Abstract
Background
The lymphatic system has been suggested to play an important role in cholesterol metabolism and cardiovascular disease. However, the relationships of vascular endothelial growth factor‐C (VEGF‐C), a central player in lymphangiogenesis, with mortality and cardiovascular events in patients with suspected or known coronary artery disease are unknown.
Methods and Results
We performed a multicenter, prospective cohort study of 2418 patients with suspected or known coronary artery disease undergoing elective coronary angiography. The primary predictor was serum levels of VEGF‐C. The primary outcome was all‐cause death. The secondary outcomes were cardiovascular death, and major adverse cardiovascular events defined as a composite of cardiovascular death, non‐fatal myocardial infarction, and non‐fatal stroke. During the 3‐year follow‐up, 254 patients died from any cause, 88 died from cardiovascular disease, and 165 developed major adverse cardiovascular events. After adjustment for established risk factors, VEGF‐C levels were significantly and inversely associated with all‐cause death (hazard ratio for 1‐SD increase, 0.69; 95% confidence interval, 0.60–0.80) and cardiovascular death (hazard ratio, 0.67; 95% confidence interval, 0.53–0.87), but not with major adverse cardiovascular events (hazard ratio, 0.85; 95% confidence interval, 0.72–1.01). Even after incorporation of N‐terminal pro‐brain natriuretic peptide, contemporary sensitive cardiac troponin‐I, and high‐sensitivity C‐reactive protein into a model with established risk factors, the addition of VEGF‐C levels further improved the prediction of all‐cause death, but not that of cardiovascular death or major adverse cardiovascular events. Consistent results were observed within 1717 patients with suspected coronary artery disease.
Conclusions
In patients with suspected or known coronary artery disease, a low VEGF‐C value may independently predict all‐cause mortality.
Keywords: all‐cause death, biomarker, cardiovascular events, coronary heart disease, prospective cohort study
Subject Categories: Biomarkers, Clinical Studies, Coronary Artery Disease, Mortality/Survival, Growth Factors/Cytokines
Clinical Perspective
What Is New?
This is the first, dedicated and large‐scale prospective cohort study to demonstrate that vascular endothelial growth factor‐C levels are significantly and inversely associated with all‐cause mortality among patients with suspected or known coronary artery disease.
This is in sharp contrast to the fact that high vascular endothelial growth factor‐C levels seem to be associated with poor prognosis in patients with malignancies.
What Are the Clinical Implications?
In patients with suspected or known coronary artery disease, a low vascular endothelial growth factor‐C value may predict all‐cause mortality independent of established risk factors and cardiovascular biomarkers.
Further investigations are necessary to elucidate the mechanisms that regulate circulating vascular endothelial growth factor‐C levels, and their effect on the homeostasis maintenance.
Introduction
Vascular endothelial growth factors (VEGFs) and their endothelial tyrosine kinase receptors are central regulators of vasculogenesis, angiogenesis, and lymphangiogenesis.1 The VEGF family includes 5 members in mammals: VEGF (or VEGF‐A), placental growth factor, VEGF‐B, VEGF‐C, and VEGF‐D. VEGF (‐A) signaling through VEGF receptor‐2 is the major angiogenic pathway. VEGF receptor‐1 seems to act as a negative regulator of VEGF‐mediated angiogenesis during development, and as a stimulator of pathological angiogenesis when activated by its specific ligands placental growth factor and VEGF‐B. VEGF‐C and VEGF‐D induce lymphangiogenesis via VEGF receptor‐3.
Of the 2 lymphangiogenic factors, VEGF‐C plays a central role in both physiological and pathological lymphangiogenesis.2, 3, 4, 5 The deletion of Vegfc in mice leads to a complete absence of lymph vessels and embryonic lethality.6 Overexpression of VEGF‐C in the skin of transgenic mice induces selective hyperplasia of the lymphatic vasculature.7 To date, clinical investigations of VEGF‐C have focused on its diagnostic possibilities for various malignancies. The expression levels in tumors and/or circulating levels of VEGF‐C correlates with lymph node and distant metastasis, and poor prognosis.8, 9
Recent basic research suggests the importance of the lymphatic vasculature as a therapeutic target in cardiovascular diseases.5, 10 Lymphatic vessels play an important role in reverse cholesterol transport, a pathway responsible for cholesterol mobilization from peripheral tissues to the liver for excretion, lipoprotein metabolism, and atherosclerotic plaque formation.5, 10, 11, 12, 13 Treatment with VEGF‐C after myocardial infarction induces lymphangiogenesis, reduces fluid retention, facilitates inflammatory cell clearance in the cardiac tissue, and improves cardiac function.14, 15
We demonstrated that serum levels of VEGF‐C are significantly associated with dyslipidemia, a causative risk factor as well as a therapeutic target of cardiovascular disease.16 However, the relationships between VEGF‐C levels and the risk of mortality and cardiovascular events are unknown. Here we thus investigated the predictive value of VEGF‐C in a large‐scale, multicenter prospective cohort study of patients with suspected or known coronary artery disease (CAD) undergoing elective coronary angiography.
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Patients with suspected or known CAD (ie, stable angina, ischemic heart disease, chest pain, positive cardiac stress test) undergoing elective coronary angiography were recruited in the ANOX (development of novel biomarkers related to angiogenesis or oxidative stress to predict cardiovascular events) study: a nationwide, multicenter, prospective cohort study to determine the predictive value of possible novel biomarkers related to angiogenesis or oxidative stress for mortality and major adverse cardiovascular events (MACE). The ANOX study group consists of 15 National Hospital Organization (NHO) institutions across Japan, and the present study was conducted by nationally certified cardiologists. The exclusion criteria included inability to consent, scheduled follow‐up angiography after coronary revascularization, and patients determined as ineligible by the attending physician. Between January 2010 and November 2013, a total of 2513 patients were consecutively enrolled. After excluding 26 patients who did not provide blood samples and 69 patients who withdrew consent, a total of 2418 patients were eligible. The prevalence of risk factors for cardiovascular disease was determined by the examining physician (as described in Data S1). Data on demographic characteristics, smoking status, medical history, and medication use were collected from medical records. Submitted data were examined for completeness and accuracy by the coordinating center (Clinical Research Institute, Kyoto Medical Center, Kyoto, Japan), and data queries were sent to study sites. The study was approved by the central ethics committee of the NHO headquarters and each institution's ethical committee. All of the patients provided written informed consent.
Outcomes and Follow‐Up
The primary outcome was all‐cause death. The secondary outcomes were cardiovascular death and MACE defined as a composite of cardiovascular death, non‐fatal myocardial infarction, and non‐fatal stroke. The patients were monitored over 3 years (1080 days) for the occurrence of all‐cause death, cardiovascular death, and/or MACE. Follow‐up was performed by personnel masked to the biomarker data through medical record/chart review, a survey letter, and/or telephone interview. Sudden death resulting from an unknown but presumed cardiovascular cause in high‐risk patients was included in cardiovascular death. All deaths and MACE were recorded in the official medical chart of hospitals where the patients received care. The reported deaths, myocardial infarctions, and strokes were reviewed and adjudicated by the expert committee (3 independent and masked cardiologists). Follow‐up continued even after non‐fatal myocardial infarction and/or non‐fatal stroke had occurred. At the end of the follow‐up (day 1080), survival status and detailed information about MACE were available in 2400 patients (99.3%) and 18 patients (0.7%) were lost to follow‐up.
Exposures, Sample Collection, and Biomarker Measurement
The primary predictor was serum levels of VEGF‐C. Fasting blood samples for serum were collected from the arterial catheter sheath at the beginning of coronary angiography. The serum was stored at −80°C for a mean of 2 years until being assayed for biomarkers. The serum levels of VEGF‐C and high‐sensitivity C‐reactive protein (hs‐CRP) were measured with specific, commercially available, ELISA kits according to the manufacturers’ instructions (Quantikine, R&D Systems, Minneapolis, MN, for VEGF‐C; CycLex, Medical & Biological Laboratories Co, Ltd [MBL], Nagano, Japan for hs‐CRP).16 The sensitivity of the assay for VEGF‐C was 4.6 pg/mL. The inter‐/intra‐assay coefficients of variation of ELISA for VEGF‐C were <9%/<7%. These assays were performed by an investigator masked to the sources of the samples. The serum levels of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) were measured using a validated, sandwich electrochemiluminescence immunoassay (Elecsys, Roche Diagnostics, Indianapolis, IN). Serum contemporary sensitive cardiac troponin‐I (cTnI) was measured with the ADVIA Centaur Troponin I Ultra assay (Siemens Healthcare Diagnostics, Los Angeles, CA). Additional details are described in Data S1.
Statistical Analyses
We divided the patients into quartiles according to their baseline VEGF‐C levels. These data were compared among quartiles of VEGF‐C for differences using the Kruskal‐Wallis and chi‐square tests, and Jonckheere‐Terpstra and Cochran‐Armitage tests for trend. The relationships between the baseline VEGF‐C level and the outcomes were investigated with the use of Cox proportional hazard regression. We evaluated the incremental predictive performance of biomarkers by calculating changes in the C‐statistic, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) metrics.17 We assessed model calibration by comparing predicted probabilities with observed probabilities. A residual analysis was used to assess model fit. We also performed stratified analyses to examine the association between VEGF‐C levels and the risk of all‐cause death. All statistical tests were 2‐sided, and P<0.05 was considered significant. Since all analyses were considered exploratory, P values were not adjusted for multiple comparisons. Analyses were performed using SPSS version 23.0 (IBM Japan, Tokyo), JMP11.2 (SAS, Cary, NC), and R, version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria). Additional details are described in Data S1.
Results
Baseline Characteristics
The baseline characteristics of the entire cohort and those divided into quartiles of VEGF‐C levels are shown in Table 1 and Table S1. The lower baseline levels of VEGF‐C were significantly associated with older age, a higher rate of male sex, lower body mass index, lower low‐density lipoprotein cholesterol and triglycerides levels, and higher rates of chronic kidney disease (CKD), anemia, and previous cardiovascular events such as myocardial infarction and heart failure hospitalization. In addition, lower baseline levels of VEGF‐C were significantly associated with higher levels of NT‐proBNP and cTnI, but not those of hs‐CRP. A low VEGF‐C level was modestly associated with the presence of CAD, multivessel or left main trunk disease, and New York Heart Association (NYHA) functional class III or IV.
Table 1.
Baseline Characteristics According to Quartiles of VEGF‐C
| Baseline Characteristics | Overall | Quartile 1 (n=604) | Quartile 2 (n=605) | Quartile 3 (n=604) | Quartile 4 (n=605) | P Value | P Value for Trend |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), y | 70.6 (10.4) | 73.3 (9.2) | 72.7 (9.3) | 70.1 (9.6) | 66.3 (11.9) | <0.001 | <0.001 |
| Male | 1624 (67.2) | 421 (69.7) | 430 (71.1) | 405 (67.1) | 368 (60.8) | <0.001 | <0.001 |
| Body mass index, mean (SD) | 24.0 (3.9) | 23.5 (3.8) | 24.1 (3.9) | 24.2 (3.6) | 25.0 (4.2) | <0.001 | <0.001 |
| Obesitya | 936 (38.7) | 207 (34.3) | 225 (37.2) | 224 (37.1) | 280 (46.3) | <0.001 | <0.001 |
| Hypertension | 1843 (76.2) | 458 (75.8) | 466 (77.0) | 461 (76.3) | 458 (75.7) | 0.948 | 0.889 |
| Dyslipidemia | 1466 (60.6) | 333 (55.1) | 369 (61.0) | 363 (60.1) | 401 (66.3) | 0.001 | <0.001 |
| Diabetes mellitus | 1087 (45.0) | 283 (46.9) | 289 (47.8) | 255 (42.2) | 260 (43.0) | 0.133 | 0.057 |
| Current smoker | 428 (17.7) | 88 (14.6) | 100 (16.5) | 111 (18.4) | 129 (21.3) | 0.016 | 0.001 |
| History of smoking habit | 1463 (60.5) | 377 (62.4) | 361 (59.7) | 363 (60.1) | 362 (59.8) | 0.740 | 0.411 |
| Previous cardiovascular eventsb | 1098 (45.4) | 318 (52.6) | 299 (49.4) | 262 (43.4) | 219 (36.2) | <0.001 | <0.001 |
| Coronary artery disease | 1392 (57.6) | 364 (60.3) | 369 (61.0) | 342 (56.6) | 317 (52.4) | 0.009 | 0.002 |
| Multivessel or LMT disease | 794 (32.8) | 213 (35.3) | 214 (35.4) | 192 (31.8) | 175 (28.9) | 0.049 | 0.008 |
| NYHA class III or IV | 252 (10.4) | 82 (13.6) | 57 (9.4) | 52 (8.6) | 61 (10.1) | 0.026 | 0.042 |
| Atrial fibrillation | 261 (10.8) | 92 (15.2) | 65 (10.7) | 64 (10.6) | 40 (6.6) | <0.001 | <0.001 |
| Chronic kidney diseasec | 999 (41.3) | 336 (55.6) | 291 (48.1) | 207 (34.3) | 165 (27.3) | <0.001 | <0.001 |
| Malignancies | 226 (9.3) | 86 (14.2) | 55 (9.1) | 42 (7.0) | 43 (7.1) | <0.001 | <0.001 |
| Anemiad | 882 (36.5) | 348 (57.6) | 251 (41.5) | 167 (27.6) | 116 (19.2) | <0.001 | <0.001 |
| NT‐proBNP, median (IQR), pg/mL | 198 (73–737) | 475 (136–1451) | 219 (85–716) | 145 (62–484) | 124 (46–373) | <0.001 | <0.001 |
| cTnI, median (IQR), pg/mL | 0.0 (0.0–11.0) | 2.0 (0.0–22.0) | 0.0 (0.0–9.0) | 0.0 (0.0–9.0) | 0.0 (0.0–6.0) | <0.001 | <0.001 |
| hs‐CRP, median (IQR), mg/L | 0.9 (0.3–3.1) | 1.0 (0.3–3.5) | 0.9 (0.3–3.1) | 0.7 (0.3–2.7) | 1.1 (0.4–3.0) | 0.012 | 0.822 |
| Anti‐hypertensive drug use | 1967 (81.3) | 505 (83.6) | 502 (83.0) | 473 (78.3) | 487 (80.5) | 0.070 | 0.048 |
| Statin use | 1222 (50.5) | 286 (47.4) | 324 (53.6) | 301 (49.8) | 311 (51.4) | 0.175 | 0.354 |
| Aspirin use | 1340 (55.4) | 324 (53.6) | 340 (56.2) | 342 (56.6) | 334 (55.2) | 0.733 | 0.572 |
Values are expressed as number (percentage) unless otherwise indicated. The quartiles of VEGF‐C levels were as follows: quartile 1, ≤2657; quartile 2, 2658 to 3543; quartile 3, 3544 to 4435; quartile 4, ≥4436 pg/mL. cTnI indicates contemporary sensitive cardiac troponin I; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LMT, left main trunk; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; VEGF‐C, vascular endothelial growth factor‐C.
Obesity is defined as the body mass index of ≥25.
Previous cardiovascular events includes myocardial infarction, stroke, heart failure hospitalization, and coronary revascularization.
Chronic kidney disease is defined as an estimated glomerular filtration rate of <60 mL/min per 1.73 m2.
Anemia is defined as a hemoglobin level of less than 13 g/dL in men and <12 g/dL in women.
Incidence of Outcomes
During the 3‐year follow‐up, 254 (10.5%) patients died from any cause, 88 (3.6%) died from cardiovascular disease, and 165 (6.8%) developed MACE. Figure 1 shows the cumulative incidence of all‐cause death, cardiovascular death, and MACE according to the quartiles of VEGF‐C levels.
Figure 1.
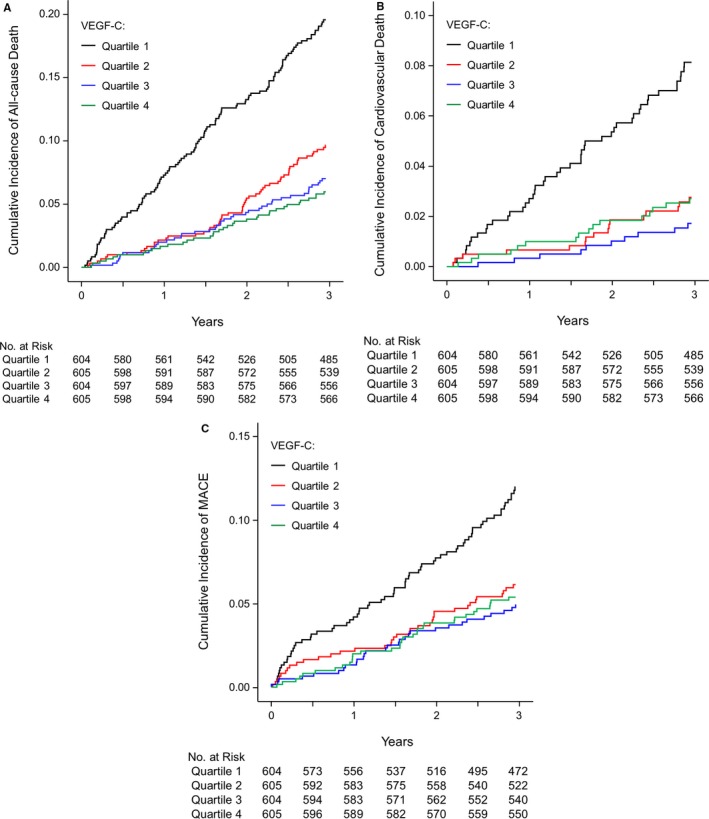
Cumulative incidence of all‐cause death (A), cardiovascular death (B), and major adverse cardiac events (C) according to the vascular endothelial growth factor‐C level at baseline. Follow‐up results are truncated after 3 years. MACE indicates major adverse cardiovascular events; VEGF‐C, vascular endothelial growth factor‐C.
The lowest quartile of VEGF‐C had the greatest risk of all‐cause death, cardiovascular death, and MACE. The incidence of outcomes in the entire cohort and according to the quartiles of VEGF‐C levels are shown in Table S1.
Multivariate Cox Regression Analyses
After adjustment for established risk factors (ie, age, sex, dyslipidemia, hypertension, diabetes mellitus, current smoker, obesity, previous cardiovascular events, CKD, CAD, multivessel or left main trunk disease, statin use, aspirin use, and anti‐hypertensive drug use), VEGF‐C levels were significantly and inversely associated with all‐cause death (P<0.001) and cardiovascular death (P=0.002), but not with MACE (P=0.071) (model 2; Figure 2). Even after additional adjustment for NT‐proBNP (>75th percentile), cTnI (>75th percentile), and hs‐CRP (>1.0 mg/L), VEGF‐C levels were significantly and inversely associated with all‐cause death (P<0.001) and cardiovascular death (P=0.017), but not with MACE (P=0.282) (model 3; Figure 2). Among other possible novel biomarkers measured as the secondary predictors, either VEGF or soluble VEGF receptor‐2 was not significantly associated with all‐cause death, cardiovascular death or MACE, after adjustment for established risk factors, NT‐proBNP, cTnI, and hs‐CRP (model 3; Table S2).
Figure 2.
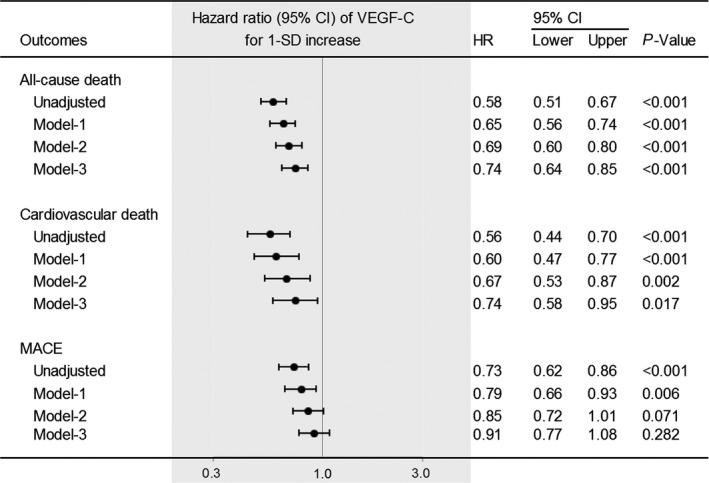
haHazard ratios for all‐cause death, cardiovascular death, and major adverse cardiovascular events according to VEGF‐C levels. Values are for 1‐SD increase. Data were adjusted for the following variables: model‐1, age and sex; model‐2, model‐1 plus dyslipidemia, hypertension, diabetes mellitus, current smoker, obesity, previous cardiovascular events, chronic kidney disease, coronary artery disease, multivessel or left main trunk disease, statin use, aspirin use, and anti‐hypertensive drug use; model‐3, model‐2 plus N‐terminal pro‐brain natriuretic peptide (>75th percentile), contemporary sensitive cardiac troponin I (>75th percentile), and high‐sensitivity C‐reactive protein (>1.0 mg/L). CI indicates confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular events; VEGF‐C, vascular endothelial growth factor‐C.
After excluding patients with known (a history of) CAD, ie, within suspected CAD patients (n=1717), VEGF‐C levels were also significantly and inversely associated with all‐cause death (hazard ratio for 1‐SD increase, 0.69; 95% confidence interval [CI], 0.58–0.83; P<0.001) and cardiovascular death (hazard ratio, 0.72; 95% CI, 0.52–0.99; P=0.046), but not with MACE (hazard ratio, 0.91; 95% CI, 0.74–1.13; P=0.40) after adjusting for established risk factors.
Discrimination, Reclassification, and Calibration
The C statistics of the model with established risk factors (base model) were 0.742 for all‐cause death, 0.771 for cardiovascular death, and 0.706 for MACE (Table 2). As expected, the combination of NT‐proBNP (>75th percentile), cTnI (>75th percentile), and hs‐CRP (>1.0 mg/L) markedly improved the prediction of all‐cause death, cardiovascular death, and MACE (Table 2). Notably, the addition of VEGF‐C levels further improved the prediction of all‐cause death (P<0.001 for NRI, P=0.003 for IDI), but not cardiovascular death (P=0.003 for NRI, P=0.090 for IDI), or MACE (P=0.151 for NRI, P=0.833 for IDI) (Table 2). Even within suspected CAD patients, the addition of VEGF‐C levels significantly improved the prediction of all‐cause death over the model with established risk factors, NT‐proBNP, cTnI, and hs‐CRP (NRI, 0.282; 95% CI, 0.121–0.443; P<0.001; IDI, 0.009; 95% CI, 0.003–0.016; P=0.005). Calibration of the models with or without VEGF‐C showed no evidence of lack of fit.
Table 2.
Model Performance Measures for All‐Cause Death, Cardiovascular Death, and Major Adverse Cardiovascular Events
| Risk Factors and Biomarkers | C Statistics | ∆C Statistics | Continuous NRI (95% CI) | P Value | IDI (95% CI) | P Value |
|---|---|---|---|---|---|---|
| All‐cause death | ||||||
| Base modela | 0.742 | ··· | ··· | ··· | ||
| Base model+NT‐proBNP+cTnI+hs‐CRPb | 0.778 | 0.037 | 0.497 (0.371–0.623) | <0.001 | 0.034 (0.024–0.044) | <0.001 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+VEGF‐C (for 1‐SD increase)c | 0.787 | 0.009 | 0.307 (0.179–0.435) | <0.001 | 0.009 (0.003–0.014) | 0.003 |
| Cardiovascular death | ||||||
| Base modela | 0.771 | ··· | ··· | ··· | ||
| Base model+NT‐proBNP+cTnI+hs‐CRPb | 0.827 | 0.055 | 0.753 (0.551–0.954) | <0.001 | 0.034 (0.022–0.047) | <0.001 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+VEGF‐C (for 1‐SD increase)c | 0.829 | 0.002 | 0.323 (0.114–0.533) | 0.003 | 0.005 (−0.001 to 0.011) | 0.090 |
| Major adverse cardiovascular events | ||||||
| Base modela | 0.706 | ··· | ··· | ··· | ||
| Base model+NT‐proBNP+cTnI+hs‐CRPb | 0.745 | 0.038 | 0.540 (0.384–0.695) | <0.001 | 0.027 (0.018–0.036) | <0.001 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+VEGF‐C (for 1‐SD increase)c | 0.746 | 0.001 | 0.116 (−0.042 to 0.273) | 0.151 | 0.000 (−0.001 to 0.002) | 0.833 |
Follow‐up results are truncated after 3 years. The ∆C statistic, continuous NRI and IDI show the change in model performance from “Base model” or “Base model+NT‐proBNP (>75th percentile)+cTnI (>75th percentile)+hs‐CRP (>1.0 mg/L)”. CI indicates confidence interval; cTnI, contemporary sensitive cardiac troponin I; hs‐CRP, high‐sensitivity C‐reactive protein; IDI, integrated discrimination improvement; NRI, net reclassification improvement; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
The base model is based on age, sex, dyslipidemia, hypertension, diabetes mellitus, current smoker, obesity, previous cardiovascular events, chronic kidney disease, coronary artery disease, multivessel or left main trunk disease, statin use, aspirin use, and anti‐hypertensive drug use.
Evaluated the change of model performance from the “base model”.
Evaluated the change of model performance from the “base model+NT‐proBNP (>75th percentile)+cTnI (>75th percentile)+hs‐CRP (>1.0 mg/L)”.
Multivariate‐Adjusted Stratified Analyses
In multivariate‐adjusted stratified analyses, VEGF‐C levels were significantly and inversely associated with all‐cause death regardless of age (≥75 or <75 years old), sex, obesity, or the presence/absence of dyslipidemia, diabetes mellitus, a history of smoking, previous cardiovascular events, CAD, multivessel or left main trunk disease, NYHA class III or IV, CKD, malignancies, statin use, aspirin use, or anti‐hypertensive drug use (Figure 3). The VEGF‐C level was not significantly associated with all‐cause death in the patients without hypertension, those with current smoking, those with atrial fibrillation, or those without anemia. There was no interaction between VEGF‐C levels and factors of strata.
Figure 3.
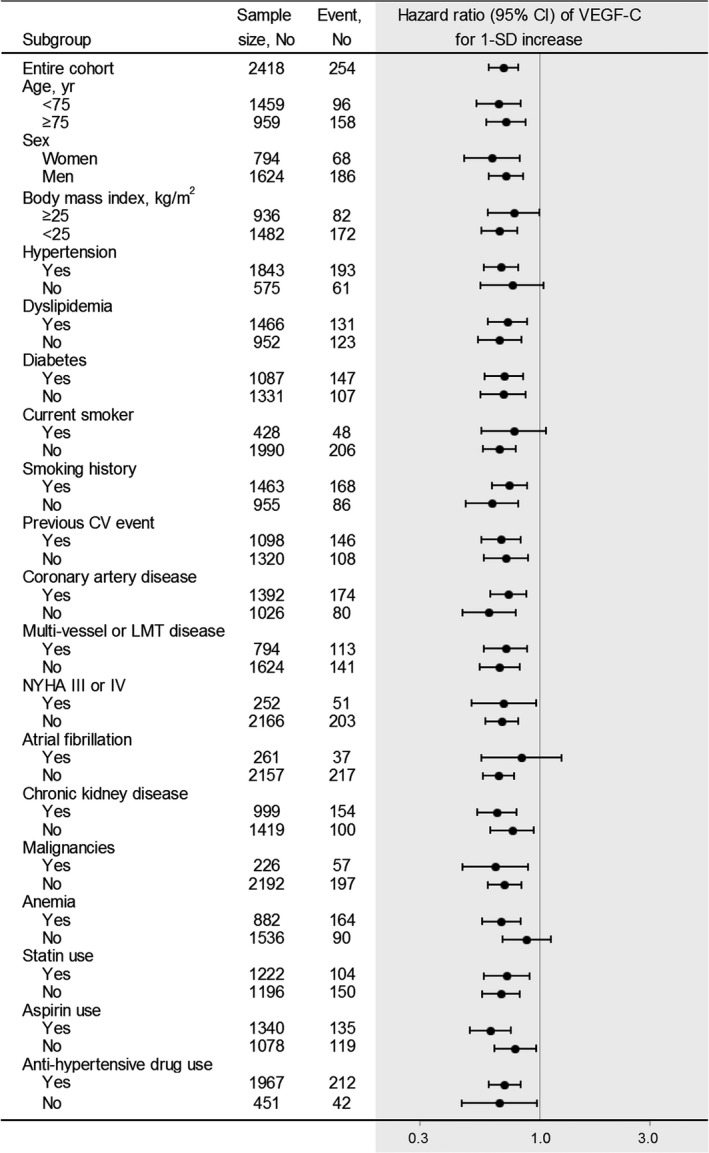
Multivariate‐adjusted stratified analyses on associations of vascular endothelial growth factor‐C with the risk of all‐cause death. The multivariable‐adjusted hazard ratios (95% CIs) of VEGF‐C levels for all‐cause death are plotted for the entire cohort and according to strata of baseline covariates. Data were adjusted for age, sex, dyslipidemia, hypertension, diabetes mellitus, current smoker, obesity, chronic kidney disease, previous cardiovascular events, coronary artery disease, multivessel or left main trunk disease, statin use, aspirin use, and anti‐hypertensive drug use. CI indicates confidence interval; LMT left main trunk; NYHA, New York Heart Association; VEGF‐C, vascular endothelial growth factor‐C.
Discussion
This is the first, dedicated and large‐scale prospective cohort study to demonstrate that VEGF‐C levels are significantly and inversely associated with all‐cause mortality among patients with suspected or known CAD, in sharp contrast to the fact that high VEGF‐C levels seem to be associated with poor prognosis in patients with malignancies. The strength of our investigation includes the large sample size, the multicenter prospective design, and a high follow‐up rate (99.3%). Moreover, the inverse correlations observed remain significant not only after adjustment for established risk factors, but also after additional adjustment for the cardiovascular biomarkers of NT‐proBNP, cTnI, and hs‐CRP, suggesting that the measurement of VEGF‐C provides prognostic information about all‐cause mortality beyond those factors in clinical settings.
The study subjects included not only patients with suspected but also those with known (a history of) CAD, and the latter patients would be at much higher risk of having the outcomes. However, even in patients with suspected (no history of) CAD, the addition of VEGF‐C levels significantly improved the prediction of all‐cause death over the model with established risk factors and cardiovascular biomarkers. Thus, the inverse relationship between VEGF‐C and all‐cause death is robust.
In comparison with NT‐proBNP, cTnI, and hs‐CRP, only VEGF‐C has an inverse association with the risk of all‐cause death. In Kaplan‐Meier analyses, there was a big difference in mortality rate between the first quartile and the others, whereas the differences were small among quartiles 2 to 4. Thus, there does not seem to be linear, but non‐linear threshold of VEGF‐C levels at around the 25th percentile for the risk of mortality. In other words, this might suggest a phenomenon where a subset of patients are relatively “VEGF‐C deficient”, and that VEGF‐C and the lymphatic system have a critical role in the maintenance of homeostasis.
In our present stratified analyses, we did not observe significant associations between the VEGF‐C level and all‐cause death in the patients without hypertension, those with current smoking, those with atrial fibrillation, or those without anemia. In addition, VEGF‐C levels did not significantly improve the prediction of cardiovascular death over the model with established risk factors and biomarkers in the entire cohort. However, we had limited statistical power to test these findings. Longer follow‐ups are needed to determine whether VEGF‐C represent a distinct cardiovascular risk factor or not, and the relationships between VEGF‐C levels and specific causes of death.
Previous experimental animal model studies have suggested that following myocardial infarction, the cardiac lymphatics underwent a profound angiogenic response, accompanied by an upregulation in the lymphatic development gene program.15 The remodeling and dysfunction of collecting ducts contribute to the development of chronic myocardial edema and inflammation aggravating cardiac fibrosis and dysfunction.14 This cardiac lymphangiogenic response was also significantly enhanced by ectopic VEGF‐C stimulation following injury, leading to improvement in cardiac function.15 If VEGF‐C have a protective role against cardiac injury in humans as well, those with low VEGF‐C levels may have an increased susceptibility to heart failure after myocardial infarction. Further clinical studies are necessary to determine the role of VEGF‐C in the left ventricular remodeling and dysfunction after myocardial infarction.
At baseline, our study population's VEGF‐C level was inversely correlated with prevalent CKD and anemia, whereas it was positively correlated with obesity and dyslipidemia, as was also the case in previous studies.16, 18 The mechanisms underlying these correlations merit consideration.
Although the sources of VEGF‐C remain unclear, the proximal renal tubules were one of the sources of VEGF‐C in a mouse model of progressive renal fibrosis.19 Because the proximal renal tubule is the primary sensor and effector in the progression of CKD,20 reduced VEGF‐C levels may—at least in part—represent the proximal renal tubular damage.
Macrophages were the other source of VEGF‐C in the above‐mentioned progressive renal fibrosis model.19, 21 Interestingly, VEGF‐C expression was higher in alternatively activated M2‐ than in classically activated M1‐polarized macrophages.19 Thus, the phenotypic modulation of macrophages induced during the progression of renal fibrosis may affect their ability to secret VEGF‐C.
Anemia is a major comorbidity of CKD, in which erythropoietin deficiency is the most significant cause of anemia. Erythropoietin may thus be involved in the regulation of VEGF‐C levels. In accordance with this idea, the systemic administration of erythropoietin increased VEGF‐C expression in macrophages.22 Conversely, VEGF‐C was critical for fetal erythropoiesis, but it did not have a major effect on adult erythropoiesis.23 Thus, erythropoietin might play a role in the regulation of VEGF‐C secretion from macrophages in human adults with renal anemia.
In the CKD subgroup, the low VEGF‐C level was associated with all‐cause death, independent of the estimated glomerular filtration rate, and anemia (data not shown). However, we did not have data on the urinary albumin‐to‐creatinine ratio (which is a gold standard of risk stratification for CKD progression and mortality) or cystatin C, a powerful predictor of mortality and cardiovascular events.24 Other cohort studies including ours (the EXCEED‐J [Establishment of the Method to Extract a High Risk Population Employing Novel Biomarkers to Predict Cardiovascular Events in Japan] study, UMIN000018807) will further clarify the prognostic value of VEGF‐C in CKD patients in comparison with the urinary albumin‐to‐creatinine ratio and cystatin C.
The VEGF‐C levels were elevated in overweight and obese subjects,18 and they decreased following bariatric surgery.25 We previously demonstrated that serum VEGF‐C levels are more closely correlated with dyslipidemia than overweightness itself,16 as is the case in the present study (data not shown). Notably, a recent study suggested that intestinal lymphatic vessels play an important role in dietary lipid absorption: the deletion of VEGF‐C in adult mice had no effect on lymphatic vasculature in the skin, trachea, or lymph nodes, but it caused a slow regression of intestinal lymphatic vessels.26 In the mice fed a high‐fat diet in that study, the atrophy of the intestinal lymphatic vessels reduced the lipid uptake, increased the lipid excretion into feces, counteracted obesity, and improved glucose metabolism.26 If circulating VEGF‐C levels partly represent the intestinal lymphatic vessel integrity required for lipid uptake, a positive correlation between VEGF‐C and lipid levels can be well explained. Further investigations are necessary to elucidate the mechanisms that regulate circulating VEGF‐C levels, and their effect on the homeostasis maintenance.
Limitations
First, the assay used for measuring VEGF‐C is a research assay that is not automated or approved for clinical use at present. Second, the blood samples were drawn from the arterial sheath. Given VEGF‐C is a key regulator of lymphatic endothelial cells, there is the potential for concentrations to differ in the arterial and venous circulation. In our preliminary data (n=40), VEGF‐C levels in sera from the arterial sheath were closely correlated with those from the peripheral vein before cardiac catheterization (β, 0.730; standard error of the mean, 0.084; P<0.001). However, to determine the potential for VEGF‐C to be used more widely in risk prediction, other cohort studies using the peripheral venous blood samples, such as the EXCEED‐J study, are necessary. Third, this was an observational study, and unmeasured confounding factors may exist. Thus, whether VEGF‐C could serve as a molecular therapeutic target or not is unclear. Future interventional studies are required to answer the question. Fourth, we have no collected data of cardiovascular imaging such as echocardiography, cardiovascular magnetic resonance, computed tomography, intravascular ultrasound/optical coherence tomography, and nuclear imaging. The relationship of VEGF‐C levels with those cardiac imaging data, including cardiac (coronary artery) inflammation/macrophage infiltration and myocardial infarct size in clinical settings, will provide valuable insight into the mechanistic role of VEGF‐C. Finally, because the ANOX study cohort includes exclusively Asian individuals with suspected or known CAD, our results may not be generalizable to general Asian populations, or other ethnic groups.
Conclusions
Nevertheless, our results clearly demonstrate that a low VEGF‐C value was independently associated with all‐cause mortality beyond the established risk factors, NT‐proBNP, cTnI, and hs‐CRP in patients with suspected or known CAD undergoing elective coronary angiography.
Sources of Funding
The ANOX study is supported by a Grant‐in‐Aid for Clinical Research from the National Hospital Organization.
Disclosures
Dr Wada reports patent pending—Vascular endothelial growth factor receptor‐C as a predictive marker of cardiovascular and all‐cause death in patients with suspected coronary artery disease (Patent application No. JP P2017‐213467). The remaining authors have no disclosures to report.
Supporting information
Appendix S1. The members of the ANOX Study Investigators.
Data S1. Supplemental methods.
Table S1. Baseline Characteristics and Incidence of Outcomes
Table S2. Hazard Ratios for All‐Cause Death, Cardiovascular Death, and a Major Adverse Cardiovascular Event According to Biomarker Levels
Acknowledgments
The authors thank the other members, cooperators, and participants of the ANOX study for their valuable contributions.
(J Am Heart Assoc. 2018;7:e010355 DOI: 10.1161/JAHA.118.010355.)
References
- 1. Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. [DOI] [PubMed] [Google Scholar]
- 2. Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF‐C, is a ligand for the Flt4 (VEGFR‐3) and KDR (VEGFR‐2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest. 2014;124:888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vuorio T, Tirronen A, Ylä‐Herttuala S. Cardiac lymphatics—a new avenue for therapeutics? Trends Endocrinol Metab. 2017;28:285–296. [DOI] [PubMed] [Google Scholar]
- 6. Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. [DOI] [PubMed] [Google Scholar]
- 7. Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF‐C transgenic mice. Science. 1997;276:1423–1425. [DOI] [PubMed] [Google Scholar]
- 8. Chen JC, Chang YW, Hong CC, Yu YH, Su JL. The role of the VEGF‐C/VEGFRs axis in tumor progression and therapy. Int J Mol Sci. 2012;14:88–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang CA, Tsai SJ. The non‐canonical role of vascular endothelial growth factor‐C axis in cancer progression. Exp Biol Med (Maywood). 2015;240:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res. 2016;118:515–530. [DOI] [PubMed] [Google Scholar]
- 11. Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci‐Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR‐BI‐mediated transport of HDL. Cell Metab. 2013;17:671–684. [DOI] [PubMed] [Google Scholar]
- 13. Kutkut I, Meens MJ, McKee TA, Bochaton‐Piallat ML, Kwak BR. Lymphatic vessels: an emerging actor in atherosclerotic plaque development. Eur J Clin Invest. 2015;45:100–108. [DOI] [PubMed] [Google Scholar]
- 14. Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards‐Lévy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. 2016;133:1484–1497. [DOI] [PubMed] [Google Scholar]
- 15. Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada H, Ura S, Kitaoka S, Satoh‐Asahara N, Horie T, Ono K, Takaya T, Takanabe‐Mori R, Akao M, Abe M, Morimoto T, Murayama T, Yokode M, Fujita M, Shimatsu A, Hasegawa K. Distinct characteristics of circulating vascular endothelial growth factor‐A and C levels in human subjects. PLoS One. 2011;6:e29351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond). 2005;29:1308–1314. [DOI] [PubMed] [Google Scholar]
- 19. Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W. Vascular endothelial growth factor‐C and ‐D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int. 2013;83:50–62. [DOI] [PubMed] [Google Scholar]
- 20. Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016;311:F145–F161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji RC. Macrophages are important mediators of either tumor‐ or inflammation‐induced lymphangiogenesis. Cell Mol Life Sci. 2012;69:897–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee AS, Kim DH, Lee JE, Jung YJ, Kang KP, Lee S, Park SK, Kwak JY, Lee SY, Lim ST, Sung MJ, Yoon SR, Kim W. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 2011;71:4506–4517. [DOI] [PubMed] [Google Scholar]
- 23. Fang S, Nurmi H, Heinolainen K, Chen S, Salminen E, Saharinen P, Mikkola HK, Alitalo K. Critical requirement of VEGF‐C in transition to fetal erythropoiesis. Blood. 2016;128:710–720. [DOI] [PubMed] [Google Scholar]
- 24. Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlöv J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. [DOI] [PubMed] [Google Scholar]
- 25. Farey JE, Fisher OM, Levert‐Mignon AJ, Forner PM, Lord RV. Decreased levels of circulating cancer‐associated protein biomarkers following bariatric surgery. Obes Surg. 2017;27:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF‐C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The members of the ANOX Study Investigators.
Data S1. Supplemental methods.
Table S1. Baseline Characteristics and Incidence of Outcomes
Table S2. Hazard Ratios for All‐Cause Death, Cardiovascular Death, and a Major Adverse Cardiovascular Event According to Biomarker Levels


