Abstract
The pathogenesis of colorectal cancer (CRC) is poorly understood. MicroRNA (miR)-32 upregulation in CRC tissues was previously reported, where it increased the proliferation, migration and invasion, and reduced apoptosis of CRC cells by inhibiting the expression of phosphatase and tensin homolog (PTEN). However, the mechanism underlying miR-32 upregulation remains unknown. miR-32 is an intronic miRNA located within intron 14 of the transmembrane protein 245 gene (TMEM245). The present study aimed to elucidate the biological pathways underlying miR-32 regulation in CRC. A truncated promoter containing the 5′-flanking region of TMEM245/miR-32 gene was constructed. The promoter region was analyzed by dual luciferase reporter assay in CRC cells. DNA pull-down assay and mass spectrometry (MS) were used to identify proteins binding to the core promoter. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and transcription factor (TF) analyses were used to identify the binding proteins. The −320 to −1 bp fragment of the 5′-flanking region exhibited the highest luciferase activity. The regions spanning −606 to −320 bp exhibited a significant decrease in luciferase activity, compared with the −320 to −1 bp fragment. DNA pull-down assay and MS revealed 403 potential miR-32 promoter binding proteins. GO and KEGG pathway analysis indicated that these proteins were involved in numerous physiological and biochemical processes, including ‘structural molecule activity’, ‘RNA binding’, ‘small molecule metabolic process’ and ‘biogenesis’. Furthermore, TF analysis revealed 10 potential interacting TFs, including SMAD family member 1 (SMAD1), signal transducer and activator of transcription 1 (STAT1) and forkhead box K1 (Foxk1). These results suggested that the core promoter region may be located within-320 to −1 bp of the 5′-flanking region of TMEM245/miR-32 gene, while the region from −606 to −320 bp may harbor repressive regulatory elements. The TFs SMAD1, STAT1 and Foxk1 may be involved in the transcriptional regulation of miR-32.
Keywords: colorectal cancer, promoter, microRNA-32, transcription factor, bioinformatics analysis
Introduction
Colorectal cancer (CRC) is a type of malignant tumor derived from the colonic epithelial mucosa. Carcinogenesis of CRC involves abnormal expression of genes associated with proliferation, apoptosis, metastasis and angiogenesis (1). To date, the molecular mechanisms underlying CRC oncogenesis are not fully understood. The pathogenesis and development of CRC is a multi-factor, multi-step process, in which gene mutations and abnormal expression may serve important roles (2). The involvement of DNA epigenetic modifications (3), non-coding RNAs including microRNAs (miRNAs/miRs) (4), long non-coding RNA (lncRNA) (5), circular RNA (cirRNA) (6) and chromatin remodeling (7) in the development of CRC are receiving increasing attention.
miRNAs are a class of non-coding, single-stranded RNAs with a length of 18–25 nucleotides. They affect gene expression by binding to specific sites at the 3′-untranslated region of target mRNAs. miRNAs are involved in tumor development and are potential biomarkers in cancer diagnostics and treatment, including CRC (8,9). Accumulating evidence has demonstrated that aberrantly expressed miRNAs acted as oncogenes or tumor suppressor genes in CRC (4,10,11).
miR-32 is an intronic miRNA located within intron 14 of transmembrane protein 245 gene (TMEM245). Our previous studies (12,13) revealed that miR-32 was upregulated in CRC tissues and that high miR-32 levels were significantly associated with lymph node and distant metastasis. Additionally, patients with high miR-32 expression had a poor overall survival. Furthermore, overexpression of miR-32 led to increased proliferation, migration and invasion, and reduced apoptosis of CRC cells via inhibition of phosphatase and tensin homolog (PTEN). However, the mechanism underlying the upregulation of miR-32 remains unknown. The aim of the current study was to elucidate the mechanisms involved in the upregulation of miR-32 in CRC by analyzing the promoter of the miR-32 gene and investigating the proteins that bind to the promoter. The results obtained may assist in the further investigation of transcriptional regulatory mechanisms of miR-32 expression.
Materials and methods
DNA cloning and construction of truncated promoter plasmids
To analyze the promoter region responsible for expression of the miR-32 gene, serially truncated fragments of five different lengths of the 5′-flanking region of host gene TMEM245 (ENST00000374586.7 from University of California Santa Cruz Genome Browser) were amplified by polymerase chain reaction (PCR) using different pairs of primers (Table I). PCR was performed with 2×HIFITaq PCR StarMix (Genstar, Beijing, China) and the conditions were as follows: Initial denaturation at 94°C for 5 min; 35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 2 min; and final extension at 72°C for 10 min. These primers also introduced a KpnI site at the 5′end and an XhoI site at the 3′end of the amplified fragments. The PCR fragments were purified, digested with KpnI and XhoI and cloned into a pGL3-basic vector (Promega Corporation, Madison, WI, USA). Five successive truncated constructs from the 5′-flanking region termed pGL3-1987 (−1987 to −1 bp), pGL3-1648 (−1648 to −1 bp), pGL3-1088 (−1088 to −1 bp), pGL3-606 (−606 to −1 bp), pGL3-320 (−320 to −1 bp) were generated. All inserts were verified by DNA sequencing (Beijing Genomics Institute, Beijing, China).
Table I.
Primer sequences.
| Sequence 5′ → 3′ | |||
|---|---|---|---|
| Primer | Forward | Reverse | Position (bp) |
| pGL3-1987 | AAAGGTACCCAGCCTGTTCA ACATGGTGAA | AAACTCGAGGTAATGGGAGTCGGGC TAGAAAC | −1987 to −1 |
| pGL3-1648 | AAAGGTACCCTCCCACC GGGAGACTGC | AAACTCGAGGTAATGGGAGTCGG GCTAGAAAC | −1648 to −1 |
| pGL3-1088 | AAAGGTACCCTTGCAAGG TTTGAAGCAATCA | AAACTCGAGGTAATGGGAGTCGG GCTAGAAAC | 1088 to −1 |
| pGL3-606 | AAAGGTACCCTTGCCTGT GCCACTTGG | AAACTCGAGGTAATGGGAGTCGG GCTAGAAAC | 606 to −1 |
| pGL3-320 | AAAGGTACCTTCTAGTATG CAGCTTGGGTTTTAATATC | AAACTCGAGGTAATGGGAGTCG GGCTAGAAAC | - 320 to −1 |
Cell transfection and dual luciferase assay
The CRC cell line HCT-116 was obtained from the Cell Bank of the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). HCT-116 cells were plated in six-well plates and cultured in RPMI-1640 medium (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin (GE Healthcare Life Sciences) at 37°C with 5% CO2. For each transfection, cells were incubated with 0.5 µg of each promoter reporter plasmid respectively, and 0.5 µg of pRL-TK (Promega Corporation), which was used as an internal control. The pGL3-basic vector was used as the negative control. Transfection was performed using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). Cells were harvested for subsequent experimentation 48 h after transfection using the dual-luciferase reporter assay system (Promega Corporation), following the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla luciferase activity and the ratio of Firefly/Renilla luciferase in each group reflected the promoter activity. Each experiment was performed in triplicate.
DNA pull-down assay
The DNA pull-down was performed with a DNA pull-down test kit (catalog no. KT401), according to the manufacturer's protocol (Gzscbio, Guangzhou, China). Briefly, the sequences of −320 to −1 bp 5′-flanking region were amplified by PCR and tagged with biotin. The biotin-labeled promoter was bound with streptavidin magnetic beads (Dynabeads™ M-280 Streptavidin; Thermo Fisher Scientific, Inc.) at 4°C for 4 h. The non-biotinylated promoter was used as the negative control. All protein extracted from HCT-116 cells in the input group was used as the positive control. The bound promoter was incubated with 1 mg protein extracted from HCT-116 cells with gentle agitation at 4°C overnight. The bound beads-promoter protein complexes were washed with wash buffer, which was included in the kit, and separated by SDS-PAGE. Gel bands were visualized by silver staining.
Mass spectrometry (MS) and bioinformatics analysis
The proteins were digested by incubating with 0.02 µg/µl trypsin at 37°C overnight. The resulting peptides were extracted, purified and processed using a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Inc.). Proteins were identified by comparing the MS data with the Uniprot human protein sequence database (https://www.uniprot.org/taxonomy/9606) using the Mascot search engine (http://www.matrixscience.com/; V2.3.02). Finally, the protein identification results were verified by analyzing the protein quality, matches of the secondary spectrum of the protein, number of peptides matching the protein, protein abundance and protein description between samples. The proteins exhibiting differential binding to the biotin-labeled promoter and negative control, identified by MS, were analyzed using Gene Ontology (GO) functional enrichment (http://www.geneontology.org/), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses (https://www.kegg.jp/) and the AnimalTFDB database (version 2; bioinfo.life.hust.edu.cn/AnimalTFDB) for possible transcription factors (TFs). Proteins were considered significantly enriched in GO terms and KEGG pathways when P<0.05. The MS and bioinformatics analyses were performed by Sagene Biotech Co., Ltd. (Guangzhou, China).
Statistical analysis
The experimental data were analyzed using one-way analysis of variance and were presented as the mean ± standard deviation from three independent experiments using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Functional analysis of the promoter of the miR-32 gene
To investigate the mechanisms involved in the expression of miR-32, the 5′-flanking region of the host gene TMEM245 was dissected into a series of deletion fragments termed pGL3-1987 (−1987 to −1 bp), pGL3-1648 (−1648 to −1 bp), pGL3-1088 (−1088 to −1 bp), pGL3-606 (−606 to −1 bp) and pGL3-320 (−320 to −1 bp) (Fig. 1A). A dual luciferase reporter assay was performed to further detect the transcriptional activity of the fragments. Compared with the pGL3-basic vector, the luciferase activity in pGL3-320, pGL3-606, pGL3-1088, pGL3-1648 and pGL3-1987 was significantly increased in HCT-116 cells (P<0.05; Fig. 1B). The fragment −320 to −1 bp exhibited the most increased activity, indicating the presence of potential positive regulatory elements, which enhance miR-32 transcription in this region. However, a decrease in transcriptional activity in the pGL3-606 group compared with the pGL3-320 group (P<0.05) was observed (Fig. 1B), suggesting the presence of repressive regulatory elements in the region between position −606 and −320 bp.
Figure 1.
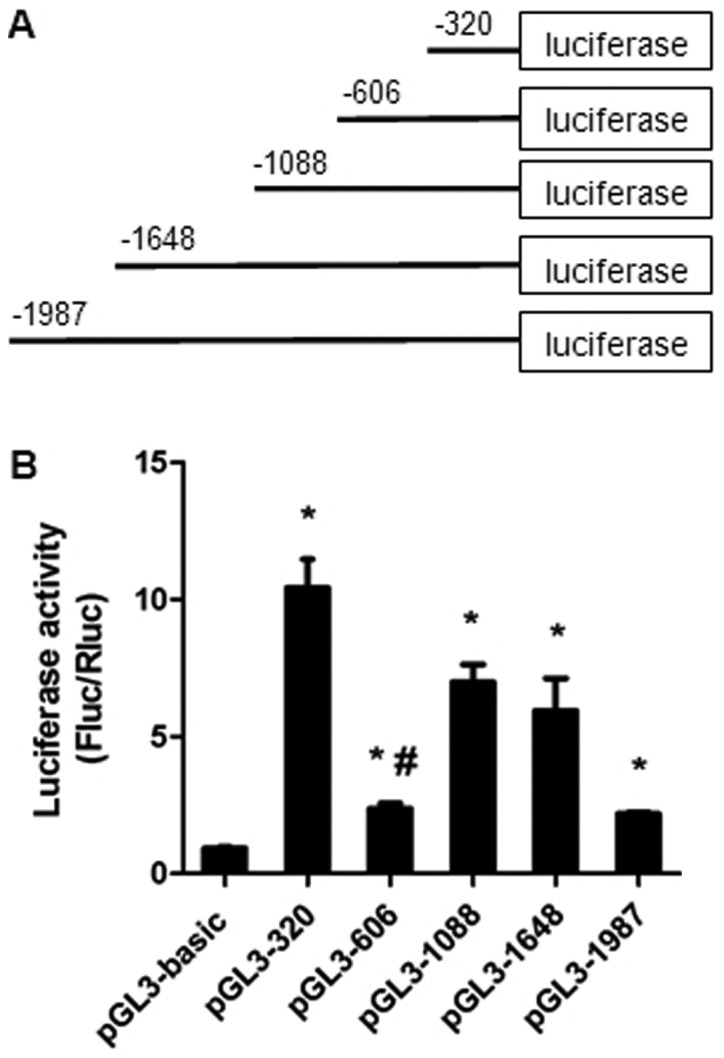
The luciferase activity of the truncated promoter of miR-32. (A) Schematics of each truncated promoter plasmids. (B) Dual luciferase reporter assays of miR-32 gene promoter constructs. Various recombinant vectors, including pGL3-1987, pGL3-1648, pGL3-1088, pGL3-606, pGL3-320 and pRL-TK, were co-transfected into HCT-116 cells. pRL-TK and pGL3-basic were used as internal and negative controls, respectively. Relative luciferase activity was determined by the ratio of Fluc/Rluc activity. Data presented as the mean ± standard deviation of three independent experiments. *P<0.05, compared with the pGL3-basic group. #P<0.05, compared with pGL3-320. Fluc, firefly luciferase; Rluc, Renilla luciferase; miR, microRNA.
Identification of promoter-binding proteins
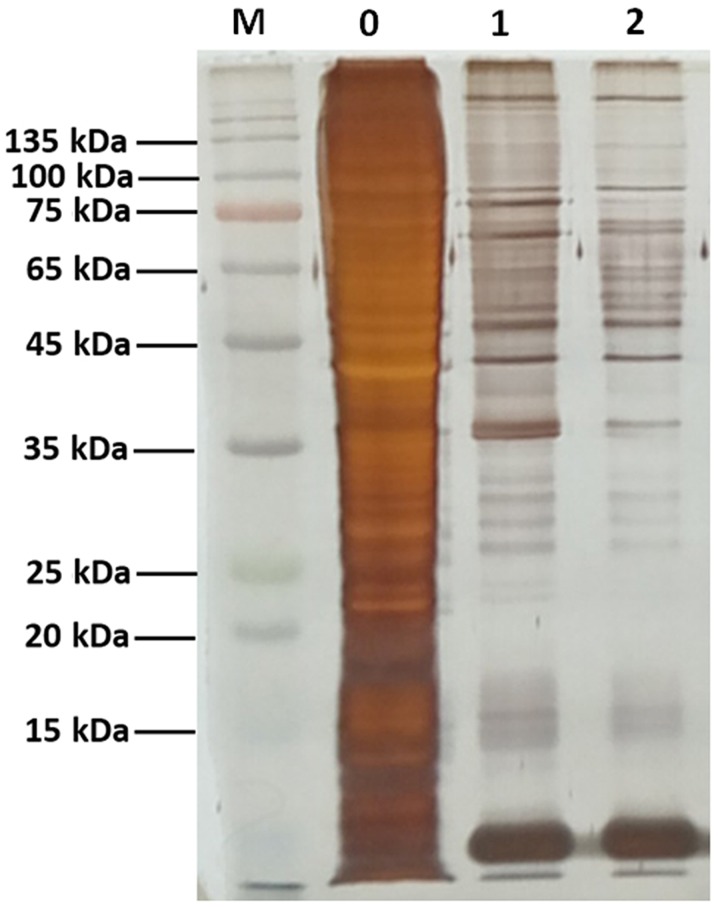
The DNA pull-down assay was used to identify putative interacting proteins binding to the −320 to −1 bp fragment using streptavidin magnetic beads coated with the biotin-labeled promoter. The proteins were subsequently analyzed by SDS-PAGE and silver staining (Fig. 2). Identification of the differentially binding proteins in the two groups by MS revealed that the binding factors of miR-32 promoter included 403 proteins. These proteins were further analyzed by bioinformatics tools.
Figure 2.
SDS-PAGE analysis of promoter-binding proteins obtained using the DNA pull-down assay. The gel was stained with silver staining. M, PageRuler pre-stained protein ladder; 0, input group, total protein extracted from HCT-116 cells; 1, biotin-labeled promoter-binding protein complexes obtained by pull-down assay; 2, non-biotinylated promoter-binding protein complexes obtained by pull-down assay (negative control).
GO, KEGG pathway and transcription factor analysis
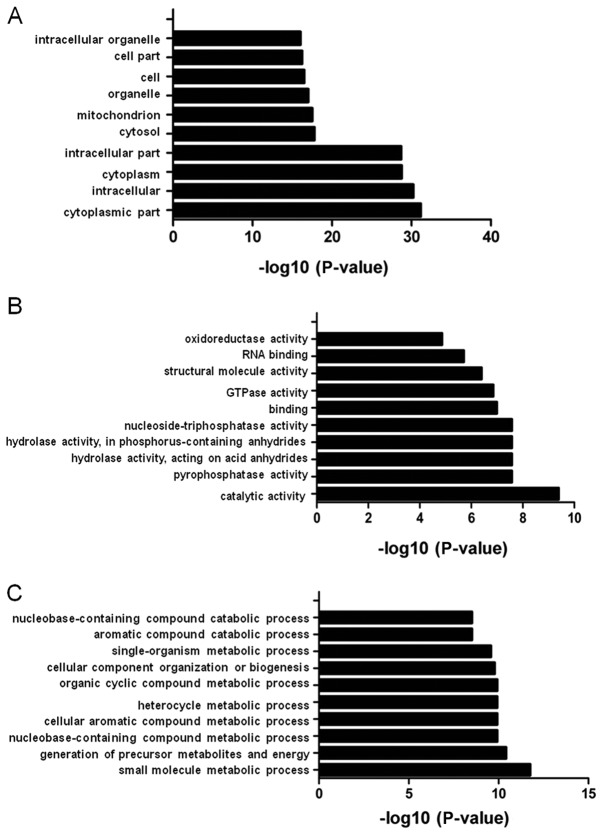
The 403 binding proteins were analyzed using GO enrichment analysis. The GO analysis classified the proteins into the following three functional categories: Biological process, cellular component and molecular function (Fig. 3). Under cellular component, the top ten GO terms were ‘cytoplasmic part’, ‘intracellular’, ‘cytoplasm’, ‘intracellular part’, ‘cytosol’, ‘mitochondrion’, ‘organelle’, ‘cell’, ‘cell part’ and ‘intracellular organelle’ (Fig. 3A). Under molecular function, the top ten GO terms were ‘catalytic activity’, ‘pyrophosphatase activity’, ‘hydrolase activity’, ‘acting on acid anhydrides’, ‘hydrolase activity’, ‘acting on acid anhydrides’, ‘in phosphorus-containing anhydrides’, ‘nucleoside-triphosphatase activity’, ‘binding’, ‘GTPase activity’, ‘structural molecule activity’, ‘RNA binding’ and ‘oxidoreductase activity’ (Fig. 3B). Under biological process, the top ten GO terms were ‘small molecule metabolic process’, ‘generation of precursor metabolites and energy’, ‘nucleobase-containing compound metabolic process’, ‘cellular aromatic compound metabolic process’, ‘heterocycle metabolic process’, ‘organic cyclic compound metabolic process’, ‘cellular component’, ‘organization or biogenesis’, ‘single-organism metabolic process’, ‘aromatic compound catabolic process’ and ‘nucleobase-containing compound catabolic process’ (Fig. 3C).
Figure 3.
Gene Ontology analysis of the binding proteins. (A) Cellular component. (B) Molecular function. (C) Biological process. GTP, guanosine triphosphate.
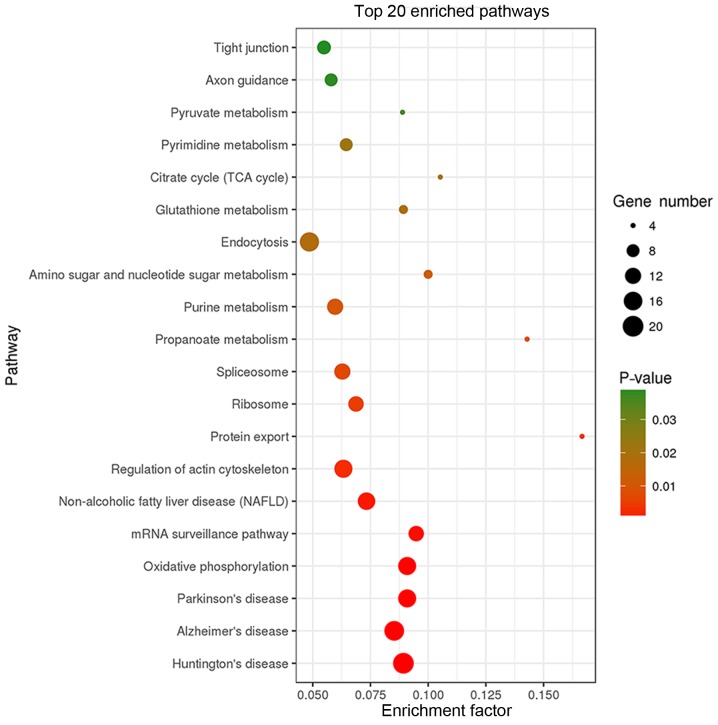
KEGG pathway analysis revealed that the 403 binding proteins identified were involved in various cellular processes, including ‘tight junction’, ‘oxidative phosphorylation’, ‘mRNA surveillance’, ‘actin cytoskeleton regulation’, ‘protein export’, and diseases, including ‘Huntington's disease’, ‘Alzheimer's disease’, ‘Parkinson's disease’, ‘non-alcoholic fatty liver disease’ (Fig. 4).
Figure 4.
Kyoto Encyclopedia of Genes and Genomes pathway analysis of the binding proteins. TCA, tricarboxylic acid.
Possible transcription factors (TFs) involved were predicted using the AnimalTFDB database. The analysis resulted in the identification of 10 potential interacting TFs of the miR-32 promoter (Table II).
Table II.
TFs potentially interacting with the microRNA-32 promoter as detected by mass spectrometry.
| Gene ID | TF symbol | Domain | TF name |
|---|---|---|---|
| ENSG00000170365 | SMAD1 | MH1 | SMAD family member 1 |
| ENSG00000061455 | PRDM6 | ZBTB | PR domain containing 6 |
| ENSG00000164916 | FOXK1 | Fork | Forkhead box K1 |
| ENSG00000165684 | SNAPC4 | MYB | Small nuclear RNA activating complex, polypeptide 4 |
| ENSG00000182359 | KBTBD3 | ZBTB | Kelch repeat and BTB (POZ) domain containing 3 |
| ENSG00000153048 | CARHSP1 | CSD | Calcium regulated heat stable protein 1 |
| ENSG00000167377 | ZNF23 | ZBTB | Zinc finger protein 23 |
| ENSG00000121297 | TSHZ3 | ZBTB | Teashirt zinc finger homeobox 3 |
| ENSG00000115415 | STAT1 | STAT | Signal transducer and activator of transcription 1 |
| ENSG00000136535 | TBR1 | T-box | T-box, brain, 1 |
TF, transcription factor.
Discussion
In recent years, a number of studies reported the potential role of miRNAs in different types of cancer (14–16). The characterization of dysregulated miRNAs in CRC may help to improve the understanding of carcinogenesis and develop treatments for the disease. Previous studies have demonstrated that the overexpression of miR-32 led to increased proliferation, migration, and invasion and reduced apoptosis of CRC cells via inhibition of the anti-oncogene PTEN (12,13). However, the regulation of miR-32 expression in CRC remains unknown. The aim of the current study was to investigate the regulation of miR-32 expression.
The expression of miRNAs is regulated by regulatory systems, including the promotors of their host genes, epigenetic regulation and TFs (17–21). Dysregulation of miRNA expression in different types of cancer may be due to an abnormal combination of TFs acting on the promoter regions or due to epigenetic changes, including aberrant DNA methylation and histone modification (22–24). Various stimuli in the external environment or signals at different stages of development may cause different TFs to bind to transcriptional regulatory elements, activating or inhibiting the transcription of miRNAs (25). Therefore, the identification of proteins interacting with the promoter of miRNAs as well as analysis of their function is important for investigating the transcriptional regulation of miRNAs. Zhu et al (21) demonstrated that TF Kruppel like factor 4 negatively regulated miR-106a expression by binding to the promoter of miR-106a. A study by Kumar et al (26) revealed that the TF myocyte enhancer factor-2 and hypermethylation and histone modifications may have contributed to the downregulation of the miR-379/miR-656 cluster in oligodendrogliomas, either acting independently or in synergy, in oligodendroglioma. Nuclear factor-κB bound to the promoter of miR-1275 and inhibited its transcription, in response to tumor necrosis factor α (TNF-α) stimulation (27). Another study reported that transforming growth factor β1 (TGFβ1) promoted the binding of mothers against decapentaplegic homolog (SMAD)4 to the miR-155 promoter at a site located 454 bp from the transcription start site, suggesting that miR-155 may be a transcriptional target of the TGFβ1/SMAD4 pathway (28).
miRNAs are transcribed by RNA polymerase II to generate the original transcript of miRNAs, called primary miRNAs (pri-miRNAs) (29–31). Drosha, an enzyme in the polymerase III family, processes the pri-miRNAs into a hairpin-like precursor miRNA (pre-miRNA) (29–31). The pre-miRNA is exported into the cytoplasm by exportin 5 and then cleaved by Dicer into 18–25 nucleotide double-stranded miRNAs, which are then unwound to generate mature miRNAs (29–31). Half of the known mammalian miRNA sequences are located in the introns of protein-coding host genes, referred to as intronic miRNAs (32). Such intron-derived miRNAs are commonly expressed coordinately and processed with their host gene transcripts (33). miR-32 is an intronic miRNA encoded by TMEM245, as described in the University of California, Santa Cruz Genome Browser (genome.ucsc.edu). Several intronic miRNAs are transcribed together with the host gene (18,34,35). Human papillomavirus type 16 E6 may regulate miR-23b, an intronic miRNA, indirectly through the methylation of its host gene TMEM245 (36). Lerner et al (37) demonstrated that deleted in lymphocytic leukemia 2 (DLEU2) acts as a host gene of miR-15a/miR-16-1, and the binding of the Myc to two alternative DLEU2 promoters represses both the host gene transcription and levels of mature miR-15a/miR-16-1. It is reported that the transcript levels of TMEM245 and miR-32 are positively correlated (38). Functional analysis of the promoter of miR-32 is required to understand the molecular mechanisms governing miR-32 gene expression. In the present study, the truncation analysis and luciferase reporter assays demonstrated that the cloned promoter fragment was capable of driving expression of the luciferase gene in transfected HCT-116 cells. The core promoter of miR-32 may be located within the −320 to −1 bp region which exhibited the highest luciferase activity. The regions spanning −606 to −320 bp potentially harbor negative regulatory elements as a significant decrease in promoter activity was observed. These data indicate that the miR-32 overexpression is due to the complex interactions between different regulatory elements and promoter.
A DNA pull-down assay in combination with MS was performed to identify the proteins that bind to the miR-32 gene promoter. In addition, bioinformatics analyses were performed to characterize the binding proteins. The binding proteins were involved in a variety of key biological processes, including ‘structural molecule activity’, ‘RNA binding’, ‘small molecule metabolic process’ and ‘biogenesis’. This suggested that these proteins may potentially serve a role in carcinogenesis. The KEGG pathway analysis revealed that the 403 binding proteins identified were involved in neuronal diseases. Yan et al (39) demonstrated that miR-32 promotes neuroinflammation and neuropathic pain development through regulation of dual-specificity phosphatase 5, and knockdown of miR-32 suppressed mechanical allodynia and heat hyperalgesia and decreased inflammatory cytokine [interleukin (IL)-1β, TNF-α and IL-6] protein expression in rats following spinal nerve ligation. Another study revealed that two single nucleotide polymorphisms in the host gene TMEM245 were involved in genetic loci strongly associated with schizophrenia (40). Since miR-32 and its host gene TMEM245 may be involved in the pathogenesis of nervous system-associated diseases, proteins binding to the miR-32 promoter may also be involved in the signaling pathways of nervous system diseases. The 403 binding proteins identified in the current study are also involved in other cellular processes, including ‘tight junction’, ‘oxidative phosphorylation’, ‘mRNA surveillance’ and ‘actin cytoskeleton regulation’. These pathways are also involved in the development of tumors (41–44), including pancreatic, ovarian and gastric cancer, which may be associated with pathogenesis of colorectal cancer.
TFs are a group of proteins that can regulate RNA transcription by binding to the promoter of the corresponding DNA sequence (45). TF analysis revealed 10 potential interacting TFs, including SMAD1, signal transducer and activator of transcription 1 (STAT1) and forkhead box K1 (Foxk1) among the binding proteins. Yang et al (46) revealed that SMAD1 promotes migration of CRC cells by inducing Snail and ajuba LIM protein expression simultaneously. The level of SMAD1 was significantly increased in CRC tissues, and was confirmed as significant predictor for overall survival (47). High STAT1 activity was significantly associated with longer patient overall survival in CRC (48). Wu et al (49) demonstrated that higher expression of Foxk1 could indicate a poor prognosis in patients with CRC since Foxk1 induces epithelial-mesenchymal transition (EMT) and promotes CRC cell invasion in vitro and in vivo; knockdown of Foxk1 inhibited TGF-β1-induced EMT. These transcription factors potentially serve a role in the pathogenesis of CRC, and further investigation is required to identify, verify and validate their involvement as binding proteins in miR-32 expression.
The current study demonstrated that the core promoter region of the human miR-32 gene is located in the region spanning −320 to −1 bp, and binding proteins, especially TFs, may be involved in the transcriptional regulation of this gene. These results provide insight into the mechanism of miR-32 gene regulation. Due to the limitations of bioinformatics, which are only based on bioinformatics predictions, it's not certain whether these proteins can affect the expression of miR-32. Further verifications, including gain-of-function and loss-of-function studies, and a chromatin immunoprecipitation assay, are required to clarify the function of possible binding proteins.
Acknowledgements
Not applicable.
Funding
This study was supported by Guangdong Natural Science Foundation of China (grant no. 2017A030313546).
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Authors' contributions
WW and YZ designed the experiments. WW, WT and SY performed the experiments. WW and JQ performed statistical analysis of the obtained data, and drafted the manuscript. YZ revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Peddareddigari V, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molnár B, Galamb O, Péterfia B, Wichmann B, Csabai I, Bodor A, Kalmár A, Szigeti KA, Barták BK, Nagy ZB, et al. Gene promoter and exon DNA methylation changes in colon cancer development-mRNA expression and tumor mutation alterations. BMC Cancer. 2018;18:695. doi: 10.1186/s12885-018-4609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai E, Nakajima A, Kaneda A. Accumulation of aberrant DNA methylation during colorectal cancer development. World J Gastroenterol. 2014;20:978–987. doi: 10.3748/wjg.v20.i4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun W, Wang X, Li J, You C, Lu P, Feng H, Kong Y, Zhang H, Liu Y, Jiao R, et al. MicroRNA-181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 2018;9:438. doi: 10.1038/s41419-018-0490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai KW, Lo YH, Liu H, Yeh CY, Chen YZ, Hsu CW, Chen WS, Wang JH. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol Cancer. 2018;17:72. doi: 10.1186/s12943-018-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taborda MI, Ramírez S, Bernal G. Circular RNAs in colorectal cancer: Possible roles in regulation of cancer cells. World J Gastrointest Oncol. 2017;9:62–69. doi: 10.4251/wjgo.v9.i2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pancione M, Remo A, Zanella C, Sabatino L, Di Blasi A, Laudanna C, Astati L, Rocco M, Bifano D, Piacentini P, et al. The chromatin remodelling component SMARCB1/INI1 influences the metastatic behavior of colorectal cancer through a gene signature mapping to chromosome 22. J Transl Med. 2013;11:297. doi: 10.1186/1479-5876-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirafkan N, Mansoori B, Mohammadi A, Shomali N, Ghasbi M, Baradaran B. MicroRNAs as novel biomarkers for colorectal cancer: New outlooks. Biomed Pharmacother. 2018;97:1319–1330. doi: 10.1016/j.biopha.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105:849–859. doi: 10.1093/jnci/djt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang PY, Chen CC, Chang YS, Tsai WS, You JF, Lin GP, Chen TW, Chen JS, Chan E. MicroRNA-223 and microRNA-92a in stool and plasma samples act as complementary biomarkers to increase colorectal cancer detection. Oncotarget. 2016;7:10663–10675. doi: 10.18632/oncotarget.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Zou K, Yu L, Zhao W, Lu Y, Mao J, Wang B, Wang L, Fan S, Song B, Li L. MicroRNA-140 inhibits the epithelial-mesenchymal transition and metastasis in colorectal cancer. Mol Ther Nucleic Acids. 2018;10:426–437. doi: 10.1016/j.omtn.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, Tan W, Wei G, Zhou Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30. doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, Yang P, Feng X, Wang H, Qiu Y, Tian T, He Y, Yu C, Yang J, Ye S, Zhou Y. The relationship between and clinical significance of MicroRNA-32 and phosphatase and tensin homologue expression in colorectal cancer. Genes Chromosomes Cancer. 2013;52:1130–1140. doi: 10.1002/gcc.22108. [DOI] [PubMed] [Google Scholar]
- 14.Porzycki P, Ciszkowicz E, Semik M, Tyrka M. Combination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognition. Int Urol Nephrol. 2018;50:1619–1626. doi: 10.1007/s11255-018-1938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Min L, Ren C, Xu X, Yang J, Sun X, Wang T, Wang F, Sun C, Zhang X. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS One. 2017;12:e0171751. doi: 10.1371/journal.pone.0171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Y, Wang KX, Xu H, Hong Y. Integrative miRNA analysis identifies hsa-miR-3154, hsa-miR-7-3, and hsa-miR-600 as potential prognostic biomarker for cervical cancer. J Cell Biochem. 2018;119:1558–1566. doi: 10.1002/jcb.26315. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Friedman JM, Chihara Y, Egger G, Chuang JC, Liang G. Epigenetic therapy upregulates the tumor suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem Biophys Res Commun. 2009;379:726–731. doi: 10.1016/j.bbrc.2008.12.098. [DOI] [PubMed] [Google Scholar]
- 18.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekala JR, Naushad SM, Ponnusamy L, Arivazhagan G, Sakthiprasad V, Pal-Bhadra M. Epigenetic regulation of miR-200 as the potential strategy for the therapy against triple-negative breast cancer. Gene. 2018;641:248–258. doi: 10.1016/j.gene.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhu M, Zhang N, Lu X, He S. Negative regulation of kruppel-like Factor 4 on microRNA-106a at upstream transcriptional level and the role in gastric cancer metastasis. Dig Dis Sci. 2018;63:2604–2616. doi: 10.1007/s10620-018-5143-z. [DOI] [PubMed] [Google Scholar]
- 22.Kuang Q, Li J, You L, Shi C, Ji C, Guo X, Xu M, Ni Y. Identification and characterization of NF-kappaB binding sites in human miR-1908 promoter. Biomed Pharmacother. 2015;74:158–163. doi: 10.1016/j.biopha.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Xu Z, Li B, Zhang Z, Luo H, Wang Y, Lu Z, Wu X. Epigenetic silencing of miRNA-9 is correlated with promoter-proximal CpG island hypermethylation in gastric cancer in vitro and in vivo. Int J Oncol. 2014;45:2576–2586. doi: 10.3892/ijo.2014.2667. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Ji G, Xiao X, Chen X, Qin WW, Yang F, Li YF, Fan LN, Xi WJ, Huo Y, et al. Epigenetically regulated miR-145 suppresses colon cancer invasion and metastasis by targeting LASP1. Oncotarget. 2016;7:68674–68687. doi: 10.18632/oncotarget.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagne JB, Mohtar OR, Campbell JD, Lakshminarayanan M, Huang J, Hinds AC, Lu J, Ramirez MI. Transcription factor and microRNA interactions in lung cells: An inhibitory link between NK2 homeobox 1, miR-200c and the developmental and oncogenic factors Nfib and Myb. Respir Res. 2015;16:22. doi: 10.1186/s12931-015-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Nayak S, Pathak P, Purkait S, Malgulawar PB, Sharma MC, Suri V, Mukhopadhyay A, Suri A, Sarkar C. Identification of miR-379/miR-656 (C14MC) cluster downregulation and associated epigenetic and transcription regulatory mechanism in oligodendrogliomas. J Neurooncol. 2018;139:23–31. doi: 10.1007/s11060-018-2840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou YF, Fu ZY, Chen XH, Cui Y, Ji CB, Guo XR. Tumor necrosis factor-α and interleukin-6 suppress microRNA-1275 transcription in human adipocytes through nuclear factor-κB. Mol Med Rep. 2017;16:5965–5971. doi: 10.3892/mmr.2017.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H, Zhang J, Shao H, Liu J, Jin M, Chen J, Huang Y. Transforming growth factor β1/smad4 signaling affects osteoclast differentiation via regulation of miR-155 expression. Mol Cells. 2017;40:211–221. doi: 10.14348/molcells.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sand M. The pathway of miRNA maturation. Methods Mol Biol. 2014;1095:3–10. doi: 10.1007/978-1-62703-703-7_1. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 31.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 33.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Zhang L, Sun T. Cohesive regulation of neural progenitor development by microRNA miR-26, its host gene ctdsp and target gene Emx2 in the mouse embryonic cerebral cortex. Front Mol Neurosci. 2018;11:44. doi: 10.3389/fnmol.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma N, Wang X, Qiao Y, Li F, Hui Y, Zou C, Jin J, Lv G, Peng Y, Wang L, et al. Coexpression of an intronic microRNA and its host gene reveals a potential role for miR-483-5p as an IGF2 partner. Mol Cell Endocrinol. 2011;333:96–101. doi: 10.1016/j.mce.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Yeung CL, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 suppresses microRNA-23b expression in human cervical cancer cells through DNA methylation of the host gene C9orf3. Oncotarget. 2017;8:12158–12173. doi: 10.18632/oncotarget.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerner M, Harada M, Lovén J, Castro J, Davis Z, Oscier D, Henriksson M, Sangfelt O, Grandér D, Corcoran MM. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp Cell Res. 2009;315:2941–2952. doi: 10.1016/j.yexcr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan T, Zhang F, Sun C, Sun J, Wang Y, Xu X, Shi J, Shi G. miR-32-5p-mediated Dusp5 downregulation contributes to neuropathic pain. Biochem Biophys Res Commun. 2018;495:506–511. doi: 10.1016/j.bbrc.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Aragam N, Li X, Villla EC, Wang L, Briones D, Petty L, Posada Y, Arana TB, Cruz G, et al. BCL9 and C9orf5 are associated with negative symptoms in schizophrenia: meta-analysis of two genome-wide association studies. PLoS One. 2013;8:e51674. doi: 10.1371/journal.pone.0051674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyuno D, Yamaguchi H, Ito T, Kono T, Kimura Y, Imamura M, Konno T, Hirata K, Sawada N, Kojima T. Targeting tight junctions during epithelial to mesenchymal transition in human pancreatic cancer. World J Gastroenterol. 2014;20:10813–10824. doi: 10.3748/wjg.v20.i31.10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo NL, Zhang JX, Wu JP, Xu YH. Isoflurane promotes glucose metabolism through up-regulation of miR-21 and suppresses mitochondrial oxidative phosphorylation in ovarian cancer cells. Biosci Rep. 2017;37(pii):BSR20170818. doi: 10.1042/BSR20170818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Geng Y, Feng R, Zhu Q, Miao B, Cao J, Fei S. The human RNA surveillance factor UPF1 modulates gastric cancer progression by targeting long non-coding RNA MALAT1. Cell Physiol Biochem. 2017;42:2194–2206. doi: 10.1159/000479994. [DOI] [PubMed] [Google Scholar]
- 44.Peng JM, Bera R, Chiou CY, Yu MC, Chen TC, Chen CW, Wang TR, Chiang WL, Chai SP, Wei Y, et al. Actin cytoskeleton remodeling drives epithelial-mesenchymal transition for hepatoma invasion and metastasis in mice. Hepatology. 2018;67:2226–2243. doi: 10.1002/hep.29678. [DOI] [PubMed] [Google Scholar]
- 45.Spitz F, Furlong EE. Transcription factors: From enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 46.Yang D, Hou T, Li L, Chu Y, Zhou F, Xu Y, Hou X, Song H, Zhu K, Hou Z, et al. Smad1 promotes colorectal cancer cell migration through Ajuba transactivation. Oncotarget. 2017;8:110415–110425. doi: 10.18632/oncotarget.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Liu Z, Tan J, Dong H, Zhang X. Multispectral imaging reveals hyper active TGF-β signaling in colorectal cancer. Cancer Biol Ther. 2018;19:105–112. doi: 10.1080/15384047.2017.1395116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordziel C, Bratsch J, Moriggl R, Knösel T, Friedrich K. Both STAT1 and STAT3 are favourable prognostic determinants in colorectal carcinoma. Br J Cancer. 2013;109:138–146. doi: 10.1038/bjc.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Peng Y, Wu M, Zhang W, Zhang M, Xie R, Zhang P, Bai Y, Zhao J, Li A, et al. Oncogene FOXK1 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Oncotarget. 2016;7:51150–51162. doi: 10.18632/oncotarget.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.