Abstract
At present, a large amount of research from experimental and theoretical points of view has been done on interpolyelectrolyte complexes formed by electrostatic attractive forces and/or interpolymer complexes stabilized by hydrogen bonds. By contrast, relatively less attention has been given to polymer–polymer complex formation with synthetic polyampholytes (PA). In this review the complexation of polyampholytes with polyelectrolytes (PE) is considered from theoretical and application points of view. Formation of intra- and interpolyelectrolyte complexes of random, regular, block, dendritic polyampholytes are outlined. A separate subsection is devoted to amphoteric behavior of interpolyelectrolyte complexes. The realization of the so-called “isoelectric effect” for interpolyelectrolyte complexes of water-soluble polyampholytes, amphoteric hydrogels and cryogels with respect to surfactants, dye molecules, polyelectrolytes and proteins is demonstrated.
Keywords: polyampholyte, polyelectrolyte, interpolyelectrolyte complexes, intrapolyelectrolyte complexes, “isoelectric effect”
1. Introduction
Formation of polyelectrolyte-polyampholyte complexes (PPC) is crucial from fundamental and practical points of view [1,2,3,4,5]. IUPAC (International Union of Pure and Applied Chemistry) defines [6] that a polyampholyte (PA) is a special type of polyelectrolyte (PE) which possesses both cationic and anionic groups. Conditionally polyampholytes can be divided into 3 classes: “annealed”, “quenched” and “betainic” (or “zwitterionic”). “Annealed” polyampholytes consist of acid-base monomers that are ionized in dependence of pH, while “quenched” polyampholytes containing strongly charged cationic and anionic monomers retain their respective charges independently on pH. “Betainic” (or “zwitterionic”) polyampholytes are macromolecules containing identical numbers of acid-base (or fully charged anionic-cationic) species in the same monomer units [7,8,9,10]. The macromolecules existing via compensation of the cationic–anionic monomer pairs without counterions also belong to ”zwitterionic” polymers. In literature the terms “amphoteric” polyelectrolytes and “amphoteric” copolymers (or macromolecules) are also used [11]. Table 1 represents examples of various polyampholytes with different chemical structures. The microstructure of polyampholytes can be represented as random, alternating, graft, diblock, triblock, multiblock, dendritic, and stars.
Table 1.
Examples of various polyampholytes.
| Chemical Structure of Monomer Units | Name Acronym | Type of Polyampholyte | Refs |
|---|---|---|---|
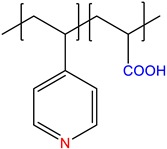
|
4-vinylpyridine-co-acrylic acid, P(4VPy-co-AA) |
Annealed | [12] |
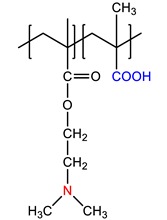
|
N,N-dimethylaminoethylmethacrylate- co-methacrylic acid, P(DMAEM-co-MAA) |
Annealed | [13] |
|
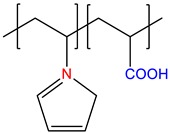
N-vinylimidazole-co-acryic acid, P(VI-co-AA) |
Annealed | [14] |
|
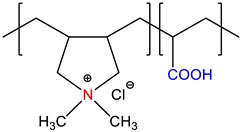
N,N’-dimethyl-N,N’-diallylammonium chloride-co-acrylic acid, P(DMDAAC-co-AA) |
Self-quenched * | [15] |
|
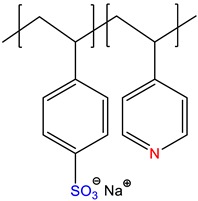
sodium styrene sulfonate-co-4-vinylpyridine, P(NaSS-co-4VPy) | Self-quenched * | [16] |

|
poly(1-methyl-4-vinylpyridinium chloride)-block-poly(methacrylic acid), P1M4VPCl-b-PMAA |
Self-quenched * | [17,18] |
|
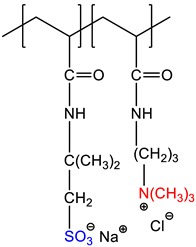
2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt-co-(3-acrylamidopropyl)trimethylammonium chloride, P(AMPSNa-co-APTAC) |
Quenched | [19] |
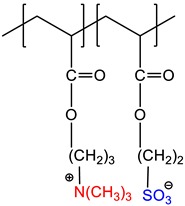
|
2-methacryloyloxyethyltrimethylammonium-co-2-methacryloyloxyethanesulfonate, P(METMA-co-MES) | Quenched or zwitterionic |
[20] |

|
Poly(carbobetaine), PCB | Annealed | [7] |
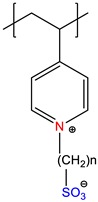
|
Poly(sulfobetaine), PSB | Quenched or zwitterionic |
[7] |

|
Poly(phosphobetaine), PPB | Quenched or zwitterionic |
[7] |
* The term “self-quenched” for polyampholytes represents polyampholytes which possess permanently ionized charged groups (anionic or cationic) in the repeating units.
Concerning the polyelectrolytes, we refer the readers to earlier publications [21,22,23,24,25,26,27,28,29], where the fundamental and applied aspects are described and to recent reviews [30,31], in which the theory of polyelectrolytes and a perspective on polyelectrolyte solutions are comprehensively outlined.
In the context of the present review, interaction of polyelectrolytes with polyampholytes leading to the formation of PPC is considered as a model reaction between biological objects, in particular between proteins and DNA [32], between sodium hyaulronate and proteins [33] on the one hand, and for protein purification and separation [34,35,36], enzyme immobilization [37], controlling delivery of DNA into the cell [38,39] on the other.
This review is mainly focused on the past, present and recent advances of intra- and interpolyelectrolyte complexes of polyampholytes. First, the theoretical achievements of polyelectrolyte–polyampholyte complexation are highlighted. The theory of polyampholytes is described briefly to introduce the readers to specific properties of amphoteric macromolecules in dependence of charge distribution. The ability of polyampholytes to form intrapolyelectrolyte complexes (Intra-PEC) at the isoelectric point (IEP) is considered. Then experimental findings on the interaction of polyelectrolytes with polyampholytes and formation of interpolyelectrolyte complexes (Inter-PEC) are reviewed. The realization of “the isoelectric effect” is discussed in the light of competition between the Intra- and Inter-PEC. Analogy between interpolyelectrolyte complexes derived from anionic and cationic polyelectrolyte pairs and polyampholytes is justified to expand our representation on amphoteric behavior of polyelectrolyte complexes.
2. Theory of Polyampholytes (PA) and Polyelectrolyte (PE)–Polyampholyte Complexation
2.1. Theory of Polyampholytes
Key problems of the protein-folding process definitely intensified the fast development of polyampholyte theory because the understanding of the protein-folding mechanism on the level of synthetic analogs can be of help to explain their structural–functional relationship [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Several attempts to simulate the folding of diblock polyampholyte chains were undertaken by authors [70,71,72,73,74]. The first theoretical work [41] considered the statistical polyampholytes containing N monomeric units with equal amounts of positive f and negative g charges and pointed out that fluctuation-induced attraction of opposite charges is the major factor determining their physicochemical behavior. This is called microelectrolyte and can be illustrated by the Debye–Huckel (DH) theory. The conformation change from collapsed state to extended one could be mathematically described by Equation (1):
| (1) |
In the above formula, b l and L represent the monomer size, the Kuhn length and the arc length, respectively, and γ represents dimensionless parameter. The rest of the physical parameters are the electron charge (e), the Boltzman constant (k), and the absolute temperature (T).
Earlier research works [59,60] used DH theory to describe the behavior of polyampholytes which contain unevenly dispersed positive and negative charges. Collapsing phenomena among alternating polyampholytes is similar to precipitation of neutral macromolecules in poor solvents which experience a conformational transformation, “coil-globule”. The DH theory is not feasible for regular polyampholytes since the nearest-neighbor interactions of opposite charges act as dipoles and eliminate charge fluctuations [44]. Diblock polyampholytes containing the same length of flexible oppositely charged blocks collapse in three regimes [72]. In the first regime is the electrostatic repulsion of oppositely charged fragments stabilized by short-range repulsion between monomers. Globules can be represented as densely packed correlation blobs. These blobs are similar to correlation blobs of polyampholytes, which have a random distribution of positive and negative charges. The third regime is related to the formation of dipoles, which are associated into quadruples and further organized into big multiplets like ionomers [75]. Figure 1 demonstrated the evolution of polyampholytes (polymerization degree: n = 256).
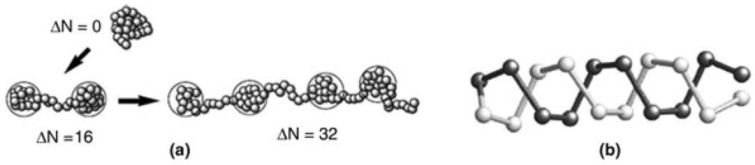
Figure 1.
Cascade type of transition of polyampholyte chain in dependence of charge asymmetry (a) and double-stranded helical conformation of the ground state of a diblock polyampholyte obtained from Monte Carlo simulation (b). Dark and light shades indicate complementary charges (Reprinted from [72]).
A balanced polyampholyte with zero charge ΔN = 0 forms dense globule (where ΔN is the difference between the amount of positive fN+ and negative fN− charges). Irrespective of positive or negative, at charge asymmetry (ΔN = 16) a globule is transformed into two halves as shown in Figure 1. Further increase in charge asymmetry (ΔN = 32) promotes the formation of “pearl” or bead strings connected by a “spring”. The simulation findings of variations in sequence and chain length of random, diblock and zwitterionic polyampholytes are consistent with experimental observations [65]. Comprehensive information on the theory of polyampholytes can be found in monograph [4].
All aforementioned theoretical conclusions successfully explain most experimental results.
(1) Polyampholytes demonstrate polycationic (if f >> g) or polyanionic (if g >> f) properties correspondingly.
(2) At the isoelectric point (IEP) (if f ≈ g) the polyampholytes become quasi-electroneutral and have a small hydrodynamic radius. If soluble, they tend to aggregate; if insoluble, they precipitate.
(3) Addition of salts causes neutral polyampholytes swelling if polyampholytes are soluble, narrowing the phase separation region or even dissolving the precipitate if they are not soluble (“antipolyelectrolyte” effect).
However all aforementioned theories developed for polyampholytes consider mostly quenched polyampholytes; disregard the specific binding among counterions and polyion; consider mostly the diluted solutions and are applicable only at low concentration of salts; and do not take into account non-electrostatic forces (hydrogen bonds, hydrophobic interactions, Van der Waals forces). Nevertheless the aforementioned principles explain the solution behavior of most polyampholytes reasonably well.
2.2. Theory of Polyelectrolyte–Polyampholyte Complexation
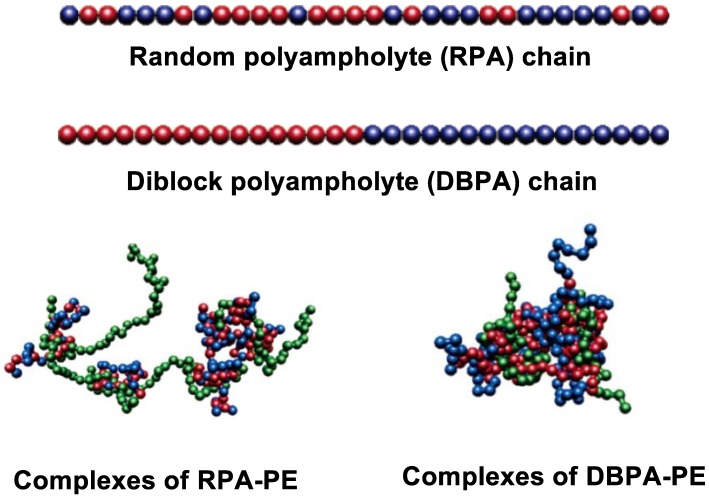
The study of polyelectrolyte-polyampholyte complexation is promising for protein separation [76] and delivery of DNA into the cell [77]. The polyelectrolyte–protein or polyampholyte–DNA interactions are similar to the adsorption of polyampholytes on charged surfaces, because polyelectrolyte chains are able to polarize protein molecules and recognize them [3,78]. The Monte Carlo method [79] and molecular dynamics simulations [80,81] have been conducted to explain the polyampholyte–polyelectrolyte complexation as a function of charge distribution of a polyampholyte, nature of solvent and salt. The structure of polyelectrolyte-polyampholyte complexes strongly depends on the charge distribution of polyampholyte chain. The typical aggregate structures formed between random polyampholytes and polyelectrolytes (RPA-PE) on the one hand, and diblock polyampholyte and polyelectrolyte (DBPA-PE) on the other, show that DBPA forms micellar aggregate while RPA forms branch polymer-like aggregate.
Molecular simulation study proved the contribution of polarization and indicated the stability order for the following complexes: block polyampholyte–polyelectrolyte > statistical polyampholyte–polyelectrolyte > alternating polyampholyte–polyelectrolyte.
Computational works were devoted to (1) Monte Carlo simulation and analytical considerations on the adsorption of flexible polyelectrolyte chains onto charged Janus nanospheres (JNS) containing two oppositely charged hemispheres [82]; and (2) critical adsorption of periodic and random polyampholytes onto charged surfaces [83]. It is concluded that the PE chain adsorbed onto JNS differs from the adsorption of PE onto uniformly charged spheres. In the case of PA-surface adsorption, increasing PA block length enhances the adsorption onto charged planar surfaces (Figure 2). At low surface charge density σ, the oppositely charged blocks are internally bound to one another by electrostatic attractions and weakly bound to the surface. At high surface charge density, the positively charged blocks of PA strongly bind to the oppositely charged surface. In other words, the positive–negative interactions among the blocks within the PA are replaced by external binding of positive blocks with the negative surface and the negative PA blocks are liberated. After reaching the critical adsorption transition, σ → σc, a rapid reduction in the binding energy occurs. It should be noted that under the same conditions and surface charge densities the binding energies for the periodic (block) PAs are significantly bigger than that of the random polyampholytes. Transformation of Inter-PEC to Intra-PEC is related to exchange of external contacts between PA blocks and PE chain to internal contacts between positively and negatively charged PA blocks. The simulation-based analysis of critical PA-surface adsorption conditions can successfully be applied for theoretical modeling of PE–PA, PE-protein, PE-RNA, and PE-DNA complexation. Electrostatic-driven binding of PE with Janus type amphoteric nano- or microgels may potentially be used as stimuli-responsive nano- and micro-actuators [84].
Figure 2.
Snapshots of complexes of random polyampholyte (RPA) and diblock polyampholyte (DBPA) with polyelectrolyte (PE). Red bead represents the positively charged beads of polyampholyte; blue bead represents negatively charged ones; green bead represents polyelectrolyte chain. (Updated from [80]).
Monte Carlo simulations were used to study the pH effect on the behavior of isolated polyampholytes and PPC in dilute solutions [85]. Simulation for the polyelectrolyte-polyampholyte system show that the resulting complexes are stable at pH ≤ 6.5. A further increase in pH results in destruction of PPC due to repulsion between the polyampholyte and the negatively charged polyelectrolyte. This theoretical finding confirms the existence of the “isoelectric effect” that is realized at the IEP of polyampholytes and discussed in Section 5.
3. Intrapolyelectrolyte Complexes (Intra-PEC) of Polyampholytes
Formation of intrapolyelectrolyte complexes (Intra-PEC) within a single chain of polyampholytes is mostly pronounced for block polyampholytes [86]. A linear diblock copolymer poly(styrenesulfonate)-b-poly(2-vinylpyridine) (PSS-b-P2VP) is the first example of formation of Intra-PEC at the isoelectric point (IEP) of polyampholytes [87]. Depending on the degree of ionization of a P2VP block, the conformation of the PSS-b-P2VP is altered from segregated structure with a nucleus of P2VP blocks to a “frozen” coil and further to globular structure at the IEP due cooperative intraionic contacts between positively and negatively charged blocks.
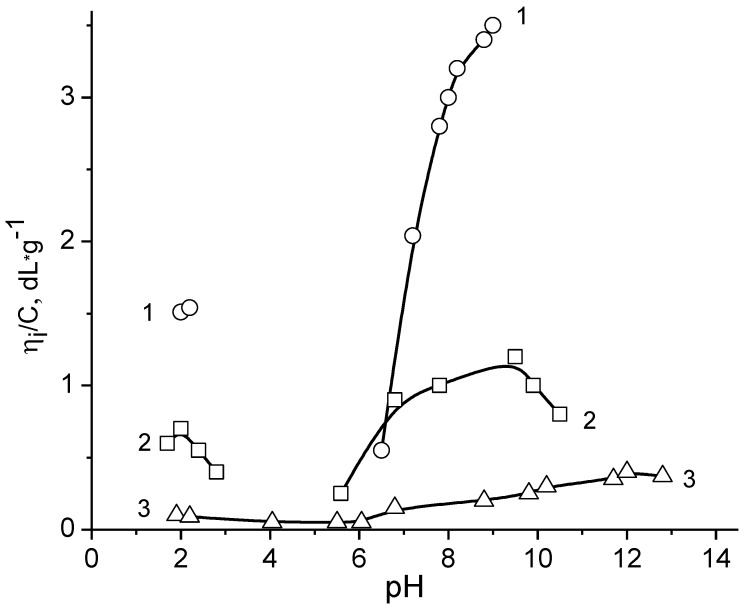
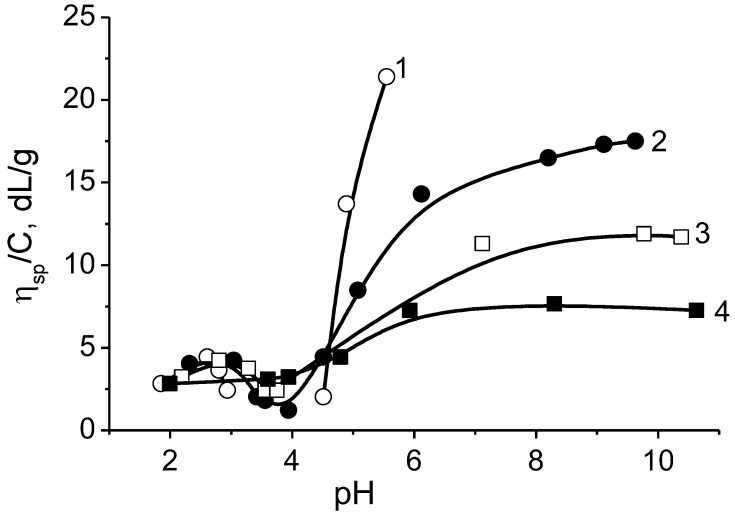
Similar behavior was observed for nearly equimolar BPA consisting of poly(methacrylic acid) (PMAA) and poly(1-methyl-4-vinylpyridinium chloride) (P1M4VPCl) [18,88]. As seen from Figure 3 in acidic and alkaline media PMAA-b-P1M4VPCl acts as polycation and polyanion, respectively; the reduced viscosity drops with the increase in the ionic strength (µ).
Figure 3.
pH-dependent reduced viscosity of PMAA-b-P1M4VPCl in aqueous solution (1) and at ionic strength µ = 0.01 (2) and 0.3 mol·L−1 KCl (3). [PMAA-b-P1M4VPCl] = 0.045 g·dL−1. (Reprinted from [18]).
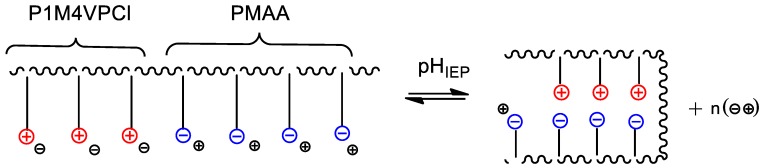
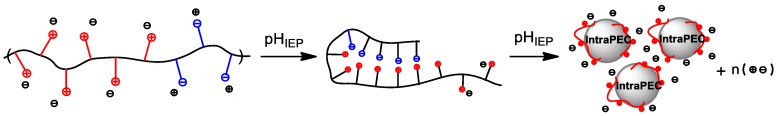
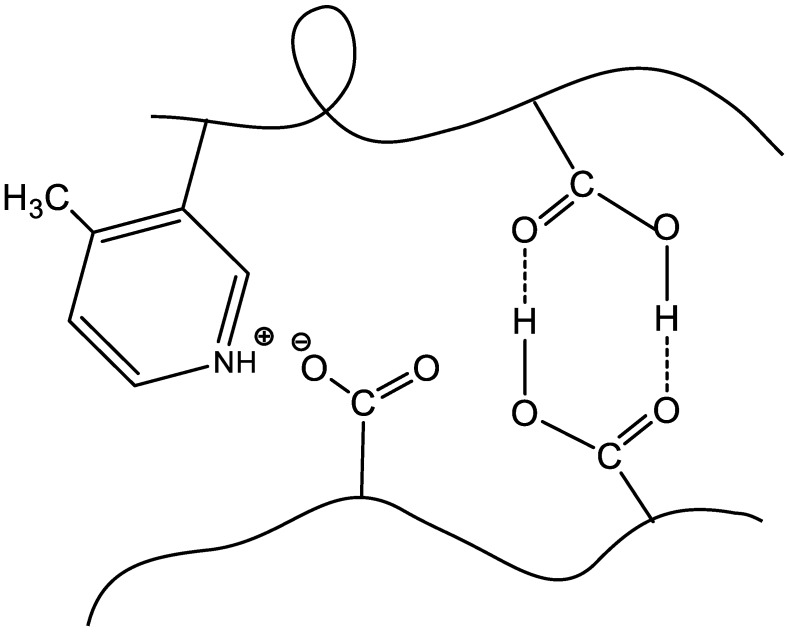
The insolubility of BPA in aqueous solution is observed at the IEP due to the formation of globular Intra-PEC because of cooperative interactions between the anionic and cationic blocks (Scheme 1).
Scheme 1.
Formation of intrapolyelectrolyte complexes (Intra-PEC) at the isoelectric point (IEP) of equimolar PMAA-b-P1M4VPCl.
By definition [89], a globule is the state of a macromolecule possessing a definite thermodynamically stable spatial structure in which the fluctuation of the density is rather small in comparison with the density itself, while the correlation radius of the density fluctuation is much smaller than the size of whole macromolecule.
Increase in the ionic strength (µ) of the solution through addition of KCl diminishes the precipitation region and at higher values of µ = 0.3 mol·L−1 the BPA is entirely dissolved. Dissolution of BPA is due to screening of the electrostatic interactions among the oppositely charged blocks on the polymers by KCl, resulting in the change of globule to coil. By definition [89], coil is the state of macromolecule possessing an adequate spatial structure. In this case, the fluctuation of the density is in the order of density itself, while correlation radius is in the order of a macromolecular size. Thus, coil and globule differ from each other by fluctuation regime. From a statistical physics point of view, such a major (or big) difference between two states is called phases, and transformation between them is defined as a phase transition. According to this terminology, the transition of globular structure of BPA at the IEP to coil structure upon addition of low-molecular-weight salts can be qualified as a phase transition.
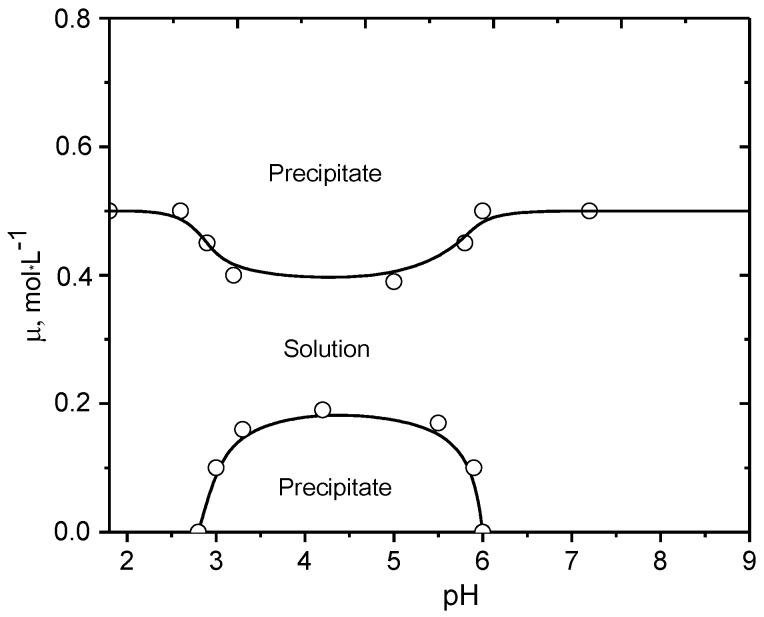
A phase diagram of PMAA-b-P1M4VPCl at constant concentration of BPA (C = 0.045 g·dL−1) and various pH and ionic strength is shown in Figure 4. At the IEP BPA has a wide region of insolubility that is narrowed upon increase in the ionic strength of the solution adjusted by addition of KCl. The dissolution of BPA at µ = 0.2–0.4 mol·L−1 KCl is related to the destruction of the globules stabilized by intraionic salt “bridges” between the oppositely charged blocks. Precipitation of PMAA-b-P1M4VPCl at µ > 0.4 mol·L−1 can be explained by the “salting out” effect. The Intra-PEC of PMAA-b-P1M4VPCl composed of the excess of anionic or cationic blocks has the specific structure at the IEP [18,88]. Its aggregate is made of Intra-PEC surrounded by anionic (or cationic) blocks, which prevent it from precipitation and preserving the whole macromolecules in water-soluble state (Scheme 2).
Figure 4.
Phase diagram of PMAA-b-P1M4VPCl (45:55 mol %) in aqueous solution. [PMAA-b-P1M4VPCl] = 0.045 g·dL−1. (Reprinted from Ref. [18]).
Scheme 2.
Schematic representation of Intra-PEC formed at the IEP of PMAA-b-P1M4VPCl with the excess of cationic block.
Figure 5 presents the phase diagram of chitosan carboxymethyl esters (CCM)/water/salt systems with parameters including the polymer concentration, pH and ionic strength of the solution [90,91]. When the concentration of polyampholyte increases, an isoelectric region is broadened and separated into 2 phases. One is a solvent and the other is a swollen precipitated Intra-PEC.
Figure 5.
Phase diagram of chitosan carboxymethyl esters (CCM)-water-salt system as a function of polymer concentration. [CCM] = 0.05 wt % (1), 0.1 wt % (2) and 0.5 wt % (3).
Formation of Intra-PEC at the IEP was compared for triblock and random polyampholytes based on N,N-dimethylaminoethylmethacrylate-methylmethacrylate-methacrylic acid (DMAEM-MMA-MAA) [92,93]. Light-scattering data of random and triblock copolymers DMAEM-MMA-MAA of 1:1:1 composition reveal that no aggregation is observed for random copolymers while large aggregates of the size 110–188 nm are formed for triblock copolymers at the IEP. The latter is due to the formation of globular Intra-PEC. The same phenomenon was observed for DMAEM-MAA containing hydrophobic spacers [94] and ABC triblock copolymers of poly(styrene)-b-poly(2- (or 4-)vinylpyridine)-b-poly(methacrylic acid) [95].
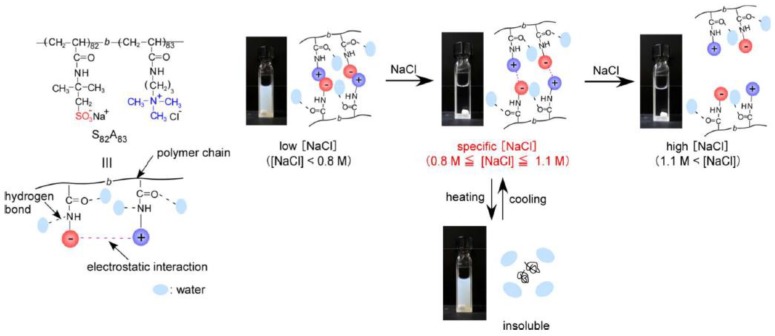
Diblock “quenched” polyampholytes composed of AMPSNa-APTAC synthesized by RAFT radical polymerization exhibit both thermo- and salt sensitive behavior [96]. At low salt concentration, the Intra-PEC is stabilized by ionic bonds (Figure 6). At 0.8 mol·L−1 ≤ [NaCl] ≤ 1.1 mol·L−1 the Intra-PEC is disrupted, becomes water-soluble and at 1.1 mol·L−1 ≤ [NaCl] transforms to unimer state with Rh = 6.0 nm. Upon heating, the saline solution of AMPSNa-APTAC shows the LCST (lower critical soluble temperature) due to enhancement of hydrophobic interactions of the polymer chains.
Figure 6.
Thermo- and salt sensitivity of diblock “quenched” polyampholyte AMPSNa-APTAC. (Reprinted from [96]).
Authors [97] studied the solution and volume-phase behavior, structural and stimuli-responsive properties of linear and crosslinked triblock polyampholytes PAA109-b-P2VP819-b-PAA109 and PAA109-b-QP2VP819-b-PAA109 (where PAA is poly(acrylic acid), P2VP is poly(2-vinylpyridine), QP2VP is quaternized P2VP) with the change in pH and ionic strength of the solution together with computer simulation of the systems. At pH > 5.0 the system PAA109-b-QP2VP819-b-PAA109 forms a viscous opaque liquid, while at pH < 3.0 it forms a stiff gel. These results are interpreted in terms of a charge-driven association between oppositely charged blocks and the formation of neutral hydrophobic complexes.
The rheology, rotational dynamic light scattering and small angle neutron scattering were used to evaluate the influence of the pH on gel and solution behavior of the triblock terpolymer: poly(methyl methacrylate)-b-poly(2-(diethylamino)ethyl methacrylate-co-methacrylic acid)-b-poly(methyl methacrylate) (PMAA-b-P(DEA-MAA)-b-PMMA) [98,99]. Existence of telechelic polyampholytes in several regimes was identified. Phase separation, or precipitation, of PMAA-b-P(DEA-MAA)-b-PMMA was observed at the IEP (in the range of pH 7.6–8.8). At pH 6.0–7.4 (just below the IEP regime) a sol–gel transition was fixed. In acidic or basic regions, amphoteric macromolecules are in stretched conformation due to predominance of positive or negative charges in the polymer chain.
Recent remarkable review [100] is focused on the comparative analysis of stimuli-responsive hydrogels made of block co-polyampholytes and block copolymers or homopolyelectrolytes. The main attention is paid to physically crosslinked hydrogels based on the intermolecular association of hydrophilic triblock copolymers end-capped by polyion sequences. Another gelling system is a mixture of triblock copolymer with ionic end blocks and an oppositely charged homopolymer. The study of the solution, rheological and structural properties of co-assembled hydrogels offers important guidance for their potential biomedical application.
The globular structure of macromolecules [89] can be considered as dense three-dimensional nucleation, consisting of a “core” from which the monomers “force out of” the solvent and surrounded by a hydrophilic “edge”. Such globular structure can be realized at the IEP of water-soluble polyampholytes and lead to formation of high ordered structures [101]. In the authors’ opinion, the appearance of sharp lines in a Raman spectrum, especially in the low-frequency region, indicates the presence of a high-ordered structure like crystalline domains in aqueous media. However, additional XRD (X-ray diffraction), SAXS (small angle X-ray scattering), WAXS (wide angle X-ray scattering), SANS (small angle neutron scattering) measurements are needed to confirm such hypothesis.
4. Interpolyelectrolyte Complexes of Polyampholytes
The pioneering works of H.A. Bixler and A.S. Michaelis [102], V.A. Kabanov [103], B. Philipp [104], E. Tsuchida [105], and E.A. Bekturov [106] made substantial contributions to the fast development of interpolyelectrolyte (or interpolymer) complexes. Various aspects of polyelectrolyte–polyelectrolyte complexes have been reviewed recently [107,108,109,110,111]. Interpolyelectrolyte complexes of polyampholytes [112] are a subject less considered in comparison with hydrogen-bonded interpolymer complexes [113,114]. The complexation between 2-methyl-5-vinylpyridine-acrylic acid (2M5VPy-co-AA) and poly(acrylic acid) (PAA) was first studied by V.A. Kabanov et al. [115]. It was established that the common cooperative system consisting of the 2M5VPy-co-AA and PAA via ionic and hydrogen bonds is responsible for PPC formation (Scheme 3).
Scheme 3.
Schematic representation of Inter-PEC between 2-methyl-5-vinylpyridine-co-acrylic acid (2M5VPy-co-AA) and poly(acrylic acid) (PAA).
The interaction between polyampholyte and 2,5-ionene bromide by turbidimetry was studied by [116] (Figure 7).
Figure 7.
Turbidimetric titration curves for ionene bromide solution by chitosan sulfate (CS) at pH = 2.5 (curve 1) and 11.5 (curve 2). [2,5-ionene] = 5·10−4 mol·L−1. Z = [CS]/[2,5-ionene].
It was found that in the alkaline region Inter-PEC while in acidic region Intra-PEC is formed.
The competition between the Intra-PEC and Inter-PEC is clearly seen when 13C nuclear magnetic resonance (NMR) spectra of N-methyldiallylamine-maleic acid (MDAA-MA) and mixture of MDAA-MA and poly(N,N-dimethyl-N,N-diallylammonium chloride) (PDMDAAC) are compared [117,118,119,120,121]. The signals of carboxylate anions of MDAA-MA participating in the formation of Intra-PEC appear at 180.5 and 180.8 ppm. The formation of Inter-PEC between carboxylate anions of MDAA-MA and quaternary nitrogen atoms of PDMDAAC shifts the same peaks to 181.3 and 181.8 ppm. At the IEP of N,N-dimethyldiallylammonium-maleic acid (DMDAA-MA) (pHIEP = 4.1), the position of the carboxylate anions peak of DMDAA-MA at 182.4 ppm does not change during the addition of PDMDAAC, e.g., the strength of Intra-PEC is higher than that of the Inter-PEC. Formation of the Inter-PEC between cellulose-based poly(zwitterion) and PDMDAAC is mentioned in [122].
A copolymer of N,N-dimethyldiallylammonium and alkyl (or aryl) derivatives of maleamic acids (abbreviated as APA series in dependence of the length of the hydrophobic moieties of maleamic acid) were involved into the complexation with PAA and poly(styrene sodium sulfonate) (NaPSS) [123] (Scheme 4)
Scheme 4.
The repeating units of APA (Alkyl (or aryl) polyampholytes) series, where R = H (APA-1); C4H9 (APA-2); C6H5 (APA-3); C6H5(CH2)3CH3 (APA-4) (Reprinted from [123]).
The PPCs with participation of PAA (or NaPSS) and polyampholytes of the APA series are very compact and represent core–shell particles that are preserved in aqueous solution due to the presence of carboxylic and amide groups of APA. Table 2 summarizes the compositions of PPC formed between APA series and PAA or NaPSS.
Table 2.
Composition of polyelectrolyte-polyampholyte complexes (PPC) formed between APA and PAA and NaPSS.
| Polyampholytes | Polyelectrolytes (PE) | PPC, n = [APA]/[PE] |
|---|---|---|
| APA-1 | PAA | 1:1 |
| NaPSS | 3:1 | |
| APA-2 | PAA | 1:4 |
| NaPSS | 5:2 | |
| APA-3 | PAA | 1:2 |
| NaPSS | 5:1 | |
| APA-4 | PAA | 1:2 |
Interaction of amphoteric dendrimers and proteins with linear and crosslinked anionic and cationic polyelectrolytes was investigated by Kabanov et al. [124,125]. In acidic and alkaline media up to pH ~ pHIEP, dendritic polyampholytes can form Inter-PEC with flexible linear polyanions and polycations. The polyampholyte dendrimers are also efficiently sorbed by a polyanionic (below the IEP) and polycationic hydrogels (above the IEP). The binding degree of polyampholyte dendrimers with oppositely charged linear or crosslinked polyelectrolytes is ascribed to the competition between intra-dendrimeric zwitterions and interionic salt bonds of functional groups of dendrimers and polyelectrolytes.
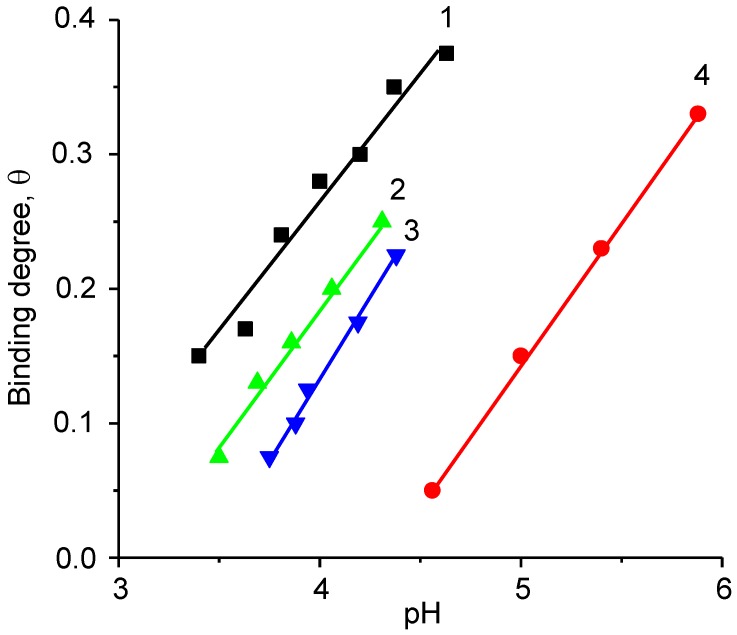
The BPA binds the anionic and cationic polyelectrolytes more cooperatively in comparison with statistical and alternating polyampholytes [17,18,88]. Complexation between BPA and anionic (or cationic) polyelectrolyte is higher than complexation between the acidic and basic blocks of BPA. It should be mentioned that BPA binds the cationic (or anionic) initially and later the anionic (or cationic) polyelectrolytes (Figure 8).
Figure 8.
pH profile of binding degree θ for systems consisting of PMAA-b-P1M4VPCl/PAA (1), PMAA-b-P1M4VPCl/PDMDAAC (2), (PMAA-b-P1M4VPCl/PDMDAAC)/PAA (3). Curve 4 corresponds to intramolecular complexation within block polyampholyte PMAA-b-P1M4VPCl. (Reprinted from Ref. [88]).
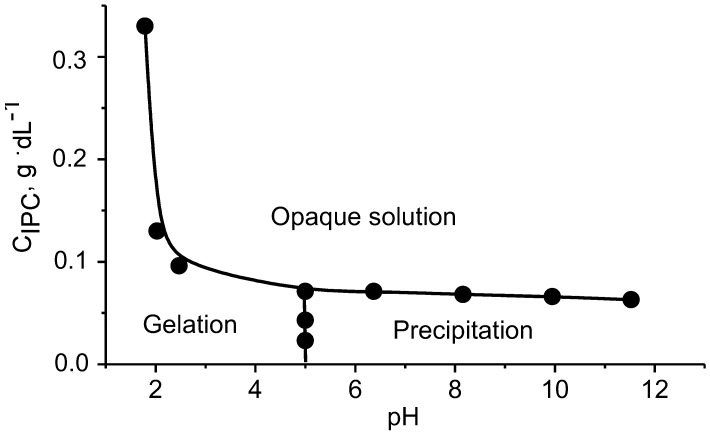
However, the cooperative character of this process is much lower than the cooperativity of block polyampholyte–polyelectrolyte pairs. Such Inter-PECs are insoluble and tend to precipitate. Figure 9 shows the phase diagram of the system consisting of PMAA-b-P1M4VPCl and PVBTMACl in dependence of PMAA-b-P1M4VPCl concentration and pH of the solution.
Figure 9.
Phase transitions of Inter-PEC consisting of block polyampholyte PMAA-b-P1M4VPCl and poly(vinylbenzyltrimethylammonium chloride) (PVBTMACl) in water with opaque solution, gelation, and precipitation regions. (Reprinted from [18]).
The Inter-PEC in the form of opaque solution is formed at CPEC > 0.075 g·dL−1 and pH = 2–12. The precipitation starts at pH ≥ 5.0 and CPEC <0.075 g·dL−1. The gelation occurs preferentially at pH < 5.0 and CPEC > 0.075 g·dL−1. Thus there are three phases in this system which are solution, gel and precipitate. They can be transformed into each other with changes in either PEC concentration or pH. Higher CPEC leads to precipitation of inter-PEC particles. At the relatively low CPEC they are more uniformly distributed to form an opaque solution. Both the phase transition between precipitate–microgel and between opaque solution–microgel at low pH are subject to the swelling of Inter-PEC particles and decrease in the interaction among the ionized carboxylic groups of acidic blocks in Inter-PEC formation. The gel formation process occurs following the release of PMAA “loops” which form interchain networks with H-bonding. On the basis of the above results, the transitions between precipitate–opaque solution, precipitate-microgel and opaque solution–microgel can be illustrated as shown in Scheme 5:
Scheme 5.
Schematic representation of phase transitions of system PMAA-b-P1M4VPCl/PVBTMACl in dependence of BPA (blockpolyampholyte) concentration and pH of the solution.
A phase diagram for triblock and random polyampholyte AA-DMAEM-MMA (0.9:1:1) and poly(vinyl alcohol) (PVA) was investigated along with polymer concentration, pH, and salt concentration [126,127]. Phase separation occurred at 10% polymer concentrations. The phase diagrams of PVA with the block polyampholytes exhibit whole miscibility at acidic and alkaline pH regardless of salt concentration. Each region of the phase diagram was interpreted in terms of the formation of cooperative hydrogen bonds between the hydroxyl groups of PVA and carboxyl groups of the undissociated methacrylic acid residue of polyampholytes. PVA and the random polyampholytes generally display one-phase behavior in the pH-(KCl) space. The phase behavior of the triblock polyampholytes with PVA is useful to apply for biological molecule separation including proteins, and organic molecule contaminants, such as halogenated aromatic hydrocarbons.
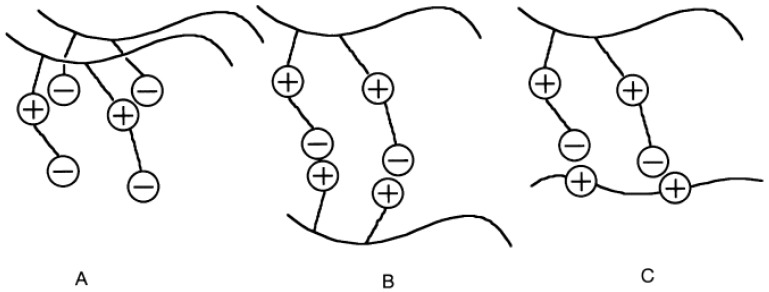
Authors [128,129] studied the complexation of polycarbobetaines with NaPSS, DNA and poly(zwitterion)—poly[3-dimethyl(methacryloyloxyethyl ammonium propane sulfonate)] (PDMAPS) with polymeric anion: poly(2-acrylamido-2-methyl propane sulfonic acid) (PAMPS) or polymeric cations: poly(3-acrylamidopropyltrimethyl ammonium chloride) (PDMAPAA-Q) and x,y-ionene bromides (x = 3, 6; y = 3,4) (Scheme 6).
Scheme 6.
Chemical structures of polymers used for complexation reactions (Reprinted from [128]).
The phase behavior of PDMAPS is totally altered with the addition of these polyelectrolytes, and the resulting UCST (upper critical soluble temperature) values are found to be different. Furthermore, the UCST decreased significantly with addition of PAMPS into the PDMAPS solution and vanished at high PAMPS concentration. This distinct variation in the UCST indicates the strong interaction between two polyelectrolytes which is correlated with the geometrical structure of polyelectrolyte complexes (Scheme 7A–C).
Scheme 7.
Scheme of formation of Inter-PEC between PDMAPS and PAMPS (A), PDMAPAA-Q (B) and x,y-ionene bromides (C). (Reprinted from [128]).
In the case of PDMAPS–PAMPS, an ammonium cation in the middle interacts with sulfonate anion of PAMPS. Such Inter-PEC is solubilized in water by free anionic groups of PDMAPS (Scheme 7A). For solubilization of Inter-PEC consisting of PDMAPAA-Q and PDMAPS, ammonium cation of PDMAPS is responsible because the sulfonates located at the end of side chain are occupied by quaternary ammonium groups of PDMAPAA-Q (Scheme 7B). Also, PDMAPS can interact with ionene through the sulfonates located at the end of side chain (Scheme 7C). Thus, complexation of PDMAPS with anionic polyelectrolyte drastically increases the viscosity via forming a network and reduces the UCST with increase in the molar ratio of PAMPS/PDMAPS, which is ascribed to presence of free sulfonates located at the end of side-chain. However, the complexation of PDMAPS-polycation first minimizes the UCST, which is also found in the complexation of PDMAPS-PAMPS, then the UCST value increases with a molar ratio of polycation/PDMAPS without forming the network due to the presence of the free ammonium cation of PDMAPS in the middle of the side chain.
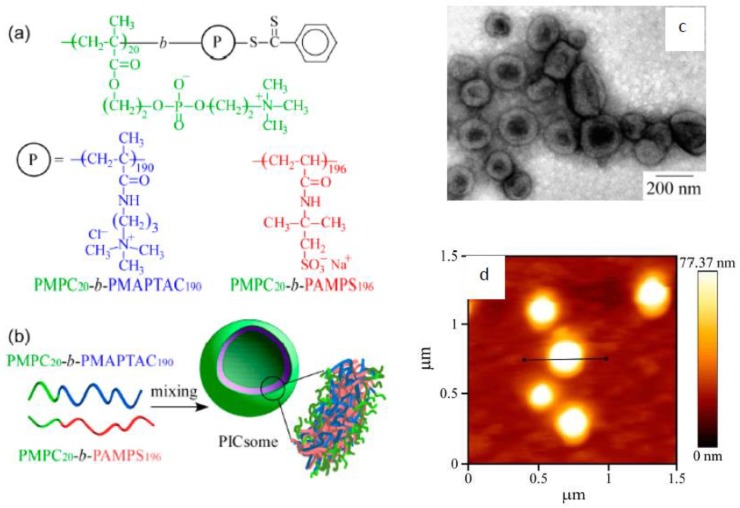
The formation of Inter-PEC micelles and vesicles was described by [130,131,132]. Micelles assembled from random copolymers of AMPSNa-co-APTAC [132] end-capped by anionic (AMPSNa) or cationic (APTAC) blocks that show protein antifouling properties (Figure 10).
Figure 10.
Chemical structure of random copolymers of AMPSNa-APTAC containing the anionic (AMPSNa) and cationic (APTAC) blocks (a) and schematic representation of Inter-PEC micelle formation together with protein antifouling properties (b). (Reprinted from Ref. [132]).
Inter-PEC vesicles were assembled by mixing aqueous solutions of diblock copolymers consisting of a hydrophilic poly(2-(methcaryloyloxy)ethylphosphorylcholine (PMPC) block and either a cationic (APTAC) or anionic (AMPSNa) blocks (Figure 11).
Figure 11.
Chemical structures of PMPC-APTAC and PMPC-AMPSNa diblock copolymers (a), conceptual illustration of stoichiometric Inter-PEC vesicle formation (b), transmission electron microsope (TEM) (c) and atomic force microscope (AFM) (d) images of vesicles. (Reprinted from [131]).
Both Inter-PEC micelles and vesicles may be appropriate carriers for bioactive compounds and used as drug delivery systems.
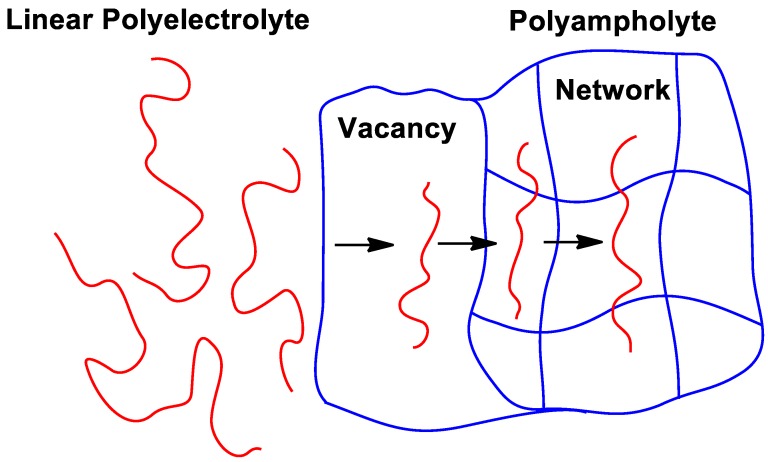
Complexation of polyampholyte gels and linear polyelectrolytes is a less considered subject [107]. Mechanism of sorption of polyelectrolytes by amphoteric gel is similar to the penetration of linear polyelectrolytes within the oppositely charged polyelectrolyte networks, as shown schematically in Figure 12.
Figure 12.
Penetration of macromolecules into hydrogel matrix via “ion-hopping transportation” mechanism.
Penetration of macromolecules into hydrogel matrix proceeds via “race-relay ion transport” (or “ion-hopping transportation”) mechanism resulting in gel contraction. The driving force of this process is electrostatic binding of anionic or cationic polyelectrolytes with positive or negative charges of hydrogels, e.g., constant migration of charged macromolecules deeply into the gel volume by exchanging one fragment of the network to another vacant place. Each transfer taking place at the gel–solution interface is directed towards the hydrogel phase and leads to the formation of a vacancy within network that in turn is accessible to other macromolecules for transfer in the same direction.
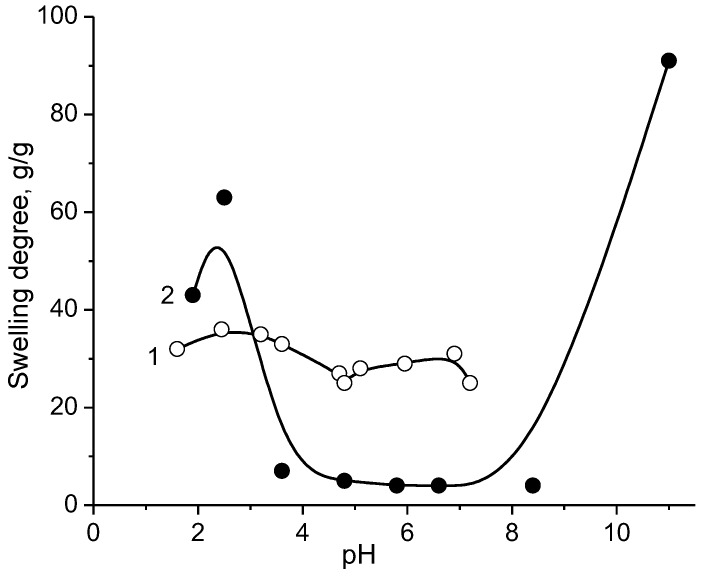
The swelling–deswelling behavior of amphoteric gel which was made of maleic acid (MA), N,N’-dimehyldiallylammonium chloride (DMDAAC) and diallylamine (DAA) was studied in the presence of NaPSS [133]. The swelling degree of the amphoteric gel–NaPSS is minimally in the pH range between 4 and 8 (Figure 13) and increases significantly in the strong acidic and alkaline regions. As distinct from amphoteric gel MA-DMDAAC-DAA, which shrinks at the IEP (pH ≈ 4.6), the complex of MA-DMDAAC-DAA with NaPSS collapses over a wide range of pH because the NaPSS entrapped in the gel network acts as additional physical crosslinker. The complexation of polyampholyte gel with linear polyelectrolyte considerably broadens the IEP of PPC in comparison with the IEP of amphoteric gel itself.
Figure 13.
pH dependent swelling of amphoteric gel MA-DMDAAC-DAA (1) and complexes of amphoteric gel with NaPSS (2). (Updated from [133]).
Layer-by-layer (LbL) assembling of rigid polyampholyte—sulfonated cardo poly(arylen ether sulfone) (SPES)—with anionic NaPSS and cationic PDMDAAC was shown by [134]. The formation of LbL between SPES and NaPSS was not observed in the pH range of 1~12. In contrast, multilayers were easily fabricated between SPES and PDMDAAC in the same pH range. The reason is that SPES contain two negative charges and one positive charge with every repeat unit exhibiting preferentially polyanionic behavior. The remarkable behavior of polyampholytes to change the net charge from positive to negative through the IEP is a powerful tool to assemble the LbL coatings or films with both cationic and anionic polyelectrolytes. Such an approach was realized by authors [135] to obtain pH-sensitive LbL films with an amphoteric copolymer, i.e., poly(diallylamine-co-maleic acid) (PDAMA). LbL films between the NaPSS and PDAMA were formed at pH 3.0, while LbL films containing PDAMA and cationic poly(N-ethyl-4-vinylpyridine) (PEVP) were observed at pH 8.0. Using this methodology, the same authors [136] were able to use PDAMA as a component to construct free-standing LbL films in combination with the NaPSS or the PDMDAAC. The resulting films were coated onto a quartz slide at a given pH value of 4.0 and 8.0. Furthermore, the precursor, or so called sacrificial layer, was covered with LbL films of poly(allylamine hydrochloride) (PAH) and NaPSS. After pH dependent dissolution of the PDAMA-PDMDAAC or PDAMA-NaPSS precursor layer, the resulting free-standing PAH-PSS films are colloidally stable in water for a few months. If the films are thick enough, they can be stable in air.
5. Realization of the ‘Isoelectric Effect’ at the Isoelectric Point (IEP) of Polyampholytes
At the IEP the compact globule structure is formed via squeezing out the solvent due to strong electrostatic attraction forces between oppositely charged groups. The isoelectric effect taking place at the IEP was first reported by authors [137,138,139] and afterwards confirmed by several researchers [140,141,142]. Moreover, it can be stated that any molecules associated with polyampholytes can potentially be discharged at the IEP via controlling their inter- and intramolecular interactions. Furthermore, if intrachain interaction of acidic and basic groups within a single macromolecule prevails the above releasing phenomena can be realized via overcoming interchain interaction between substance and polyampholytes. The realization of the “isoelectric effect” for a series of water-soluble polyampholytes was demonstrated for metal ions [143], dye molecules [144], polyelectrolytes and proteins [145].
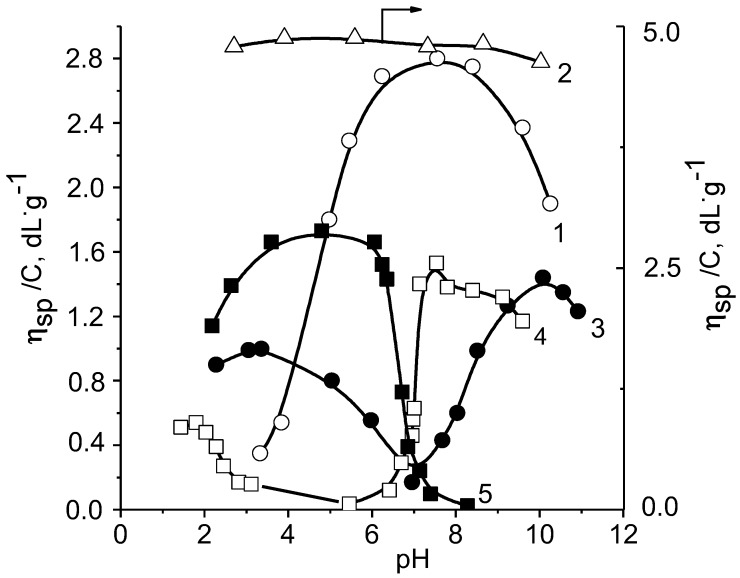
The existence of the “isoelectric effect” was clearly shown for systems consisting of polyampholyte based on 2,5-dimethyl-4-vinylethynylpiperidinol-4 and acrylic acid (DMVEP-co-AA) and anionic or cationic polyelectrolytes [145]. The composition of Inter-PEC derived from the potentiometric, conductimetric and turbidimetric curves is identified to be at the ratio of [polyampholyte] to [polyelectrolyte] = 3:1. Figure 14 shows the correlation of the reduced viscosity with pH for the individual components and Inter-PEC.
Figure 14.
Effect of pH on the reduced viscosity of PAA (curve 1), PDMDAAC (curve 2), DMVEP-co-AA (curve 3), and Inter-PEC composed of DMVEP-co-AA/PAA (curve 4) and DMVEP-co-AA/PDMDAAC (curve 5) in water.
Phase separation for the following two systems occurs at the different pH ranges. For the system composed of DMVEP-co-AA and PAA it occurs at 3.0 < pH < 5.5 and it occurs at pH > 8.2 for the mixture of DMVEP-co-AA and PDMDAAC. Near the IEP of DMVEP-co-AA (pHiep = 7.0) the reduced viscosity of associates increases sharply. This is due to destruction of polycomplex particles into individual components. A cooperative destruction of polycomplex particles near the IEP is confirmed by an independent sedimentation experiment. Figure 15 shows the sedimentation diagrams of DMVEP-co-AA, PAA and polycomplexes at various pH.
Figure 15.
Sedimentograms of PAA (curve 1), DMVEP-co-AA (curve 2) and polyelectrolyte complexes composed of [DMVEP-co-AA]/[PAA] = 3:1 (curves 3-5) at pH = 6.1 (curve 3), 6.9 (curve 4), and 7.1 (curves 1, 2, 5).
The sedimentograms of the polyampholyte–polyelectrolyte complex demonstrate a single peak with sedimentation coefficient S = 4.75 and 4.18 at pH = 6.1 and 6.9, respectively. However at pH = 7.1 two peaks with S = 1.9 (“slow peak”) and 5.6 (“fast peak”) show up. The sedimentation coefficients for individual PAA and DMVEP-co-AA at pH = 7.1 are 1.06 and 3.53, respectively. Thus the presence of “slow” and “fast” sedimentation peaks at narrow interval of pH (∆pH = 0.2) is ascribed to the destruction of Inter-PEC into the singular macromolecules. Inter-PEC consisting of polyampholyte and polyelectrolyte is expressed as double strand sequences of pairs formed via ionic and hydrogen bonds [108]. At the near IEP some acidic and basic groups of polyampholytes displayed on loops can interact with each other and form Intra-PEC. Scheme 8 represents the speculative mechanism of destruction of polyampholyte–polyelectrolyte associates near the IEP.
Scheme 8.
Proposed mechanism for destruction of Inter-PEC near the IEP of polyampholyte.
The dependence of Inter-PEC on pH provides further evidence for the validity of the proposed mechanism [141]. The yield of Inter-PEC composed of polyampholyte-poly(styrenesulfonate) (PA-NaPSS) and polyampholyte-poly(N,N-dimethyldiallylammonium chloride) (PA-PDMDAAC) is close to zero at the IEP of polyampholyte.
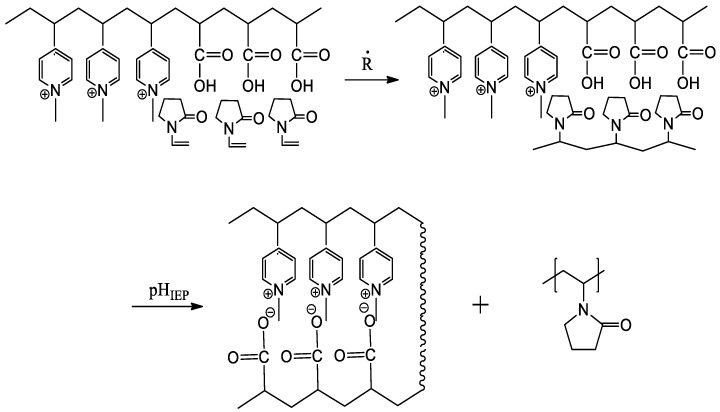
The “isoelectric effect” can also smartly be used for matrix polyreactions [146]. Scheme 9 explains the synthesis reaction of poly(N-vinylpyrrolidone) on block polyampholyte matrix. Application of block polyampholyte as polymerization matrix has the following advantages in comparison with polyacids and polybases alone: (1) each block (anionic or cationic) can serve as polymerization matrix; (2) the product of matrix polymerization is water-soluble and preserved from the precipitation due to free (non-loaded) acidic or basic blocks; (3) the “daughter” chain can easily be separated from the matrix as a result of competition between inter- and intramacromolecular complexation; (4) molecular weight of products can be to some degree controlled by the length of polymer matrix; (5) block polyampholyte can be used for matrix polyreactions several times.
Scheme 9.
Realization of the “isoelectric effect” in synthesis of poly(N-vinylpyrrolidone) on blockpolyampholyte matrix.
The method of protein separation by water-soluble polyampholytes is selective complexation of polyampholyte with one of the proteins, which has predominating positive or negative charge [93,147,148,149,150]. Upon addition of polyampholyte to a mixture of proteins, some proteins form a complex with the polyampholyte, as the others stay in the supernatant phase [151]. The precipitated protein–polyampholyte complex may be isolated from the system and then redissolved at a different pH or else can be destroyed at the IEP, where the full precipitation of polyampholyte itself takes place [152].
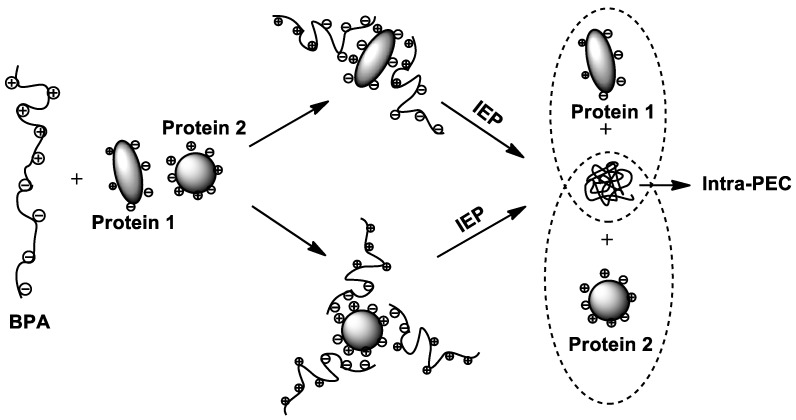
Unique separation of protein mixture was performed at the IEP of block polyampholytes [153]. In this case one of the blocks of polyampholyte interacts with one charge of protein, which is opposite to its charge, while the main chain (or other block)—with another protein charge as illustrated in Scheme 10.
Scheme 10.
Separation of protein mixture at the IEP of BPA.
In both cases protein release process occurs at the IEP of polyampholyte due to the formation of intraionic polyelectrolyte complexes (Intra-PEC).
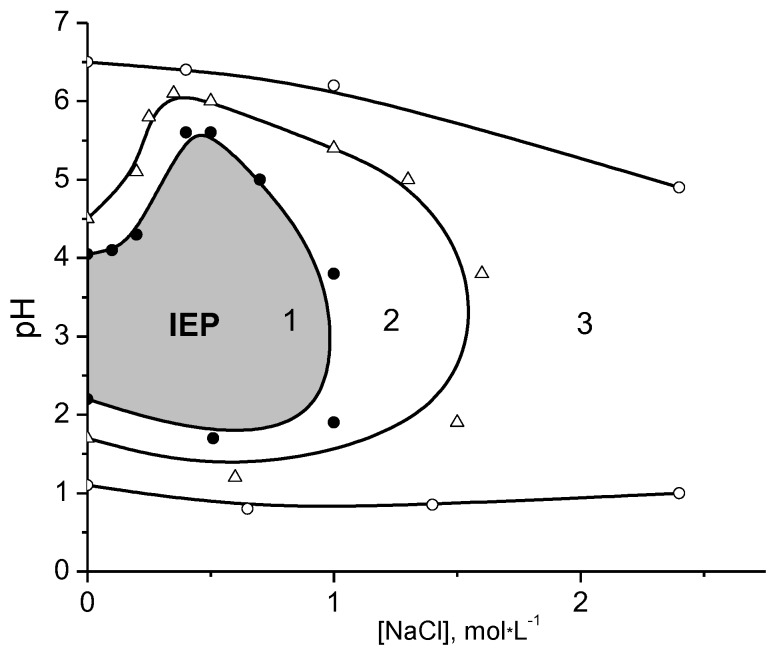
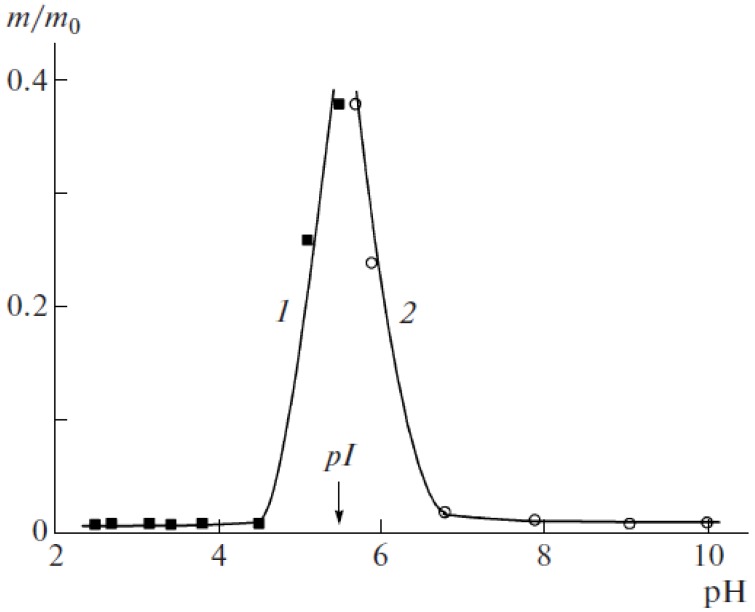
It is interesting to note that the “isoelectric effect” was observed for complexes of polyampholyte dendrimers with highly swelling sodium poly(2-acrylamido-2-methylpropanesufonate) (PAMPSNa) and PDMDAAC used as polyelectrolyte networks [154]. Polyampholyte dendrimers represent the carboxylated poly(propylenimine) dendrimers (PPID) of five generations abbreviated as PPID-(COOH)x, where x = 4, 8, 16, 32 and 64. As seen from Figure 16 at the IEP of polyampholyte dendrimer the relative weight of Inter-PEC samples m/m0 (where m is the equilibrium weight of the product of completed sorption at a given pH of the equilibrium solution, m0 is the weight of initial equilibrium swollen gel in water) increases significantly due to destruction of Inter-PEC and release of polyampholyte dendrimer to outer solution from the polyelectrolyte network. If such a sample is washed with distilled water, its relative weight becomes close to unity.
Figure 16.
pH dependence of relative weight m/m0 for polyampholyte dendrimer-PAMPSNa (1) and polyampholyte dendrimer-PDMDAAC (2) complexes. pI denotes the isoelectric point. (Reprinted from [154]).
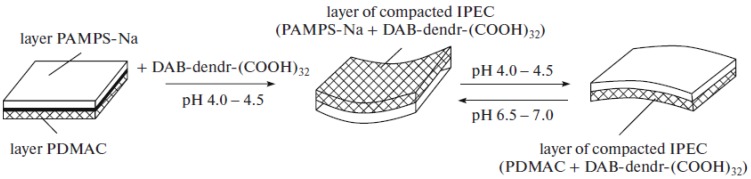
Thus at the IEP of polyampholyte dendrimer the detachment of amphoteric macromolecules from the gel matrix of polyelectrolytes takes place. This statement is very important because the “isoelectric effect” can be realized not only for linear polyampholyte–polyelectrolyte complexes, but also for water-soluble dendrimeric polyampholyte-polyelectrolyte gels. The mechanism of release of polyelectrolytes or polyampholytes can be explained by competition between Intra-PEC and Inter-PEC formation. The data of Figure 16 suggest that the polyampholyte dendrimer can pass from the Inter-PEC containing the anionic gel to a new Inter-PEC formed by the cationic gel, and vice versa, with fine-tuning of pH near the IEP. The transfer of polyampholyte dendrimer from anionic to cationic gel in dependence of pH is schematically illustrated in Scheme 11.
Scheme 11.
pH dependent transfer of polyampholyte dendrimer from anionic gel to cationic one through the IEP. (Reprinted from [154]).
At pH 4.0–4.5 sorption of polyampholyte dendrimer by anionic gel AMPSNa leads to shrinking of the gel layer and concavation of gel sample. At pH 6.5–7.0 the polyampholyte dendrimer is desorbed from AMPSNa and absorbed by PDMDAAC. The formation of Inter-PEC between polyampholyte dendrimer and PDMDAAC gel results in gel contraction and concavation of sample.
The feasibility of the “isoelectric effect” was tested for crosslinked amphoteric macroporous cryogels consisting of N,N-dimethylaminoethylmethacrylate and methacrylic acid (DMAEM-co-MAA) with respect to surfactant—sodium dodecylbenzene sulfonate (SDBS), dyes methylene blue (MB) and methyl orange (MO), and protein lysozyme [155]. It was found that the release efficiency of dye, surfactant and protein at the IEP from amphoteric cryogel (pHIEP = 7.1) can reach up to 98%. Figure 17 shows release mechanism of low- and high-molecular compounds at the IEP of amphoteric cryogel.
Figure 17.
Schematic representation of adsorption of sodium dodecylbenzene sulfonate (SDBS) at pH 5.3 (route 1), lysozyme at pH 9.5 (route 2) and their release at pH 7.1, eg. at the IEP of amphoteric cryogel (route 3). (Reprinted from [155]).
At the IEP of amphoteric cryogel the cooperativity of intrachain interactions between the acidic and basic groups within a cryogel chain exceeds those of interchain interactions between cryogel and protein (surfactant and dye molecules). As a result, at the IEP of amphoteric cryogel (pHIEP = 7.1) the lysozyme, MB, MO and SDBS are released from the cryogel matrix to the outer solution.
An alternative way of protein separation is molecularly imprinted polyampholyte (MIP) hydrogels and cryogels that are synthesized by crosslinking acid-base monomers in the presence of template proteins forming polyampholyte–protein complexes within the network [156,157,158]. After removing of the template a cavity with a complementary shape and selective recognition properties to template molecules is maintained [159]. The stimuli sensitive ability of MIP networks to switch on/off the affinity of the networks for the imprinted molecules by fine-tuning of temperature, pH, and ionic strength is promising for biomedical applications [160,161].
6. Amphoteric Behavior of Interpolyelectrolyte Complexes
Polyelectrolyte complexes (PEC) are the products of interaction of weak or strong polyelectrolytes in various combinations [102,103,104,105,106,107,162]. The formation of PEC has no analogues in chemistry of low-molecular-weight compounds because the stabilization of the final products is mainly due to the entropy factor. From the thermodynamic point of view, the complementarity of macromolecules bearing cationic and anionic groups is the driving force of PEC formation that is also responsible for self-organization of biopolymers. Depending on the charge density of interacting polyelectrolytes, concentration, stoichiometry, pH, ionic strength, organic solvent additive PEC particles can be maintained as clear solution, opaque solution, precipitation and gel state. The preparation procedure of PEC films, fibers or coatings includes the dissolution of PEC in ternary system consisting of water, salt and organic solvent. A thin interfacial PEC film can rapidly be formed when two high concentrated polyelectrolytes are mixed [102]. PEC membranes can also be designed on the boundary of water–organic solvents [163,164,165,166,167,168,169]. In recent years the layer-by-layer deposition technique received a lot of interest [170,171,172,173]. The existence of stoichiometric (SPEC) and non-stoichiometric (NPEC) polyelectrolyte complexes is commonly recognized today [162]. As distinct from SPEC, where the molar ratio of oppositely charged polyelectrolytes involved into complexation reaction is 1:1, NPEC are the products of cooperative interpolyelectrolyte reactions between oppositely charged polyelectrolytes with different polymerization degrees or chain lengths taken in non-equivalent ratios.
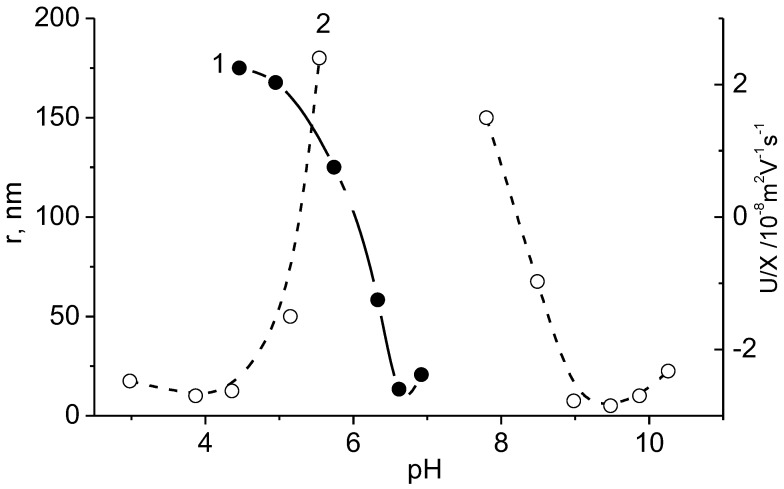
Block polyampholytes with the equal number of oppositely charged polyelectrolyte blocks are very close to SPEC, while block polyampholytes with the excess of positively or negatively charged blocks are analogous to NPEC [17,18]. However the main difference between block polyampholytes and PECs is that the interactions between two blocks result in a loss of conformational and translational entropy. The electrophoretic mobilities and radius of the particles of block polyampholyte PDMAEM-b-PMANa (the molar ratio of base/acid segment is 1/1.8) and the mixture of PDMAEM and sodium salt of PMAA with the same molar ratio of polybase:polyacid = 1:1.8 are compared in Figure 18 [86].
Figure 18.
Radius and electrophoretic mobilities of the block polyampholyte PDMAEM-b-PMANa (1) and polyelectrolyte complexes of PDMAEM-PMANa (2) as a function of pH at µ = 10−3 mol·L−1. (Reprinted from Ref. [86]).
The IEPs of both systems are 6.0. The instability regions for the mixture of homopolyelectrolytes and the blockpolyampholytes are also similar and displaced near the IEP. However, for the mixture of homopolyelectrolytes the strong complexation starts at 4.5 < pH < 9.0, and for block polyampholyte at 5.5 < pH < 8.0. This fact is probably explained by additional hydrophobic stabilization of interpolyelectrolyte complex particles. The stable homopolymer complexes at pH below 4.5 and above pH 9.0 have smaller size than the copolymer does even if the homopolymer molecules have longer length.
Isotachophoretic and viscometric results obtained for SPEC consisting of sodium salt of PAA (NaPAA) and poly[2-(N,N-diethyl-N-methylamino)ethyl acrylate] show positive and negative charges at pH = 4.6 and 8.3, respectively, and neutrality at pH = 7.0 [167,168,174,175]. Whereas for NPEC consisting of poly(sodium acrylate-co-acrylamide) and poly[2-(N,N-diethyl-N-methylamino)ethyl acrylate] the isoelectric pH corresponds to 4.0. The value of the reduced viscosity at the IEP increases with the growth of the ionic strength as in the case of polyampholytes (Figure 19).
Figure 19.
The relationship between the reduced viscosity of the system [poly(sodium acrylate-co-acrylamide)]/[poly-2-(N,N-diethyl-N-methylamino)ethyl acrylate] and pH at µ = 0.05 (curve 1), 0.075 (curve 2), 0.25 (curve 3), and 0.5 (curve 4). (Reprinted from [174]).
The conformational behavior of equimolar polyampholytes on the basis of vinyl- and styrenesulfonic acids and aminoalkylmethacrylates [176] repeats the properties of semi-interpenetrating polyelectrolyte networks (semi-IPPN) composed of crosslinked polybase and linear polyacid [177,178,179]. The invariability of the reduced viscosity at 2.8 < pH < 6.0 is an indication of the constant hydrodynamic volume of polyampholyte particles at the IEP [176]. The semi-IPPN [174] also collapses in the range of 3 < pH < 8 due to formation of interpolyelectrolyte complexes between oppositely charged groups. The swelling of semi-IPPN [179] at the IEP with increasing of the ionic strength can be accounted for the screening of ionic network by small ions. PEC membranes derived from the polyelectrolyte complexes [166,167,168,169,180,181] also show amphoteric character [182]. A minimum swelling degree of the membranes is ensured by Coulombic attractions between long sequences of acidic and basic groups of polyelectrolytes (Figure 20). The isoelectric point of PEC solution and membrane where the macromolecule is electroneutral is observed at pH ≅ 6.5.
Figure 20.
The swelling as a function of pH for semi-IPPN composed of crosslinked chitosan and linear PAA. (Reprinted from [180]).
Shashua [183] observed the oscillation of the electric potential across an ultrathin PEC membrane designed from co(acrylic acid-acrylamide) and PDMAEA in the presence of 0.15M NaCl. Shashua’s membrane consists of a cation selective polyacidic zone (a), an anion-selective polybasic zone (b) and a neutral polyampholytic zone (c). Under an externally imposed potential across the membrane, the cations will migrate from left to right while the anions will act oppositely. The salt accumulation takes place in the ampholytic region. Such salt accumulation invokes two effects. The generated osmotic pressure in the ampholytic zone (c) will cause water flow and induce an increase of hydrostatic pressure. Simultaneously, the accumulated salt will cause the contraction of polyanionic (a) and polycationic (b) zones. If the hydrostatic pressure generated from the polyelectrolyte configurational change overcomes the osmotic pressure, the solvent flow will be reversed. The process can be regenerated with the addition of salt which subsequently forces solvent from the zone c, thereby driving the concentration yet higher. In the regenerative step, salt will move out of the ampholytic region because of its high concentration gradient, and will continue its flow out until the membrane has reached the point of maximum contraction. Thus the process moves into the stage of diminishing salt concentration, following the relaxation of the PEC membrane matrix, and eventual return of the membrane to its initial state. The analytical solution of this phenomenon has been presented.
7. Conclusions
Intra- and interpolyelectrolyte complexes of polyampholytes represent an unusual category of the polyelectrolyte complexes (PEC) that are the products of interaction of oppositely charged polyelectrolytes. The formation of polyelectrolyte-polyampholyte complexes (PPC) with participation of random, regular, block, dendritic polyampholytes has no analogues in chemistry of low-molecular-weight compounds because the stabilization of final products is mainly due to electrostatic attraction between the opposite charges and entropy factor arising from the release of counterions. As evidenced in recent experiments [184,185], the driving entropy forces also originate from the rearrangement of water molecules around the polymer chains during the complexation. The complementarity of interacting macromolecules bearing charged groups is the driving force of PPC formation that is also responsible for the self-organization of biopolymers. In a dependence with charge density of interacting components, concentration, stoichiometry, pH, ionic strength, organic solvent additives, the PPC can be formed as clear solution, opaque solution, precipitation and gel state. As distinct from homopolyelectrolytes, polyampholytes can interact with both anionic and cationic macromolecules. This is especially specific for block polyampholytes. In turn the complexation of the equal number of oppositely charged polyelectrolyte blocks are very close to the formation of stoichiometric PEC, while block polyampholytes with the excess of positively or negatively charged blocks are able to form non-stoichiometric PEC. However the main difference between blockpolyampholytes and PECs is a loss of conformational and translational entropy caused by the interaction between two blocks. The stability order of PPC is theoretically predicted and experimentally confirmed as shown in the following order: block polyampholyte–polyelectrolyte > statistical polyampholyte–polyelectrolyte > alternating polyampholyte–polyelectrolyte. In most cases the suggested theoretical models describe the solution behavior and complexation properties of polyampholytes reasonably well. Several fundamental properties of polyampholytes were discovered in the last century. One of them is the so-called “antipolyelectrolyte” effect predicted by Ehrlich and Doty [186], theoretically concluded by Higgs and Joanny [42] and confirmed experimentally by many research groups [187,188,189,190]. The “antipolyelectrolyte” effect is related to the hydrodynamic size (or unfolding) of amphoteric macromolecules at the isoelectric point (IEP) upon addition of low-molecular-weight salts. Afterwards, this statement was confirmed for statistical-, alternating- and block-polyampholytes of various natures and structures as well as for polyampholyte hydrogels. Another fundamental discovery is the so called “isoelectric effect” described in subsection 5. Briefly, any substances bound to polyampholytes may be discharged at the IEP via governing forces among inter- and intramolecular paths. Furthermore, as the intrachain interaction of acidic and basic groups within a single macromolecule is superior to the interchain interaction, releasing the loaded substance from the polyampholytes carrier can be fulfilled. The realization of the “isoelectric effect” for a series of water-soluble polyampholytes, amphoteric hydrogels and cryogels was demonstrated with respect to surfactants, dye molecules, polyelectrolytes and proteins. The third finding is the structural and behavioral similarity of stoichiometric and non-stoichiometric polyelectrolyte complexes as well as interpenetrating polyelectrolyte networks to polyampholytes that is formulated as follows: (1) stoichiometric and nonstoichiometric PEC can serve as model of balanced or neutral (the same number of negative and positive charges) and unbalanced or non-neutral (an excess of positive or negative charges) polyampholytes; (2) stoichiometric and nonstoichiometric PEC have a compact structure and minimum solubility at the isoelectric pH; (3) near the IEP both PECs and polyampholytes are able to swell at high salinity media; (4) the oscillation mechanism of PEC membranes and polyampholyte gels has the same nature and explanation; (5) the “isoelectric” effect found at the IEP of polyampholytes is also observed for PEC. An open problem in the behavior of polyampholytes is the globular structure of macromolecules that can be considered as dense three-dimensional nucleations consisting of a “core” from which the monomers “force out” the solvent and surrounded by a hydrophilic “edge”. The possibility of formation of a highly ordered (crystaline) structure at the IEP of water-soluble polyampholytes is also a challenging task. Here, theoretical, computational and simulation approaches for better understanding of intra- and interpolyelectrolyte complexation of polyampholytes are required.
Acknowledgments
Financial support from the Ministry of Education and Science of the Republic of Kazakhstan (IRN AP05131003, 2018–2020) is greatly acknowledged. N.N greatly acknowledges the financial support of ACSPRF (57095-DN17).
Author Contributions
Writing-Original Draft Preparation, S.E.K; Writing-Review & Editing, N.N.
Funding
This research was funded by Ministry of Education and Science of the Republic of Kazakhstan IRN AP05131003, 2018–2010 and ACSPRF (57095-DN17).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Candau F., Joanny J.F. Polyampholytes (Properties in aqueous solution) In: Salamone J.C., editor. Polymeric Materials Encyclopedia. CRC Press; Boca Raton, FL, USA: New York, NY, USA: 1996. pp. 5476–5488. [Google Scholar]
- 2.Kudaibergenov S. Polyampholytes: Synthesis, Characterization and Application. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. p. 220. [Google Scholar]
- 3.Dobrynin A.V., Colby R.H., Rubinstein M. Polyampholytes. J. Polym. Sci. Part B. 2004;42:3513–3538. doi: 10.1002/polb.20207. [DOI] [Google Scholar]
- 4.Kudaibergenov S. Encyclopedia of Polymer Science and Technology. John Wiley Interscience; Hoboken, NJ, USA: 2008. Polyampholytes; pp. 1–30. [Google Scholar]
- 5.Ciferri A., Kudaibergenov S. Natural and synthetic polyampholytes. 1. Theory and basic structure. Macromol. Rapid Commun. 2007;28:1953–1968. doi: 10.1002/marc.200700162. [DOI] [Google Scholar]
- 6.Hess M., Jones R.G., Kahovec J., Kitayama T., Kratochvíl P., Kubisa P., Mormann W., Stepto R.F., Tabak D., Vohlídal J., et al. Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC Recommendations 2006) Pure Appl. Chem. 2006;78:2067–2074. doi: 10.1351/pac200678112067. [DOI] [Google Scholar]
- 7.Kudaibergenov S., Jaeger W., Laschewsky A. Polymeric betaines: Synthesis, characterization and application. Adv. Polym. Sci. 2006;201:157–224. [Google Scholar]
- 8.Lowe A.B., McCormick C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002;102:4177–4189. doi: 10.1021/cr020371t. [DOI] [PubMed] [Google Scholar]
- 9.Laschewsky A. Structures and synthesis of zwitterionic polymers. Polymers. 2014;6:1544–1601. doi: 10.3390/polym6051544. [DOI] [Google Scholar]
- 10.Luca C., Neagu V., Vasiliu S., Barboiu V. Synthetic polybetaines. Synthesis and properties. In: Dragan E.S., editor. Focus on Ionic Polymers. Research Signpost; Kerala, India: 2005. pp. 1–36. [Google Scholar]
- 11.Ascoli F., Botre C. Amphoteric behavior of a copolymer: N,N-diethylallylamine-acrylic acid. J. Polym. Sci. 1962;62:S56–S59. doi: 10.1002/pol.1962.1206217422. [DOI] [Google Scholar]
- 12.Masuda S., Minagawa K., Tsuda M., Tanaka M. Spontaneous copolymerization of acrylic acid with 4-vinylpyridine and microscopic acid dissociation of the alternating copolymer. Eur. Polym. J. 2001;37:705–710. doi: 10.1016/S0014-3057(00)00159-2. [DOI] [Google Scholar]
- 13.Merle Y. Synthetic polyampholytes. 5. Influence of nearest-neighbor interaction on potentiometric curves. J. Phys. Chem. 1987;91:3092–3098. doi: 10.1021/j100295a089. [DOI] [Google Scholar]
- 14.Kudaibergenov S.E., Zhaimina G.M., Sigitov V.B., Bekturov E.A. Complexation of polyampholyte based on 1-vinylimidazole and acrylic acid with transition metal ions; Proceedings of the 32nd International Symposium on Macromolecules; Kyoto, Japan. 1–5 August 1988; p. 741. [Google Scholar]
- 15.Su S., Wu R., Huang X., Cao L., Wang J. Effect of the anionic-group/cationic group ratio on the swelling behavior and controlled release of agrochemicals of the amphoteric, superabsorbent polymer poly(acrylic acid-co-diallyldimethylammonium chloride. J. Appl. Polym. Sci. 2006;102:986–989. doi: 10.1002/app.23990. [DOI] [Google Scholar]
- 16.Lica C.G., Segărceanu M., Pleşca M., Rikabi A.A., Nechifor G. Synthesis of a new polymer poly(styrene sulfonic acid-co-4-vinyl pyridine) for proton exchange membrane for fuel cell. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014;76:151–158. [Google Scholar]
- 17.Kudaibergenov S.E., Frolova V.A., Bekturov E.A., Rafikov S.R. Interpolymer reactions with participation of block polyampholyte poly(1-methyl-4-vinylpyridinium chloride)-poly(methacrylic acid) Dokl. Akad. Nauk SSSR. 1990;311:396–398. [Google Scholar]
- 18.Bekturov E.A., Kudaibergenov S.E., Khamzamulina R.E., Frolova V.A., Nurgalieva D.E., Schulz R.C., Zöller J. Phase behaviour of block-polyampholytes based on poly(methacrylic acid)-block-poly(1-methyl-4-vinylpyridinium chloride) in aqueous salt solutions. Makromol. Chem. Rapid Commun. 1992;13:225–229. doi: 10.1002/marc.1992.030130407. [DOI] [Google Scholar]
- 19.Nakahata R., Yusa S. Preparation of water-soluble polyion complex (PIC) micelles covered with amphoteric random copolymer shells with pendant sulfonate and quaternary amino groups. Polymers. 2018;10:205. doi: 10.3390/polym10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salamone J.C., Rice W.C. Polyampholytes. In: Mark H.F., Bikales N.M., Overberger C.G., Menges G., editors. Encyclopedia of Polymer Science and Engineering. Volume 11. Wiley; New York, NY, USA: 1987. pp. 514–530. [Google Scholar]
- 21.Tanford C. Physical Chemistry of Macromolecules. John Wiley and Sons; New York, NY, USA: 1961. p. 710. [Google Scholar]
- 22.Rice S.A., Nagasawa M. Polyelectrolyte Solutions. Academic Press; London, UK: New York, NY, USA: 1961. p. 568. [Google Scholar]
- 23.Armstrong R.W., Strauss U.P. Polyelectrolytes. In: Mark H.F., Gaylord N.G., Bikales N.M., editors. Encyclopedia of Polymer Science and Technology. 1st ed. Volume 10. Interscience Publishers; New York, NY, USA: 1969. pp. 781–861. [Google Scholar]
- 24.Selegny E., Mandel M., Strauss U.P., editors. Polyelectrolytes. D. Reidel; Dordrecht, The Netherlands: Boston, MA, USA: 1974. p. 533. [Google Scholar]
- 25.Eisenberg H. Biological Macromolecules and Polyelectrolytes. Clarendon Press; Oxford, UK: 1976. p. 272. [Google Scholar]
- 26.Dautzenberg H., Jaeger W., Koetz J., Philipp B., Seidel C., Stscherbina D. Polyelectrolytes: Formation, Characterization, Application. Hanser; Munich, Germany: 1994. p. 343. [Google Scholar]
- 27.Mandel M. Aqueous solutions of polyelectrolytes. In: Editor Finch C.A., editor. Chemistry and Technology of Water-Soluble Polymers. Springer; Boston, MA, USA: 1983. pp. 179–192. [Google Scholar]
- 28.Mandel M. Polyelectrolytes. In: Mark H.F., Bikales N.M., Overberger C.G., Menges G., Kroschwitz J.I., editors. Encyclopedia of Polymer Science and Engineering. 2nd ed. Volume 11. Wiley Interscience; New York, NY, USA: 1985. pp. 739–829. [Google Scholar]
- 29.Bekturov E.A., Bimendina L.A., Kudaibergenov S.E. Polyelectrolytes (Chain Models of Polyions) In: Salamone J.C., editor. Concise Polymeric Materials Encyclopedia. CRC Press; Boca Raton, FL, USA: 1996. pp. 5141–5146. [Google Scholar]
- 30.Dobrynin A.V., Rubinstein M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005;30:1049–1118. doi: 10.1016/j.progpolymsci.2005.07.006. [DOI] [Google Scholar]
- 31.Muthukumar M. 50th Anniversary Perspective: A perspective on polyelectrolyte solutions. Macromolecules. 2017;50:9528–9560. doi: 10.1021/acs.macromol.7b01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews C.K., Van Holde K.E. Biochemistry. Benjamin/Cummings Publishing; Redwood City, CA, USA: 1990. [Google Scholar]
- 33.Swann D.A. In: The Joints and Synovial Fluid. Sokoloff L., editor. Academic Press; New York, NY, USA: 1978. p. 407. [Google Scholar]
- 34.Mattison K.W., Brittain I.J., Dubin P.L. Protein-polyelectrolyte phase boundary. Biotechnol. Prog. 1995;11:632–637. doi: 10.1021/bp00036a005. [DOI] [Google Scholar]
- 35.Jiang J., Prausnitz J.M. Molecular thermodynamics for protein precipitation with a polyelectrolyte. J. Phys. Chem. 1999;103:5560–5569. doi: 10.1021/jp990455e. [DOI] [Google Scholar]
- 36.Kokufuta E. Complexation of proteins with polyelectrolytes in a salt-free system and biochemical characteristics of the resulting complexes. In: Dubin P., Block J., Davis R., Schulz D.N., Thies C., editors. Macromolecular Complexes in Chemistry and Biology. Springer; Berlin, Germany: 1995. pp. 300–325. [Google Scholar]
- 37.Kokufuta E. Functional immobilized biocatalysts. Prog. Polym. Sci. 1992;17:647–697. doi: 10.1016/0079-6700(92)90004-I. [DOI] [Google Scholar]
- 38.Trubetskoy V.S., Hagstrom J.E., Budker V.G., Wolff J.A., Rozema D.B., Monahan S.D. Compositions and methods for drug delivery using pH sensitive molecules. 6,919,091. U.S. Patent. 2005 Jul 19;
- 39.Zurick K.M., Bernards M. Recent biomedical advances with polyampholyte polymers. J. Appl. Polym. Sci. 2014 doi: 10.1002/app.40069. [DOI] [Google Scholar]
- 40.Khan M.O., Akesson T., Jonsson B. Adsorption of polyampholytes to charged surfaces. Macromolecules. 2001;34:4216–4221. doi: 10.1021/ma001437u. [DOI] [Google Scholar]
- 41.Edwards S.F., King P.R., Pincus P. Phase change in polyampholytes. Ferroelectrics. 1980;30:3–6. doi: 10.1080/00150198008209479. [DOI] [Google Scholar]
- 42.Higgs P.G., Joanny J.F. Theory of polyampholyte solutions. J. Chem. Phys. 1991;94:1543–1554. doi: 10.1063/1.460012. [DOI] [Google Scholar]
- 43.Higgs P.G., Orland H. Scaling behavior of polyelectrolytes and polyampholytes: Simulation by an ensemble growth method. J. Chem. Phys. 1991;96:4506–4518. doi: 10.1063/1.461774. [DOI] [Google Scholar]
- 44.Wittmer J., Johner A., Joanny J.F. Random and alternating polyampholytes. Europhys. Lett. 1993;24:263–268. doi: 10.1209/0295-5075/24/4/005. [DOI] [Google Scholar]
- 45.Everaers R., Johner A., Joanny J.F. Complexation and precipitation in polyampholyte solutions. Europhys. Lett. 1997;37:275–280. doi: 10.1209/epl/i1997-00143-x. [DOI] [Google Scholar]
- 46.Kantor Y., Kardar M. Polymers with random self-interactions. Europhys. Lett. 1991;14:421–426. doi: 10.1209/0295-5075/14/5/006. [DOI] [Google Scholar]
- 47.Kantor Y., Li H., Kardar M. Conformations of polyampholytes. Phys. Rev. Lett. 1992;69:61–64. doi: 10.1103/PhysRevLett.69.61. [DOI] [PubMed] [Google Scholar]
- 48.Kantor Y., Kardar M. Excess charge in polyampholytes. Europhys. Lett. 1994;27:643–648. doi: 10.1209/0295-5075/27/9/002. [DOI] [Google Scholar]
- 49.Kantor Y., Kardar M. Collapse of randomly self-interacting polymers. Europhys. Lett. 1994;28:169–174. doi: 10.1209/0295-5075/28/3/003. [DOI] [Google Scholar]
- 50.Kantor Y., Kardar M., Li H. Statistical mechanics of polyampholytes. Phys. Rev. E. 1994;49:1383–1392. doi: 10.1103/PhysRevE.49.1383. [DOI] [PubMed] [Google Scholar]
- 51.Kantor Y., Kardar M. Instabilities of charged polyampholytes. Phys. Rev. E. 1995;51:1299–1312. doi: 10.1103/PhysRevE.51.1299. [DOI] [PubMed] [Google Scholar]
- 52.Kantor Y., Kardar M. Randomly charged polymers: An exact enumeration study. Phys. Rev. E. 1995;52:835–846. doi: 10.1103/PhysRevE.52.835. [DOI] [PubMed] [Google Scholar]
- 53.Kantor Y., Kardar M., Ertas D. Necklace model of randomly charged polymers. Phys. A Stat. Mech. Appl. 1998;249:301–306. doi: 10.1016/S0378-4371(97)00478-0. [DOI] [Google Scholar]
- 54.Ertas D., Kantor Y. Randomly charged polymers, random walks, and their extremal properties. Phys. Rev. E. 1996;53:846–860. doi: 10.1103/PhysRevE.53.846. [DOI] [PubMed] [Google Scholar]
- 55.Levin Y., Barbosa M.C. Conformational phase transition of a polyampholyte in a low dielectric solvent. Europhys. Lett. 1995;31:513–518. doi: 10.1209/0295-5075/31/9/002. [DOI] [Google Scholar]
- 56.Diehl A., Barbosa M.C., Levin Y. Neutral polyampholyte in an ionic solution. Phys. Rev. A. 1996;54:6516–6525. doi: 10.1103/PhysRevE.54.6516. [DOI] [PubMed] [Google Scholar]
- 57.Gutin A.M., Shakhnovich E.I. Effect of a net charge on the conformation of polyampholytes. Phys. Rev. E. 1994;50:R3322–R3325. doi: 10.1103/PhysRevE.50.R3322. [DOI] [PubMed] [Google Scholar]
- 58.Angerman H.J., Shakhnovich E. Freezing in polyampholyte globules: Influence of the long-range nature of the interaction. J. Chem. Phys. 1999;111:772–785. doi: 10.1063/1.479356. [DOI] [Google Scholar]
- 59.Dobrynin A.V., Rubinstein M. Flory theory of a polyampholyte chain. J. Phys. II. 1995;5:677–695. doi: 10.1051/jp2:1995157. [DOI] [Google Scholar]
- 60.Bratko D., Chakraborty A.K. A numerical study of polyampholyte configuration. J. Phys. Chem. 1996;100:1164–1173. doi: 10.1021/jp951506k. [DOI] [Google Scholar]
- 61.Desouza A.R., Degreve L. Monte Carlo simulation of polyampholyte chains. Mol. Simul. 1993;11:337–344. doi: 10.1080/08927029308022518. [DOI] [Google Scholar]
- 62.Srivastava D., Muthukumar M. Sequence dependence of conformations of polyampholytes. Macromolecules. 1996;29:2324–2326. doi: 10.1021/ma951204g. [DOI] [Google Scholar]
- 63.Rabin Y., Panyukov S., Wilhelm M. Energy landscapes of interacting randomly and periodically charged rigid polyampholytes. Acta Polym. 1998;49:544–548. doi: 10.1002/(SICI)1521-4044(199810)49:10/11<544::AID-APOL544>3.0.CO;2-C. [DOI] [Google Scholar]
- 64.Shimizu H., Uehara K., Yamamoto K., Hiwatari Y. Structural phase transition of di-block polyampholyte. Mol. Simul. 1999;22:285–301. doi: 10.1080/08927029908022102. [DOI] [Google Scholar]
- 65.Lee N., Obukhov S. Multiple conformations in polyampholytes. Eur. Phys. J. B. 1998;1:371–376. doi: 10.1007/s100510050196. [DOI] [Google Scholar]
- 66.Long D., Dobrynin A.V., Rubinstein M., Ajdari A. Electrophoresis of polyampholytes. J. Chem. Phys. 1998;108:1234–1240. doi: 10.1063/1.475485. [DOI] [Google Scholar]
- 67.Moldakarimov S.B., Kramarenko E.Y., Khokhlov A.R., Kudaibergenov S.E. Formation of salt bonds in polyampholyte chains. Macromol. Theory Simul. 2001;10:780–788. doi: 10.1002/1521-3919(20011001)10:8<780::AID-MATS780>3.0.CO;2-Q. [DOI] [Google Scholar]
- 68.Muller-Nedebock K.K., Vilgis T.A. Collective dynamics of random polyampholytes. J. Chem. Phys. 1999;110:4651–4657. doi: 10.1063/1.478348. [DOI] [Google Scholar]
- 69.Gonzalez-Mozuelos P., de la Cruz M.O. Random phase approximation for complex charged systems: Application to copolyelectrolytes (polyampholytes) J. Chem. Phys. 1994;100:507–517. doi: 10.1063/1.466965. [DOI] [Google Scholar]
- 70.Baumketner A., Shimizu H., Isobe M., Hiwatari Y. Helix transition in di-block polyampholyte. J. Phys. Condens. Matter. 2001;13:10279–10291. doi: 10.1088/0953-8984/13/46/302. [DOI] [Google Scholar]
- 71.Yamada Y., Ueda Y., Kataoka Y. Replica Exchange Monte Carlo simulations for folding of di-block polyampholyte. J. Comput. Chem. Jpn. 2005;4:127–130. doi: 10.2477/jccj.4.127. [DOI] [Google Scholar]
- 72.Shusharina N.P., Zhulina E.B., Dobrynin A.V., Rubinstein M. Scaling theory of diblock polyampholyte solutions. Macromolecules. 2005;38:8870–8881. doi: 10.1021/ma051324g. [DOI] [Google Scholar]
- 73.Guskova O.A., Ryabova O.A., Pavlov A.S. Computer modeling of coil-globule phase transition in polyampholyte systems. Struct. Dyn. Mol. Syst. 2003;10:269–272. [Google Scholar]
- 74.Jiang J., Feng J., Liu H., Hu Y. Phase behavior of polyampholytes from charged hard-sphere chain model. J. Chem. Phys. 2006;124:144908. doi: 10.1063/1.2186316. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z., Rubinstein M. Regimes of conformational transitions of a diblock polyampholyte. Macromolecules. 2006;39:5897–5912. doi: 10.1021/ma0607517. [DOI] [Google Scholar]
- 76.Dubin P., Block J., Davis R., Schulz D.N., Thies C., editors. Macromolecular Complexes in Chemistry and Biology. Springer; Berlin, Germany: 1995. [Google Scholar]
- 77.Izumrudov V.I., Zhiryakova M.V., Kudaibergenov S.E. Controllable stability of DNA-containing polyelectrolyte complexes in water-salt solutions. Biopolymers. 1999;52:94–108. doi: 10.1002/1097-0282(1999)52:2<94::AID-BIP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 78.Joanny J.F. Adsorption of a polyampholyte chain. J. Phys. II. 1994;4:1281–1288. doi: 10.1051/jp2:1994199. [DOI] [Google Scholar]
- 79.Van der Eijik J.M., Nolte R.J.M., Drenth W., Hezemans A.M.F. Optically active polyampholytes derived from L- and D-carbylanayl-L.-histidine. Macromolecules. 1980;13:1391–1397. doi: 10.1021/ma60078a012. [DOI] [Google Scholar]
- 80.Jeon J., Dobrynin A.V. Molecular dynamics simulations of polyampholyte-polyelectrolyte complexes in solutions. Macromolecules. 2005;38:5300–5312. doi: 10.1021/ma050303j. [DOI] [Google Scholar]
- 81.Jeon J., Dobrynin A.V. Molecular dynamics simulations of polyampholyte-polyelectrolyte complexes. Effect of solvent quality and salt concentration. J. Phys. Chem. 2006;110:24652–24665. doi: 10.1021/jp064288b. [DOI] [PubMed] [Google Scholar]
- 82.de Carvalho S.J., Metzler R., Cherstvy A.G. Critical adsorption of polyelectrolytes onto charged Janus nanospheres. Phys. Chem. Chem. Phys. 2014;16:15539–15550. doi: 10.1039/C4CP02207F. [DOI] [PubMed] [Google Scholar]
- 83.Caetano D.L.Z., de Carvalho S.J., Metzler R., Cherstvy A.G. Critical adsorption of periodic and random polyampholytes onto charged surfaces. Phys. Chem. Chem. Phys. 2017;19:23397–23413. doi: 10.1039/C7CP04040G. [DOI] [PubMed] [Google Scholar]
- 84.Kudaibergenov S.E., Nuraje N., Khutoryanskiy V.V. Amphoteric nano-, micro-, and macrogels, membranes, and thin films. Soft Matter. 2012;8:9302–9321. doi: 10.1039/c2sm25766a. [DOI] [Google Scholar]
- 85.Nair A.K.N., Jimenez A.M., Sun S. Complexation behavior of polyelectrolytes and polyampholytes. J. Phys. Chem. B. 2017;121:7987–7998. doi: 10.1021/acs.jpcb.7b04582. [DOI] [PubMed] [Google Scholar]
- 86.Goloub T., Keizer A., Stuart M.A.C. Association behavior of ampholytic diblock copolymers. Macromolecules. 1999;32:8441–8446. doi: 10.1021/ma9907441. [DOI] [Google Scholar]
- 87.Varoqui R., Tran Q., Pefferkorn E. Polycation-polyanion complexes in the linear diblock copolymer of poly(styrene sulfonate)/poly(2-vinylpyridinium) salt. Macromolecules. 1979;12:831–835. doi: 10.1021/ma60071a008. [DOI] [Google Scholar]
- 88.Bekturov E.A., Kudaibergenov S.E., Frolova V.A., Schultz R.S., Zoller J. Conformational and complex formation ability of poly(methacrylic acid)-block-poly(1-methyl-4-vinylpyridinium chloride) Makromol. Chem. 1990;191:457–463. doi: 10.1002/macp.1990.021910220. [DOI] [Google Scholar]
- 89.Lifshits I.M., Grosberg A.Y., Khokhlov A.R. Volume interactions in the statistical physics of a polymer macromolecule. Sov. Phys. Usp. 1979;22:123–142. doi: 10.1070/PU1979v022n03ABEH005423. [DOI] [Google Scholar]
- 90.Bazt M.R., Skorikova E.E., Vikhoreva L.S., Galbraikh L.S. Dilute solution properties of carboxymethyl ester of chitosan. Vysokomol. Soed. Ser. A. 1990;32:805–809. [Google Scholar]
- 91.Waldo A.M., Carlos P.C. Preparation of a novel polyampholyte from chitosan and citric acid. Makromol. Chem. Rapid Commun. 1993;14:73–78. [Google Scholar]
- 92.Patrickios C.S., Hertler W.R., Abbot N.L., Hatton T.A., Diblock A.B.C. Triblock, and random methacrylic polyampholytes: Synthesis by group transfer polymerization and solution behavior. Macromolecules. 1994;27:930–937. doi: 10.1021/ma00082a008. [DOI] [Google Scholar]
- 93.Patrickios C.S., Sharma L.R., Armes S.P., Billingham N.C. Precipitation of a water-soluble ABC triblock methacrylic polyampholyte: Effects of time, pH, polymer concentration, salt type and concentration, and presence of a protein. Langmuir. 1999;15:1613–1620. doi: 10.1021/la970662a. [DOI] [Google Scholar]
- 94.Santos-Rosas R., Licea-Claverie A., Arndt K.F. Statistical copolymers of methacrylic acid derivatives with hydrophobic spacers and N,N’- dimethylaminoethylmethacrylate: New associating polyampholytes. J. Mex. Chem. Soc. 2006;50:164–174. [Google Scholar]
- 95.Giebeler E., Stadler R. ABC triblock polyampholytes containing a neutral hydrophobic block, a polyacid and a polybase. Macromol. Chem. Phys. 1997;198:3816–3825. doi: 10.1002/macp.1997.021981204. [DOI] [Google Scholar]
- 96.Kawata Y., Kozuka S., Yusa S. Thermo-responsive behavior of amphoteric diblock copolymers bearing sulfonate and quaternary amino pendant groups. Langmuir. 2018 doi: 10.1021/acs.langmuir.8b01684. [DOI] [PubMed] [Google Scholar]
- 97.Dyakonova M.A., Berezkin A.V., Kyriakos K., Gkermpoura S., Popescu M.T., Filippov S.K., Stepanek P., Di Z., Tsitsilianis C., Papadakis C.M. Salt-induced changes in triblock polyampholyte hydrogels: Computer simulations and rheological, structural and dynamic characterization. Macromolecules. 2015;48:8177–8189. doi: 10.1021/acs.macromol.5b01746. [DOI] [Google Scholar]
- 98.Dyakonova M.A., Gotzamanis G., Niebuur B.-J., Vishnevetskaya N.S., Raftopoulos K.N., Di Z., Filippov S.K., Tsitsilianis C., Papadakis C.M. pH responsiveness of hydrogels formed by telechelic polyampholytes. Soft Matter. 2017;13:3568–3579. doi: 10.1039/C7SM00315C. [DOI] [PubMed] [Google Scholar]
- 99.Gotzamanis G., Papadimitriou K., Tsitsilianis C. Design of a C-b-(A-co-B)-b-C telechelic polyampholyte pH-responsive gelator. Polym. Chem. 2016;7:2121–2129. doi: 10.1039/C5PY02066B. [DOI] [Google Scholar]
- 100.Papadakis C.M., Tsitsilianis C. Responsive hydrogels from associative block copolymers: Physical gelling through polyion complexation. Gels. 2017;3:3. doi: 10.3390/gels3010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kudaibergenov S. Recent advances in the study of synthetic polyampholytes in solutons. Adv. Polym. Sci. 1999;144:116–197. [Google Scholar]
- 102.Bixler H.A., Michaelis A.S. Polyelectrolyte complexes. In: Mark H.F., Gaylord N.G., Bikales N.M., editors. Encyclopedia of Polymer Science and Technology. Volume 10. Interscience Publishers; New York, NY, USA: 1969. p. 765. [Google Scholar]
- 103.Kabanov V.A., Zezin A.B. Soluble interpolymeric complexes as a new class of synthetic polyelectrolytes. Pure Appl. Chem. 1984;56:343–354. doi: 10.1351/pac198456030343. [DOI] [Google Scholar]
- 104.Philipp B., Dautzenberg H., Linow K.J., Koetz J., Dawydoff W. Polyelectrolyte complexes. Recent development, open problems. Prog. Polym. Sci. 1989;14:91–172. doi: 10.1016/0079-6700(89)90018-X. [DOI] [Google Scholar]
- 105.Tsuchida E., Abe K. Interactions between macromolecules and intermacromolecular complexes. Adv. Polym. Sci. 1982;45:1–119. [Google Scholar]
- 106.Bekturov E.A., Bimendina L.A. Interpolymer complexes. Adv. Polym. Sci. 1981;43:100–147. [Google Scholar]
- 107.Kabanov V.A. Polyelectrolyte complexes in solution and in the condensed phase. Uspekhi Khimii. 2005;74:5–23. doi: 10.1070/RC2005v074n01ABEH001165. [DOI] [Google Scholar]
- 108.van der Gucht J., Spruijt E., Lemmers M., Stuart A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011;361:407–422. doi: 10.1016/j.jcis.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 109.De Morais W.A., Silva G.T.M., Nunes A.O., Neto W., Pereira M.R., Fonseca J.L.S. Interpolyelectrolyte complex formation: From lyophilic to lyophobic colloids. Colloids Surf. A. 2016;498:112–120. doi: 10.1016/j.colsurfa.2016.03.052. [DOI] [Google Scholar]
- 110.Pergushov D.V., Muller A.H.E., Schacher F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012;41:6888–6901. doi: 10.1039/c2cs35135h. [DOI] [PubMed] [Google Scholar]
- 111.Kaur J., Harikumar S.L., Kaur A. Interpolyelectrolyte complexes as prospective carriers for controlled drug deliver. Int. Res. J. Pharm. 2012;3:58–62. doi: 10.7897/2230-8407. [DOI] [Google Scholar]
- 112.Bekturov E.A., Kudaibergenov S.E., Rafikov S.R. The properties of solutions and complex formation reactions of amphoteric polyelectrolytes. Russ. Chem. Rev. 1991;60:410–419. doi: 10.1070/RC1991v060n04ABEH001085. [DOI] [Google Scholar]
- 113.Khutoryanskiy V.V., Staikos G., editors. Hydrogen Bonded Interpolymer Complexes: Formation, Structure and Applications. World Scientific Pub Co Inc.; Singapore: 2009. p. 376. [Google Scholar]
- 114.Khutoryanskiy V.V., Smyslov R.Y., Yakimansky A.V. Modern methods for studying polymer complexes in aqueous and organic solutions. Polym. Sci. Ser. A. 2018;60:533–576. doi: 10.1134/S0965545X18050085. [DOI] [Google Scholar]
- 115.Savinova I.V., Fedoseeva N.A., Evdakov V.P., Kabanov V.A. Polymer-polymer complexes with participation of synthetic polyampholytes. Vysokomol. Soedin. Ser. A. 1976;18:2050–2057. [Google Scholar]
- 116.Skorikova E.E., Vikhoreva G.A., Kalyuzhnaya R.I., Zezin A.B., Gal’braikh L.S., Kabanov V.A. Polyelectrolyte complexes based on chitosan. Vysokomol. Soedin. Ser. A. 1988;30:44–49. doi: 10.1016/0032-3950(88)90253-5. [DOI] [Google Scholar]
- 117.Legkunetz R.E., Dzhagiparova A.T., Kudaibergenov S.E., Bekturov E.A., Shayakhmetov S.S. Study of complexation reaction of polyampholytes based on 1,2,5-trimethyl-4-vinylethynylpiperidinol-4 and acrylic acid with poly(acrylic acid) in aqueous solution. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1982;6:54–59. [Google Scholar]
- 118.Kudaibergenov S.E., Sigitov V.B., Bekturov E.A., Koetz J., Philipp B. Studing of novel polyampholytes based on allylamines and maleic acid by 13C NMR and Raman spectroscopy. Vysokomol. Soedin. Ser. B. 1989;31:132–136. [Google Scholar]
- 119.Khamzamulina R.E., Kudaibergenov S.E., Bekturov E.A., Koetz J., Philipp B., Hahn M. Study of interaction of synthetic polyampholyte on the basis of maleic acid and allylamine. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1990;1:45–48. [Google Scholar]
- 120.Hahn M., Koetz J., Ebert A., Schmolke R., Philipp B., Kudaibergenov S., Sigitov V., Bekturov E. Synthese, charakterisierung und symplexbildung von polyampholyten aus ungesattigten dicarbonsaure und allylaminderivaten. 2.Mitt.: Spektroskopische untersuchungen an polymeren aus maleinsaure und allylaminderivaten. Acta Polym. 1989;40:331–335. doi: 10.1002/actp.1989.010400508. [DOI] [Google Scholar]
- 121.Koetz J., Philipp B., Hahn M., Evert A., Schmolke R., Kudaibergenov S.E., Sigitov V.B., Bekturov E.A. Synthese, charakterisierung und symplexbildung von polyampholyten aus ungesattigten dicarbonsaure und allylaminderivaten, 4. Mitt.: Spektroskopische untersuchungen und symplexbildung zwischen poly(dimethyldiallylammoniumchlorid) und einem ampholytischen copolymer aus maleinsaure und methyldiallylamin. Acta Polym. 1989;40:405–407. [Google Scholar]
- 122.Elschner T., Heinze T. A promising cellulose-based polyzwitterion with pH-sensitive charges. Beilstein J. Org. Chem. 2014;10:1549–1556. doi: 10.3762/bjoc.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ibraeva Z.E., Hahn M., Jaeger W., Bimendina L.A., Kudaibergenov S.E. Solution Properties and Complexation of Polyampholytes based on N,N-Dimethyldiallylammonium Chloride and Maleic Acid or Alkyl (Aryl) Derivatives of Maleamic Acids. Macromol. Chem. Phys. 2004;205:2464–2472. doi: 10.1002/macp.200400242. [DOI] [Google Scholar]
- 124.Zezin A., Rogacheva V., Skobeleva V., Kabanov V. Controlled uptake and release of proteins by polyelectrolyte gels. Polym. Adv. Technol. 2001;13:919–925. doi: 10.1002/pat.244. [DOI] [Google Scholar]
- 125.Zansokhova M.F., Rogacheva V.B., Gulyaeva Zh.G., Zezin A.B., Joosten J., Brekman J. Interaction of ampholyte dendrimers with linear polyelectrolytes. Polym. Sci. Ser. A. 2008;50:656–664. doi: 10.1134/S0965545X08060096. [DOI] [Google Scholar]
- 126.Patrickios C.S., Abbot N.L., Foss R.P., Hatton T.A. Synthetic polyampholytes for protein partitioning in two-phase aqueous polymer systems. In: Ataai M.M., Sikdar S.K., editors. New Developments in Bioseparation. Volume 88. American Institute of Chemical Engineers; New York, NY, USA: 1992. pp. 80–88. (AIChE Symposium Series). [Google Scholar]
- 127.Patrickios C.S., Hertler W.R., Hatton T.A. Phase behavior of random and ABC triblock methacrylic polyampholytes with poly(vinyl alcohol) in water: Effect of pH and salt. Fluid Phase Equilib. 1995;108:243–254. doi: 10.1016/0378-3812(95)02709-N. [DOI] [Google Scholar]
- 128.Chen L., Honma Y., Mizutani T., Liaw D.-J., Gong J.P., Osada Y. Effect of polyelectrolyte complexation on the UCST of zwitterionic polymer. Polymer. 2000;41:141–147. doi: 10.1016/S0032-3861(99)00161-5. [DOI] [Google Scholar]
- 129.Izumrudov V.A., Zelikin A.N., Jaeger W., Bohrisch J. Interpolyelectrolyte reactions in solutions of polycarboxybetaines. J. Phys. Chem. B. 2003;107:7982–7988. doi: 10.1021/jp027400o. [DOI] [PubMed] [Google Scholar]
- 130.Nakai K., Nishiuchi M., Inoue M., Ishihara K., Sanada Y., Sakurai K., Yusa S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir. 2013;29:9651–9661. doi: 10.1021/la401063b. [DOI] [PubMed] [Google Scholar]
- 131.Nakai K., Ishihara K., Kappl M., Fujii S., Nakamura Y., Yusa S. Polyion complex vesicles with solvated phosphobetaine shells formed from oppositely charged diblock copolymers. Polymers. 2017;9:49. doi: 10.3390/polym9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakahata R., Yusa S. Solution properties of amphoteric random copolymers bearing pendant sulfonate and quaternary ammonium groups with controlled structures. Langmuir. 2018 doi: 10.1021/acs.langmuir.7b03785. [DOI] [PubMed] [Google Scholar]
- 133.Ibraeva Z.E., Hahn M., Jaeger W., Laschewsky A., Bimendina L.A., Kudaibergenov S.E. Swelling behavior and complex formation ability of ternary amphoteric gels based on allylamine derivatives and maleic acid. Macromol. Mater. Eng. 2005;290:769–777. doi: 10.1002/mame.200500080. [DOI] [Google Scholar]
- 134.Guang C., Guojun W., Liming W., Zhang S., Su Z. Layer-by-layer assembly of single-charged ions with a rigid polyampholyte. Chem. Commun. 2008;15:1741–1743. doi: 10.1039/B801784K. [DOI] [PubMed] [Google Scholar]
- 135.Tokuda Y., Miyaqishima T., Tomida K., Wanq B., Takahashi S., Sato K., Anzai J. Dual pH-sensitive layer-by-layer films containing amphoteric poly(diallylamine-co-maleic acid) J. Colloid Interface Sci. 2013;399:26–32. doi: 10.1016/j.jcis.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 136.Wang B., Tokuda Y., Tomida K., Takahashi S., Sato K. Use of amphoteric copolymer films as sacrificial layers for constructing free-standing layer-by-layer films. Materials. 2013;6:2351–2359. doi: 10.3390/ma6062351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kudaibergenov S.E., Bekturov E.A. Peculiarities of synthetic polyampholytes at the isoelectric point. Vestnik Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1985;4:36–38. [Google Scholar]
- 138.Kudaibergenov S.E., Bekturov E.A. New properties of synthetic polyampholytes in solution. Vestnik Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1988;12:41–44. [Google Scholar]
- 139.Bekturov E.A., Kudaibergenov S.E., Dzhumadilov T.K. Ionic Polymers; Ordered Polymers for High Performance Materials, Biomaterials. Materials Research Society; Pittsburg, CA, USA: 1989. Complexes of water-soluble polymers; pp. 19–33. [Google Scholar]
- 140.Koetz J., Philipp B., Kudaibergenov S.E., Bekturov E.A. Untersuchungen zur gleichzeitigen homo-und heterosymplexbildung mit synthetischen polyampholyten vom pendant typ. Acta Polym. 1991;42:181–185. doi: 10.1002/actp.1991.010420412. [DOI] [Google Scholar]
- 141.Koetz J., Hahn M., Philipp B., Bekturov E.A., Kudaibergenov S.E. Inter- and intramolecular interactions in polyelectrolyte complex formation with polyampholytes. Makromol. Chem. 1993;194:397–409. doi: 10.1002/macp.1993.021940203. [DOI] [Google Scholar]
- 142.Kulagina E.M. Ph.D. Thesis. Kazan State Technological University; Kazan, Russia: 1995. Intermolecular Association of Water-Soluble Amphoteric Polyelectrolytes Based on (Meth)acrylic Acid with Cationic Surfactants. [Google Scholar]
- 143.Kudaibergenov S.E., Zhaimina G.M., Bekturov E.A. Recovery of Transition Metal Ions by Polyampholytes. Moscow, Russia: 1984. pp. 1–5. Author Certificate of the USSR No.1231810. [Google Scholar]
- 144.Bekturov E.A., Kudaibergenov S.E., Saltybaeva S.S., Kyaturka V.G., Radzhunas L.V. Study of complex formation properties of synthetic polyampholyte based on N,N-dimethylaminoethylmethacrylate and methacrylic acid. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1990;1:33–38. [Google Scholar]
- 145.Kudaibergenov S.E., Bekturov E.A. Influence of the coil-globule confromational transition in polyampholytes on sorption and desorption of polyelectrolytes and human serum albumin. Vysokomol. Soedin. Ser. A. 1989;31:2614–2617. [Google Scholar]
- 146.Kudaibergenov S.E. Ph.D. Thesis. Moscow State University; Moscow, Russia: 1991. Complexation reactions with participation of synthetic polyampholytes. [Google Scholar]
- 147.Patrickios C.S., Jang C.J., Hertler W.R., Hatton T.A. Protein interactions with acrylic polyampholytes. Polym. Prepr. 1993;34:954–955. [Google Scholar]
- 148.Patrickios C.S., Jang C.J., Hertler W.R., Hatton T.A. Protein interactions with acrylic polyampholytes. In: Schmitz K.S., editor. Macro-Ion Characterization from Dilute Solutions to Complex Fluids. Volume 548. American Chemical Society; Washington, DC, USA: 1994. pp. 257–267. (ACS Symposium Series). Chapter 19. [Google Scholar]
- 149.Nath S., Patrickios C.S., Hatton T.A. A turbidimetric titration study of the interaction of proteins with block and random acrylic polyampholytes. Biotechnol. Prog. 1995;11:99–103. doi: 10.1021/bp00031a014. [DOI] [Google Scholar]
- 150.Nath S. Complexation behavior of proteins with polyelectrolytes and random acrylic polyampholytes using turbidimetric titration. J. Chem. Technol. Biotechnol. 1995;62:295–300. doi: 10.1002/jctb.280620313. [DOI] [Google Scholar]
- 151.Patrickios C.S., Hertler W.R., Hatton T.A. Protein complexation with acrylic polyampholytes. Biotechnol. Bioeng. 1994;44:1031–1039. doi: 10.1002/bit.260440903. [DOI] [PubMed] [Google Scholar]
- 152.Kudaibergenov S.E., Bekturov E.A. Polymeric Electrolytes, Hydrogels, Complexes and Catalysts. Gylym; Almaty, Kazakhstan: 2007. Natural and synthetic polyampholytes: From fundamentals to application; pp. 7–44. [Google Scholar]
- 153.Kudaibergenov S., Ciferri A. Natural and synthetic polyampholytes. 2. Functions and applications. Macromol. Rapid Commun. 2007;28:1969–1986. doi: 10.1002/marc.200700197. [DOI] [Google Scholar]
- 154.Rogacheva V.B., Panova T.V., Bykova E.V., Zezin A.B., Joosten J., Brekman J. Interaction of ampholyte dendrimers with network polycations and polyanions. Polym. Sci. Ser. A. 2009;51:242–249. doi: 10.1134/S0965545X0903002X. [DOI] [Google Scholar]
- 155.Kudaibergenov S.E., Tatykhanova G.S., Klivenko A.N. Complexation of macroporous amphoteric cryogels based on N,N-dimethylaminoethylmethacrylate and methacrylic acid with dyes, surfactant, and protein. J. Appl. Polym. Sci. 2016;133:43784. doi: 10.1002/app.43784. [DOI] [Google Scholar]
- 156.Janiak D.S., Ayyub O.B., Kofinas P. Effects of charge density on the recognition properties of molecularly imprinted polyampholyte hydrogels. Polymer. 2010;51:665–670. doi: 10.1016/j.polymer.2009.12.022. [DOI] [Google Scholar]
- 157.Lago M.A., Grinberg V.Y., Burova T.V., Concheiro A., Alvarez-Lorenzo C. Ionic and polyampholyte N-isopropylacrylamide-based hydrogels prepared in the presence of imprinting ligands: Stimuli-responsiveness and adsorption/release properties. J. Funct. Biomater. 2011;2:373–390. doi: 10.3390/jfb2040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang C., Zhou X.-L., Liu Y.-R., Wang J., Tian L.-L., Zhang Y., Hu X.-Y. Charged groups synergically enhance protein imprinting in amphoteric polyacrylamide cryogels. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.43851. [DOI] [Google Scholar]
- 159.Huang J.T., Zhang J., Zhang J.Q., Zheng S.H. Template imprinting amphoteric polymer for the recognition of proteins. J. Appl. Polym. Sci. 2005;95:358–361. doi: 10.1002/app.21262. [DOI] [Google Scholar]
- 160.Haag S.L., Barnards M.T. Polyampholyte hydrogels in biomedical applications. Gels. 2017;3:41. doi: 10.3390/gels3040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Su E., Okay O. Polyampholyte hydrogels formed via electrostatic and hydrophobic interactions. Eur. Polym. J. 2017;88:191–204. doi: 10.1016/j.eurpolymj.2017.01.029. [DOI] [Google Scholar]
- 162.Zezin A.B., Kabanov V.A. A new class of water-soluble polyelectrolytes-polyelectrolyte complexes. Uspekhi Khimii. 1982;51:1447–1483. [Google Scholar]
- 163.Kudaibergenov S.E., Khamzamulina R.E., Bekturov E.A., Bimendina L.A., Frolova V.A., Askarova M.Z. Polyelectrolyte complex formation on a dimeric interface. Macromol. Chem. Rapid Commun. 1994;15:943–947. doi: 10.1002/marc.1994.030151206. [DOI] [Google Scholar]
- 164.Bimendina L.A., Iskaraeva S.B., Kudaibergenov S.E., Bekturov E.A. Polyelectrolyte complexes on a dimeric interface. Int. J. Polym. Anal. Charact. 1996;2:135–140. doi: 10.1080/10236669608233903. [DOI] [Google Scholar]
- 165.Bimendina L.A., Kudaibergenov S.E., Bekturov E.A. Polymer complexes at interfaces. J. Macromol. Sci. Rev. Macromol. Chem. Phys. C. 2003;43:27–44. doi: 10.1081/MC-120018020. [DOI] [Google Scholar]
- 166.Kudaibergenov S.E., Bimendina L.A., Zhumadilova G.T. Advanced Macromolecular and Supramolecular Materials and Processes. Kluwer Academic/Plenum Publishers; Dordrecht, The Netherlands: 2003. Design, structure and behavior of interpolymer complex membranes; pp. 149–162. Chapter 10. [Google Scholar]
- 167.Zhumadilova G.T., Gazizov A.D., Bimendina L.A., Kudaibergenov S.E. Properties of polyelectrolyte complex membranes based on some weak polyelectrolytes. Polymer. 2001;42:2985–2989. doi: 10.1016/S0032-3861(00)00442-0. [DOI] [Google Scholar]
- 168.Gazizov A.D., Zhumadilova G.T., Bimendina L.A., Kudaibergenov S.E. Interpolymer complexes of some vinyl copolymers in a solution and on the boundary of two liquid phases. Polymer. 2000;41:5793–5797. doi: 10.1016/S0032-3861(99)00767-3. [DOI] [Google Scholar]
- 169.Kudaibergenov S.E., Mun G.A., Khutoryanskiy V.V., Gazizov A.D. Design of Composite Films and Ultrathin Membranes of Interpolymer Complexes. Polym. Adv. Technol. 2000;11:15–19. doi: 10.1002/(SICI)1099-1581(200001)11:1<15::AID-PAT925>3.0.CO;2-P. [DOI] [Google Scholar]
- 170.Bertrand P., Jonas A., Laschewsky A., Legras R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000;21:319–348. doi: 10.1002/(SICI)1521-3927(20000401)21:7<319::AID-MARC319>3.0.CO;2-7. [DOI] [Google Scholar]
- 171.Arys X., Jonas A.M., Laschewsky A., Legras R. Supramolecular polyelectrolyte assemblies. In: Ciferri A., editor. Supramolecular Polymers. Marcel Dekker Inc.; New York, NY, USA: 2000. [Google Scholar]
- 172.Richardson J.J., Cui J., Bjornmalm M., Braunger J.A., Ejima H., Caruso F. Innovation in layer-by-layer assembly. Chem. Rev. 2016;116:14828–14867. doi: 10.1021/acs.chemrev.6b00627. [DOI] [PubMed] [Google Scholar]
- 173.Izumrudov V.A., Mussabayeva B.K., Murzagulova K.B. Polyelectrolyte multilayers: Preparation and application. Russ. Chem. Rev. 2018;87:192–200. doi: 10.1070/RCR4767. [DOI] [Google Scholar]
- 174.Koetz J., Philipp B., Schultz W., Kudaibergenov S.E., Bekturov E.A. Amphoteric character of polyelectrolyte complex particles as revealed by isotachophoresis and viscometry. Colloid Polym. Sci. 1988;266:906–912. doi: 10.1007/BF01410845. [DOI] [Google Scholar]
- 175.Koetz J., Philipp B., Kudaibergenov S.E., Sigitov V.B., Bekturov E.A. Influence of the charge density of polyelectrolytes on the properties of polyelectrolyte complexes. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1989;3:54–58. [Google Scholar]
- 176.Fedorova I.P., Korshunov M.A., Varkholeva N.F., Gorpenyuk L.V., Senitch V.M. Conformational behavior of equimolar polyampholytes on the basis of vinyl- and styrenesulfonic acids and aminoalkylmethacrylates. Vestnik Kievskogo Universiteta. Khimiya. 1986;27:18–25. [Google Scholar]
- 177.Kudaibergenov S.E., Nurgalieva D.E., Bekturov E.A. Study of polyampholyte hydrogels and interpenetrating polyelectrolyte networks based on 4(but-3-en-1-ynyl)-1-methylpiperidin-4-ol. Macromol. Chem. Phys. 1994;195:3033–3038. doi: 10.1002/macp.1994.021950903. [DOI] [Google Scholar]
- 178.Nurgalieva D.E., Sigitov V.B., Kudaibergenov S.E., Bekturov E.A. Synthesis and investigation of polyampholyte hydrogels and interpenetrating networks based on 1-methyl-4vinylethynylpiperidinol-4. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia. 1993;4:71–74. [Google Scholar]
- 179.Kudaibergenov S.E. Synthesis and characterization of polyampholyte gels. Ber. Bunsenges. Phys. Chem. 1996;100:1079–1082. doi: 10.1002/bbpc.19961000662. [DOI] [Google Scholar]
- 180.Wang H., Li W., Wang Z. Studies on chitosan and poly(acrylic acid) interpolymer complex. 1. Preparation, structure, pH-sensitivity, and salt sensitivity of complex forming-poly(acrylic acid): Chitosan semi-interpenetrating polymer network. J. Appl. Polym. Sci. 1997;65:1445–1450. doi: 10.1002/(SICI)1097-4628(19970822)65:8<1445::AID-APP1>3.0.CO;2-G. [DOI] [Google Scholar]
- 181.Bimendina L.A., Zhumadilova G.T., Kudaibergenov S.E. Phase transitions in interpolymer complexes of some linear and crosslinked polymers. In: Bohidar H.B., Dubin P., Osada Y., editors. Polymer Gels. Fundamentals and Applications. Volume 833. ACS; Washington, DC, USA: 2002. pp. 137–148. (ACS Symposium Series). Chapter 9. [Google Scholar]
- 182.Chen W.Y., Alexandridis P., Su C.K., Patrickios C.S., Hertler W.R., Hatton T.A. Effect of block size and sequence on the micellization of ABC triblock methacrylic polyampholytes. Macromolecules. 1995;28:8604–8611. doi: 10.1021/ma00129a020. [DOI] [Google Scholar]
- 183.Katchalsky A., Spangler R. Dynamics of membrane processes. Q. Rev. Biophys. 1968;1:137–175. doi: 10.1017/S0033583500000524. [DOI] [PubMed] [Google Scholar]
- 184.Wang Q., Schlenoff J.B. The polyelectrolyte complex/Coacervate continuum. Macromolecules. 2014;47:3108–3116. doi: 10.1021/ma500500q. [DOI] [Google Scholar]
- 185.Fu J., Schlenoff J.B. Driving forces for oppositely charged polyion association in aqueous solutions: Enthalpic, entropic, but not electrostatic. J. Am. Chem. Soc. 2016;138:980–990. doi: 10.1021/jacs.5b11878. [DOI] [PubMed] [Google Scholar]
- 186.Ehrlich G., Doty P. Macro-ions. III. The solution behavior of a polymeric ampholyte. J. Am. Chem. Soc. 1954;76:3764–3777. doi: 10.1021/ja01643a053. [DOI] [Google Scholar]
- 187.Kudaibergenov S.E., Shayakhmetov S.S., Rafikov S.R., Bekturov E.A. On hydrodynamic properties of amphoteric copolymers. Dokl. Acad. Nauk SSSR. 1979;246:147–149. [Google Scholar]
- 188.Puglia G.P. Solution properties of polymers. 1. A measure of the ionic strength dependence of polyampholyte solutions. Annu. Tech. Conf. Soc. Plast. Eng. 1992:2280–2281. [Google Scholar]
- 189.Zheng G.-Z., Meshitsuka G., Ishizu A. Inter- and intramolecular ionic interactions of polyampholyte: Carboxymethyl-2-diethylaminoethylcellulose. Polym. Int. 1994;34:241–248. doi: 10.1002/pi.1994.210340301. [DOI] [Google Scholar]
- 190.Ali S.A., Rasheed A., Wazeer M.I. Synthesis and solution properties of a quaternary ammonium polyampholyte. Polymer. 1999;40:2439–2446. doi: 10.1016/S0032-3861(98)00448-0. [DOI] [Google Scholar]