Abstract
Recombinant human growth hormone (GH) is commonly used to treat short stature in children. However, GH treatment has limited efficacy, particularly in severe, non-GH-deficient conditions such as chondrodysplasias, and potential off-target effects. Because short stature results from decreased growth plate chondrogenesis, we developed a cartilage-targeting single-chain human antibody fragment (CaAb) aiming to deliver therapeutic molecules to the growth plate, thereby increasing treatment efficacy while minimizing adverse effects on other tissues. To this end, we created fusion proteins of these CaAbs conjugated with insulin-like growth factor 1 (IGF-1), an endocrine and/or paracrine factor that positively regulates chondrogenesis. These CaAb-IGF-1 fusion proteins retained both cartilage binding and IGF-1 biological activity, and they were able to stimulate bone growth in an organ culture system. Using a GH-deficient (lit) mouse model, we found that subcutaneous injections of these CaAb-IGF-1 fusion proteins increased overall growth plate height without increasing proliferation in kidney cortical cells, suggesting on-target efficacy at the growth plate and less off-target effect on the kidney than IGF-1 alone. Alternate-day injections of these fusion proteins, unlike IGF-1 alone, were sufficient to produce a therapeutic effect. Our findings provide proof of principle that targeting therapeutics to growth plate cartilage can potentially improve treatment for childhood growth disorders.
Keywords: drug targeting, drug delivery, therapeutics, cartilage, growth plate, growth hormone, short stature, growth disorder, fusion protein, antibody
Graphical Abstract
Short stature results from decreased growth plate chondrogenesis. Lui and colleagues created fusion proteins combining cartilage-targeting antibody fragments with insulin-like growth factor 1 to provide targeted growth plate therapy in mice. The findings provide proof of principle that targeting therapeutics to growth plate cartilage can potentially improve treatment for childhood growth disorders.
Introduction
The growth plate is a cartilaginous structure found near the ends of juvenile long bones and vertebrae. Chondrogenesis at the growth plate drives longitudinal bone growth and, therefore, height gain in children.1 Growth plate chondrogenesis is a complex process, which is extensively regulated by multiple endocrine, paracrine, and intracellular pathways.2 Consequently, many acquired or genetic disorders that affect these pathways impair growth plate chondrogenesis, resulting in short stature. Conversely, each of the endocrine, autocrine, or paracrine factors that positively regulates chondrogenesis might be exploited to treat disorders of linear growth. For example, growth hormone (GH), acting in part through insulin-like growth factor 1 (IGF-1), stimulates growth plate chondrogenesis, and, consequently, both recombinant human GH and IGF-1 administration can increase linear growth in a variety of disorders. However, these treatments have limited efficacy in severe disorders, and they have significant known and potential adverse effects.
We hypothesized that targeting chondrogenesis-promoting factors specifically to the growth plate might augment the therapeutic skeletal effect while diminishing undesirable effects on non-target tissues. To develop cartilage-targeting therapy, we recently identified several antibody fragments3 that bind with high affinity to human and mouse matrilin-3, which is an extracellular matrix protein expressed with high tissue specificity in cartilage.4
In this study, we generated fusion proteins conjugating these antibody fragments with IGF-1, and then we tested the hypothesis that the fusion protein would target the growth plate and, thereby, increase therapeutic efficacy and decrease off-target actions on other tissues. We assessed the therapeutic and off-target effects in a GH-deficient (lit) mouse model.5 The findings provide proof of principle that targeting therapeutic molecules to growth plate cartilage has the potential to improve treatment for childhood growth disorders.
Results
IGF-1 Fusion Proteins Retained Cartilage-Binding Ability
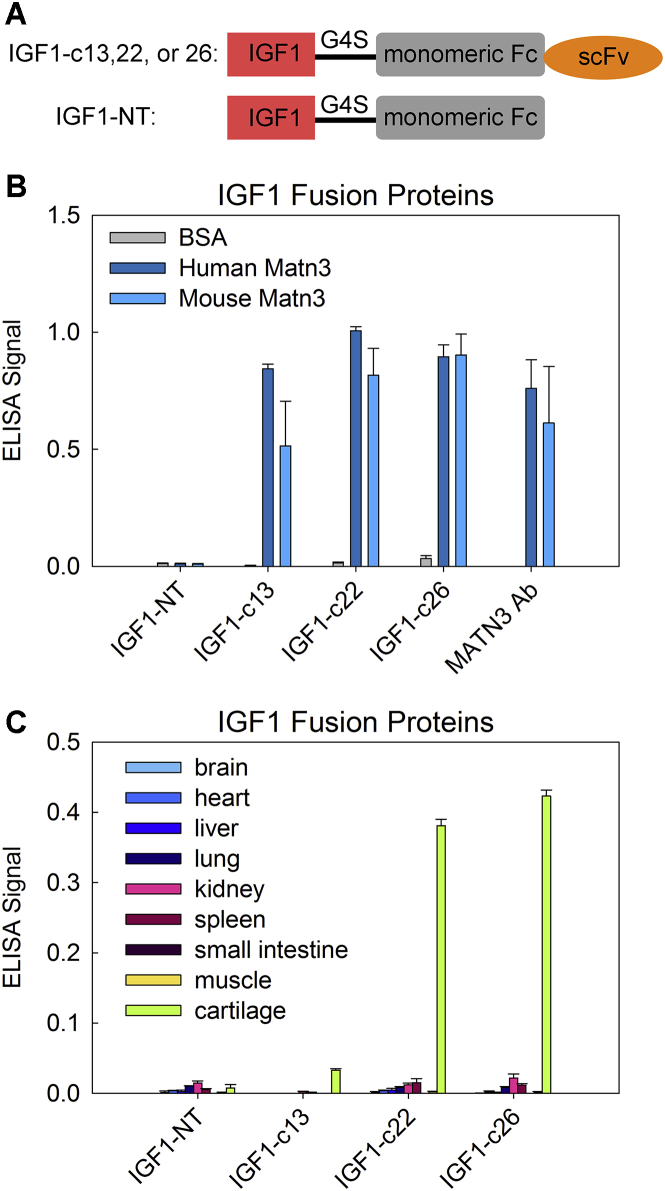
We previously identified three clones of cartilage-targeting single-chain variable (scFv) antibody fragments (c13, c22, and c26).3 In the current study, we used these cartilage-targeting scFvs to generate fusion proteins of cartilage-targeting IGF-1 (hereafter referred to as IGF1-c13, IGF1-c22, and IGF1-c26; Figure 1A), and we tested their potential to deliver IGF-1 to the growth plate cartilage. To this end, we first used ELISA to assess the ability of our fusion proteins to bind to matrilin-3 (both human and mouse). All three clones (IGF1-c13, -c22, and -c26) of IGF-1 fusion proteins bound strongly to human and mouse matrilin-3. In contrast, the non-targeted fusion protein (IGF1-NT) did not bind to matrilin-3 (Figure 1B). We also assessed the binding specificity of our fusion proteins toward growth plate cartilage with a panel of nine different mouse organ lysates, and we found that IGF1-c22 and -c26 showed highly specific binding to cartilage protein with minimal binding to other organ lysates. IGF1-c13 also preferably bound to cartilage protein lysate, but the signal was weaker compared with IGF1-c22 and -c26. As expected, IGF1-NT did not bind specifically to cartilage proteins (Figure 1C). We also assessed whether conjugation of antibody fragment to IGF-1 might affect the intrinsic binding affinity of IGF-1 toward different tissues using an ELISA detecting IGF-1. We found that IGF-1 itself showed minimal binding to any tissue lysate, similar to IGF-NT (Figure S1). In contrast, IGF1-c22 and -c26 again showed substantial, specific binding toward cartilage protein lysate, similar to that observed in Figure 1C.
Figure 1.
Cartilage-Targeting IGF-1 Fusion Proteins Bind to Matrilin-3 and Cartilage Protein Lysate In Vitro
(A) Schematic diagram depicting the design of cartilage-targeting IGF-1 fusion proteins IGF1-c13, -c22, and -c26 and non-targeted IGF-1 (IGF1-NT). (B) To assess binding ability to matrillin-3 (Matn3), ELISA plates were coated with BSA and human or mouse Matn3, and they were incubated with different IGF-1 fusion proteins or a commercially available anti-Matn3 antibody (R&D Systems); binding was detected using an anti-Fc antibody (n = 6). (C) To assess binding specificity to cartilage, ELISA plates were coated with different tissue lysates, incubated with different IGF-1 fusion proteins, and detected with an anti-Fc antibody. All three cartilage-targeting IGF-1 fusion proteins (c13, c22, and c26) bound primarily to cartilage protein lysate and showed far less binding to lysates of other tissues (n = 6). Bar graph depicts mean ± SEM.
IGF-1 Fusion Proteins Retained IGF-1 Biological Activity
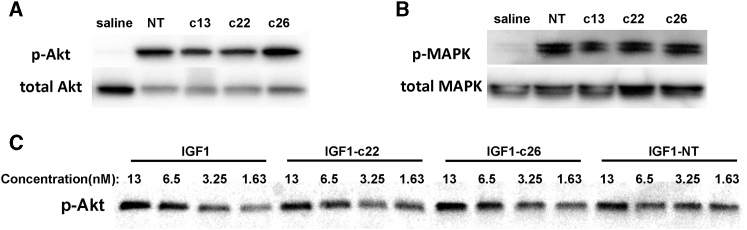
We next performed cell-based in vitro assays to test whether the antibody fragments in the fusion protein interfere with the biological activities of IGF-1. Previous studies have shown that IGF-1 treatment in MCF7 breast cancer cells leads to phosphorylation of Akt.6 After 30 min of treatment with IGF1-c13, IGF1-c22, IGF1-c26, or IGF1-NT (but not with saline), Akt phosphorylation was detected in MCF7 cells (Figure 2A). Similarly, ERK phosphorylation, which is another downstream event of IGF receptor signaling, was also detected (Figure 2B), suggesting that all four IGF-1 fusion proteins retained their ability to initiate IGF signaling in vitro. We also compared the dose-response relationship of IGF-1 itself and the fusion proteins on Akt phosphorylation in MCF7 cells, and we found that IGF1-c22, IGF1-c26, and IGF1-NT all have similar potencies compared with IGF-1 itself (Figure 2C).
Figure 2.
IGF-1 Fusion Proteins Triggered IGF-1 Receptor Signaling
MCF7 cells were serum starved for 16 h and treated with saline or 15 nM of different IGF-1 fusion proteins for 30 min and assessed for the phosphorylation of Akt (A) and Erk (B). All four IGF-1 fusion proteins were able to induce phosphorylation of Akt and Erk in MCF7 cells. (C) Potencies of IGF-1 fusion proteins (IGF1-c22, -c26, and -NT) were compared with IGF-1; all showed similar concentration-dependent responses.
IGF-1 Fusion Proteins Stimulated Metatarsal Bone Growth Ex Vivo
The results described above demonstrate that the IGF-1 fusion proteins bind specifically to cartilage proteins and can initiate IGF-1 receptor signaling. We next sought to test whether the IGF-1 fusion proteins can stimulate longitudinal bone growth in a metatarsal organ culture system. Because our in vitro-binding test (Figure 1C) suggested better binding ability of IGF1-c22 and -c26 on cartilage proteins compared with IGF1-c13, we decided to focus our attention on IGF1-c22 and -c26. We found that IGF-1 alone and our fusion proteins IGF-c22, -c26, and NT all stimulated metatarsal bone growth after 3 days of treatment, compared with saline control (Figure 3), suggesting that these fusion proteins are likely to be biologically active not only in cell culture but also on whole bone. Because the metatarsals are in direct contact with the culture medium in this organ culture system, cartilage targeting is unlikely to be necessary for the IGF-1 to elicit a biological response. As expected, we did not see a difference in metatarsal bone growth between IGF1-c22 or -c26 and the IGF1-NT.
Figure 3.

IGF-1 Fusion Proteins Triggered Metatarsal Bone Growth Ex Vivo
Fetal metatarsal bones were isolated from mouse embryos at embryonic day (E)18 and cultured for 3 days in the presence of IGF-1 itself or IGF-1 fusion proteins (IGF1-c22, -c26, and -NT). Compared with saline, IGF-1 or IGF-1 fusion proteins increased final bone length, suggesting that these fusion proteins are biologically active not only in cell culture but also in whole-bone culture. Line graph depicts mean ± SEM. *p < 0.05 versus saline (n = 6).
In Vivo Administration of IGF-1 Fusion Proteins in GH-Deficient (lit) Mice
Encouraged by the effect on metatarsal bone growth, we next sought to test the ability of the IGF-1 fusion proteins to stimulate the growth plate in vivo. To this end, we used a lit mouse model,5 which carries a homozygous mutation in the GH-releasing hormone receptor (Ghrhr) gene, leading to disruption of Ghrhr function, severe GH deficiency, and dwarfism. Previous studies have shown that injection of IGF-1 (15 μg/injection) twice daily over a 1-month period significantly increased growth in body weight, body length, and bone length.7 We aimed to develop a bioassay for growth plate effect that would be more rapid and, therefore, require production and purification of the recombinant proteins on a moderate scale. Measuring bone length after a short treatment regimen would likely have low sensitivity, because change in bone length is a gradual, cumulative effect of IGF-1 action on the growth plate. We therefore measured other, more rapid effects on growth plate activity such as the height of the proliferative zone cell columns, the size attained by hypertrophic chondrocytes, and the overall growth plate height.
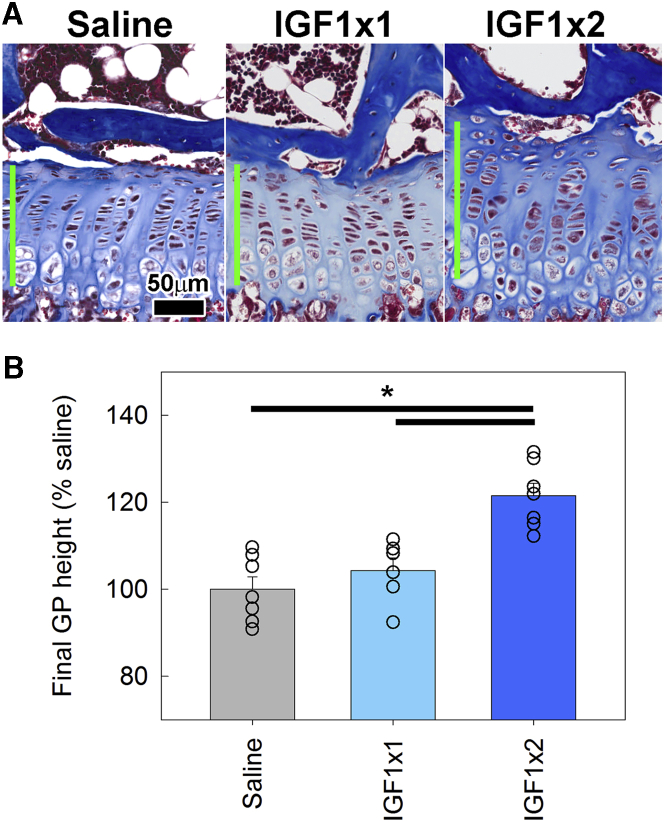
As a test of the model system, we injected 5-week-old lit mice twice a day with IGF-1 (15 μg/injection) or normal saline for 5 days. We found that 5 days of IGF-1 injection significantly increased all studied growth parameters of the growth plate (Table 1), with overall growth plate height providing the most convenient and precise readout. Therefore, we chose to test the IGF-1 fusion proteins with a 5-day injection treatment regimen and use overall growth plate height as the endpoint. Using this model system, we confirmed that twice-daily injection of IGF-1 significantly increased growth plate height compared with saline injection (Figures 4A and 4B), which is consistent with our findings shown in Table 1; but, we found that once-daily injection of IGF-1 did not significantly affect the growth plate height compared with saline (Figures 4A and 4B).
Table 1.
Growth Plate Measurements after IGF-1 Treatment
| Normal Saline (n = 6) | IGF-1 (30 μg/day) (n = 6) | t Test p Value | |
|---|---|---|---|
| Overall growth plate height (μm) | 110 ± 3 | 131 ± 5 | 0.0068 |
| Proliferative zone height (μm) | 45.6 ± 1.5 | 54.5 ± 2.3 | 0.0095 |
| Hypertrophic zone height (μm) | 43.0 ± 2.3 | 50.8 ± 2.2 | 0.036 |
| Terminal hypertrophic cell size (μm) | 17.3 ± 0.8 | 20.2 ± 0.7 | 0.022 |
IGF-1 treatment was 15 μg/injection twice daily for 5 days.
Figure 4.
Effect of Once- or Twice-Daily Injection of IGF-1 on Growth Plate Height in lit Mice
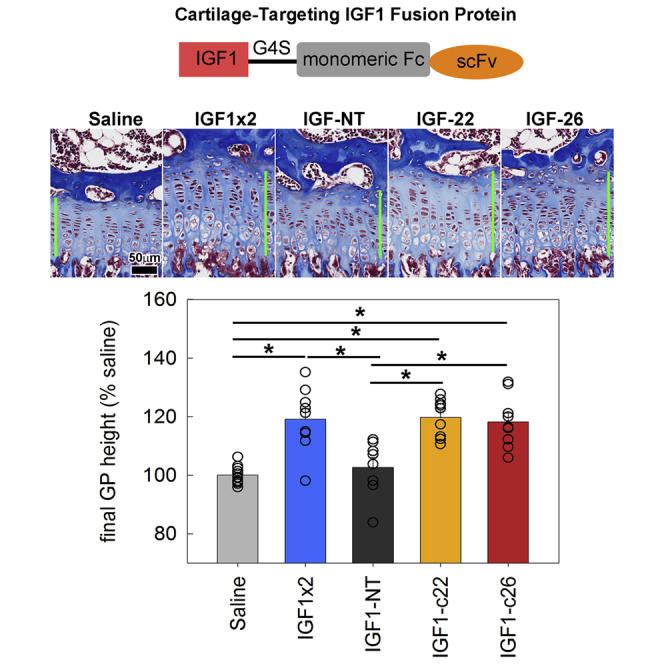
5-week-old lit mice were injected with normal saline (once daily) or 15 μg IGF-1 (once or twice daily) for 5 days. On day 5, mice were sacrificed, and growth plates were dissected, fixed, decalcified, and sectioned for quantitative histology. (A) Histology of post-treatment growth plate. Green bars show the measured growth plate height. (B) Average growth plate height for each animal was normalized to the average measurement in the saline-injected group. Bar graph depicts mean ± SEM. Individual data points (average of a single animal) are shown. *p < 0.05, Holm-Sidak-corrected pairwise comparison (overall ANOVA p < 0.05) (n = 6–7).
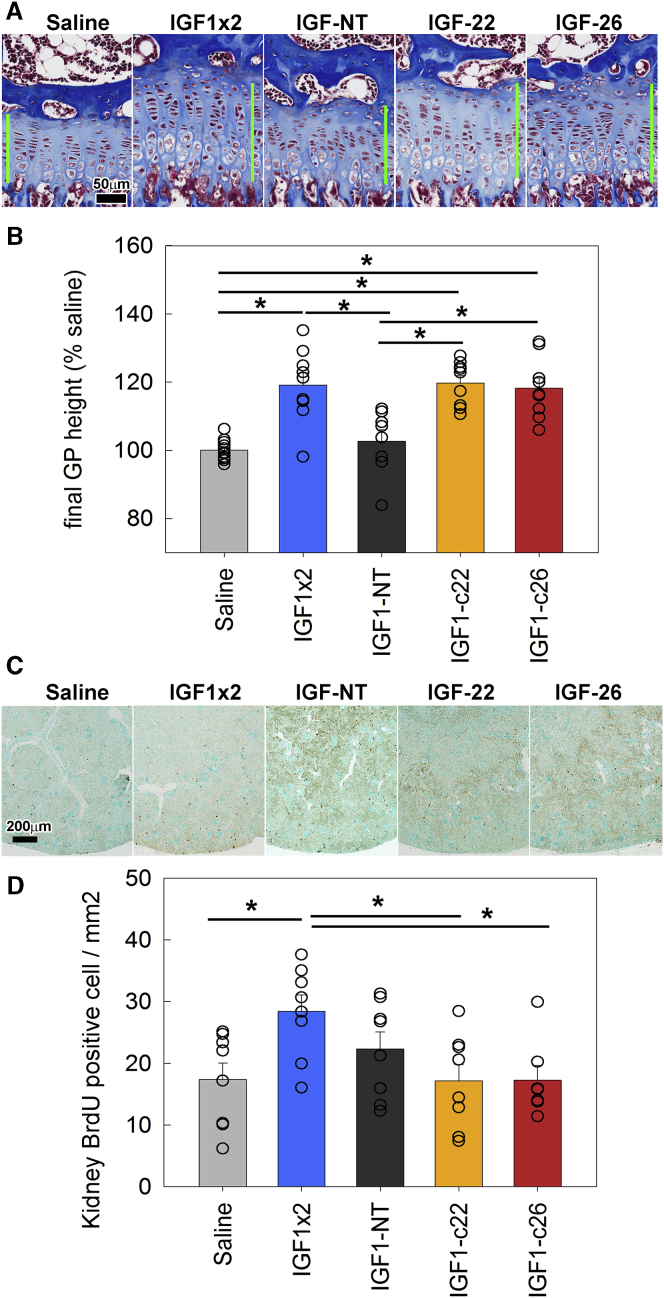
We hypothesized that the IGF-1 fusion proteins would home to growth plate cartilage after injection, thus show increased efficacy and duration of action, and, therefore, that once-daily injection of the fusion proteins, unlike IGF-1 itself, would elicit an effect on growth. To test this hypothesis, we injected IGF1-NT, -c22, or -c26 fusion proteins (molar equivalent of 15 μg IGF-1/injection) once daily in lit mice for 5 days. Neither saline nor IGF1-NT significantly affected growth plate height (Figures 5A and 5B, light gray bar versus dark gray bar). In contrast, once-daily injection of either IGF1-c22 or -c26 significantly increased growth plate height after 5 days (Figure 5B, yellow and orange bars). In these mice, we also measured the number of chondrocytes per column in the proliferative and hypertrophic zones and the height of the terminal hypertrophic cell (the last hypertrophic chondrocyte in the column, adjacent to the metaphyseal bone), and we found that once-daily injection of either IGF1-c22 or -c26, similar to twice-daily IGF-1, significantly increased the number of chondrocytes per column in the proliferative zone (but not the hypertrophic zone) and increased the height of the terminal hypertrophic cell (Figure S2). Our findings therefore suggest that the two targeted IGF-1 fusion proteins, at equimolar dosage, were more effective at stimulating the growth plate compared with IGF1-NT, presumably because the anti-matrilin constructs were preferentially binding to cartilage and, therefore, had a higher local concentration or longer retention at the growth plate.
Figure 5.
Effect of Injection of IGF-1 Fusion Proteins on Growth Plate Height of lit Mice
5-week-old lit mice were injected with normal saline (once daily), 15 μg IGF-1 (twice daily), or the molar equivalent of IGF-1 fusion proteins (once daily) for 5 days. On day 5, mice were sacrificed, and growth plates were dissected, fixed, decalcified, and sectioned for quantitative histology. (A) Histology of post-treatment growth plate. Green bars show the measured growth plate height. (B) Average growth plate height for each animal was normalized to the average measurement in the saline-injected group. Bar graph depicts mean ± SEM. Individual data points (average of a single animal) were included. *p < 0.05, Holm-Sidak-corrected pairwise comparison (overall ANOVA p < 0.05). (C) Histology of post-injection kidneys stained for BrdU incorporation (scattered discrete dark brown cells). (D) Number of BrdU-positive cells in the kidney was counted and normalized to tissue area analyzed. IGF-1 twice-daily injection increased cell proliferation compared with saline, whereas once-daily IGF1-c22 or -c26 injection did not show increased cell proliferation, thus suggesting the lack of this off-target effect. *p < 0.05, Holm-Sidak-corrected pairwise comparison (overall ANOVA p < 0.05) (n = 7–9). Bar graph depicts mean ± SEM.
Reduced Off-Target Effects of IGF-1 Fusion Proteins on Kidney
One major concern with the use of IGF-1 as a therapeutic agent to treat growth disorder is off-target effects on tissues other than the growth plate. We therefore measured cell proliferation in the kidney cortex and medulla after IGF-1 injections (Figures 5C and 5D) to assess off-target effects of IGF-1. Twice-daily IGF-1 injection (15 μg/injection) for 5 days significantly increased 5-bromo-2-deoxyuridine (BrdU) labeling in the kidney compared with saline injection (Figure 5D, blue bar). Once-daily IGF1-NT (molar equivalent of 15 μg IGF-1/injection) showed a tendency for increased proliferation but did not reach statistical significance, compared either with saline or with IGF-1 twice daily (Figure 5D, dark gray bar). Importantly, neither daily IGF1-c22 nor -c26 injection (molar equivalent of 15 μg IGF-1/injection; Figure 5D, yellow and orange bars) significantly increased proliferation in kidney compared with saline, suggesting that cartilage targeting of these IGF-1 fusion proteins provided growth-promoting effect specifically at the growth plate (Figures 5A and 5B), without causing significant off-target effects on other tissues (Figures 5C and 5D).
IGF-1 Fusion Proteins Showed Prolonged Efficacy In Vivo
Because once-daily injections of the cartilage-targeting IGF-1 fusion proteins showed a growth-stimulating effect similar to that of twice-daily injections of IGF-1 itself, we explored whether we could further reduce the treatment frequency of the fusion proteins without compromising their effects, which could be beneficial when used as a therapeutic approach in humans. To this end, we injected IGF1-c22 or -c26 (molar equivalent of 15 μg IGF-1/injection) in 5-week-old lit mice either once only on day 1, or once on day 1 and 3, or once daily for 4 consecutive days (days 1–4). Once-daily injection of saline was used as the control, and all mice were sacrificed on day 5 for histological assessment of the growth plate (Figure 6). As expected, daily injection of IGF1-c22 or IGF1-c26 for 4 days both significantly increased growth plate height compared with saline injection. Interestingly, every-other day injection of IGF-1 fusion proteins, once on day 1 and once on day 3, was sufficient to achieve a growth-promoting effect similar to that of daily injections for 4 days (Figure 6, compare IGF1-c22x2 [every other day] and IGF1-c22x4 [daily]). This represents a substantial improvement over IGF-1, which requires twice-daily injection of the same molar dose per injection (thus four times the dose per day) to achieve a significant increase in growth plate height.
Figure 6.
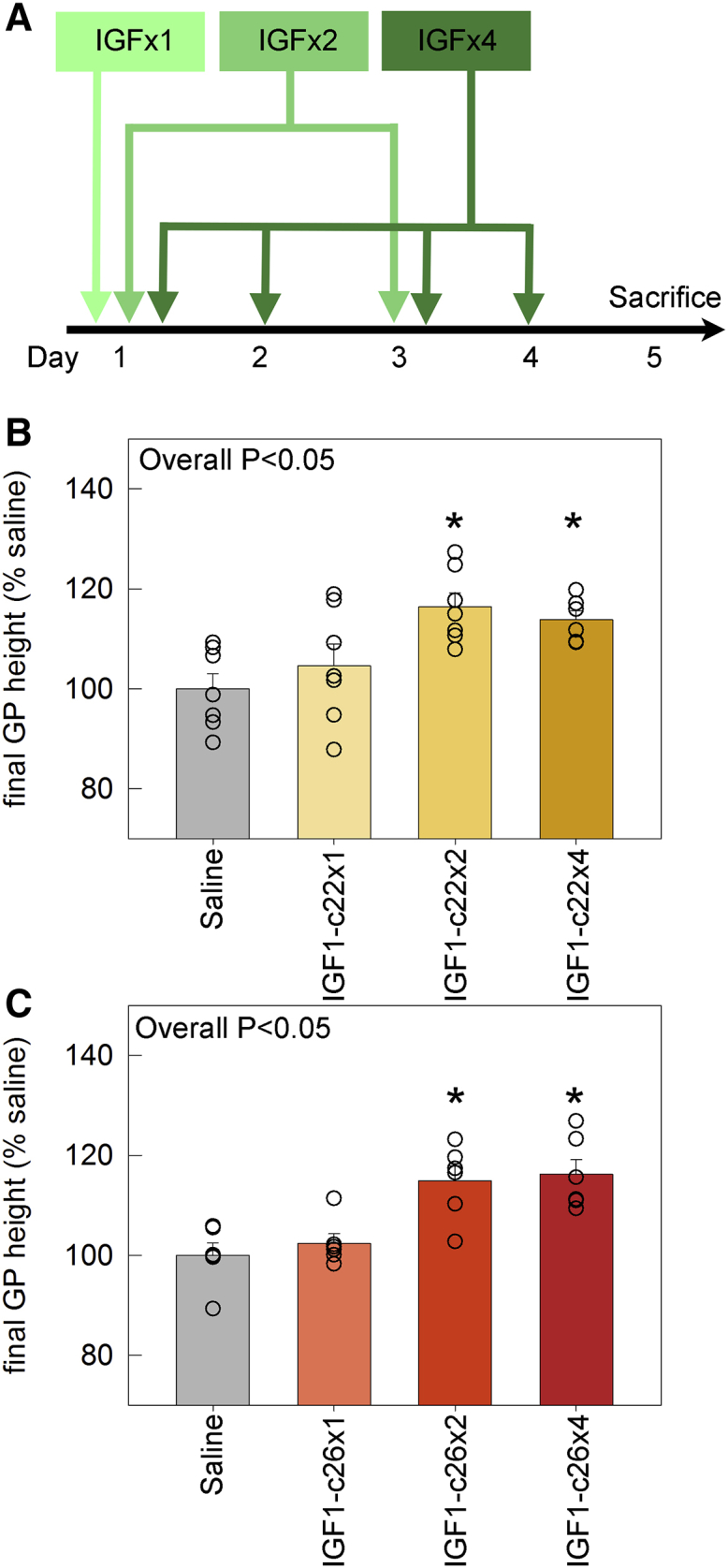
Effect on Growth Plate Height of Increasing the Dose Interval for IGF1-c22 and -c26 Fusion Proteins
(A) Schematic diagram depicting the injection schedule. 5-week-old lit mice were injected with IGF1-c22 (B) or -c26 (C) at various frequencies: either once only on day 1 (IGF1-c22x1 or -c26x1), once on both day 1 and day 3 (IGF1-c22x2 or -c26x2), or once daily for 4 consecutive days (IGF1-c22x4 or -c26x4). Once-daily injection of saline was used as the control group. All mice were sacrificed on day 5 for histological measurement of growth plate height. ANOVA was used to calculate overall statistical significance between groups (p < 0.05), and p values for pairwise comparison (versus saline) were corrected for multiple comparisons (Holm-Sidak). Bar graph depicts mean ± SEM. *p < 0.05 versus saline (n = 6–7).
Discussion
The overall aim of our study was to develop cartilage-targeting therapy to treat skeletal growth disorders. We previously used yeast display to identify scFv antibody fragments with high affinity and specificity toward the cartilage-specific protein matrilin-3, and we showed that these antibody fragments can home to growth plate cartilage when administered in vivo.3 In the current study, we created fusion proteins combining these antibody fragments with human IGF-1, and we showed that at least two of these fusion proteins (IGF1-c22 and -c26) retained both cartilage-binding ability and IGF-1 biological activity. Using an ex vivo metatarsal bone culture system, we found that these IGF-1 fusion proteins can stimulate whole-bone growth. Then using a GH-deficient (lit) mouse model, we found that these IGF-1 fusion proteins can increase growth plate height in vivo when administered once daily for 5 days, similar to a twice-daily IGF-1 injection. Importantly, non-targeted IGF-1 fusion protein did not show any significant effect on growth, suggesting the effects of IGF1-c22 and -c26 fusion proteins are due to their ability to target the growth plate to attain a higher local concentration and/or longer retention time at the cartilage. For the fusion proteins, the treatment frequency could be reduced even further to an injection once every other day, and it still achieved a similar growth-stimulating effect.
We also assessed off-target effects of these fusion proteins by measuring cell proliferation in the kidney. Daily injection of the fusion proteins IGF1-c22 and -c26 did not increase kidney cell proliferation, whereas twice-daily injection of IGF-1 did increase kidney proliferation. Thus, for IGF-1 itself, the minimal dosage regimen required to produce a growth plate effect (15 μg/injection, twice daily) also produced an off-target effect, whereas, for the fusion proteins, a dosage regimen able to produce an effect on the growth plate (the same molar dose per injection but given only once daily) showed no discernible off-target effect. Even when the same dose of fusion protein per injection was given every other day, a growth plate effect was seen. Therefore, the fusion proteins show a substantially wider therapeutic window.
We did not rigorously establish that the effect of the fusion proteins was mediated by the activation of IGF1R. It is possible, for example, that the antibody construct binding to matrilin-3 might stimulate growth plate chondrogenesis. However, several lines of evidence would support an IGF1R-mediated mechanism. First, we demonstrated that the fusion proteins retain the ability to stimulate Akt and Erk phosphorylation in vitro, suggesting retained ability to act through IGF1R. Second, the effects of the fusion proteins in vivo matched precisely with the effects of twice-daily IGF-1—stimulating an increase in overall growth plate height (Figures 5A and 5B), proliferative zone height, and hypertrophic cell size (Figure S2) (a well-established effect of IGF-1). It seems unlikely that a non-specific effect of an antibody construct would match the IGF-1 effect so precisely.
The development of cartilage-targeting proteins opens up new potential approaches to treat growth plate disorders, including chondrodysplasias, secondary growth failure due to disease or treatment, and severe idiopathic short stature. Current growth plate therapy generally involves systemic treatment with GH or, less often, IGF-1. Recombinant human GH is used in children to treat both GH deficiency and also to stimulate growth in certain non-GH-deficient causes of short stature. However, GH treatment has limited efficacy, particularly in severe conditions, and it has potential off-target effects on tissues other than the growth plate, such as increased intracranial pressure, slipped capital femoral epiphysis, insulin resistance, type II diabetes mellitus,8, 9 and possibly an increased risk of cancer. Systemic treatment with IGF-1 also has limited efficacy and has significant potential adverse effects, which are due to actions on tissues other than the growth plate and which may include hypoglycemia, lymphoid overgrowth, benign intracranial pressure, coarsening of facial features, and possible increased risk of malignancy.10 It is approved by the U.S. Food and Drug Administration only for severe IGF-1 deficiency, for example, caused by mutations in the GH receptor, IGF1 gene, or the post-GH receptor-signaling pathway,11 and not to augment growth in other causes of growth failure.12
Coupling growth-regulating endocrine factors to cartilage-binding antibody fragments has the potential to direct endocrine therapeutic agents to cartilage, thereby achieving higher local concentrations of the therapeutic molecules at the growth plate, leading to increased efficacy and reduced off-target effects. However, it is important to note that this study involved a mouse model with GH deficiency; whether or not this treatment would produce a similar effect in growth failure due to other causes, such as FGFR3 activation, is not known.
In addition to IGF-1, which acts both in endocrine and paracrine fashions, there are multiple other paracrine factors that positively regulate growth plate chondrogenesis and, therefore, might be used therapeutically, including Indian hedgehog, bone morphogenetic proteins, and C-type natriuretic peptide. Because these growth factors are produced locally and act locally in the growth plate, they do not lend themselves to systemic therapeutic approaches. To overcome this challenge, we envision that these locally acting molecules could also be targeted to the growth plate by linking them to the antibody fragments described here.
Our approach using antibody fragments that target matrilin-3 is designed to increase efficacy at the growth plate while decreasing adverse effects due to off-target actions on tissues other than cartilage. However, matrilin-3 is expressed in both growth plate and articular cartilage. Therefore, our approach is unlikely to decrease any off-target effects on articular cartilage relative to growth plate cartilage.
In conclusion, we have developed cartilage-targeting IGF-1 fusion proteins, and we showed that these fusion proteins have a greater stimulatory effect on the growth plate compared with IGF-1 alone or a non-targeted fusion protein. As a result, unlike IGF-1 itself, the fusion proteins were able to stimulate growth plate activity without any significant off-target effect on kidney. Our findings provide early proof of principle for the use of cartilage-targeted therapy as a novel treatment approach for skeletal growth disorders.
Materials and Methods
Animals
C57BL/6 mice were obtained from Charles River Laboratory and GHRHR-mutated (lit) mice were obtained from Jackson Laboratory. The lit mice were genotyped by Sanger sequencing. All animal procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee.
Construction of IGF-1 Fusion Proteins
To generate fusion proteins of cartilage-targeting IGF-1, we used three (scFv) antibody fragments that bound to human and mouse matrilin-3, an extracellular matrix protein specifically expressed in cartilage tissue, with high affinity. These antibody fragments had previously been derived using yeast display technology.3 To generate fusion proteins, these antibody fragments were each placed on the C-terminal side of a sequence encoding the human Fc antibody region and cloned into plasmid pSecTAG designed for the secretion and purification of fusion proteins. The DNA sequence for mature human IGF-1, which is 70 amino acids long and differs from the mouse IGF-1 by 2 amino acids at the C terminus, was constructed by overlapping PCR and inserted into pSecTAG at the N terminus of the human Fc, separated by a short linker Gly-Gly-Gly-Ser)x3 (G4S). A non-targeting IGF-1 fusion protein was constructed by similarly connecting IGF-1 with the human Fc separated by a G4S linker, but without the scFv fragment at the C-terminal end. All fusion protein sequences were confirmed by Sanger sequencing. The Fc regions of all fusion proteins have been engineered to remain monomeric,13 unlike the native human Fc that tends to dimerize. The use of monomeric Fc antibody has the potential benefit of improved penetration in tissues, especially when trying to target molecules to cartilage.
Expression of IGF-1 Fusion Proteins in Expi293 Cells
For production and purification, antibody fragments were expressed in Expi293 cells, a modified HEK293 suspension cell line that is adapted to serum-free medium and, thus, avoids serum immunoglobulin G (IgG), which may interfere with antibody fragment purification. Transfection was performed using ExpiFectamine 293 Transfection reagent (Life Technologies), according to the manufacturer’s instructions. Medium was collected after 4 days of protein expression.
Purification of Antibody Fragments by Protein A Column
Protein A resin (GenScript, Piscataway, NJ) slurry (2 mL) was packed into a glass column and equilibrated with 50 mL binding-washing buffer (0.15 M NaCl and 20 mM Na2HPO4 [pH 8.0]). Culture medium was loaded onto the column. Unbound proteins were washed away with 100 mL binding-washing buffer. Bound antibodies were then eluted with 8 mL elution buffer (100 mM acetic acid [pH 3.0]). The eluate was neutralized by 1/10 vol neutralization buffer (1 M Tris-HCl [pH 9.0]) and dialyzed twice against 100 vol PBS at 4°C overnight, before the purified proteins were concentrated to the working concentration for injection. The purity of the antibodies was checked by SDS-PAGE.
Assessment of the Binding Ability and Specificity of Fusion Proteins
To assess the ability of the fusion proteins to bind to purified matrilin-3, 100 μL human or mouse matrilin-3 protein (2 μg/mL) was coated onto 96-well plates at 4°C overnight. To assess binding to cartilage and non-cartilaginous tissues, heart, liver, lung, kidney, spleen, small intestine, muscle, and distal femoral and proximal tibial epiphyseal cartilage were dissected from 1-week-old C57BL/6 mice and homogenized in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], 1 NP-40, 0.1% SDS, and 0.5% sodium deoxycholate) at 4°C. Tissue debris was removed by centrifugation. 100 μL tissue lysate was coated onto 96-well plates at 4°C overnight. After blocking with 3% non-fat milk (200 μL/well), 100 nM of the fusion protein (100 μL) was introduced to each well and incubated at room temperature for 2 h. Commercial anti-matrilin-3 polyclonal antibody recognizing a 13-amino acid peptide near the center of human matrilin-3 (Thermo Scientific, Rockford, IL) served as a positive control. The wells were then washed with 0.05% Tween-PBS (PBST) four times and incubated with 50 μL horseradish peroxidase (HRP)-conjugated anti-Fc antibody (Millipore, Temecula, CA) (diluted 1:5,000 in 3% non-fat milk) at room temperature for 1 h. Finally, tetramethybezidine (TMB) substrate reagent (eBioscience, San Diego, CA) was added for color development, and absorbance was read at 450 nm. Each fusion protein was tested in triplicate wells, and the experiment was repeated. To assess whether the IGF-1 fusion protein affected the intrinsic binding of IGF-1 to tissues, we performed another ELISA test where we similarly coated 96-well plates with different tissue lysates. The wells were then incubated with 100 nM fusion proteins or 100 nM IGF-1 alone (R&D Systems, Minneapolis, MN) for 2 h. Instead of using an anti-Fc antibody, an anti-human IGF-1 antibody was used (ab100545, Abcam). Subsequent procedures were performed according to the manufacturer’s protocol for the human IGF-1 ELISA kit (ab100545, Abcam). Wells only coated with tissue lysate but without incubation of exogenous IGF-1 were included for the subtraction of IGF-1 protein present inside the tissue (background).
Assessment of IGF-1 Activity in Fusion Proteins
Breast cancer cell line MCF7 cells were plated at 3 × 105 cells/1,000 mm2 and serum starved for 16 h. Cells were treated with 15 nM IGF-1 (R&D Systems, Minneapolis, MN) or IGF-1 fusion protein for 30 min at 37°C, before proteins were extracted with RIPA buffer supplemented with proteinase inhibitor cocktail (Sigma-Aldrich) and PhosSTOP (Sigma-Aldrich). Cell lysates were incubated on ice for 30 min, followed by a 10-min centrifugation at 21,000 × g to remove cell debris. Western blotting was performed as previously described14 using phospho-Akt (Cell Signaling Technology, 9271S), total Akt (Cell Signaling Technology, 9272S), phosphor-Erk (Cell Signaling Technology, 4376S), or total Erk (Cell Signaling Technology, 4695S) antibody.
Metatarsal Culture
Mouse metatarsal culture was performed as previously described.15 Briefly, the middle three metatarsals were aseptically dissected from wild-type C57BL/6 mice at embryonic day 18. Bones were maintained in 500 μL α-minimum essential medium (MEM) supplemented with 0.2% BSA, 0.1 mM b-glycerophosphate, 50 μg/mL ascorbic acid, 1% penicillin-streptomycin, and 0.1% Fungizone. 15 nM IGF-1 (R&D Systems) protein or IGF-1 fusion protein was added to the medium. Bones were incubated individually in 24-well plates in a humidified incubator at 37°C and 5% CO2 for 3 days, and the medium, containing IGF-1 or IGF-1 fusion protein, was refreshed daily. Metatarsal bone lengths were measured daily under a dissecting microscope using an eyepiece micrometer.15
Injection of IGF-1 Fusion Proteins in lit Mice
As an in vivo model system, we used GH-releasing hormone-deficient (lit) mice, which are deficient in GH and, therefore, have decreased circulating IGF-1, longitudinal bone growth, and body size.7 Beginning at 5 weeks of age (age range: 33–38 days old), they received subcutaneous injections of IGF-1 (R&D Systems, 15 μg/injection) or IGF-1 fusion protein (molar equivalent of 15 μg IGF-1/injection). For twice-daily injection of IGF-1, mice were injected once at 9 a.m. and once at 6 p.m. For once-daily injections, mice were injected in the morning at 24-h intervals.
After mice were sacrificed, growth plates were dissected, fixed, and decalcified. Samples were embedded in paraffin, and 10-μm sections were mounted on Superfrost Plus slides (Thermo Fisher Scientific). Histological evaluations were performed on Masson Trichrome-stained epiphyseal sections, which were visualized using a ScanScope CS digital scanner (Aperio Technologies) under bright-field microscopy. All histological measurements were performed in the central two-thirds of the growth plate sections, as previously described.16 Overall growth plate height was defined as the distance between the edge of the secondary ossification center to the bottom of the hypertrophic zone where trabecular bone started to form. For each growth plate section, we performed 5 measurements of growth plate height, number of proliferative cells per column, and number of hypertrophic cells per column and 10 measurements of terminal hypertrophic cell height, and then averages were taken from 8 growth plate sections for each animal.
BrdU Staining for the Measurement of Proliferation in Kidney
To assess cell proliferation, BrdU (Sigma-Aldrich, St. Louis, MO; 0.1 mg/g body mass, intraperitoneally [i.p.]) was injected 2 h before mice were killed. Whole kidneys were dissected, formalin fixed, and paraffin embedded. Sagittal sections were made close to the middle of the kidney and mounted on Superfrost Plus slides. BrdU labeling was detected by immunohistochemistry using the BrdU In Situ Detection Kit (BD Biosciences, San Jose, CA) and counterstained with methyl green. Sections were visualized using a ScanScope CS digital scanner (Aperio Technologies) under bright-field microscopy. BrdU-positive cells were counted in the whole kidney section. For each animal, averages were taken from 4 kidney sections. BrdU-positive counts were then normalized to the area of measurement.
Statistical Analysis
Data are presented as mean ± SEM. Line graphs and bar graphs were generated with SigmaPlot11. ANOVA analyses were performed in SigmaPlot 11. One-way ANOVA was used when comparing a single parameter (such as growth plate height) among multiple groups (such as saline versus IGF-1 versus fusion protein 22 versus fusion protein 26). p values were corrected for multiple comparisons, whenever appropriate, using the Holm-Sidak method.
Author Contributions
Conceptualization, J.C.L., C.S.F.C., and J.B.; Methodology, J.C.L., M.C., C.S.F.C., Z.Z., D.S.D., and K.M.B.; Investigation, J.C.L., M.C., C.S.F.C., M.A., A.L., and K.M.B.; Formal Analysis, J.C.L., M.C., and C.S.F.C.; Data Curation, J.C.L. and K.M.B.; Writing – Original Draft, J.C.L., M.A., and J.B.; Writing – Review and Editing, J.C.L. and J.B.; Supervision, J.C.L., D.S.D., and J.B.; Project Administration, J.C.L.; Funding Acquisition, J.C.L. and J.B.
Conflicts of Interest
J.C.L., C.S.F.C., Z.Z., D.S.D., and J.B. are co-inventors in a patent application (U.S. Patent Application 61/927,904) submitted by the NIH. Merck KGaA has performed a scientific review of the publication, but the views and opinions described in the publication do not necessarily reflect those of Merck KGaA.
Acknowledgments
This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute, NIH. J.C.L. received additional funding through Grant for Growth Innovation from EMD Serono, Inc., which is a biopharmaceutical business of Merck KGaA, Darmstadt, Germany. J.C.L. also received an Endocrine Scholars Award in Growth Hormone Research from the Endocrine Society.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2019.01.017.
Supplemental Information
References
- 1.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Wood A.R., Esko T., Yang J., Vedantam S., Pers T.H., Gustafsson S., Chu A.Y., Estrada K., Luan J., Kutalik Z., Electronic Medical Records and Genomics (eMEMERGEGE) Consortium. MIGen Consortium. PAGEGE Consortium. LifeLines Cohort Study Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung C.S., Zhu Z., Lui J.C., Dimitrov D., Baron J. Human monoclonal antibody fragments targeting matrilin-3 in growth plate cartilage. Pharm. Res. 2015;32:2439–2449. doi: 10.1007/s11095-015-1636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatt A.R., Nitsche D.P., Kobbe B., Mörgelin M., Paulsson M., Wagener R. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J. Biol. Chem. 2000;275:3999–4006. doi: 10.1074/jbc.275.6.3999. [DOI] [PubMed] [Google Scholar]
- 5.Wajnrajch M.P., Gertner J.M., Harbison M.D., Chua S.C., Jr., Leibel R.L. Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat. Genet. 1996;12:88–90. doi: 10.1038/ng0196-88. [DOI] [PubMed] [Google Scholar]
- 6.Dufourny B., Alblas J., van Teeffelen H.A., van Schaik F.M., van der Burg B., Steenbergh P.H., Sussenbach J.S. Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J. Biol. Chem. 1997;272:31163–31171. doi: 10.1074/jbc.272.49.31163. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie C., Read L.C., Bagley C.J., Ballard F.J. Enhanced potency of truncated insulin-like growth factor-I (des(1-3)IGF-I) relative to IGF-I in lit/lit mice. J. Endocrinol. 1990;127:401–405. doi: 10.1677/joe.0.1270401. [DOI] [PubMed] [Google Scholar]
- 8.Cutfield W.S., Wilton P., Bennmarker H., Albertsson-Wikland K., Chatelain P., Ranke M.B., Price D.A. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355:610–613. doi: 10.1016/S0140-6736(99)04055-6. [DOI] [PubMed] [Google Scholar]
- 9.Darendeliler F., Karagiannis G., Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm. Res. 2007;68(Suppl 5):41–47. doi: 10.1159/000110474. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld R.G. IGF-I therapy in growth disorders. Eur. J. Endocrinol. 2007;157(Suppl 1):S57–S60. doi: 10.1530/EJE-07-0187. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbloom A.L. Insulin-like growth factor-I (rhIGF-I) therapy of short stature. J. Pediatr. Endocrinol. Metab. 2008;21:301–315. doi: 10.1515/jpem.2008.21.4.301. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbloom A.L. Is there a role for recombinant insulin-like growth factor-I in the treatment of idiopathic short stature? Lancet. 2006;368:612–616. doi: 10.1016/S0140-6736(06)69205-2. [DOI] [PubMed] [Google Scholar]
- 13.Ying T., Feng Y., Wang Y., Chen W., Dimitrov D.S. Monomeric IgG1 Fc molecules displaying unique Fc receptor interactions that are exploitable to treat inflammation-mediated diseases. MAbs. 2014;6:1201–1210. doi: 10.4161/mabs.29835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lui J.C., Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc. Natl. Acad. Sci. USA. 2013;110:6181–6186. doi: 10.1073/pnas.1219079110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui J.C., Garrison P., Nguyen Q., Ad M., Keembiyehetty C., Chen W., Jee Y.H., Landman E., Nilsson O., Barnes K.M., Baron J. EZH1 and EZH2 promote skeletal growth by repressing inhibitors of chondrocyte proliferation and hypertrophy. Nat. Commun. 2016;7:13685. doi: 10.1038/ncomms13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lui J.C., Jee Y.H., Garrison P., Iben J.R., Yue S., Ad M., Nguyen Q., Kikani B., Wakabayashi Y., Baron J. Differential aging of growth plate cartilage underlies differences in bone length and thus helps determine skeletal proportions. PLoS Biol. 2018;16:e2005263. doi: 10.1371/journal.pbio.2005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.