Abstract
Recently, a tumor-autonomous cytochrome P450 (CYP)-3A5-mediated resistance to cancer therapy has been demonstrated in pancreatic ductal adenocarcinoma. Expression of CYP3A5, which is involved in the degradation of irinotecan, has also been reported in colorectal cancer (CRC). The aim of the present study was to analyze CYP3A5 expression in the normal colon, colon adenoma, CRC and normal tissues, as well as to examine whether CYP3A5 expression in CRC has an impact on tumor response to irinotecan treatment. Immunohistochemistry was used to assess 85 tissue samples from 65 patients with CRC, along with 15 samples of normal colon and 45 samples of colon adenoma (including tubular, tubulovillous, and sessile serrated adenomas), and a tissue microarray (TMA) comprised of 26 different normal tissue types. Expression of CYP3A5 was evaluated with a semi-quantitative score. Tumor response to irinotecan therapy was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines. In normal tissues, CYP3A5 was expressed in epithelial cells of the colon, gallbladder, kidney, liver, small intestine, stomach, thyroid gland and tonsil, as well as in nerves. Expression in colon mucosa was heterogeneous, with only weak staining in the minority of specimens. CYP3A5 exhibited markedly higher expression in adenomas compared with normal colon tissues. A statistically significant inverse correlation was identified between CYP3A5 expression in CRC tissues and tumor response to irinotecan therapy. Irinotecan treatment itself did not alter CYP3A5 expression in CRC tissues. As CYP3A5 is involved in the degradation of irinotecan, the significantly higher intratumoral expression of CYP3A5 in patients with CRC who do not respond to irinotecan-based chemotherapy may indicate a causal role of CYP3A5 in tumor resistance.
Keywords: colorectal cancer, cytochrome P450 3A5, irinotecan, resistance, colorectal adenoma, immunohistochemistry, RECIST
Introduction
Cytochrome P450 (CYP) enzymes are involved in the metabolism of the majority of therapeutic drugs (1). While the majority of CYP enzymes are expressed in the endoplasmic reticulum and inner mitochondrial membrane of liver cells, these enzymes can also be found in extrahepatic tissues (2,3) contributing to the metabolism of xenobiotic compounds (4). The tissue-specific expression of CYP enzymes has a major influence on the sensitivity and exposure of a particular organ to a given drug (3).
The role of CYP enzymes in the metabolism of anticancer agents and the efficacy of cancer therapy has been the subject of investigation for years (5). Inter- and intraindividual differences in the expression and activity of CYP enzymes have been demonstrated in several tumor tissues (6,7).
Recently, Noll et al (8) demonstrated a tumor-autonomous CYP-mediated resistance to therapy with paclitaxel, dasatinib, and erlotinib in pancreatic ductal adenocarcinoma. Further research revealed that CYP3A5 was also expressed in other malignancies including rectal adenocarcinoma and colon adenoma (8), suggesting that CYP3A5 played a similar role in these malignancies.
With an estimated 1.4 million new cases in 2012, colorectal cancer (CRC) is one of the most commonly diagnosed cancer types (9). The main goal for localized CRC therapy is surgical resection; however, advanced stages are treated with different chemotherapy protocols using the agents 5-fluorouracil (+/−leucovorin), irinotecan (CPT-11), and oxaliplatin, as well as monoclonal antibodies (10–12).
Irinotecan is part of the standard treatment regimen for metastatic CRC (13) and is usually administered as part of a combination therapy, e.g., with 5-fluorouracil/folinic acid (or capecitabine) (FOLFIRI/XELIRI), 5-fluorouracil/folinic acid/oxaliplatin (FOLFOXIRI) and/or with monoclonal antibodies against vascular endothelial growth factor (VEGF; bevacizumab) or epidermal growth factor receptor (EGFR; cetuximab, panitumumab) in selected patients with RAS wild-type CRC (14). Irinotecan can be administered as part of first-line treatment, but also in all later lines of sequential CRC therapy. First approved in France in 1995, the topoisomerase I inhibitor irinotecan has since been approved in ~80 countries (13).
Although no deterioration in quality-of-life scores has been reported with chemotherapy including irinotecan (13), administration of irinotecan is often associated with potentially lethal side effects, mainly diarrhea and neutropenia (13,15).
Irinotecan acts as a prodrug and is activated by carboxylesterases into 7-ethyl-10-hydroxy-camptothecin (SN-38), which is ~100- to 1,000-fold more toxic and inhibits topoisomerase I, leading to deoxyribonucleic acid (DNA) breaks. The active metabolite SN-38 can be deactivated either by glucuronidation in hepatic and extrahepatic tissues, or by CYP3A4- and CYP3A5-dependent oxidation, forming the inactive metabolites 7-ethyl-10 [4-N-(5-aminopentanoicacid)-1-piperidino] carbonyloxycamptothecin (APC) and 7-ethyl-10 [4-amino-1-piperidino] carbonyloxycamptothecin (NPC). CYP3A induction has been demonstrated to cause decreased formation of SN-38 (15).
CYP3A5 is the most frequently expressed CYP3A isoform in extrahepatic tissues, suggesting an important role for this isoform in local metabolism (16). The presence of CYP3A5 has been demonstrated in normal colon (3,17,18), colon adenoma (19), and CRC (20). The CYP3A5*3 polymorphism, which can lead to reduced enzyme activity, has been associated with significantly longer progression-free survival in patients with metastatic CRC (21).
The potential of cancer therapy is impaired by difficulties in predicting both tumor response and adverse events (6). The purpose of the present study was to systematically analyze CYP3A5 protein expression in normal colon, colon adenomas, CRC, and additional normal tissues, and to evaluate whether CYP3A5 expression in CRC tissue determines tumor response to irinotecan therapy.
Materials and methods
Patient material
Tissue samples were stored in the tissue bank of the National Center for Tumor Diseases (NCT; Heidelberg, Germany) and used with the approval of the ethics committee of Heidelberg University (206/2005). All tissues were fixed in formalin and embedded in paraffin.
We examined tissue microarrays (TMAs) comprising normal tissues (Table I). Duplicate cores were available for each specimen on the TMA. Whole tissue slides of normal colon of 15 patients and colon adenomas of 45 patients (15 tubular adenomas, 15 tubulovillous adenomas, 15 sessile serrated adenomas) were also examined.
Table I.
Composition of tissue microarrays.
| Tissue type | Number of specimens (n) |
|---|---|
| Adipose tissue | 10 |
| Adrenal gland | 3 |
| Appendix | 4 |
| Colon | 17 |
| Endometrium | 2 |
| Esophagus | 13 |
| Gallbladder | 10 |
| Heart | 13 |
| Kidney | 17 |
| Liver | 14 |
| Lung | 10 |
| Muscle | 7 |
| Myometrium | 5 |
| Ovary | 6 |
| Pancreas | 7 |
| Prostate | 13 |
| Salivary gland | 8 |
| Skin | 5 |
| Small intestine | 13 |
| Spleen | 14 |
| Stomach | 14 |
| Testis | 12 |
| Thymus | 6 |
| Thyroid gland | 11 |
| Tonsil | 5 |
| Urinary bladder | 4 |
The examined normal tissue types and number of specimens per tissue type on tissue microarrays. Duplicate cores were available for each specimen.
In addition to the abovementioned tissues, 85 whole tissue slides of CRC tissues from 65 patients undergoing surgery or biopsy (population P1), were also obtained from the tissue bank of the NCT. Of these tissue samples, 48 were specimens from primary CRC and 37 were specimens from metastases. Regarding treatment exposure, 68 CRC tissues were irinotecan-naïve, while 17 were procured following treatment with irinotecan. The present study was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg (reference no. 206/2005).
To assess a possible correlation between CYP3A5 expression and tumor response to irinotecan treatment, tissues of a subpopulation of P1 including 61 patients (population P2) for whom clinical data were available were examined. Specimens were obtained before irinotecan treatment in 53 cases (in 40 cases before any kind of chemotherapy), and after irinotecan therapy in 8 cases.
Immunohistochemistry
Immunohistochemistry was performed using the ZytoChem Plus AP Polymer System kit (Zytomed Systems, Berlin, Germany). Tissue sections were deparaffinized and rehydrated. Antigen retrieval was achieved by incubating the specimens in Target Retrieval Solution (0.01 M citrate buffer, PH 6.0) (Dako; Agilent Technologies GmbH, Waldbronn, Germany) at 99°C for 25 min followed by cooling at room temperature for 15 min. The blocking solution was applied for 5 min. The sections were then incubated with the rabbit monoclonal anti-CYP3A5 antibody (Abcam, Cambridge, UK) at 4°C overnight. The PostBlock was added to the slides for 20 min. The specimens were then incubated with AP-polymer for 30 min. Liquid Permanent Red (Dako; Agilent Technologies GmbH) was used for staining. After 17 min, the reaction was terminated with distilled water. The slides were washed with phosphate-buffered saline after each incubation, and the tissue was counterstained with hematoxylin.
Scoring
A semi-quantitative score was used to evaluate the immunohistochemical staining of each specimen by estimating the staining intensity (negative, weak, moderate, strong; Fig. 1) and the percentage of stained cells, assigning a score of 0–300. The score was calculated using the equation 1 × (% of cells with weak staining) + 2 × (% of cells with moderate staining) + 3 × (% of cells with strong staining), as previously established and used (22). Scoring was performed by researchers blinded to therapy response.
Figure 1.
Examples of semiquantitative scoring of CYP3A5 expression. Magnification, ×400. (A) CYP3A5-negative. (B) CYP3A5-positive with weak staining. (C) CYP3A5-positive with moderate staining. (D) CYP3A5-positive with strong staining. CYP3A5, cytochrome P450 3A5.
Assessment of therapy response
Best overall tumor response was assessed for the first chemotherapy containing irinotecan. Protocols included fluoropyrimidine and oxaliplatin as well as the monoclonal antibodies bevacizumab, cetuximab, and panitumumab.
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines (23) on the basis of contrast-enhanced computed tomography (CT) scans. Unidimensional measurements of target lesions and qualitative assessment of non-target disease allowed categorization of overall tumor response as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The best overall response recorded from the beginning until the end of the treatment was used for further analysis.
Statistical analysis
Spearman's rank correlation was used to assess a possible correlation between CYP3A5 expression and tumor response to irinotecan therapy. We used the Wilcoxon signed-rank test to analyze differences between paired specimens and the Kruskal-Wallis test to investigate whether CYP3A5 expression differed among the different categories of tumor response and among certain clinical parameters. The Mann-Whitney U test was used to assess differences in CYP3A5 expression between two groups. Due to the nature of the study as an explorative data analysis no alpha adjustment was performed.
In cases of more than one specimen per patient, a specimen was selected randomly. The final score for each specimen on the TMA was determined as the mean score of the duplicate cores.
All analyses were performed using SPSS for Windows version 24 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
CYP3A5 expression in normal tissues
Among the 26 examined normal tissues, adipose tissue, adrenal gland, appendix, endometrium, esophagus, heart muscle, lung epithelial cells, skeletal muscle, myometrium, ovary, exocrine pancreas, prostate, salivary gland, squamous epithelium of the skin, spleen, testis, thymus, and urothelium were CYP3A5-negative.
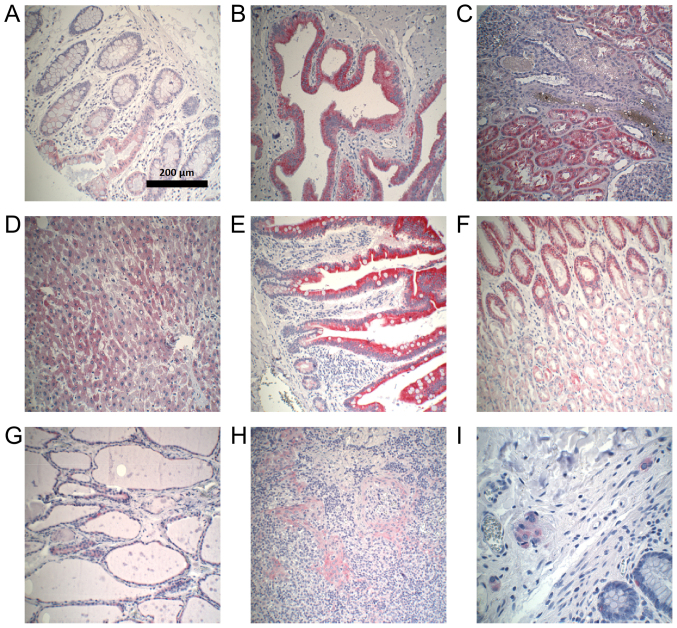
Colon epithelium, gallbladder epithelium, kidney, liver, epithelium of the small intestine, stomach, thyroid gland, and lymphatic tissue of the tonsil expressed CYP3A5 to different extents (Fig. 2). All CYP3A5-positive organs demonstrated a heterogeneous distribution of CYP3A5 expression. Examples of CYP3A5-positive staining in normal tissues are shown in Fig. 3. CYP3A5-negative sections could be found in every organ. The highest CYP3A5 expression was observed in gallbladder epithelium. In addition, expression of CYP3A5 was observed in ganglion cells located in normal colon.
Figure 2.
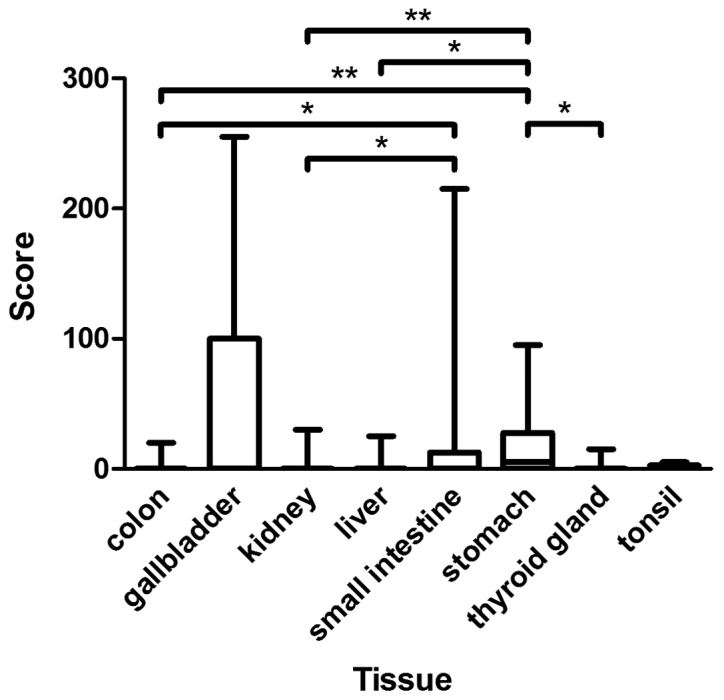
Assessment of immunohistochemical staining in CYP3A5-positive normal tissues included in TMA. The figure presents the medians, 25 and 75th percentiles, and the minimums and maximums of the scores in CYP3A5-positive normal tissues of TMA. Medians: Colon 0, gallbladder 0, kidney 0, liver 0, small intestine 0, stomach 5, thyroid gland 0, and tonsil 0. *P<0.05 and **P<0.01, as indicated. CYP3A5, cytochrome P450 3A5; TMA, tissue microarrays.
Figure 3.
Examples of CYP3A5-positive immunohistochemical staining in normal tissues. Magnification, ×100. (A) Colon. (B) Gallbladder. (C) Kidney. (D) Liver. (E) Small intestine. (F) Stomach. (G) Thyroid gland. (H) Tonsil. (I) Ganglion cells. CYP3A5, cytochrome P450 3A5.
CYP3A5 expression in normal colon, colorectal adenoma, and colorectal cancer
Expression of CYP3A5 was heterogeneously distributed in normal colon, colorectal adenoma, and CRC. In principle, expression was higher in apical epithelial cells compared to that in epithelial cells located in the depths of the colonic crypts. The results of the immunohistochemical assessment of CYP3A5 expression in these tissues are shown in Table II.
Table II.
Assessment of CYP3A5 staining in normal colon, colorectal adenoma and colorectal cancer.
| Tissue type | Median score | Score range | CYP3A5-positive specimens, n (%) |
|---|---|---|---|
| Normal colon | 0 | 0–40 | 2 (13.3) |
| Colorectal adenoma | 0 | 0–140 | 19 (42.2) |
| Sessile serrated adenoma | 0 | 0–110 | 7 (46.7) |
| Tubular adenoma | 10 | 0–120 | 8 (53.3) |
| Tubulovillous adenoma | 0 | 0–140 | 4 (26.6) |
| Colorectal cancer | 0 | 0–130 | 14 (21.5) |
| CRC: Primary tumor | 0 | 0–130 | 9 (23.1) |
| CRC: Metastasis | 0 | 0–180 | 5 (19.2) |
This table lists the median scores, ranges of score, and the number and percentage of CYP3A5-positive specimens in the normal colon, colorectal adenoma and colorectal cancer. CYP3A5, cytochrome P450 3A5; CRC, colorectal cancer.
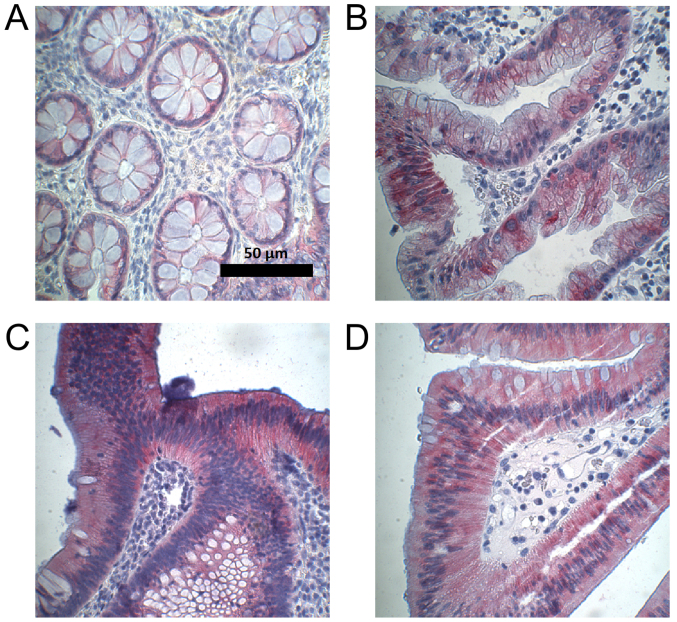
Medians of CYP3A5 expression in normal colon, sessile serrated adenoma, and tubulovillous adenoma did not differ (median, 0). However, expression of CYP3A5 in tubular adenoma (median, 10) differed significantly from that in normal colon (P=0.04). Expression of CYP3A5 was higher in colorectal adenoma than in normal colon mucosa, with a difference that was close to statistically significant (P=0.06). Figs. 4 and 5 provide an overview of the assessment of CYP3A5 expression in normal colon and colorectal adenoma, as well as examples of CYP3A5 staining.
Figure 4.
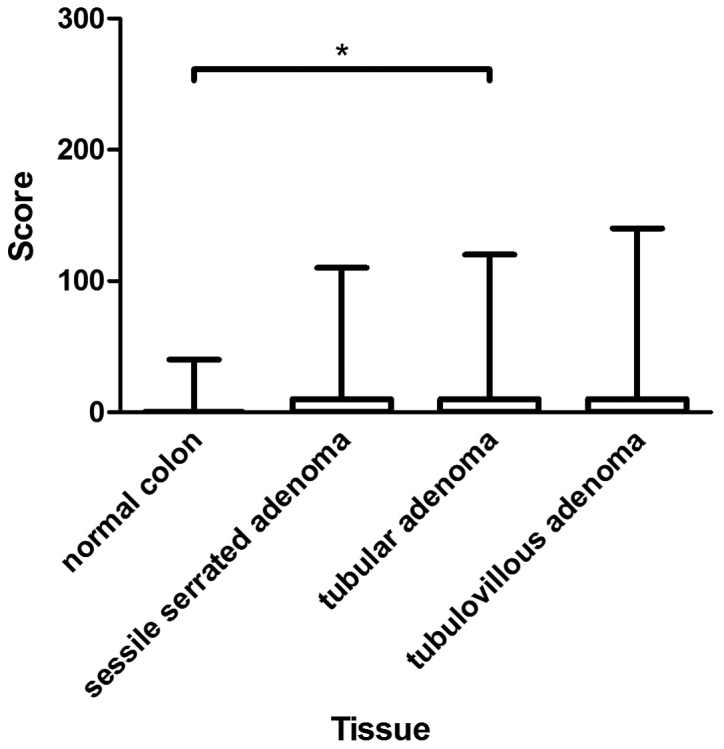
Assessment of immunohistochemical staining in normal colon and colon adenomas. The figure presents the medians, 25 and 75th percentiles, and the minimums and maximums of the scores in the normal colon (median, 0), sessile serrated adenoma (median, 0), tubular adenoma (median, 10), and tubulovillous adenoma (median, 0). *P<0.05, as indicated.
Figure 5.
Examples of CYP3A5-positive immunohistochemical staining in the normal colon and colon adenomas. Magnification, ×400. (A) Normal colon. (B) Sessile serrated adenoma. (C) Tubular adenoma. (D) Tubulovillous adenoma. CYP3A5, cytochrome P450 3A5.
In population P1, originating from 65 patients, CYP3A5 was heterogeneously expressed in CRC tissues (Table II). Most CYP3A5-positive tissues demonstrated spotty CYP3A5 expression. Individual CYP3A5-positive cells were found in otherwise CYP3A5-negative tissues.
Primary cancer specimens and corresponding metastases from 17 patients were compared. No statistically significant differences in CYP3A5 expression were found.
CYP3A5 expression correlates with tumor response to irinotecan therapy
Tissues of 61 patients were examined to determine whether CYP3A5 expression correlated with tumor response to irinotecan therapy (population P2). The clinical data of population P2 are shown in Table III. Among patients in this population, 4 (6.6%) showed CR, 22 (36.1%) PR, 17 (27.9%) SD, and 18 (29.5%) PD following the first irinotecan treatment. Table IV lists the distribution of CYP3A5-negative and -positive tissues as well as the percentage of responders and non-responders for each chemotherapy protocol.
Table III.
Clinical data of population P2.
| Variable | CYP3A5-negative tissue (n=47), n (%) | CYP3A5-positive tissue (n=14), n (%) | Responders (CR + PR) (n=26), n (%) | Non-responders (SD + PD) (%) (n=35), n (%) |
|---|---|---|---|---|
| Age at first irinotecan therapy | mean, 61 years (range, 23–76) | mean, 60 years (range, 32–77) | mean, 61 years (range, 43–76) | mean, 60 years (range, 23–77) |
| Sex | ||||
| Male | 31 (66.0) | 9 (64.3) | 20 (76.9) | 20 (57.1) |
| Female | 16 (34.0) | 5 (35.7) | 6 (23.1) | 15 (42.9) |
| Primary tumor site | ||||
| Caecum | 6 (12.8) | 2 (14.3) | 2 (7.7) | 6 (17.1) |
| Colon | 11 (23.4) | 3 (21.4) | 3 (11.5) | 11 (31.4) |
| Sigmoid | 15 (31.9) | 3 (21.4) | 11 (42.3) | 7 (20.0) |
| Rectosigmoid | 1 (2.1) | 0 (0) | 1 (3.8) | 0 (0) |
| Rectum | 14 (29.8) | 6 (42.9) | 9 (34.6) | 11 (31.4) |
| KRAS status | ||||
| Mutated | 16 (34.0) | 7 (50) | 6 (23.1) | 17 (48.6) |
| Wild type | 30 (63.8) | 7 (50) | 20 (76.9) | 17 (48.6) |
| Unknown | 1 (2.1) | 0 (0) | 0 (0) | 1 (2.9) |
| Chemotherapy prior to irinotecan treatment | ||||
| No prior therapy | 31 (66.0) | 9 (64.3) | 14 (53.8) | 26 (74.3) |
| Prior chemotherapy | 16 (34.0) | 5 (35.7) | 12 (46.2) | 9 (25.7) |
This table presents the patients' mean age at first irinotecan therapy, sex, primary tumor site, KRAS status, and whether patients received chemotherapy prior to irinotecan treatment, categorized by CYP3A5 expression and tumor response to irinotecan. CYP3A5, cytochrome P450 3A5; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table IV.
Protocols of the first irinotecan-based chemotherapy in population P2.
| Chemotherapy protocol | CYP3A5-negative tissue, n (%) | CYP3A5-positive tissue, n (%) | Responders (CR + PR), n (%) | Non-responders (SD + PD), n (%) |
|---|---|---|---|---|
| Fluoropyrimidine, irinotecan | 6 (12.8) | 1 (7.1) | 1 (3.8) | 6 (17.1) |
| Fluoropyrimidine, irinotecan, bevacizumab | 23 (48.9) | 7 (50.0) | 13 (50.0) | 17 (48.6) |
| Fluoropyrimidine, irinotecan, cetuximab | 15 (31.9) | 5 (35.7) | 11 (42.3) | 9 (25.7) |
| Fluoropyrimidine, irinotecan, oxaliplatin | 0 (0) | 1 (7.1) | 0 (0) | 1 (2.9) |
| Fluoropyrimidine, irinotecan, oxaliplatin, bevacizumab | 2 (4.3) | 0 (0) | 0 (0) | 2 (5.7) |
| Fluoropyrimidine, irinotecan, panitumumab | 1 (2.1) | 0 (0) | 1 (3.8) | 0 (0) |
This table lists the chemotherapeutic agents and antibodies used in first irinotecan-based chemotherapy as well as the number of patients it was administered to, categorized by CYP3A5 expression and tumor response to irinotecan therapy, respectively. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CYP3A5, cytochrome P450 3A5.
Spearman's rank correlation revealed a statistically significant correlation (P=0.03) between CYP3A5 expression in CRC tissue and tumor response to irinotecan treatment in population P2 (correlation coefficient, 0.276). A statistically significant difference (P=0.02) in CYP3A5 expression was demonstrated between responders (CR + PR) and non-responders (SD + PD) (Figs. 6 and 7).
Figure 6.
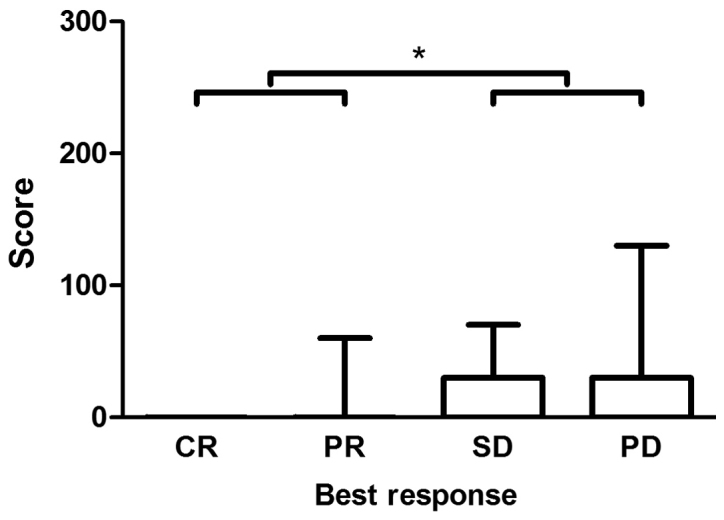
Assessment of immunohistochemical staining as a function of therapy response under irinotecan treatment. The figure presents the medians, 25 and 75th percentiles, and minimums and maximums of the scores in every category of the tumor response under treatment with irinotecan. Medians in all categories: 0. Analysis included 61 patients; a total of 53 specimens were irinotecan-naïve and 8 were obtained following treatment with irinotecan. *P<0.05, as indicated. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
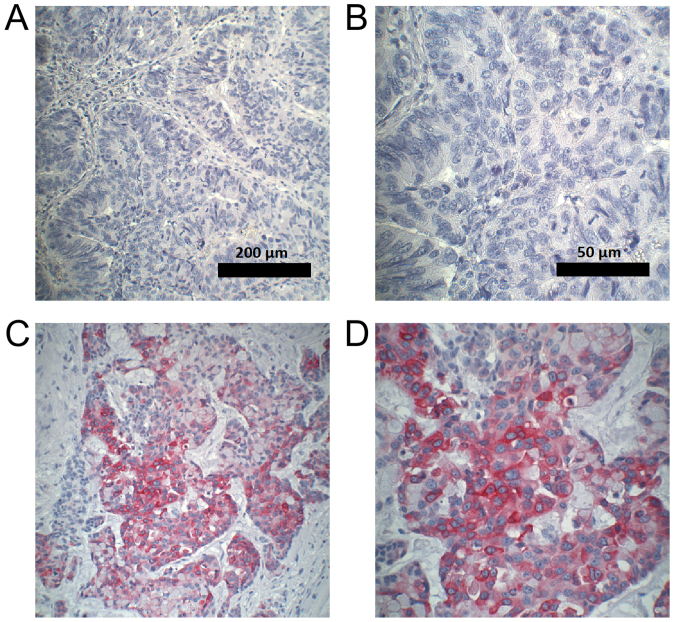
Figure 7.
Examples of CYP3A5 immunohistochemical staining in colorectal carcinoma. CRC tissue with best response to irinotecan: CR (responder), at magnification (A) ×100 and (B) ×400. PD (non-responder), at magnification (C) ×100 and (D) ×400. CYP3A5, cytochrome P450 3A5. CR, complete response; PD, progressive disease; CRC, colorectal cancer.
Interestingly, no CYP3A5 was expressed in CRC tissues with complete response to irinotecan therapy. The highest CYP3A5 expression was observed in tumors progressing under treatment.
In CRC tissues, no statistically significant difference was observed in CYP3A5 expression before and after treatment with irinotecan.
Discussion
Owing to the complex regulation of cytochrome enzymes, the quantity of CYP3A5 mRNA does not correlate with the expressed amount of cytochrome enzyme in normal colon and colorectal adenoma tissue (19). Therefore, we selected immunohistochemistry for detection of CYP3A5 protein expression.
Immunohistochemical assessment of CYP3A5 expression in normal tissues had previously only been performed in stomach and lung tissue. In the present study, we found that more than half of the examined stomach tissues expressed CYP3A5 at low to moderate intensity. In a previous study, Kolars et al (24) were not able to demonstrate CYP3A5 expression through immunohistochemical examination of the stomach tissue of only one patient. In lung tissue, immunohistochemistry-based studies found CYP3A5 to be the predominant CYP3A enzyme (25,26), while all of our lung specimens were CYP3A5-negative. These conflicting results might result from methodological differences in immunohistochemistry and the use of different antibodies.
In the present study, we observed the highest expression of CYP3A5 in the small intestine, gallbladder, and stomach. CYP3A5 was also expressed to a small extent in the liver, kidney, and thyroid gland. CYP3A5 expression data in the small intestine (27), liver (28–30), kidney (31,32), and thyroid gland (29,30) is so far limited to CYP3A5 mRNA analyses and/or CYP3A5 protein immunoblots.
In accordance with the existing data (24), we observed no CYP3A5 expression in the pancreas. While we could not demonstrate CYP3A5 expression in the adrenal gland, endometrium, esophagus, heart, muscle, myometrium, ovary, prostate, salivary gland, skin, spleen, testis, thymus, or urothelium of the urinary bladder, expression of CYP3A5 mRNA has been described in these sites (30,33,34). Expression of CYP3A5 in the gallbladder, tonsil, neurons, adipose tissue, and appendix was examined for the first time in the present study.
CYP3A5 expression in normal colon has been investigated with a variety of different methods in the past (17,18,27,35–37). Consistent with the results of the present study, Kumarakulasingham et al (20) observed weak CYP3A5-positive staining in 25% of the examined colonic tissues using immunohistochemistry.
CYP enzymes can be involved in the activation of procarcinogens (38). Therefore, it could be hypothesized that CYP3A5 accumulation in adenomas reflects the dysplastic character of these lesions and facilitates the transition to malignant tumors. Indeed, CYP3A5 expression in colorectal adenoma was higher than that in normal colon, although this difference did not reach statistical significance, possibly owing to the small number of specimens in our analysis.
In contrast to the findings of the present study, Bergheim et al (19) reported 54% lower expression of CYP3A5 in adenoma tissue and the surrounding normal colon tissue compared to the expression in patients without adenomas. Furthermore, CYP3A5 expression did not differ between adenoma and surrounding normal colon tissue (19). As colon adenomas and normal colon tissue showed a patchy distribution of CYP3A5 expression, quantification of CYP3A5 expression by western blot analysis of lysates from the whole specimens, as performed by Bergheim et al (19), might miss high expression of the enzyme when restricted to small tissue patches.
The risk of invasive carcinoma in colorectal adenomas depends on the type of adenoma (39,40). However, these different risks were not reflected by different CYP3A5 expression levels in our study. The present study is the first to compare CYP3A5 expression in different adenoma subtypes. However, we did not find any obvious trends, suggesting that differences in expression might instead by caused by inter-individual differences.
Using immunohistochemistry, Kumarakulasingham et al (20) reported CYP3A5 expression in ~65% of the CRC tissues, with most specimens demonstrating low, and <5% showing high, CYP3A5 expression. In an immunohistochemical analysis performed by Noll et al (8), weak CYP3A5 expression was found in 3 out of 8 specimens of adenocarcinoma of the rectum.
In the current study, the percentage of CYP3A5-negative CRC tissues was greater than that previously reported, possibly owing to our decision to consider specimens CYP3A5-positive only when expression was observed in at least 10% of the tissue.
While we found expression of CYP3A5 to be the same in primary cancer and metastasis, Kumarakulasingham et al (20) reported that expression in primary cancer did not correspond to that in the respective lymph node metastases. These conflicting observations could be caused by the small, possibly not representative, specimens on the TMAs used by Kumarakulasingham et al (20).
Non-responders to irinotecan expressed significantly more CYP3A5 than responders. Therefore, a tumor-autonomous CYP3A5-mediated resistance to irinotecan therapy is possible. However, tumor samples in each response category contained CYP3A5-negative tissues, suggesting that tumor response to irinotecan therapy does not solely depend on CYP3A5 expression. Further studies are required to validate a higher metabolism of irinotecan in tumor tissue of non-responders to irinotecan therapy.
We were unable to observe an induced CYP3A5 expression in CRC tissue as a response to irinotecan treatment resulting in a secondary resistance in the clinical setting of our study.
Although similar in histology, colorectal cancer is a heterogeneous disease owing to molecular differences (11). The identification of biomarkers and mutation analyses are becoming increasingly important for prediction of outcome and therapy selection.
In the present study, we demonstrated for the first time a statistically significant correlation between CYP3A5 expression and tumor response to irinotecan therapy, suggesting a tumor-autonomous resistance to treatment with irinotecan through increased CYP3A5-mediated metabolism.
Acknowledgements
The authors would like to thank Mrs. Jutta Mohr (Department of Gastroenterology, University Hospital Heidelberg, Heidelberg, Germany) for their technical support.
Glossary
Abbreviations
- APC
7-ethyl-10 [4-N-(5-aminopentanoicacid)-1-piperidino] carbonyloxycamptothecin
- CR
complete response
- CRC
colorectal cancer
- CYP
cytochrome P450
- DNA
deoxyribonucleic acid
- EGFR
epidermal growth factor receptor
- NPC
7-ethyl-10 [4-amino-1-piperidino] carbonyloxycamptothecin
- PD
progressive disease
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- SN-38
7-ethyl-10-hydroxy-camptothecin
- TMA
tissue microarray
- VEGF
vascular endothelial growth factor
Funding
The present study was supported by the ‘Stiftung für Krebs-und Scharlachforschung Mannheim’.
Availability of data and materials
The data generated and analyzed during this study are available from the corresponding author on reasonable request.
Authors' contributions
EB performed the immunohistochemical staining, collected the patient information and analyzed the data. EB and RK wrote the manuscript. MS established the immunohistochemistry. MMG and EH provided the tissue samples and performed the pathological analysis of the examined tissues. CG obtained the clinical data of patients treated at the NCT Heidelberg. TFW and EB performed the assessment of the radiological tumor response to treatment. TB and EB performed the statistical analysis. RK was responsible for the planning and supervision of the project, as well as the interpretation of the results. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Due to the retrospective nature of the present study and the use of archive material, written informed consent from patients was not required. The study, including the use of tissues as well as patient data, was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg (reference no. 206/2005; Heidelberg, Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.MacLeod SL, Nowell S, Massengill J, Jazieh A, McClure G, Plaxco J, Kadlubar FF, Lan NP. Cancer therapy and polymorphisms of cytochromes P450. Clin Chem Lab Med. 2000;38:883–887. doi: 10.1515/CCLM.2000.128. [DOI] [PubMed] [Google Scholar]
- 2.Pavek P, Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr Drug Metab. 2008;9:129–143. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- 3.Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 4.Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet. 1994;26:144–160. doi: 10.2165/00003088-199426020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Rochat B. Role of cytochrome P450 activity in the fate of anticancer agents and in drug resistance: Focus on tamoxifen, paclitaxel and imatinib metabolism. Clin Pharmacokinet. 2005;44:349–366. doi: 10.2165/00003088-200544040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Michael M, Doherty MM. Tumoral drug metabolism: Overview and its implications for cancer therapy. J Clin Oncol. 2005;23:205–229. doi: 10.1200/JCO.2005.02.120. [DOI] [PubMed] [Google Scholar]
- 7.Yu LJ, Matias J, Scudiero DA, Hite KM, Monks A, Sausville EA, Waxman DJ. P450 enzyme expression patterns in the NCI human tumor cell line panel. Drug Metab Dispos. 2001;29:304–312. [PubMed] [Google Scholar]
- 8.Noll EM, Eisen C, Stenzinger A, Espinet E, Muckenhuber A, Klein C, Vogel V, Klaus B, Nadler W, Rösli C, et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat Med. 2016;22:278–287. doi: 10.1038/nm.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S, Johnson K, Ahmed O, Iqbal N. Advances in the management of colorectal cancer: From biology to treatment. Int J Colorectal Dis. 2014;29:1031–1042. doi: 10.1007/s00384-014-1928-5. [DOI] [PubMed] [Google Scholar]
- 11.Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Glimelius B. Benefit-risk assessment of irinotecan in advanced colorectal cancer. Drug Saf. 2005;28:417–433. doi: 10.2165/00002018-200528050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 15.Paulik A, Grim J, Filip S. Predictors of irinotecan toxicity and efficacy in treatment of metastatic colorectal cancer. Acta Medica (Hradec Kralove) 2012;55:153–159. doi: 10.14712/18059694.2015.39. [DOI] [PubMed] [Google Scholar]
- 16.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/S0169-409X(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 17.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergheim I, Bode C, Parlesak A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clin Pharmacol. 2005;5:4. doi: 10.1186/1471-2210-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergheim I, Bode C, Parlesak A. Decreased expression of cytochrome P450 protein in non-malignant colonic tissue of patients with colonic adenoma. BMC Gastroenterol. 2005;5:34. doi: 10.1186/1471-230X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumarakulasingham M, Rooney PH, Dundas SR, Telfer C, Melvin WT, Curran S, Murray GI. Cytochrome p450 profile of colorectal cancer: Identification of markers of prognosis. Clin Cancer Res. 2005;11:3758–3765. doi: 10.1158/1078-0432.CCR-04-1848. [DOI] [PubMed] [Google Scholar]
- 21.Dong N, Meng F, Wu Y, Wang M, Cui Y, Zhang S. Genetic polymorphisms in cytochrome P450 and clinical outcomes of FOLFIRI chemotherapy in patients with metastatic colorectal cancer. Tumour Biol. 2015;36:7691–7698. doi: 10.1007/s13277-015-3492-1. [DOI] [PubMed] [Google Scholar]
- 22.Koschny R, Krupp W, Xu LX, Mueller WC, Bauer M, Sinn P, Keller M, Koschny T, Walczak H, Bruckner T, et al. WHO grade related expression of TRAIL-receptors and apoptosis regulators in meningioma. Pathol Res Pract. 2015;211:109–116. doi: 10.1016/j.prp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Kolars JC, Lown KS, Schmiedlin-Ren P, Ghosh M, Fang C, Wrighton SA, Merion RM, Watkins PB. CYP3A gene expression in human gut epithelium. Pharmacogenetics. 1994;4:247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Anttila S, Hukkanen J, Hakkola J, Stjernvall T, Beaune P, Edwards RJ, Boobis AR, Pelkonen O, Raunio H. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am J Respir Cell Mol Biol. 1997;16:242–249. doi: 10.1165/ajrcmb.16.3.9070608. [DOI] [PubMed] [Google Scholar]
- 26.Raunio H, Hakkola J, Hukkanen J, Lassila A, Päivärinta K, Pelkonen O, Anttila S, Piipari R, Boobis A, Edwards RJ. Expression of xenobiotic-metabolizing CYPs in human pulmonary tissue. Exp Toxicol Pathol. 1999;51:412–417. doi: 10.1016/S0940-2993(99)80031-1. [DOI] [PubMed] [Google Scholar]
- 27.Thörn M, Finnström N, Lundgren S, Rane A, Lööf L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br J Clin Pharmacol. 2005;60:54–60. doi: 10.1111/j.1365-2125.2005.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westlind-Johnsson A, Malmebo S, Johansson A, Otter C, Andersson TB, Johansson I, Edwards RJ, Boobis AR, Ingelman-Sundberg M. Comparative analysis of CYP3A expression in human liver suggests only a minor role for CYP3A5 in drug metabolism. Drug Metab Dispos. 2003;31:755–761. doi: 10.1124/dmd.31.6.755. [DOI] [PubMed] [Google Scholar]
- 29.Koch I, Weil R, Wolbold R, Brockmöller J, Hustert E, Burk O, Nuessler A, Neuhaus P, Eichelbaum M, Zanger U, Wojnowski L. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab Dispos. 2002;30:1108–1114. doi: 10.1124/dmd.30.10.1108. [DOI] [PubMed] [Google Scholar]
- 30.Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 31.Haehner BD, Gorski JC, Vandenbranden M, Wrighton SA, Janardan SK, Watkins PB, Hall SD. Bimodal distribution of renal cytochrome P450 3A activity in humans. Mol Pharmacol. 1996;50:52–59. [PubMed] [Google Scholar]
- 32.Givens RC, Lin YS, Dowling AL, Thummel KE, Lamba JK, Schuetz EG, Stewart PW, Watkins PB. CYP3A5 genotype predicts renal CYP3A activity and blood pressure in healthy adults. J Appl Physiol (1985) 2003;95:1297–1300. doi: 10.1152/japplphysiol.00322.2003. [DOI] [PubMed] [Google Scholar]
- 33.Baron JM, Höller D, Schiffer R, Frankenberg S, Neis M, Merk HF, Jugert FK. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Invest Dermatol. 2001;116:541–548. doi: 10.1046/j.1523-1747.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 34.Lechevrel M, Casson AG, Wolf CR, Hardie LJ, Flinterman MB, Montesano R, Wild CP. Characterization of cytochrome P450 expression in human oesophageal mucosa. Carcinogenesis. 1999;20:243–248. doi: 10.1093/carcin/20.2.243. [DOI] [PubMed] [Google Scholar]
- 35.Gervot L, Carrière V, Costet P, Cugnenc PH, Berger A, Beaune PH, de Waziers I. CYP3A5 is the major cytochrome P450 3A expressed in human colon and colonic cell lines. Environ Toxicol Pharmacol. 1996;2:381–388. doi: 10.1016/S1382-6689(96)00075-0. [DOI] [PubMed] [Google Scholar]
- 36.McKinnon RA, Burgess WM, Gonzalez FJ, McManus ME. Metabolic differences in colon mucosal cells. Mutat Res. 1993;290:27–33. doi: 10.1016/0027-5107(93)90029-F. [DOI] [PubMed] [Google Scholar]
- 37.Windmill KF, McKinnon RA, Zhu X, Gaedigk A, Grant DM, McManus ME. The role of xenobiotic metabolizing enzymes in arylamine toxicity and carcinogenesis: Functional and localization studies. Mutat Res. 1997;376:153–160. doi: 10.1016/S0027-5107(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 38.Sheweita SA. Drug-metabolizing enzymes: Mechanisms and functions. Curr Drug Metab. 2000;1:107–132. doi: 10.2174/1389200003339117. [DOI] [PubMed] [Google Scholar]
- 39.Erichsen R, Baron JA, Hamilton-Dutoit SJ, Snover DC, Torlakovic EE, Pedersen L, Frøslev T, Vyberg M, Hamilton SR, Sørensen HT. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology. 2016;150:895–902.e5. doi: 10.1053/j.gastro.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 40.Nusko G, Mansmann U, Partzsch U, Altendorf-Hofmann A, Groitl H, Wittekind C, Ell C, Hahn EG. Invasive carcinoma in colorectal adenomas: Multivariate analysis of patient and adenoma characteristics. Endoscopy. 1997;29:626–631. doi: 10.1055/s-2007-1004268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during this study are available from the corresponding author on reasonable request.