Abstract
Background
Despite growing use of the subcutaneous implantable cardioverter‐defibrillator (S‐ICD), its clinical role in arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) patients remains undefined. We aim to elucidate the cardiac phenotype, implant characteristics, and long‐term efficacy regarding appropriate therapy and complications in ARVC/D patients with an S‐ICD implant.
Methods and Results
A transatlantic cohort of ARVC/D patients who underwent S‐ICD implantation was analyzed for clinical characteristics, S‐ICD therapy, and long‐term outcome including device‐related complications. The cohort included 29 patients (52% male, 76% probands, 59% with ARVC/D‐associated mutation, 59% primary prevention [no prior sustained ventricular arrhythmias], and 45% first‐generation S‐ICD devices). At implant, all inducible patients (27/29) had conversion of induced ventricular fibrillation. Two patients (7%) had superficial infections of the incision site that were treated conservatively. Over a median follow‐up of 3.16 years (interquartile range: 2.21–4.51 years), all episodes (6 patients, 4% per year) of sustained ventricular arrhythmias were appropriately detected and treated. Six patients (21%) experienced 39 inappropriate shocks, with 3 requiring device explantation. Oversensing of noncardiac signal (n=4; especially myopotentials) and cardiac signal (n=4) was the most frequent etiology. No lead or device dislodgement, infection, skin erosion, or explantation related to need for antitachycardia pacing was noted.
Conclusions
S‐ICD can effectively treat both induced and spontaneous ventricular arrhythmias in patients with ARVC/D. The rate of inappropriate shocks, although considerable, is comparable to that in ARVC/D patients treated with transvenous ICDs. When they occurred, inappropriate shocks were primarily due to cardiac and, uniquely, noncardiac oversensing. We suggest potential strategies for minimizing inappropriate therapy.
Keywords: arrhythmogenic right ventricular cardiomyopathy, implanted cardioverter defibrillator, long‐term follow‐up, ventricular tachycardia
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Sudden Cardiac Death, Cardiomyopathy
Clinical Perspective
What Is New?
This study highlights the fact that the subcutaneous implantable cardioverter‐defibrillator can effectively treat both induced and spontaneous ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia.
Inappropriate shock caused by cardiac or noncardiac oversensing is the major limitation, albeit comparable to inappropriate shock in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia treated with transvenous implantable cardioverter‐defibrillators.
What Are the Clinical Implications?
The data from this study suggest that the subcutaneous implantable cardioverter‐defibrillator remains a viable option for prevention of sudden cardiac death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia.
Inappropriate therapy might be minimized by the application of careful patient selection, R‐wave amplitude screening at implant, and use of the SMART Pass filter (Boston Scientific).
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited cardiomyopathy characterized by an increased risk of ventricular arrhythmias and sudden cardiac death (SCD).1 Implantable cardioverter‐defibrillator (ICD) therapy for SCD prevention is an integral and effective therapeutic option for ARVC/D patients.2, 3 Although proven effective in the prevention of SCD, transvenous ICD (TV‐ICD) systems are associated with considerable morbidity and long‐term lead‐related risks, especially in the younger population.3, 4, 5 To tackle this drawback, an entirely subcutaneous ICD (S‐ICD) was developed to lessen or even eliminate these complications associated with TV‐ICDs.6 Several studies have demonstrated that the S‐ICD provides reliable and effective detection and termination of ventricular arrhythmias.7, 8, 9 Over the past 5 years, the S‐ICD has emerged as a viable alternative to TV‐ICD systems for many inherited disease states.10, 11 Despite widespread adoption of the S‐ICD,12 its specific clinical role for ARVC/D patients remains undefined. ARVC/D patients are unique because of their younger age, high arrhythmic burden, phenotypic relationship to vigorous activity, specific ECG changes, and right ventricular (RV) cardiomyopathy.1, 2 Given the need for life‐long SCD protection, a detailed appraisal of the distinctive benefits and risks in this population is warranted. Our study aims to addresses this important issue by utilizing a transatlantic cohort of ARVC/D patients who underwent S‐ICD implantation. Our first objective was to report the clinical characteristics, cardiac phenotype, and implant characteristics of ARVC/D patients with S‐ICD implants. Our second goal was to delineate the long‐term efficacy regarding appropriate therapy and to understand complications, especially inappropriate ICD therapy, in ARVC/D patients with S‐ICD implantation.
Methods
Patient Population
The study population was derived from patients diagnosed with definite ARVC/D13 and enrolled in either the Johns Hopkins ARVC/D registry or in the ARVC/D registry maintained by the inherited arrhythmogenic cardiomyopathy unit of the University of Padua (Italy). All patients in these ARVC/D registries who had an S‐ICD implant for SCD prevention and were followed for at least 30 days after implantation were included in the study. The study was approved by the respective institutional review boards, and all participants provided written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Detailed clinical information regarding demographics, indications for implantation, medication use, and preimplant noninvasive and invasive evaluation (12‐lead ECG, 24‐hour Holter monitoring, magnetic resonance imaging, echocardiography, and electrophysiology study) was reviewed.
S‐ICD Implantation
S–ICD implant characteristics including device and electrode type, defibrillator threshold testing, and device settings (number of zones, rate cutoff) at implant were collected. Hospital records were reviewed to ascertain any periprocedural complications. Decisions regarding S‐ICD implantation (incision technique, preimplant screening protocol) and programming of these devices were made by the managing cardiologist and/or electrophysiologist. Surface ECG screening using the customized screening tool was performed and deemed acceptable in all patients at implant.
Clinical outcomes
S‐ICD interrogations and captured surface ECG were obtained from referring institutions and individual patients throughout the duration of follow‐up. Captured surface ECG tracings from all shock episodes stored in the S‐ICD were obtained and reviewed for details (device setting, gain setting, sensing configuration, shock polarity, and device software version at the time of ICD shock). The surface ECGs were reviewed and collaboratively adjudicated by 2 electrophysiologists from each registry. Inappropriate shocks were defined as shocks for any reason other than ventricular tachyarrhythmia at or above the programmed rate zone. Inappropriate shock episodes were reviewed regarding precipitating activity, surface ECG R‐wave amplitude, concurrent medication, and clinical management including sensing vector change and/or filtering software update. Inappropriate episodes were classified in the following categories as per prior registry data7, 14: (1) supraventricular tachycardia, defined as any supraventricular tachycardia in the conditional or shock zone; (2) cardiac oversensing, defined as T‐wave oversensing, QRS oversensing, P‐wave oversensing, oversensing caused by a low‐amplitude signal, and other/combined types of cardiac oversensing; and (3) noncardiac oversensing, defined as any kind of oversensing caused by noncardiac causes (eg, electromagnetic interference and myopotentials). Long‐term outcomes including need for device explanation and/or replacement by transvenous systems were ascertained.
Episodes of inappropriate therapy were reviewed and verified with the Boston Scientific technical support team. Simulation analysis (included in the Supplementary Data) evaluating the efficacy of SMART Pass Technology (Insight; Boston Scientific) was also performed for the oversensing episodes.
Statistical Analyses
Continuous variables are summarized as either mean±SD or median (interquartile range), as appropriate, and compared using the Student t test or Mann–Whitney test. Categorical data are displayed as frequencies and percentages and compared using a χ2 test. The cumulative probabilities of survival free from first appropriate and inappropriate S‐ICD therapy were determined by the Kaplan–Meier method and compared with those of previously published patients in the Hopkins registry who received a TV‐ICD,3 using a log rank test. All statistical analyses were performed using STATA (v14.2; StataCorp). A 2‐sided P≤0.05 was considered significant.
Results
Baseline Phenotype
The patient population consisted of 29 definite13 ARVC/D patients who received an S‐ICD between March 2011 and June 2017 for SCD prevention. The indication was primary prevention for 17 patients (59%), whereas 12 (41%) received a device for secondary prevention of SCD. Table 1 shows the clinical characteristics of patients who underwent S‐ICD implant. The study population was mostly white (97%) and about half female (48%). The mean age at S‐ICD implantation was 31.4±16 years (range: 13–78 years), with 21 patients (72%) aged ≤40 years and 5 aged ≤20 years. Figure S1 shows the age distribution of the patients who received an S‐ICD, stratified by indication. The overall mean body mass index (BMI; calculated as kg/m2) was 24.3±3.4 (range: 18–31), with 1 patient (3%) underweight (BMI ≤18) and 1 (3%) obese (BMI >30). Twenty‐seven patients (93%) had genetic testing performed, with 16 (59%) harboring a pathogenic mutation. Twenty‐two patients were probands ascertained independent of family history of ARVC/D, whereas 7 patients came to attention through cascade family screening. At the time of ICD implantation, 19 (65%) patients were being treated with a β‐blocker and 4 (14%) were receiving a membrane‐active antiarrhythmic agent. Left ventricular dysfunction (ejection fraction ≤50%) was noted in 10 patients (34%).
Table 1.
Baseline Clinical Features of ARVC/D Patients With an S‐ICD
| Clinical Variables | Overall Population (N=29) | Primary Prevention (n=17) | Secondary Prevention (n=12) |
|---|---|---|---|
| Demographics | |||
| Male | 15 (52) | 10 (53) | 5 (42) |
| White | 28 (97) | 16 (94) | 12 (100) |
| Proband | 22 (76) | 10 (59) | 12 (100) |
| Mutation carrier | 16/27 (59) | 11/16 (69) | 5/11 (45) |
| Age at ICD implantation, y | 34±15 | 32±15 | 38±16 |
| Height, cm | 170 (165–175) | 168 (164–175) | 173 (167–182) |
| Weight, lb | 155 (135–176) | 146 (132–165) | 168 (149–181) |
| BMI, kg/m2 | 24.32±3.42 | 23.43±3.48 | 25.59±3.01 |
| Clinical characteristics at implant | |||
| Syncope | 10 (34) | 5 (29) | 5 (42) |
| Inducibility at EPS | 7/17 (41) | 1/9 (11) | 6/8 (75) |
| ≥1000 PVCs on Holter monitoring | 15/22 (68) | 8/13 (62) | 7/9 (78) |
| Inverted T waves in ≥3 precordial leads | 24/28 (86) | 14/16 (88) | 10/12 (83) |
| QRS duration, ms | 92 (82–100) | 90 (83–99) | 92 (80–103) |
| NSVT | 10 (34) | 2 (12) | 8 (67) |
| Major RV structural abnormality on CMR | 21/27 (72) | 12/15 (80) | 9/12 (75) |
| RVEF, % | 41 (35–49) | 40 (33–46) | 44 (35–52) |
| LVEF, % | 56 (45–63) | 50 (45–63) | 58 (56–63) |
| Medications at implant | |||
| Any medication | 24 (83) | 14 (82) | 10 (83) |
| ACEi/ARB | 12 | 8 | 4 |
| β‐Blocker | 19 | 12 | 7 |
| Flecainide | 2 | … | 2 |
| Amiodarone | 1 | … | 1 |
| Sotalol | 1 | … | 1 |
Values are mean±SD, n (%), n/N (%), or median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; BMI, body mass index; CMR, cardiac magnetic resonance; EPS, electrophysiology study; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular contraction; RV, right ventricular; RVEF, right ventricular ejection fraction; S‐ICD, subcutaneous implantable cardioverter‐defibrillator.
Among 12 patients who underwent S‐ICD implantation for secondary prevention (100% probands), 10 had previously experienced sustained ventricular tachycardia (VT) and 2 had presented with SCD. Electrophysiology study inducibility (75%), frequent Holter ectopy (78%), and nonsustained VT (67%) were seen in most patients in this group. Among those who underwent primary prevention implantation, the majority (69%) had a pathogenic mutation along with major RV structural abnormalities (80%).
S‐ICD Implant Characteristics
Thirteen patients were implanted with a first‐generation S‐ICD (model SQ‐RX 1010; Boston Scientific), and 16 patients were implanted the subsequent S‐ICD (model A209 and A219; Boston Scientific; Table 2). Twenty patients underwent implantation at hospitals in the United States, and 9 were implanted at the University of Padua. At S‐ICD implantation, defibrillation testing was attempted in all patients, with ventricular fibrillation (VF) induced in 27 patients (93%). VF could not be induced, even after several attempts, in 2 patients (Table S1). Among those with inducible VF, all patients had conversion of the induced VF at an output of 65 J, with 1 patient converting to sinus rhythm at 50 J. Dual‐zone programming for tachyarrhythmia detection was performed in all patients.
Table 2.
S‐ICD Implant Characteristics in ARVC/D Patients
| Clinical Variables | Overall Population (N=29) | Primary Prevention (n=17) | Secondary Prevention (n=12) |
|---|---|---|---|
| S‐ICD device model | |||
| First generation | 13 (45) | 6 (35) | 7 (58) |
| Second generation | 16 (55) | 11 (65) | 5 (42) |
| Defibrillation testing attempted | 29 (100) | 17 (100) | 12 (100) |
| VF induced | 27(93) | 17 (100) | 10 (83) |
| Acute VF conversion | 27/27 (100) | 17/17 (100) | 10/10 (100) |
| S‐ICD programming | |||
| Conditional zone, bpm, mean (range) | 207 (190–230) | 208 (190–230) | 207 (190–220) |
| Shock zone, bpm, mean (range) | 242 (220–250) | 243 (230–250) | 241 (220–250) |
| Perioperative complications | |||
| S‐ICD system infection | 0 | 0 | 0 |
| Hematoma | 0 | 0 | 0 |
| Inappropriate shock: oversensing | 0 | 0 | 0 |
| Superficial/incisional‐related infection | 2 (7) | 0 | 2 (17) |
| Need for revision because of lead/device movement | 0 | 0 | 0 |
| Suspected device malfunction | 0 | 0 | 0 |
| Pneumothorax | 0 | 0 | 0 |
Values are n (%), n/N (%), or median (interquartile range). ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; VF, ventricular fibrillation.
As shown in Figure 1, S‐ICD implantation was performed as an initial ICD implant in 25 (86%) patients and following explant of a TV‐ICD in 4 patients (14%). Three of these patients underwent placement of an S‐ICD after having an endocardial lead fracture with subsequent extraction, and 1 patient had the initial TV‐ICD extracted because of a device‐related infection. Two patients (7%) had superficial infections of the incision site related to the implantation of the S‐ICD; one occurred within the first few days after implantation, and the other occurred 5 weeks after S‐ICD implant. Both infections were treated with a short course of antibiotics, without the need for surgical revision or explantation of the S‐ICD.
Figure 1.
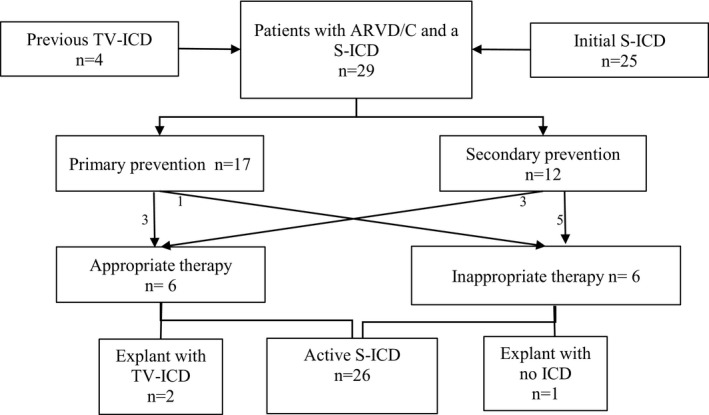
Long‐term outcomes of 29 ARVC/D patients with S‐ICD implant. ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; ICD, implantable cardioverter‐defibrillator; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; TV‐ICD, transvenous implantable cardioverter‐defibrillator.
Follow‐up
Over a median follow‐up of 3.16 years (interquartile range: 2.21–4.51 years), 6 patients (20%) received 43 appropriate ICD interventions for a sustained ventricular arrhythmia (4% per year). The median time from ICD implantation to first S‐ICD shock was 6 months (range: 1.7–13.9 months). The mean cycle length for the first appropriate intervention was 300.5±43.41 ms (range: 250–352 ms). The average number of shocks experienced was 7 (range: 1–13), with 2 patients facing VT storms (one successfully treated with amiodarone dose adjustment, the other noted to have severe hypokalemia with arrhythmia‐free course after repletion (Table 3). Table S2 shows the clinical characteristics of the patients experiencing appropriate S‐ICD interventions. Figure 2A depicts the appropriate S‐ICD therapy–free survival with comparison to patients in the Hopkins registry that have previously been reported to receive a TV‐ICD system.3 S‐ICD event‐free survival at 1 and 2 years was 84% and 78%, respectively. Figure S2 depicts captured surface ECG tracing from 3 patients who received appropriate S‐ICD therapy.
Table 3.
S‐ICD Therapy and Clinical Outcomes in ARVC/D Patients
| Clinical Variables | Overall Population (N=29) | Primary Prevention (n=17) | Secondary Prevention (n=12) |
|---|---|---|---|
| Number of patients with AS (90) | 6 (20) | 3 (17) | 3 (25) |
| Time to first AS from implant, mo | 6 (1.7–13.9) | 4.7 (0.46–23.8) | 7.3 (1.76–13.9) |
| Total number of AS during follow‐up | 43 | 28 (7,8,13) | 15 (1,3,11) |
| Patients with VT/VF storm | 2 (7) | 1 (13) | 1 (11) |
| Management of AS | |||
| Medication adjustment (90) | 3 (10) | 2 (12%) | 1 (8) |
| Electrolyte replacement (90) | 1 (3) | 0 (0) | 1 (8) |
| Ablation (90) | 1 (3) | 1 (6) | 0 |
| Number of patients with IAS (90) | 6 (21) | 1 (6) | 5 (42) |
| Time to first IAS from implant | 4.6 (1.9–12.6) | 12.6 (12.6–12.6) | 3.7 (1.9–5.5) |
| Total number of IAS during follow‐up | 39 | 8 | 31 |
| Cause of IAS, n (% patients) | |||
| SVT | 0 | 0 | 0 |
| Cardiac oversensing | |||
| T‐wave oversensing | 1 | 0 | 1 |
| P‐wave oversensing | 2 | 1 | 1 |
| R‐wave double counting | 1 | 0 | 1 |
| Noncardiac oversensing | |||
| Myopotential | 3 | 1 | 2 |
| EMI | 0 | 0 | 0 |
| Movement artifact | 1 | 0 | 1 |
| Active S‐ICD at last follow‐up (90) | 26 (90) | 16 (94) | 10 (83) |
| S‐ICD explant with TV‐ICD (90) | 2 (7) | 1 (6) | 1 (8) |
| S‐ICD explant with no ICD (90) | 1 (3) | 0 (0) | 1 (8) |
Values are n (%), n/N (%), or median (interquartile range). ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; AS, appropriate shock; EMI, electromagnetic interference; IAS, inappropriate shock; ICD, implantable cardioverter‐defibrillator; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; SVT, supraventricular tachycardia, TV‐ICD, transvenous implantable cardioverter‐defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 2.
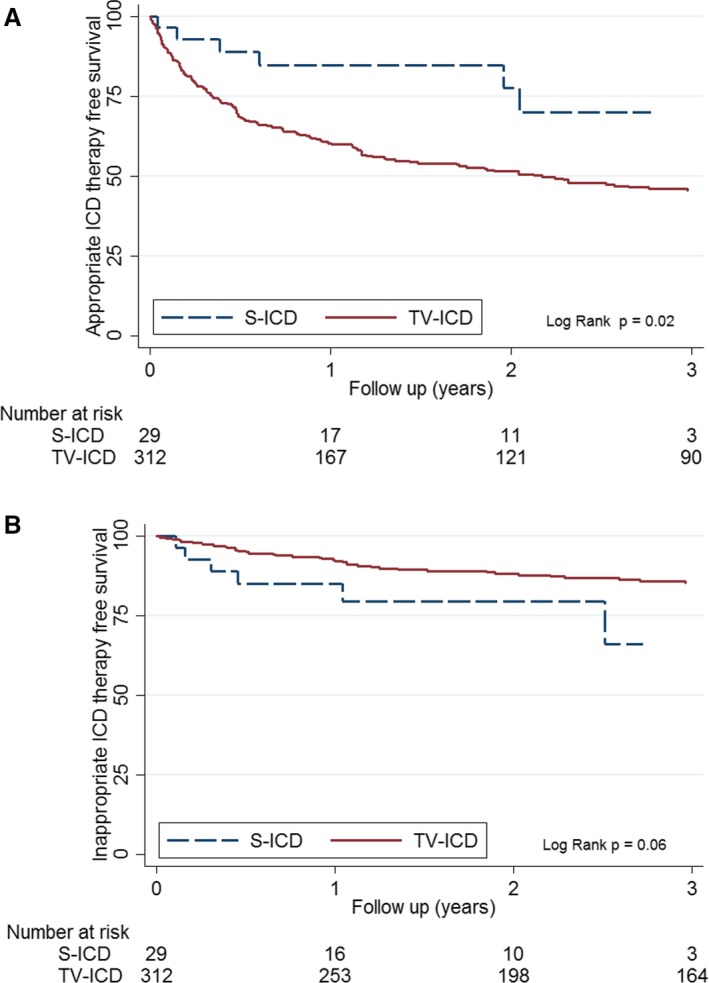
A, Kaplan–Meier analysis of cumulative survival free from first appropriate S‐ICD interventions in ARVC/D patients. B, Kaplan–Meier analysis of cumulative survival free from first inappropriate S‐ICD interventions in ARVC/D patients. ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; ICD, implantable cardioverter‐defibrillator; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; TV‐ICD, transvenous implantable cardioverter‐defibrillator.
During follow‐up, 6 patients (21%) experienced 39 inappropriate shocks at an incidence of 12.3 per 100 patient‐years (4.5% per year; Table 3). The median time from ICD implant to first inappropriate shock was 4.6 months (interquartile range: 1.9–12.6 months). A second inappropriate shock was experienced by 4 patients (14%). Figure 2B depicts the inappropriate S‐ICD therapy–free survival compared with patients in the Hopkins registry who were previously reported as receiving a TV‐ICD system.3 The S‐ICD inappropriate shock–free survival at 1 and 2 years was 83% and 77%, respectively. Patients with inappropriate shocks are more likely to be probands with major RV structural abnormalities receiving secondary prevention devices (Table 4). Oversensing of noncardiac signal (n=4) and cardiac signal (n=4) was the abnormality seen most frequently (Table 3 and Figure 3). Table S3 provides device characteristics, sensing and software details, precipitating activity, and physician response to the inappropriate shocks in these 6 patients. Surface ECG tracing from 3 patients with no inappropriate shocks showing ≥1 mV R wave on implant surface ECG (best vector) and 3 patients with myopotential oversensing (<1 mV R wave on surface ECG) are depicted in Figures S3 and S4, respectively. Surface 12‐lead ECG from a patient with myopotential oversensing–related inappropriate shock exemplifies low precordial and limb lead QRS amplitude (Figure S5). Simulation studies performed by Boston Scientific technical services to evaluate the utility of the SMART Pass filter during oversensing episodes showed improvement for movement artifact and P/T wave oversensing but no clear resolution for myopotential‐related episodes (Figure S6A–S6D).
Table 4.
Clinical Characteristics of Patients With ARVC/D Experiencing Inappropriate S‐ICD Therapy
| Clinical Variables | Overall Population (N=29) | No Inappropriate shock (n=23) | Inappropriate Shock (n=6) |
|---|---|---|---|
| Demographics | |||
| Male | 15 (52) | 13 (57) | 2 (33) |
| White | 28 (97) | 22 (96) | 6 (100) |
| Proband | 22 (76) | 16 (70) | 6 (100) |
| Mutation carrier | 16/27 (59) | 12/23 (57) | 4/6 (67) |
| Age at ICD implantation, y | 34±15 | 33±14 | 40±20 |
| Height, cm | 170 (165–175) | 173 (167–177) | 168 (164–175) |
| Weight, lb | 155 (135–176) | 150 (132–184) | 161 (147–175) |
| BMI, kg/m2 | 24±3 | 24±4 | 24±2 |
| Clinical characteristics at implant | |||
| Syncope | 10 (34) | 6 (26) | 4 (67) |
| Inducibility at EPS | 7/17 (41) | 3/11 (27) | 4/6 (67) |
| ≥1000 PVCs on Holter monitoring | 15/22 (68) | 11/17 (64) | 4/5 (80) |
| Inverted T‐waves in ≥3 precordial leads | 24/28 (86) | 18/22 (82) | 6/6 (100) |
| QRS duration, ms | 92 (82–100) | 90 (82–100) | 94 (79–100) |
| NSVT | 10 (34) | 11 (48) | 3 (50) |
| Major RV structural abnormality on CMR | 21/27 (72) | 16/21 (76) | 5/6 (83) |
| RVEF, % | 41 (35–49) | 43 (35–49) | 36 (34–48) |
| LVEF, % | 56 (45–63) | 57 (56–62) | 56 (45–63) |
| Device characteristics | |||
| First‐generation S‐ICD (Cameron Health; 1010) | 13/29 (45) | 10/13 (77) | 3/13 (23) |
| Second‐generation S‐ICD (Emblem A209, A219) | 16/29 (55) | 13/16 (81) | 3/16 (19) |
| Medications at implant | 24 (83) | ||
| ACEi/ARB | 24 (83) | 19 (83) | 5 (83) |
| β‐Blocker | 12 | 10 | 2 |
| Flecainide | 19 | 16 | 3 |
| Amiodarone | 2 | 1 | 1 |
| Sotalol | 1 | 1 | … |
Values are mean±SD, n (%), n/N (%), or median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; BMI, body mass index; CMR, cardiac magnetic resonance; EPS, electrophysiology study; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular contraction; RV, right ventricular; RVEF, right ventricular ejection fraction; S‐ICD, subcutaneous implantable cardioverter‐defibrillator.
Figure 3.
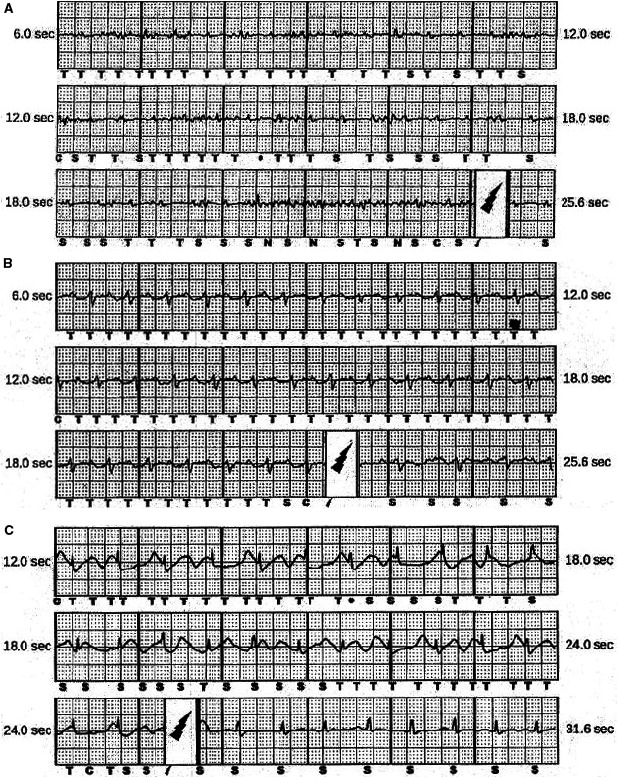
Surface ECG racing of inappropriate subcutaneous implantable cardioverter‐defibrillator interventions due to myopotentials (A), P/T wave oversensing (B), and movement artifact (C).
During follow‐up, no lead or device dislodgement, infection, skin erosion, or explantation because of need for antitachycardia pacing was noted. At the end of follow‐up, 3 patients had the device explanted and opted to have either no ICD (n=1) or a TV‐ICD (n=2; Figure 2). No death or need for transplant occurred in this cohort.
Discussion
Despite expanding use of the S‐ICD in clinical practice,12 lack of systematic data on unique patient and device characteristics and long‐term efficacy of this system in ARVC/D patients has hampered the ability to advise patients adequately. Published literature has been limited to a very small number of ARVC/D patients embedded in larger cohorts.11, 12 We describe, for the first time, the clinical experience and value of S‐ICD therapy in a transatlantic cohort of 29 ARVC/D patients.
The clinical phenotype of ARVC/D patients receiving S‐ICD implants with regard to sex, presence of pathogenic mutations, amount of ectopy on Holter, and presence of structural abnormality on magnetic resonance imaging is similar to patients previously considered for a TV‐ICD.3 This finding bolsters generalizability of the study observations toward a target population of ARVC/D patients who qualify for an ICD. The average age at implant in the study group was 34 years, in contrast to published clinical data that are largely confined to older patients (aged 49–53 years),8, 9, 12 although data exist for small numbers of younger patients with hypertrophic cardiomyopathy10 and channelopathies11 (aged 35–42 years). This has implications because younger patients may derive the most benefit from avoidance of transvenous leads, but prior reports suggest a higher inappropriate shock frequency in younger patients.15 No sex‐ or race/ethnicity‐based differences were present.
Of note, ARVC/D patients receiving an S‐ICD had lower BMI, with women receiving nearly half of the implants in contrast to major cohorts.8, 9, 12 It is likely that the female preponderance and younger age of these patients accounts for the lower BMI in our cohort. Similarly, in a prior cohort16 that included 140 S‐ICD patients of whom nearly half had some genetic risk and 40% were women, the mean BMI was 25.2. Though most S‐ICD implants are in patients with structural heart disease having an ejection fraction <35%,12 the average left ventricular ejection fraction in ARVC/D patients was normal. Lower BMI and higher ejection fraction are factors that predict passing only 1 vector versus multiple vectors12 on preimplant screening, indicating that these ARVC/D patients may not have as many sensing vector options at implant.
This study demonstrates that the implantation of an S‐ICD system is safe and well tolerated in ARVC/D patients, with only incision‐related infection seen in 2 female patients (BMIs of 20 and 25) with the larger, first‐generation device. This finding is consistent with prior published 30‐day complication data (3.8–4.5%).9, 12 The results of this study also show that the S‐ICD is effective in terminating induced VF in ARVC/D patients. Comparable to prior experience in unselected cohorts, a hypertrophic cardiomyopathy population, and channelopathy patients,4, 8, 10, 12 all induced tachyarrhythmias during defibrillation threshold testing were successfully converted.
All episodes of VT/VF during follow‐up were appropriately detected and successfully treated with no episodes of syncope or SCD/sudden cardiac arrest during >4 years of average follow‐up. This demonstrates that the S‐ICD is an effective long‐term option for this population with high arrhythmic risk. The high S‐ICD programmed rate cutoff and the multistep discriminative sensing algorithm may minimize therapy for self‐limited episodes, allowing many ventricular arrhythmias to self‐terminate yet protecting against life threatening VT/VF. In fact, in a matched Dutch cohort,16 appropriate ICD intervention rates (shocks and antitachycardia pacing) were lower in the S‐ICD group, at 17.0% versus 31.3% in the TV‐ICD group, without reduction in overall efficacy. No patient in the study had the device removed because of a perceived need for antitachycardia pacing. Patients with slow sustained VT that can be reliably pace terminated before implant on an electrophysiology study may benefit from the antitachycardia pacing ability of a TV‐ICD. However, the risk of S‐ICD shocks for future slow monomorphic VT (as opposed to successful antitachycardia pacing therapy17) must be weighed against the benefit of circumventing long‐term lead‐related complications in ARVC/D patients. In addition, the lowest available VT rate cutoff (170 bpm) for the current S‐ICD system precludes programming therapy for slow (<170 bpm) ventricular arrhythmias.
ARVC/D patients with TV‐ICDs are noted to have considerable risk of inappropriate therapy.3.16 In comparison, a significant minority (21%) of S‐ICD patients also experienced inappropriate shocks at a similar rate (4.5% per year; 12.3 per 100 patient‐years), leading to device explantation in 3 patients (10%). However, the etiology of the inappropriate shocks was markedly different in the S‐ICD recipients. Unlike prior reports,7, 14 the uniformity of 2‐zone programming and the S‐ICD sensing algorithm resulted in the absence of supraventricular tachycardia–related inappropriate shock. A high proportion (17%) of patients experienced cardiac oversensing (T/P oversensing and R double counting) in contrast to previous studies.7, 8, 14 The high number of patients with noncardiac, especially myopotential, oversensing is also unique to this report. Noncardiac oversensing is rarely reported (0.09%)14 in the S‐ICD literature, with a handful of patients noted to have electromagnetic interference7 and lead migration related myopotential oversensing.8 Isolated case reports describe myopotential oversensing with heavy lifting and clapping, likely caused by pectoral muscle contraction.18, 19 The RV cardiomyopathy in ARVC/D is associated with low R‐wave amplitudes at implantation and R‐wave amplitude decline during follow‐up in TV‐ICD systems.20 As noted in the surface ECG and 12‐lead ECG tracings from implant, it is likely that a low surface ECG R‐wave amplitude at implant or follow‐up due to underlying RV cardiomyopathy results in an unfavorable signal/noise ratio for appropriate signal processing and filtering. This may uniquely predispose this population to cardiac and/or myopotential oversensing and subsequent inappropriate therapy.
Potential Strategy to Avoid Inappropriate Shocks
First, identification of ARVC/D patients at risk might provide an opportunity to minimize the occurrence of inappropriate shocks. Although not statistically significant because of small numbers, probands with S‐ICD implantation for secondary prevention with concomitant significant RV structural abnormalities were more likely to experience inappropriate shocks. It is conceivable that a unique combination of this clinical phenotype would be at a higher risk for future inappropriate shocks. Second, the 2‐incision intermuscular technique has been recently shown to be a safe and effective alternative to the standard technique and may help to reduce complications, including inappropriate interventions.21 Third, noninvasive reprogramming (gain or vector changes) were successful in 50% of the patients in this study. Consequently, it may be desirable to have at least 2 or 3 leads suitable in the S‐ICD screening template in ARVC/D patients to facilitate future management of potential device‐related oversensing. Fourth, postoperative follow‐up visits should include repetitive and rhythmic activity simulation to unmask myopotential oversensing. This may help provide adequate counsel to patients and subsequently reduce future psychological stress. Fifth, targeting a surface ECG R‐wave amplitude >1 mV at implant may allow for better discrimination. In a case report of S‐ICD repositioning,22 1‐mV R waves led to an acceptable R wave/noise ratio. Furthermore, it will also be important to track the sensed R‐wave amplitude in various vectors on follow‐up. Finally, SMART Pass is a recently introduced filter that has been reported to reduce oversensing.23 Our simulation data suggest that many ARVC/D patients with T/P oversensing would benefit from this technology, and enabling this feature along with future software updates may reduce the likelihood of inappropriate therapy.
Limitations
Inclusion in this study required screening success, and the proportion of patients who failed ECG screening cannot be ascertained. In addition, programming and intraoperative management of patients, such as surgical technique and conversion testing, were not prescriptive. The roles of preimplantation ECG and surface QRS amplitude to screen for potential future oversensing issues require further evaluation in larger cohorts. Similarly, small sample size precludes accurate statistical comparison. The comparison to a TV‐ICD population3 is limited by variable clinical phenotype, absence of matching, and small numbers. Larger prospective studies with a predefined comparable TV‐ICD cohort would be needed to accurately define the net clinical benefit or harm from device choice because the current study involves small numbers. Despite these limitations, the data presented are unique in several ways and make an important contribution to the limited published data regarding the clinical performance of these devices in patients with ARVC/D.
Conclusion
In this first systematic analysis of S‐ICD performance in the ARVC/D population, pooled data from 29 patients show that the S‐ICD can effectively treat both induced and spontaneous VT/VF. Of note is the lack of significant complications requiring intervention. Although the rate of inappropriate shocks is considerable, it is comparable to patients treated with the TV‐ICD. When they occurred, inappropriate shocks were primarily caused by cardiac and, uniquely, noncardiac oversensing. We suggest potential strategies for minimizing inappropriate therapy with the application of careful patient selection, R‐wave amplitude screening at implant, and provocative testing at follow‐up and provide proof of the utility of the SMART Pass filter.
Sources of Funding
The authors wish to acknowledge funding from the Dr Francis P. Chiaramonte Private Foundation, the St. Jude Medical Foundation, and Boston Scientific Corp (for ICD studies only), and the Leducq Foundation–RHYTHM Network (all to Calkins). The Johns Hopkins arrhythmogenic right ventricular cardiomyopathy/dysplasia program is supported by the Leyla Erkan Family Fund for ARVD (arrhythmogenic right ventricular dysplasia) Research, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella Family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Disclosures
The Johns Hopkins arrhythmogenic right ventricular cardiomyopathy/dysplasia program receives research support from Boston Scientific. Dr Calkins has received honoraria for lectures from Boston Scientific and is a consultant to Medtronic. The remaining authors have no disclosures to report.
Supporting information
Table S1. Clinical Phenotype of the 2 Patients With Noninducible Ventricular Fibrillation at DFT Testing on Implant
Table S2. Clinical Features of Patients With Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia Experiencing Appropriate Subcutaneous Implantable Cardioverter‐Defibrillator Therapy
Table S3. Clinical Characteristics and Course of the 6 Patients that Received Inappropriate Shocks
Figure S1. Age distribution of patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia that received an subcutaneous implantable cardioverter‐defibrillator for prevention of sudden cardiac death.
Figure S2. Surface ECG images depicting appropriate subcutaneous implantable cardioverter‐defibrillator therapy.
Figure S3. Surface ECG tracing from 3 patients without inappropriate shocks during follow‐up with ≥1 mV R wave on implant (best vector).
Figure S4. Surface ECG tracing from 3 patients with <1‐mV R wave on implant (best vector) and inappropriate shocks due to myopotential oversensing on follow‐up.
Figure S5. Surface 12‐lead ECG from a patient with myopotential oversensing–related inappropriate shock showing low precordial and limb lead QRS amplitude.
Figure S6. A and B, Simulation analysis provided by Boston Scientific where SMART Pass filter was observed to have eliminated charging during episodes of putative movement artifact. C and D, SMART Pass was observed to have relatively limited improvement (if any) in these 3 particular episodes. SMART Pass is not expected to resolve these particular characteristics (P waves or myopotential noise). Of note, the episodes did not simulate exactly as observed in the field. The device memory episodes are stored in 0.25 resolution of actual/event surface ECG. In presence of noise especially, this can cause slight variations in simulated vs actual episode behavior.
Acknowledgments
We are grateful to the arrhythmogenic right ventricular cardiomyopathy/dysplasia patients and families who made this work possible. We thank Steven Donnelley and the Boston Scientific engineering department for their support in the simulation analysis of the SMART Pass filter.
(J Am Heart Assoc. 2018;7:e008782 DOI: 10.1161/JAHA.118.008782.)
References
- 1. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NM III, McKenna WJ, Thiene G, Marcus FI, Calkins H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, Bhonsale A, Kamel IR, Zimmerman SL, Judge DP, Crosson J, Tandri H, Calkins H. Implantable cardioverter‐defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. J Am Heart Assoc. 2017;6:6 DOI: 10.1161/JAHA.117.006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter‐defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. [DOI] [PubMed] [Google Scholar]
- 5. Korte T, Köditz H, Niehaus M, Paul T, Tebbenjohanns J. High incidence of appropriate and inappropriate ICD therapies in children and adolescents with implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2004;27:924–932. [DOI] [PubMed] [Google Scholar]
- 6. Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA. An entirely subcutaneous implantable cardioverter‐defibrillator. N Engl J Med. 2010;363:36–44. [DOI] [PubMed] [Google Scholar]
- 7. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, Kremers M, Crozier I, Lee KL, Smith W, Burke MC. Safety and efficacy of a totally subcutaneous implantable‐cardioverter defibrillator. Circulation. 2013;128:944–953. [DOI] [PubMed] [Google Scholar]
- 8. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ, Theuns DA, Jordaens LJ, Wilde AA, Knops RE. The entirely subcutaneous implantable cardioverter‐defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939. [DOI] [PubMed] [Google Scholar]
- 9. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DAMJ, Boersma LVA, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 10. Lambiase PD, Gold MR, Hood M, Boersma L, Theuns DA, Burke MC, Weiss R, Russo AM, Kääb S, Knight BP. Evaluation of subcutaneous ICD early performance in hypertrophic cardiomyopathy from the pooled EFFORTLESS and IDE cohorts. Heart Rhythm. 2016;13:1066–1074. [DOI] [PubMed] [Google Scholar]
- 11. Rudic B, Tülümen E, Berlin V, Röger S, Stach K, Liebe V, El‐Battrawy I, Dösch C, Papavassiliu T, Akin I, Borggrefe M, Kuschyk J. Low prevalence of inappropriate shocks in patients with inherited arrhythmia syndromes with the subcutaneous implantable defibrillator single center experience and long‐term follow‐up. J Am Heart Assoc. 2017;6:10 DOI: 10.1161/JAHA.117.006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gold MR, Aasbo JD, El‐Chami MF, Niebauer M, Herre J, Prutkin JM, Knight BP, Kutalek S, Hsu K, Weiss R, Bass E, Husby M, Stivland TM, Burke MC. Subcutaneous implantable cardioverter‐defibrillator Post‐Approval Study: clinical characteristics and perioperative results. Heart Rhythm. 2017;14:1456–1463. [DOI] [PubMed] [Google Scholar]
- 13. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB, Neuzil P, Wilde AA, Carter N, Husby M, Lambiase PD, Knops RE. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol. 2015;195:126–133. [DOI] [PubMed] [Google Scholar]
- 15. Jarman JW, Lascelles K, Wong T, Markides V, Clague JR, Till J. Clinical experience of entirely subcutaneous implantable cardioverter‐defibrillators in children and adults: cause for caution. Eur Heart J. 2012;33:1351–1359. [DOI] [PubMed] [Google Scholar]
- 16. Brouwer TF, Yilmaz D, Lindeboom R, Buiten MS, Olde Nordkamp LR, Schalij MJ, Wilde AA, van Erven L, Knops RE. Long‐term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J Am Coll Cardiol. 2016;68:2047–2055. [DOI] [PubMed] [Google Scholar]
- 17. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NA III. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol. 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corzani A, Ziacchi M, Biffi M, Diemberger I, Martignani C, Boriani G. Inappropriate shock for myopotential over‐sensing in a patient with subcutaneous ICD. Indian Heart J. 2015;67:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Álvarez‐Acosta L, Romero‐Garrido R, Hernández‐Afonso J. Inappropriate defibrillator shock in a subcutaneous device secondary to repetitive muscle contractions. Rev Esp Cardiol (Engl Ed). 2014;67:496–498. [DOI] [PubMed] [Google Scholar]
- 20. Mugnai G, Tomei R, Dugo C, Tomasi L, Morani G, Vassanelli C. Implantable cardioverter‐defibrillators in patients with arrhythmogenic right ventricular cardiomyopathy: the course of electronic parameters, clinical features, and complications during long‐term follow‐up. J Interv Card Electrophysiol. 2014;41:23–29. [DOI] [PubMed] [Google Scholar]
- 21. Migliore F, Allocca G, Calzolari V, Crosato M, Facchin D, Daleffe E, Zecchin M, Fantinel M, Cannas S, Arancio R, Marchese P, Zanon F, Zorzi A, Iliceto S, Bertaglia E. Intermuscular two‐incision technique for subcutaneous implantable cardioverter defibrillator implantation: results from a Multicenter Registry. Pacing Clin Electrophysiol. 2017;40:278–285. [DOI] [PubMed] [Google Scholar]
- 22. Berne P, Viola G, Motta G, Marziliano N, Carboni V, Casu G. Changing place, changing future: repositioning a subcutaneous implantable cardioverter‐defibrillator can resolve inappropriate shocks secondary to myopotential oversensing. HeartRhythm Case Rep. 2017;3:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santini L, Pappalardo A, Schirripa V, Danisi N, Forleo GB, Ammirati F. Oversensing of an unexpected atrial flutter. A new tool to improve detection of supraventricular arrhythmias in subcutaneous implantable cardioverter‐defibrillators. HeartRhythm Case Rep. 2017; 3:286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Phenotype of the 2 Patients With Noninducible Ventricular Fibrillation at DFT Testing on Implant
Table S2. Clinical Features of Patients With Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia Experiencing Appropriate Subcutaneous Implantable Cardioverter‐Defibrillator Therapy
Table S3. Clinical Characteristics and Course of the 6 Patients that Received Inappropriate Shocks
Figure S1. Age distribution of patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia that received an subcutaneous implantable cardioverter‐defibrillator for prevention of sudden cardiac death.
Figure S2. Surface ECG images depicting appropriate subcutaneous implantable cardioverter‐defibrillator therapy.
Figure S3. Surface ECG tracing from 3 patients without inappropriate shocks during follow‐up with ≥1 mV R wave on implant (best vector).
Figure S4. Surface ECG tracing from 3 patients with <1‐mV R wave on implant (best vector) and inappropriate shocks due to myopotential oversensing on follow‐up.
Figure S5. Surface 12‐lead ECG from a patient with myopotential oversensing–related inappropriate shock showing low precordial and limb lead QRS amplitude.
Figure S6. A and B, Simulation analysis provided by Boston Scientific where SMART Pass filter was observed to have eliminated charging during episodes of putative movement artifact. C and D, SMART Pass was observed to have relatively limited improvement (if any) in these 3 particular episodes. SMART Pass is not expected to resolve these particular characteristics (P waves or myopotential noise). Of note, the episodes did not simulate exactly as observed in the field. The device memory episodes are stored in 0.25 resolution of actual/event surface ECG. In presence of noise especially, this can cause slight variations in simulated vs actual episode behavior.


