the combined application of oxaliplatin and endostar were higher than those in the application of oxaliplatin alone, and the objective response rate in oxaliplatin group (74.51%) was significantly lower than that in combined therapy group (92.00%), showing a statistically significant difference (P<0.05). The effect of combined application was obviously superior to that of single application. Oxaliplatin can directly kill tumor cells and inhibit the activity of tumor cells, affect the neovascularization in tumor tissues (16), and strengthen the anti-angiogenesis effect of endostar.
Endostar can reduce the migration and regeneration of vascular endothelial cells, reducing new vessels in tumor tissues and increasing vascular permeability (17), thereby enhancing the killing effect of oxaliplatin on tumor cells. Findings in this study are basically consistent with those in the report of Maj et al (18) that oxaliplatin combined with endostar strengthens the ability of endostar to inhibit endothelial factor receptor, resulting in revascularization disorders in tumors. Then relevant toxic reactions were compared between the two groups. The main toxic reactions included pain (30.00%), increased bilirubin (24.00%) and neurotoxicity (20.00%) in combined therapy group, and pain (27.45%), hypoproteinemia (21.57%) and thrombocytopenia (19.61%) in oxaliplatin group, which could be restored after drug withdrawal. Clinical reactions mostly occurred in stage I–II, the toxic effect was basically consistent and general symptoms were mild and tolerable in combined therapy group and oxaliplatin group. Lyu et al (19) studied and compared the curative effect of oxaliplatin and endostar in the treatment of hepatic carcinoma, and found that the curative effect was comparable with tolerance to toxic side reactions. Results of this study showed that the median PFS was 8.6 months in combined therapy group and 6.3 months in oxaliplatin group, while the median OS was 12.9 months in combined therapy group and 10.6 months in oxaliplatin group, and there were statistically significant differences (P<0.05). The improvement rates of PFS and OS in combined therapy group were higher than those in oxaliplatin group. The curative effect in combined therapy group was significantly superior to that in oxaliplatin group, and the survival time of patients was longer in combined therapy group. The incidence rate of adverse reactions was lower in both groups.
Jin et al (20) showed that the hematological toxicity, digestive tract toxicity and renal toxicity are mild in combined therapy group and oxaliplatin group, and the quality of life of patients is improved after treatment, especially that in combined therapy group, so the combined therapy is appropriate in clinic. The CD3+ molecule is connected to T cell antigen receptor (TCR) via the salt bridge and involved in T cell signal transduction. CD4+ is an important immune cell in the body's immune system. CD4 is mainly expressed on helper T cells, which is a co-receptor for the antigen recognition of TCR. CD8+ is a lymphocyte subset, which plays an important role in the antigen recognition and presentation in specific immune response (21). According to results in this study, CD4+ and CD3+ levels in the peripheral blood in both groups were lower after treatment than those before treatment, but the CD8+ level was higher than that before treatment. At the same time, changes in the ratio of T lymphocyte subsets in combined therapy group were superior to those in oxaliplatin group, displaying statistically significant differences (P<0.05), indicating that oxaliplatin combined with endostar can significantly improve the immune function of T lymphocytes and increase the immunity in patients with hepatic carcinoma. The above results are basically consistent with those of Zhou et al (22) that oxaliplatin can inhibit DNA synthesis, and, combined with endostar, can activate the body's immune system after promoting the endometrium swallowed by the mononuclear phagocytic endothelial system, so as to improve the immune function of patients more effectively.
There are certain limitations in this study. Due to the retrospective comparative analysis, there may be subjective selection bias, reducing the reliability of results. The sample size in this study was small, limiting the number of patients that could be used for subgroup analysis, so clinical features failed to be displayed fully and reliably. Whether condition limitations and regional differences affected results of this study remains unknown. Therefore, it will be further verified in future research.
In conclusion, oxaliplatin combined with endostar has a good curative effect in the treatment of PHC with mild adverse reactions, which can prolong the survival time of patients, improve the levels of T lymphocyte subsets and increase the immunity of patients, so it is worthy of promotion and application in clinic.
Acknowledgements
Not applicable.
Funding
This study was supported by the Wuhan City Health Bureau of Medical Research project (no. WX18C02), Hubei Province health and family planning scientific research project (no. WJ2019M008).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CL and DR collected and analyzed the general data of patients. MF and CS were responsible for ELISA. QP and YL interpreted curative effect. HR helped with toxicity assessment. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). Patients who participated in this research had complete clinical data. The signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ang C, O'Reilly EM, Abou-Alfa GK. Targeted agents and systemic therapy in hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:225–246. doi: 10.1007/978-3-642-16037-0_15. [DOI] [PubMed] [Google Scholar]
- 3.Chirikov VV, Mullins CD, Hanna N, Breunig IM, Seal B, Shaya FT. Multispecialist care and mortality in hepatocellular carcinoma. Am J Clin Oncol. 2015;38:557–563. doi: 10.1097/COC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 4.Gomes MA, Priolli DG, Tralhão JG, Botelho MF. Hepatocellular carcinoma: Epidemiology, biology, diagnosis, and therapies. Rev Assoc Med Bras 1992. 2013;59:514–524. doi: 10.1016/j.ramb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri G, Marech I, Lorusso V, Goffredo V, Paradiso A, Ribatti D, Gadaleta CD. Molecular targeting agents associated with transarterial chemoembolization or radiofrequency ablation in hepatocarcinoma treatment. World J Gastroenterol. 2014;20:486–497. doi: 10.3748/wjg.v20.i2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tampaki M, Doumba PP, Deutsch M, Koskinas J. Circulating biomarkers of hepatocellular carcinoma response after locoregional treatments: New insights. World J Hepatol. 2015;7:1834–1842. doi: 10.4254/wjh.v7.i14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Barni S. Oxaliplatin-based chemotherapy: A new option in advanced hepatocellular carcinoma. A systematic review and pooled analysis. Clin Oncol (R Coll Radiol) 2014;26:488–496. doi: 10.1016/j.clon.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Rahman O. Revisiting oxaliplatin-based regimens for advanced hepatocellular carcinoma. Curr Oncol Rep. 2014;16:394. doi: 10.1007/s11912-014-0394-0. [DOI] [PubMed] [Google Scholar]
- 9.Hong L, Han Y, Zhou Y, Nita A. Angiogenesis-related microRNAs in colon cancer. Expert Opin Biol Ther. 2013;13:77–84. doi: 10.1517/14712598.2013.727391. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CH, Kang YK, Yang TS, Shun CT, Shao YY, Su WC, Sandoval-Tan J, Chiou TJ, Jin K, Hsu C, et al. Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: A multicenter phase II study. Oncology. 2013;85:44–52. doi: 10.1159/000350841. [DOI] [PubMed] [Google Scholar]
- 11.Takayasu K. Chemoembolization for unresectable hepatocellular carcinoma in Japan. Oncology. 2010;78(Suppl 1):135–141. doi: 10.1159/000315242. [DOI] [PubMed] [Google Scholar]
- 12.Khan JA, Maki RG, Ravi V. Pathologic angiogenesis of malignant vascular sarcomas: Implications for treatment. J Clin Oncol. 2017;36:194–201. doi: 10.1200/JCO.2017.74.9812. [DOI] [PubMed] [Google Scholar]
- 13.Yang CH, Pfeffer SR, Sims M, Yue J, Wang Y, Linga VG, Paulus E, Davidoff AM, Pfeffer LM. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem. 2015;290:6037–6046. doi: 10.1074/jbc.M114.632125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yodono H, Matsuo K, Shinohara A. A retrospective comparative study of epirubicin-lipiodol emulsion and cisplatin-lipiodol suspension for use with transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma. Anticancer Drugs. 2011;22:277–282. doi: 10.1097/CAD.0b013e328342231d. [DOI] [PubMed] [Google Scholar]
- 15.Wang-Yuan Z, Jiang-Zheng Z, Lu YD, Hao XB, Hong T, Huang F, Lei JH, He ZH, Huang MZ. Clinical efficacy of metronomic chemotherapy after cool-tip radiofrequency ablation in the treatment of hepatocellular carcinoma. Int J Hyperthermia. 2016;32:193–198. doi: 10.3109/02656736.2015.1099168. [DOI] [PubMed] [Google Scholar]
- 16.Marinelli S, Granito A, Piscaglia F, Renzulli M, Stagni A, Bolondi L. Metronomic capecitabine in patients with hepatocellular carcinoma unresponsive to or ineligible for sorafenib treatment: Report of two cases. Hepat Mon. 2013;13:e11721. doi: 10.5812/hepatmon.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He SL, Shen J, Sun XJ, Zhu XJ, Liu LM, Dong JC. Efficacy of capecitabine and oxaliplatin regimen for extrahepatic metastasis of hepatocellular carcinoma following local treatments. World J Gastroenterol. 2013;19:4552–4558. doi: 10.3748/wjg.v19.i28.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: The great discovery and greater complexity. Int J Oncol. 2016;49:1773–1784. doi: 10.3892/ijo.2016.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, Zhang Z, Zheng L, Deng H, Li S, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60–69. doi: 10.1016/j.jhep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Jin F, Ji H, Jia C, Brockmeier U, Hermann DM, Metzen E, Zhu Y, Chi B. Synergistic antitumor effects of endostar in combination with oxaliplatin via inhibition of HIF and CXCR4 in the colorectal cell line SW1116. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0047161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beringer DX, Kleijwegt FS, Wiede F, van der Slik AR, Loh KL, Petersen J, Dudek NL, Duinkerken G, Laban S, Joosten A, et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol. 2015;16:1153–1161. doi: 10.1038/ni.3271. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Yang T, Liu L, Lu B. Chemotherapy oxaliplatin sensitizes prostate cancer to immune checkpoint blockade therapies via stimulating tumor immunogenicity. Mol Med Rep. 2017;16:2868–2874. doi: 10.3892/mmr.2017.6908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


