Abstract
The aim of the present study was to explore the expression of the cancer testis antigens New York-esophageal squamous cell carcinoma (NY-ESO)-1 and melanoma-associated antigen (MAGE)-A4 in high-grade soft-tissue sarcoma and to evaluate their association with the standard clinical-pathological features of surgically treated high-grade sarcoma patients. The study included 82 patients, and NY-ESO-1 and MAGE-A4 antigen expression was analyzed immunohistochemically. The results revealed NY-ESO-1- and MAGE-A4-positive staining in 58.8 and 52.9% of synovial sarcomas, and 55.6 and 0% of myxoid liposarcomas, respectively. In patients with synovial sarcoma, NY-ESO-1 and MAGE-A4 were expressed in 7 patients, only NY-ESO-1 was expressed in 3 patients, and only MAGE-A4 was expressed in 2 patients. Univariate analysis indicated that a significantly higher MAGE-A4 expression was observed in younger patients (P<0.001) and those with synovial sarcoma (P<0.001). Multivariate analysis indicated that significantly higher NY-ESO-1 expression was observed in patients with synovial sarcoma (P<0.01) and myxoid liposarcoma (P<0.01), and significantly higher MAGE-A4 expression was observed in patients with synovial sarcoma (P<0.01). In high-grade sarcomas, the 2- and 5-year overall survival rates based on Kaplan-Meier estimates were 100 and 81.3% in the NY-ESO-1-positive group, and 69.7 and 53.0% in the NY-ESO-1-negative group, respectively (P=0.049). It was also demonstrated that either NY-ESO-1 or MAGE-A4 was positive in 70.6% of synovial sarcomas. These results indicate that NY-ESO-1 and MAGE-A4 may be useful for the diagnosis of synovial sarcoma. The independent expression of NY-ESO-1 and MAGE-A4, which may help expand the pool of candidates for molecular-targeted immunotherapy, will be beneficial for synovial sarcoma patients.
Keywords: New York-esophageal squamous cell carcinoma-1, melanoma-associated antigen-4, high grade soft tissue sarcoma, cancer testis antigens, synovial sarcoma
Introduction
Soft-tissue sarcomas (STS) are classified into more than 100 different histologic subtypes by the World Health Organization, all of which are relatively rare (1). Surgical excision is the most effective treatment if the tumor is resectable. Over the past few decades, neoadjuvant/adjuvant chemotherapy has been introduced to obtain a better prognosis in select sarcoma patients. In particular, in the advanced setting, chemotherapy with doxorubicin and/or ifosfamide is a common approach. However, some sarcoma subtypes are resistant to chemotherapy, and despite appropriate treatment, approximately 50% of patients die from recurrent disease. After failure of anthracycline-based chemotherapy, novel therapeutic agents such as trabectedin, eribulin and pazopanib have recently emerged and improved the progression-free/overall survival (2). However, these therapeutic approaches have no long-term impact on the patients' survival. Thus, to improve the poor prognosis of STS, it is necessary to develop a new treatment strategy involving immunotherapy.
Cancer/testis antigen (CTA) is a category of tumor antigens with a normal expression restricted to male germ cells in the testis, not being found in adult somatic tissues. Characteristics commonly shared by CTAs, aside from the highly tissue-restricted expression profile, include their existence as multigene families, frequent mapping to chromosome X, the heterogeneous protein expression in a proportion of tumors of various types, a likely correlation with tumor progression, the induction of expression by hypomethylation and/or histone acetylation, and immunogenicity in cancer patients (3). Of note, the epitopes of CTAs are recognized by autologous T lymphocytes, which target the cancer cells. Therefore, CTAs have emerged over the last decade as a therapeutic target in cases of malignant disease (4).
Recently, the aberrant expression of New York esophageal squamous cell cancer-1 (NY-ESO-1) has been reported in a variety of neoplasms, including esophageal carcinoma, hepatocellular carcinoma, non-small cell lung cancer, and melanoma. There have been some reports of the NY-ESO-1 expression in STS. Endo reported that, among STS, myxoid liposarcomas showed the highest positivity for NY-ESO-1 (88%) (5), followed by synovial sarcomas (49%). Another study found that the NY-ESO-1 antigen was expressed in approximately 80% of synovial sarcoma patients (6).
Robbins et al (7) performed the first-in-man clinical trial using the adoptive transfer of autologous peripheral blood mononuclear cells that had been retrovirally transduced with an NY-ESO-1-reactive T-cell receptor (TCR) to heavily pretreated patients with advanced synovial sarcoma. Eleven of 18 patients with NY-ESO-1-positive synovial sarcomas (61%) who received autologous T cells transduced with an NY-ESO-1-reactive TCR demonstrated objective clinical responses (7). However, this treatment strategy is restricted to HLA-A*0201+ patients with sarcoma showing NY-ESO-1 expression. HLA-A*0201 was found in 96.3% of Caucasian patients but in only 49.5% of Japanese patients. Thus, less than half of Japanese synovial sarcoma patients are eligible for this adoptive cell therapy targeting NY-ESO-1. Therefore, another target molecule for adoptive cell therapy is necessary (8).
The melanoma antigen gene (MAGE) protein family is a large, highly conserved group of proteins that share a common MAGE homology domain. MAGE-A is a Type I MAGE, which are CTAs and in humans include the MAGE-A, MAGE-B, and MAGE-C subfamily members that are clustered on the X chromosome. Of note, the expression of many MAGE proteins is restricted to reproductive tissues, but they are aberrantly expressed in a wide variety of cancer types. Among MAGEs, MAGE-A4 has been found to be broadly expressed in many tumor types, including lung cancer (19–35%), breast cancer (13%), ovarian cancer (47%), colon cancer (22%), and esophageal cancer (60%) (9,10).
The MAGE protein family was originally discovered as antigens on tumor cells and developed as cancer immunotherapy targets. Kageyama et al (11) conducted a first-in-man Phase I clinical trial of TCR gene-transduced T-cell transfer in patients with recurrent MAGE-A4-expressing esophageal cancer. They showed that 3 of 10 patients who had minimal tumor lesions at the baseline survived for more than 27 months.
However, few reports have described the NY-ESO-1 and MAGE-A4 expression in STS. Therefore, the purpose of this study is to examine the NY-ESO-1 and MAGE-A4 expression in high-grade STS, to clarify whether or not the expression of MAGE-A4 was observed independently from that of NY-ESO-1, and to evaluate the relationship between the CTA expression and various clinicopathological features.
Patients and methods
Patient characteristics
A total of 82 patients with high-grade STS were included. All patients underwent surgical excision at the Department of Orthopaedic Surgery, Graduate School of Medicine, Mie University, between 1999 and 2010. The median age of the patients was 56.7 years, with a range of 12 to 94 years. The median follow-up period for all patients was 64.3 months, with a range of 2 to 201 months. The histological diagnoses included 23 undifferentiated pleomorphic sarcomas, 17 synovial sarcomas, 11 leiomyosarcomas, 10 malignant peripheral nerve sheath tumors, 9 myxoid liposarcomas, 7 myxofibrosarcomas, 2 rhabdomyosarcomas, 1 dedifferentiated liposarcoma, 1 pleomorphic liposarcoma, and 1 angiosarcoma. The mean size of these tumors was 8.0 cm (range 1–30 cm). The locations of the STS were as follows: The upper extremities in 8 patients, the trunks in 29, and the lower extremities in 45. The tumor depth of the STS was as follows: Superficial in 12 patients and deep-seated in 70 patients. Eighteen patients were classified as stage IIA, 9 as stage IIB, 41 as stage III and 14 as stage IV at the time of the diagnosis according to the AJCC staging guidelines (7th edition) (12). Eleven patients received adjuvant chemotherapy, 16 received radiation therapy, 18 received both adjuvant chemotherapy and radiation therapy, and 38 received no adjuvant treatment.
Clinical information was obtained by reviewing medical records. The study protocol was approved by the Ethics Committee of Mie University Hospital (Mie, Japan).
Antibodies
Monoclonal antibody against human NY-ESO-1, clone E978 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was diluted at 1:200. The monoclonal antibodies against human MAGE-A4 MCV (Mie Cancer Vaccine)-1 and MCV-4 were generated at our university from hybridomas made by cell fusion of the mouse myeloma cell line SP2/0 and splenocytes harvested from C/B F1 mice (CLEA Japan, Inc., Tokyo, Japan) immunized with recombinant MAGE-A4 protein. MCV-1 and MCV-4 recognize amino acids 255–277 and 71–95 of MAGE-A4, respectively. MCV-1 cross-reacts with MAGE-A2, MAGE-A4, and MAGE-A12 protein, and MCV-4 covers MAGE-A1 and MAGE-A4 protein. As the secondary antibody, peroxidase-labeled anti-rabbit or anti-mouse antibody (Histofine Simplestain Max PO; Nichirei, Tokyo, Japan) was used.
Tissue preparation and immunohistochemistry
Tissues specimens were made from biopsied or surgically excised samples obtained before adjuvant treatment. The tumor samples were immediately fixed in 10% phosphate-buffered formalin after excision and embedded in paraffin. A serial section from each specimen was stained with hematoxylin and eosin (H&E) for a histologic diagnosis. The NY-ESO-1 and MAGE-A4 expression was examined via immunohistochemical staining using the streptavidin-biotinylated complex method. After neutralization with endogenous peroxidase, specimens on glass slides were preincubated with blocking serum and then incubated overnight with the primary antibody. After washing three times with phosphate-buffered saline (PBS), the specimens were incubated with peroxidase-labeled anti-rabbit or anti-mouse antibody for 30 min at room temperature. The peroxidase activity was detected with diaminobenzidine (DAB; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and the slides were counterstained with hematoxylin. The specimens with >5% NY-ESO-1-positive tumor cells were classified as ‘NY-ESO-1-positive’ according to the previous study (13). Regarding the MAGE-A4, the specimens were classified as ‘positively stained’ when >5% of the area of the specimens was stained in a similar fashion by both anti-MAGE antibodies (MCV-1 and MCV-4).
Statistical analyses
The chi-squared test, extended Fisher's exact test and Mann-Whitney U test were used to analyze the correlation between the CTA expression and the patient parameters, including the histopathological findings. A multivariate analysis was performed using the logistic regression model. A survival analysis was performed using Kaplan-Meier curves. The survival was compared by the log-rank test. Statistical significance was determined if the 2-sided value of a test was <0.05. Statistical analyses were performed using the IBM SPSS Statistics software program, version 22 (IBM Corp., Armonk, NY, USA).
Results
The expression of NY-ESO-1 and MAGE-A4 protein in high-grade STS patients
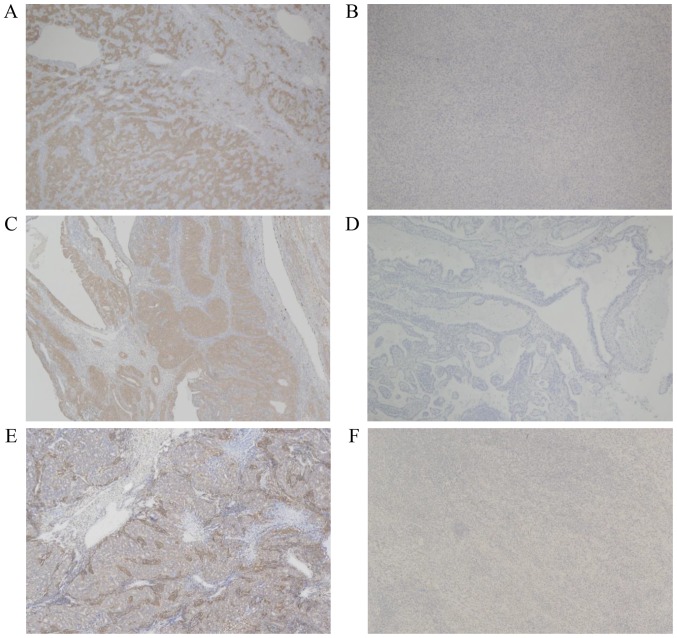
Both NY-ESO-1 and MAGE-A4 protein was stained in the cytoplasm of sarcoma cells, as previously reported (11) (Fig. 1). The tumor specimens of 16 patients (19.5%) were categorized as positively stained for NY-ESO-1. MAGE-A4 was expressed in 10 patients (12.2%). The NY-ESO-1 expression was observed in more than half of patients with synovial sarcoma (10/17, 58.8%) and myxoid liposarcoma (5/9, 55.6%), while the MAGE-A4 expression was observed in more than half of patients only with synovial sarcoma (9/17, 52.9%). The MAGE-A4 expression was not observed in patients with myxoid liposarcoma. The relationship between the histologic type and the expression of NY-ESO-1 and MAGE-A4 is summarized in Table I. In synovial sarcoma patients, both NY-ESO-1 and MAGE-A4 were expressed in seven patients, only NY-ESO-1 was expressed in three patients, and only MAGE-A4 was expressed in two patients.
Figure 1.
Microscopic results presenting the expression of NY-ESO-1 and MAGE-A4 in synovial sarcoma (magnification, ×40). (A and B) The clone E978 recognizes the NY-ESO-1 protein. (C and D) MCV-1 recognizes the MAGE-A2, MAGE-A4, and MAGE-A12 protein, and (E and F) MCV-4 recognizes the MAGE-A1 and MAGE-A4 protein. A case of positive staining with clone (A) E978, (C) MCV-1 and (E) MCV-4, respectively. A case of negative staining with clone (B) E978, (D) MCV-1 and (F) MCV-4, respectively. NY-ESO-1, New York-esophageal squamous cell carcinoma-1; MAGE-A4, melanoma-associated antigen-4; MCV, Mie Cancer Vaccine.
Table I.
NY-ESO-1 and MAGE-A4 expression among the sarcoma subtypes.
| Tumors examined, n/total n (%) | ||
|---|---|---|
| Pathological diagnoses | NY-ESO-1-positive | MAGE-A4-positive |
| Undifferentiated pleomorphic sarcoma | 0/23 (0) | 0/23 (0) |
| Synovial sarcoma | 10/17 (58.8) | 9/17 (52.9) |
| Leiomyosarcoma | 1/11 (9.1) | 1/11 (9.1) |
| Malignant peripheral nerve sheath tumor | 0/10 (0) | 0/10 (0) |
| Myxoid liposarcoma | 5/9 (55.6) | 0/9 (0) |
| Myxofibrosarcoma | 0/7 (0) | 0/7 (0) |
| Rhabdomyosarcoma | 0/2 (0) | 0/2 (0) |
| Dedifferentiated liposarcoma | 0/1 (0) | 0/1 (0) |
| Pleomorphic liposarcoma | 0/1 (0) | 0/1 (0) |
| Angiosarcoma | 0/1 (0) | 0/1 (0) |
| Total | 16/82 (19.5) | 10/82 (12.2) |
NY-ESO-1, New York-esophageal squamous cell carcinoma-1; MAGE-A4, melanoma-associated antigen-4.
Univariate and multivariate analyses investigating the correlation between the CTA expression and clinical features
A possible association between the immunohistochemical expression of NY-ESO-1 and MAGE-A4 antigens and the clinicopathological features of sarcoma, including patients' age, sex, tumor location, tumor depth, tumor stage, tumor size, and histological tumor type (synovial sarcoma or myxoid liposarcoma), is presented in Table II. The univariate analysis indicated that a significantly higher NY-ESO-1 expression was observed in younger patients (P<0.001) and those with synovial sarcoma (P<0.001) and myxoid liposarcoma (P=0.004). Furthermore, a significantly higher MAGE-A4 expression was observed in younger patients (P<0.001) and those with synovial sarcoma (P<0.001). The multivariate analysis indicated that a significantly higher NY-ESO-1 expression was observed in patients with synovial sarcoma [odds ratio (OR): 78.57, 95% confidence interval (CI): 8.698–709.79, P<0.01] and myxoid liposarcoma (OR: 68.75, 95% CI: 6.396–739.03, P<0.01), and a significantly higher MAGE-A4 expression was observed in patients with synovial sarcoma (OR: 72.0, 95% CI: 8.035–645.16, P<0.01). There were no significant relationships between the CTA expression and patients' sex, tumor location, tumor depth, tumor stage, or tumor size.
Table II.
Univariate analysis investigating the correlation between cancer/testis antigen expression and the patients' clinical features.
| NY-ESO-1 | MAGE-A4 | |||||
|---|---|---|---|---|---|---|
| Factors | (+) | (−) | P-value | (+) | (−) | P-value |
| Mean age, years | 36.7 | 61.4 | <0.001 | 35.8 | 60 | <0.001 |
| Sex, n | ||||||
| Male | 7 | 42 | 0.146 | 6 | 43 | 0.987 |
| Female | 9 | 24 | 4 | 29 | ||
| Tumor location, n | ||||||
| Extremity | 10 | 43 | 0.842 | 5 | 48 | 0.302 |
| Trunk | 6 | 23 | 5 | 24 | ||
| Tumor depth, n | ||||||
| Superficial | 1 | 11 | 0.29 | 0 | 12 | 0.162 |
| Deep-seated | 15 | 55 | 10 | 60 | ||
| Tumor stage, n | ||||||
| I or II | 4 | 24 | 0.39 | 3 | 25 | 0.768 |
| III or IV | 12 | 42 | 7 | 47 | ||
| Tumor size, cm | ||||||
| ≤5 | 5 | 27 | 0.477 | 4 | 28 | 0.946 |
| >5 | 11 | 39 | 6 | 44 | ||
| Synovial sarcoma | ||||||
| Yes | 10 | 7 | <0.001 | 9 | 8 | <0.001 |
| No | 6 | 59 | 1 | 64 | ||
| Myxoid liposarcoma | ||||||
| Yes | 5 | 4 | 0.004 | 0 | 9 | 0.236 |
| No | 11 | 62 | 10 | 73 | ||
| Total n | 16 | 66 | 10 | 72 | ||
NY-ESO-1, New York-esophageal squamous cell carcinoma-1; MAGE-A4, melanoma-associated antigen-4.
Relationship between the patients' survival and the CTA expression
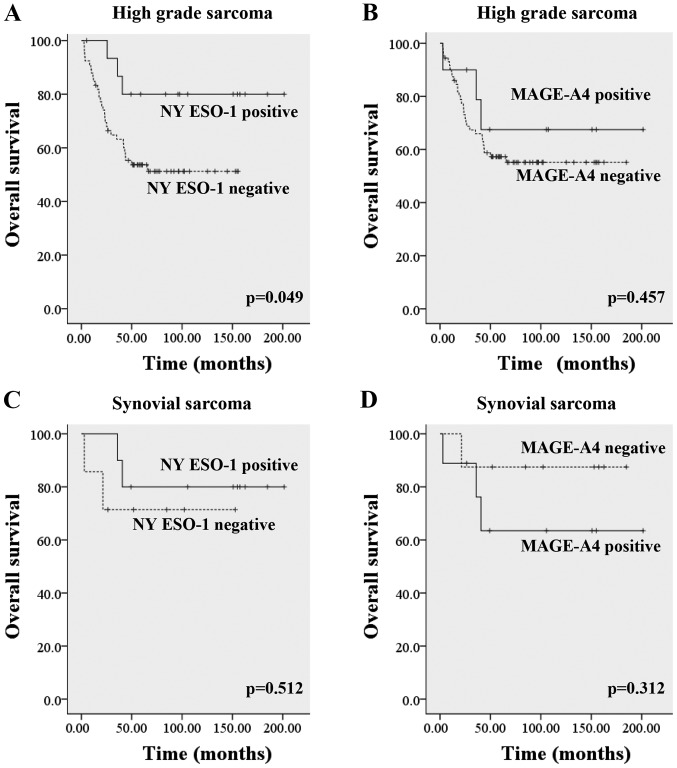
In high-grade sarcomas, the 2- and 5-year overall survival rates based on Kaplan-Meier estimates were 100 and 81.3% in the NY-ESO-1-positive group and 69.7 and 53.0% in the NY-ESO-1-negative group, respectively (P=0.049). The 2-, 5-years-overall survival rates were 90.0 and 70.0% in MAGE-A4 positive group, and 73.6 and 56.9% in MAGE-A4 negative group, respectively (P=0.457). When the histologic diagnosis was restricted to synovial sarcoma, the 2-, 5-years-overall survival rates were 100 and 80% in NY-ESO-1-positive group, and 71.4 and 71.4% in NY-ESO-1-negative group (P=0.512). The 2-, 5-years-overall survival rate of MAGE-A4 positive synovial sarcoma was 88.9 and 66.7% and those of MAGE-A4 negative synovial sarcoma were 87.5 and 87.5% (P=0.312) (Fig. 2). There were no significant differences between CTA expression and metastasis free survival rates/recurrent free survival rates (data not shown).
Figure 2.
Overall survival in regard to the expression of NY-ESO-1 and MAGE-A4 antigens. (A) The overall survival in regard to the expression of the NY-ESO-1 antigen in patients with high-grade sarcoma. (B) The overall survival in regard to the expression of the MAGE-A4 antigen in patients with high-grade sarcoma. (C) The overall survival in regard to the expression of the NY-ESO-1 antigen in patients with synovial sarcoma. (D) The overall survival in regard to the expression of the MAGE-A4 antigen in patients with synovial sarcoma. NY-ESO-1, New York-esophageal squamous cell carcinoma-1; MAGE-A4, melanoma-associated antigen-4.
Discussion
In this study, we found that NY-ESO-1 was expressed in more than half of synovial sarcomas and myxoid liposarcomas. Furthermore, we showed that MAGE-A4 was expressed in more than half of synovial sarcomas. The expression of MAGE-A4 was not observed in other types of STS, including myxoid liposarcomas. This specific expression of MAGE-A4 in the synovial sarcoma has not been described in previous reports. Either NY-ESO-1 or MAGE-A4 was positive in 70.6% of synovial sarcomas.
Jungbluth et al (14) first investigated the NY-ESO-1 expression in synovial sarcoma and found that 80% (20/25) of samples expressed NY-ESO-1. Recently, Endo et al (5) and Lai et al (6) reported that the NY-ESO-1 gene expression was observed in approximately 49–76% of synovial sarcoma tissue samples. Previous studies reported the NY-ESO-1 expression in 88–100% of myxoid liposarcomas; these results were based on sample sizes ranging from 25 to 158 cases (15,16). Our study revealed that 10 of 17 (58.8%) untreated synovial sarcoma and 5 of 9 (55.6%) untreated myxoid liposarcomas were positive for NY-ESO-1. Our data concerning the NY-ESO-1 expression in STS are almost identical to the previously reported findings.
Recently, Iura et al (17) showed that MAGE-A4 was expressed in 89 of 108 (82%) synovial sarcoma patients. However, there seems to be a difference in the rate of the MAGE-A4 expression between our study (52.9%) and Iura's (82%). This may be due to differences in the antibodies used in the immunostaining. Because we were unable to obtain stable results with several commercially available antibodies against MAGE-A4 protein, we generated two types of monoclonal antibodies against human MAGE-A4: MCV-1 and MCV-4. MCV-1 and MCV-4 recognize amino acids 255–277 and 71–95 of MAGE-A4, respectively. Since the MAGE-A family consists of 12 members (1.2B, 3,4,5,6,7,8,9,10,11,12), the cross-reactivity was strictly checked. MCV-1 cross-reacted to MAGE-A2, MAGE-A4, and MAGE-A12 protein, while MCV-4 cross-reacted to MAGE-A1 and MAGE-A4 protein. We therefore carefully observed the specimens and defined ‘positive staining’ as the sample being stained with both antibodies. We suspect that the cross-reactivity of the antibody may have led to the high positive rate of MAGE-A4 expression in Iura's report. To our knowledge, there have been no reports about the MAGE-A4 expression in various types of STS. We showed for the first time that MAGE-A4 was expressed in more than half of synovial sarcoma patients, although these results were limited to this particular lesion.
The univariate analyses showed that the age of the patients was a factor significantly correlated with the expression of NY-ESO-1. However, this finding is due to tendencies associated with each specific type of sarcoma, as the NY-ESO-1-positive group contained many young patients with synovial sarcoma. Of note, the multivariate analyses showed that the expression of NY-ESO-1 were correlated with only synovial sarcoma and myxoid liposarcoma. These results suggest that immunohistochemical staining of NY-ESO-1 and MAGE-A4 is useful for the diagnosis of synovial sarcoma and myxoid liposarcoma.
In synovial sarcoma patients, both NY-ESO-1 and MAGE-A4 were expressed in seven patients, only NY-ESO-1 was expressed in three patients, and only MAGE-A4 was expressed in two patients. The expression of either NY-ESO-1 or MAGE-A4 was observed in 70.6% of synovial sarcoma patients. These findings suggest that NY-ESO-1 and MAGE-A4 are regulated under different as-yet-unidentified mechanisms and are independently expressed in synovial sarcoma cells. The independent expression of NY-ESO-1 and MAGE-A4, which may help expand the pool of candidates for molecular-targeted immunotherapy, will be beneficial for synovial sarcoma patients.
In high-grade sarcomas, the overall survival rate was significantly better in patients with NY-ESO-1-positive tumors than in those with NY-ESO-1-negative tumors. A tendency toward a favorable prognosis was found in MAGE-A4-positive patients compared with MAGE-A4-negative patients. These findings are most likely due to the fact that the NY-ESO-1-positive and MAGE-A4-positive groups contained high proportions of synovial sarcoma patients and myxoid liposarcoma patients with relatively good prognoses. Among synovial sarcoma patients, those with NY-ESO-1-positive lesions tended to have a more favorable prognosis than those with NY-ESO-1-negative lesions. The expression of NY-ESO-1 may induce the activation of T cell activity, leading to an anti-tumor response. Moreover, expression of human leukocyte antigen (HLA) class I and tumor infiltrating lymphocyte (TIL) are important. Downregulation of HLA class I was associated with poor CD8+ infiltration and unfavorable prognosis with Ewing's sarcoma (18). HLA class I expression was associated with improved overall and event-free survival compared to HLA class I-negative osteosarcoma which underscores the importance of the immune systems response to sarcoma (19). Since CD8+ lymphocytes can kill tumor cells presenting tumor associated antigen, CD8+ lymphocytes are essential for adaptive tumor immunity. As a result, a high infiltration of CD8+ TILs has a positive impact on the clinical course of many types of cancer (20). Fujii et al (21) reported that a higher infiltration of CD8+ lymphocytes was associated with a favorable prognosis and correlated with distant metastasis-free survival in patients with angiosarcoma. Oike et al (22) reported that negative expression of HLA class I showed a trend toward lower CD8+ infiltration and unfavorable prognosis with synovial sarcoma patients. In the current study, the infiltration of TIL and HLA class I expression were not assessed. Further study is necessary to clarify the relationship between NY-ESO-1/MAGE-A4 expression and intra-tumoral immunologic status.
In contrast, among synovial sarcoma patients, those with MAGE-A4-negative lesions tended to have a more favorable prognosis than those with NY-ESO-1-positive lesions. The MAGE-A4 expression has been reported to correlate with a poor prognosis in many tumor types, including non-small cell lung carcinoma and bladder cancer. (9,23,24). Doyle et al (25) showed that a defining biochemical function of MAGEs is their ability to bind to specific E3 RING ubiquitin ligases through their MHDs. Thus, the aberrant expression of MAGEs in tumor cells can lead to changes in cellular processes and signaling pathways through ubiquitination, along with other activities, which may contribute to tumorigenesis.
This study has several limitations that need to be discussed. First, this was a retrospective study; as such, there may have been a selection bias when enrolling patient. Second, the current series included various stages and sarcoma subtypes. Future investigations should focus on synovial sarcomas and mxyoid liposarcoma. Third, given the small number of samples tested in the current study, larger numbers of STS patients are required to determine the clinical implications of the CTA expression.
Although the number of cases was small in our study, the present findings are the first to suggest that MAGE-A4 may be an attractive target for adoptive T cell therapy in synovial sarcoma patients. A large, prospective cohort study should be conducted in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B) (grant no. JP15K199930.
Availability of date and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TK and AM conceived and designed the study, and wrote, edited and reviewed the manuscript. TK, AM and TI researched and analyzed data. KA, TM and TN collected the data. SK, HI, HS generated the many types of clones of anti-MAGE mouse monoclonal antibody, and examined the specificity for the MAGE-A4 antigen. AS made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. TK, AM, TI, KA, TM, TN, SK, HI, HS and AS gave final approval for publication. AM takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Clinical information was obtained by reviewing medical records. The study protocol was approved by the Ethics Committee of Mie University Hospital (Mie, Japan). Written informed consent was obtained from all individual participants included in the study.
Patient consent for publication
Written informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rosenberg AE. WHO classification of soft tissue and bone, fourth edition: Summary and commentary. Curr Opin Oncol. 2013;25:571–573. doi: 10.1097/01.cco.0000432522.16734.2d. [DOI] [PubMed] [Google Scholar]
- 2.Azvolinsky A. Heterogeneous and rare: Toward histology-specific treatment of soft-tissue sarcoma. J Natl Cancer Inst. 2016;108:djw161. doi: 10.1093/jnci/djw161. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 4.Burgess M, Tawbi H. Immunotherapeutic approaches to sarcoma. Curr Treat Options Oncol. 2015;16:26. doi: 10.1007/s11864-015-0345-5. [DOI] [PubMed] [Google Scholar]
- 5.Endo M, de Graaff MA, Ingram DR, Lim S, Lev DC, Briaire-de Bruijn IH, Somaiah N, Bovée JV, Lazar AJ, Nielsen TO. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol. 2015;28:587–595. doi: 10.1038/modpathol.2014.155. [DOI] [PubMed] [Google Scholar]
- 6.Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, Rosenberg SA, Miettinen MM, Lee CC. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: Significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol. 2012;25:854–858. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Viña MA, Falco M, Sun Y, Stastny P. DNA typing for HLA class I alleles: I. Subsets of HLA-A2 and of -A28. Hum Immunol. 1992;33:163–173. doi: 10.1016/0198-8859(92)90068-X. [DOI] [PubMed] [Google Scholar]
- 9.Weon JL, Potts PR. The MAGE protein family and cancer. Curr Opin Cell Biol. 2015;37:1–8. doi: 10.1016/j.ceb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M, Doki Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine. 2014;32:5901–5907. doi: 10.1016/j.vaccine.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kageyama S, Ikeda H, Miyahara Y, Imai N, Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, et al. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin Cancer Res. 2015;21:2268–2277. doi: 10.1158/1078-0432.CCR-14-1559. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 13.Balafoutas D, zur Hausen A, Mayer S, Hirschfeld M, Jaeger M, Denschlag D, Gitsch G, Jungbluth A, Stickeler E. Cancer testis antigens and NY-BR-1 expression in primary breast cancer: Prognostic and therapeutic implications. BMC Cancer. 2013;13:271. doi: 10.1186/1471-2407-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, Chen YT, Stockert E, Ladanyi M, Old LJ. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 15.Pollack SM, Jungbluth AA, Hoch BL, Farrar EA, Bleakley M, Schneider DJ, Loggers ET, Rodler E, Eary JF, Conrad EU, III, et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer. 2012;118:4564–4570. doi: 10.1002/cncr.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemminger JA, Iwenofu OH. NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms. Mod Pathol. 2013;26:1204–1210. doi: 10.1038/modpathol.2013.65. [DOI] [PubMed] [Google Scholar]
- 17.Iura K, Maekawa A, Kohashi K, Ishii T, Bekki H, Otsuka H, Yamada Y, Yamamoto H, Harimaya K, Iwamoto Y, Oda Y. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum Pathol. 2017;61:130–139. doi: 10.1016/j.humpath.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Yabe H, Tsukahara T, Kawaguchi S, Wada T, Torigoe T, Sato N, Terai C, Aoki M, Hirose S, Morioka H, Yabe H. Prognostic significance of HLA class I expression in Ewing's sarcoma family of tumors. J Surg Oncol. 2011;103:380–385. doi: 10.1002/jso.21829. [DOI] [PubMed] [Google Scholar]
- 19.Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006;97:1374–1380. doi: 10.1111/j.1349-7006.2006.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, et al. CD8+ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 22.Oike N, Kawashima H, Ogose A, Hotta T, Hatano H, Ariizumi T, Sasaki T, Yamagishi T, Umezu H, Endo N. Prognostic impact of the tumor immune microenvironment in synovial sarcoma. Cancer Sci. 2018;109:3043–3054. doi: 10.1111/cas.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida N, Abe H, Ohkuri T, Wakita D, Sato M, Noguchi D, Miyamoto M, Morikawa T, Kondo S, Ikeda H, Nishimura T. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens and T cell infiltration in non-small cell lung carcinoma and their prognostic significance. Int J Oncol. 2006;28:1089–1098. [PubMed] [Google Scholar]
- 24.Bergeron A, Picard V, LaRue H, Harel F, Hovington H, Lacombe L, Fradet Y. High frequency of MAGE-A4 and MAGE-A9 expression in high-risk bladder cancer. Int J Cancer. 2009;125:1365–1371. doi: 10.1002/ijc.24503. [DOI] [PubMed] [Google Scholar]
- 25.Doyle JM, Gao J, Wang J, Yang M, Potts PR. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–974. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]