Abstract
Background
The prognostic interrelationship between natriuretic peptide (NP) levels and body mass index (BMI) among patients with chronic stable heart failure with preserved ejection fraction is not well characterized.
Methods and Results
Participants from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial enrolled in the Americas meeting inclusion by the NP stratum were stratified into 4 data‐derived categories by BMI and standardized NP‐z score. Adjusted Cox‐proportional models determined the independent association of BMI, NP‐z score, and BMI/NP categories with composite primary end point, heart failure hospitalization, and all‐cause mortality. The study population included 997 participants. There was a U‐shaped relationship between BMI and NP with elevated NP levels noted at extremes of BMI distribution. There was also a U‐shaped relationship between BMI and risk of adverse clinical outcomes with the lowest risk among patients approximating a BMI of 25 kg/m2. In contrast, higher NP levels were linearly associated with higher risk of adverse clinical outcomes. For BMI/NP‐based categories, participants in the high BMI/high NP group had greater prevalence of cardiac structural and functional abnormalities and the highest risk of adverse clinical outcomes (hazard ratio for primary end point; 95% confidence interval: 2.29 [1.36–3.84] Reference: low BMI/low NP).
Conclusions
There is a U‐shaped association between BMI and NP levels among patients with chronic heart failure with preserved ejection fraction. Higher NP levels are independently associated with a higher risk of mortality across both high and low BMI strata. Among obese patients with heart failure with preserved ejection fraction, elevated NP levels identify a higher risk phenotype with a significantly increased incidence of both mortality and heart failure hospitalization.
Keywords: brain natriuretic peptide, heart failure, obesity
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
The prognostic interrelationship between natriuretic peptide levels and body mass index among patients with chronic stable heart failure with preserved ejection fraction is not well known.
Our study findings demonstrate a U‐shaped association between body mass index and natriuretic peptide levels among patients with chronic stable heart failure with preserved ejection fraction.
What Are the Clinical Implications?
Higher natriuretic peptide levels among obese patients with heart failure with preserved ejection fraction identifies a higher‐risk phenotype with a significantly increased risk of both mortality and HF hospitalization.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is common, increasing in prevalence, and associated with significant morbidity and mortality.1 Unlike HF with reduced ejection fraction, HFpEF has no clearly beneficial treatment.2 A major challenge to developing effective therapies for HFpEF is the heterogeneous nature of this disease process and the existence of distinct phenotypes that may require specific tailored therapies.3 Obesity is a well‐established risk factor for HFpEF, and recent studies have identified a distinct obesity phenotype in HFpEF that is associated with greater impairment in hemodynamic reserve.4, 5, 6 However, large‐scale observational studies have reported the phenomenon of an obesity paradox whereby obese patients with HFpEF have better long‐term survival than normal‐to‐overweight patients.7, 8, 9 This discordance in the hemodynamic and clinical implications of obesity in HFpEF highlights the need to better understand the heterogeneity within this phenotype and identify the highest‐risk obese patients.
Elevation in NP levels is a surrogate measure of hemodynamic stress in patients with HFpEF.10, 11 However, the contribution of elevation in NP levels toward clinical outcomes across different body mass index (BMI) categories is not known. This is particularly relevant considering the previously reported inverse association between BMI and NP levels.12 Against this background, we sought to determine the interrelationship between BMI and NP levels for predicting the risk of adverse clinical outcomes among patients with HFpEF.8, 13 We hypothesize that elevated NP levels among obese patients with chronic HFpEF will identify the subset that have the highest risk of adverse clinical outcomes such as HF hospitalization and death.
Methods
Study Design and Population
The present study was done as a secondary analysis of the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial using a publicly released version of the trial database obtained from the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) via an approved proposal. The present study does not necessarily reflect the opinions and views of the TOPCAT investigators or the National Heart, Lung, and Blood Institute. The authors will not make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure. TOPCAT was a multicenter, randomized, double‐blind, placebo‐controlled trial that evaluated the effects of spironolactone in patients with symptomatic HFpEF. The details about the study design, rationale, inclusion criteria, and primary findings have been reported before.14, 15 Briefly, the trial included patients older than 50 years with signs and symptoms of heart failure, left ventricular ejection fraction >45%, controlled systolic blood pressure, serum potassium levels <5 mmol/L, serum creatinine <2.5 mg/dL, and who fulfilled at least 1 of the following inclusion criteria: (1) history of hospitalization for HF within the past 12 months; or (2) brain natriuretic peptide (BNP) ≥100 pg/mL or an N‐terminal‐proBNP (NT‐proBNP) ≥360 pg/mL within 60 days before randomization. The study included 3445 participants from 233 sites across the United States, Canada, South America (n=1767 participants), and Europe (Russia and Georgia, n=1678 participants). Each site approved the protocols and all patients signed informed consent before randomization. The present analysis was limited to participants enrolled from centers in the Americas. This was done because of the previously reported heterogeneity in clinical characteristics, outcome event rates, and physiological response to spironolactone between the patients enrolled in Americas versus Russia and Georgia.16, 17, 18 We also excluded participants who would not have met inclusion in the NP stratum and with missing BMI and BNP/NT‐ProBNP levels at baseline. The final study population included 997 participants. The Institutional Review Board at the University of Texas Southwestern Medical Center, Dallas, Texas, approved the present analysis.
Primary Exposure Variables of Interest
Primary exposure variables of interest for the present analysis were BMI and serum BNP/NT‐ProBNP levels. BMI at baseline was calculated as the ratio of weight in kilograms and height squared (meter2). The data on BNP or NT‐ProBNP levels at the baseline were obtained from the case report forms as reported previously.19
Outcomes of Interest
Consistent with the primary trial, the primary outcome of interest for the present study was the composite of cardiovascular death, HF hospitalization, or aborted cardiac arrest. Secondary outcomes analyzed were all‐cause death and HF hospitalization. As described previously,14 HF hospitalization was defined as an unexpected presentation to an acute care facility requiring an overnight (change in calendar day) hospitalization with exacerbation of HF meeting the criteria with at least 1 symptom (worsening dyspnea, orthopnea, paroxysmal nocturnal dyspnea, increased fatigue/exercise intolerance) and at least 1 sign (peripheral edema, elevated jugular venous pressure, radiological signs of HF, ascites, pulmonary edema, rales, rapid weight gain) for HF. The clinical outcomes committee, using prespecified protocols, adjudicated the outcome events independently.15
Echocardiographic Measurements
A subset of the study participants had echocardiographic assessment done at baseline. The details about the echocardiographic methods and baseline echocardiographic characteristics have been published before.15, 20
Statistical Analysis
Because NPs were measured as either BNP or NT‐proBNP, a single combined, standardized log‐transformed NP Z‐score was calculated as previously reported.19 The study participants were stratified into data‐derived quartiles of BMI and NP‐Z scores and grouped into the following 4 categories: low BMI low NP (BMI and NP Z‐score below median); low BMI‐high NP (BMI below median and NP Z‐score above median); high BMI‐low NP (BMI above median and NP Z‐score below median); and high BMI‐high NP (BMI and NP Z‐score above median). Baseline demographic, clinical, and echocardiographic characteristics were reported across the 4 study groups with continuous variables represented by median and 25th to 75th percentile and categorical as n (%). Differences in baseline characteristics across the BMI/NP categories were tested via the Kruskal‐Wallis or Pearson's χ2 tests. Kaplan–Meier estimates of the time‐to‐event outcomes were generated to compare the unadjusted risk of primary and secondary clinical end points. Differences in failure curves were compared via the log‐rank test. Adjusted associations between the exposure variables of interest and key clinical outcomes were assessed using multivariable adjusted Cox models. The proportional hazards assumption was tested using scaled Schoenfeld residuals. However, the proportional hazards assumption was not met over the entire follow‐up duration, with a stronger association between BMI/NP and outcomes early on during follow‐up. As a result, consistent with a previously reported approach in this data set, this analysis was limited to the first 2 years of follow‐up to satisfy the proportional hazards assumption.21 Separate models were constructed for continuous measures of BMI and NP Z‐scores (both in the same model) and the BMI/NP Z‐score categories (High BMI/Low NP, Low BMI/High NP, High BMI/High NP versus Low BMI/Low NP [referent group]). Linearity with respect to the outcome was assessed by visual inspection of each variable's risk relationship plotted as a fractional polynomial. If nonlinear, the variables were accordingly transformed. For example, the association between continuous measures of BMI and risk of primary composite and secondary outcomes was nonlinear and it was included in the Cox model as a linear spline with knot at 25 kg/m2. Following a priori selected variables were included in the Cox models for multivariable adjustment: sex, blood urea nitrogen levels, systolic blood pressure (linear spline with knot at 140 mm Hg), New York Heart Association class, sodium level, history of atrial fibrillation, and history of diabetes mellitus. Interaction testing was also performed to determine the impact of treatment assignment (spironolactone versus placebo) on the risk of clinical outcomes across different BMI/NP groups. For the models evaluating the associations of BMI and NP levels (continuous as well as BMI/NP groups) with risk of HF hospitalization, competing risk analysis was performed accounting for death as a competing risk in the updated Cox models using the Fine and Grey approach of survival regression modeling with cumulative incidence and subdistribution hazard functions.22
Several sensitivity analyses were performed to assess the robustness of our study findings. First, the association of BMI and NP Z‐score‐based categories with the risk of primary composite outcome was assessed using alternative data‐derived tertiles‐based cutoffs to define high and low BMI/NP groups as follows: low BMI low NP (BMI tertile 1 and NP Z‐score tertile 1); low BMI‐high NP (BMI tertile 1 and NP Z‐score tertile 3); high BMI‐low NP (BMI tertile 3 and NP Z‐score tertile 1); and high BMI‐high NP (BMI and NP Z‐score both tertile 3). Second, sensitivity analysis was also performed including participants enrolled from European centers to evaluate the association between BMI, NP levels, and risk of primary composite end point. Finally, the association between waist circumference (WC), a measure of central adiposity, and the risk of adverse clinical outcomes after adjustment for potential confounders including NP levels, was also assessed using multivariable adjusted Cox models as described above. Two‐sided P<0.05 were considered statistically significant. Statistical analyses were performed using Stata version 13.1 software (Stata Corp LP, College Station, TX).
Results
The final study population included 997 patients with both BMI and NP values at baseline. The distribution of NP Z‐scores across different BMI levels in the study population are shown in Figure 1. There was a nonlinear U‐shaped relationship between BMI (median BMI: 31.9 kg/m2 [interquartile range: 27.2–37.4]) and NP levels (median NP Z‐score: −0.16 [interquartile range: −0.82 to 0.66]; median BNP [n=659]: 267 [interquartile range: 157–459]; median NT‐ProBNP [n=341]: 984 [631–2054]). The lowest NP levels were associated with an approximate BMI of 35 (kg/m2) and NP levels increased from there towards both extremes of the BMI spectrum. The distribution of absolute measures of NT‐ProBNP and BNP levels across BMI categories are shown in Figure S1.
Figure 1.
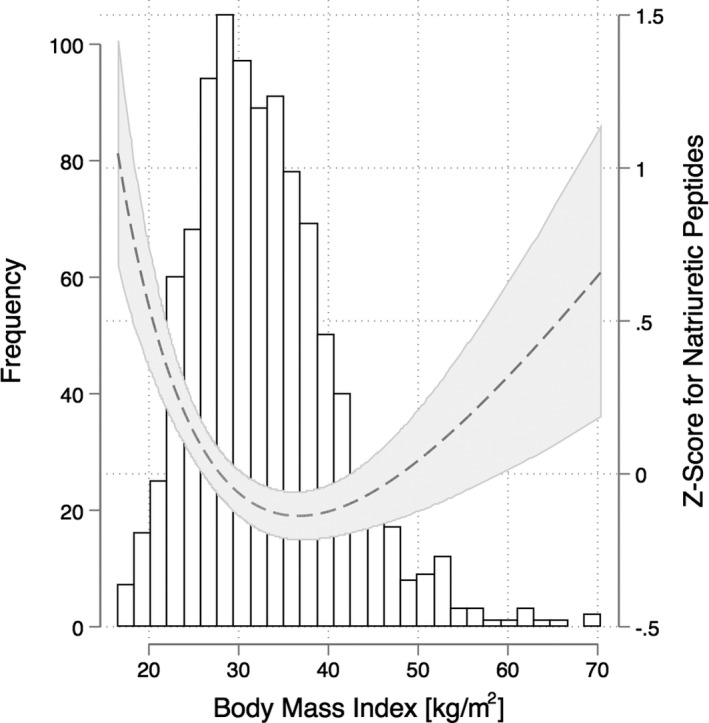
Association between body mass index and natriuretic peptide Z‐score in the study population.
The baseline characteristics of study participants across the 4 BMI/NP‐based groups are shown in Table 1. Participants with higher BMI were younger, more commonly black, had higher blood pressure levels, and higher prevalence of diabetes mellitus irrespective of the NP strata. The prevalence of atrial fibrillation was higher in both high BMI as well as high NP groups as compared with the low BMI/low NP group. Baseline characteristics of the study participants across BMI and NP Z‐score quartiles are shown in Table 2.
Table 1.
Baseline Characteristics of the Study Participants Stratified by BMI and Standardized NP Z‐Score
| Low BMI/Low NP (N=236) | Low BMI/High NP (N=263) | High BMI/Low NP (N=263) | High BMI/High NP (N=235) | P Value | |
|---|---|---|---|---|---|
| Age, y | 77 (71–82) | 78 (71–83) | 68 (62–77) | 69 (63–76) | 0.0001 |
| Males, % | 50.8 | 52.1 | 49 | 56.6 | 0.38 |
| Race, % | |||||
| White | 84.7 | 79.8 | 77.6 | 74.5 | 0.007 |
| Black | 9.3 | 12.9 | 18.6 | 20.8 | |
| Others | 5.9 | 7.2 | 18.5 | 4.7 | |
| BMI, kg/m2 | 27.3 (3.0) | 26.3 (3.6) | 38.9 (6.4) | 39.2 (6.3) | 0.0001 |
| SBP, mm Hg | 126 (118–138) | 124 (114–138) | 130 (118–140) | 130 (118–139) | 0.07 |
| HR, bpm | 64 (56–72) | 67 (60–76) | 67 (60–75) | 67 (60–75) | 0.003 |
| Cr, mg/dL | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) | 1.2 (1.0–1.5) | 0.0001 |
| BUN, mg/dL | 21 (17–29) | 25 (20–34) | 22 (17–30) | 24.0 (17–35) | 0.0005 |
| BNP, pg/mL (N=656) | 156 (133–203) | 494 (348–725) | 156 (128–202) | 425 (323–623) | 0.0001 |
| NT‐ProBNP, pg/mL (N=341) | 636 (475–815) | 2258 (1628–3814) | 618 (468–784) | 1937 (1336–2833) | 0.0001 |
| β‐Blockers, % | 75.4 | 81.7 | 80.6 | 86.4 | 0.02 |
| Diuretic, % | 78.8 | 90.1 | 90.9 | 94 | 0.0001 |
| ACEi/ARB, % | 68.2 | 73.3 | 81.8 | 82.5 | 0.0001 |
| Warfarin, % | 29.7 | 43 | 36.5 | 38.7 | 0.02 |
| Diabetes mellitus, % | 27.2 | 31.2 | 54 | 60.4 | <0.0001 |
| Afib, % | 38.7 | 52.8 | 43.3 | 50.6 | 0.005 |
| Revasc (%) CABG | 21.3 | 21.3 | 20.1 | 21.3 | 0.98 |
| PCI | 19.5 | 19.0 | 23.6 | 24.7 | 0.31 |
Study groups are defined based on the median cutoffs for BMI and NP Z‐score (median BMI: 31.95 kg/m2, median NP Z‐score: −0.16): low BMI low NP (BMI and NP Z‐score below median); low BMI‐high NP (BMI below median and NP Z‐score above median); high BMI‐low NP (BMI above median and NP Z‐score below median); and high BMI‐high NP (BMI and NP Z‐score above median). ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; Afib, atrial fibrillation; BMI, body mass index; BNP, brain natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; Cr, creatinine; HR, heart rate; NP, natriuretic peptide; NT‐ProBNP, N‐terminal brain natriuretic peptide; PCI, percutaneous coronary interventions; Revasc, revascularization; SBP, systolic blood pressure.
Table 2.
Baseline Characteristics of the Study Participants Across BMI and NP Z‐Score Quartiles
| Body Mass Index Quartiles (BMI, kg/m2) | Natriuretic Peptides (NP) Z‐Score Quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (N=250) | Q2 (N=249) | Q3 (N=249) | Q4 (N=249) | P Value | Q1 (N=249) | Q2 (N=249) | Q3 (N=250) | Q4 (N=250) | P Value | |
| Age, y | 76.9 (9.2) | 74.8 (8.8) | 71.3 (8.8) | 67.3 (8.4) | 0 | 70.7 (9.3) | 73.2 (9.5) | 73.2 (9.2) | 73.3 (9.3) | 0.004 |
| Males (%) | 48 | 55 | 58.6 | 46.6 | 0.97 | 48.8 | 50.8 | 59.2 | 49.2 | 0.49 |
| Race (%) | ||||||||||
| White | 78.4 | 85.9 | 81.1 | 71.1 | 79.2 | 82.4 | 79.2 | 75.2 | ||
| Black | 12 | 10.4 | 13.6 | 25.7 | 0.06 | 17.2 | 11.6 | 16 | 17.2 | 0.15 |
| Others | 9.6 | 3.6 | 5.2 | 3.2 | 3.6 | 6 | 4.8 | 7.6 | ||
| BMI, kg/m2 | 24.0 (2.4) | 29.6 (1.4) | 34.5 (1.6) | 43.6 (6.0) | 33.7 (7.6) | 33.2 (7.8) | 32.7 (7.1) | 32.0 (9.1) | 0.002 | |
| SBP, mm Hg | 124 (16) | 128 (14) | 129 (15) | 128 (16) | 0.003 | 128 (14) | 127 (16) | 126 (17) | 127 (16) | 0.45 |
| HR, bpm | 68 (13) | 66 (12) | 68 (12) | 68 (11) | 0.24 | 66 (13) | 67 (12) | 68 (12) | 69 (12) | 0.0008 |
| Cr, mg/dL | 1.1 (0.32) | 1.2 (0.32) | 1.2 (0.35) | 1.2 (0.35) | 0.04 | 1.1 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | <0.0001 |
| BUN, mg/dl | 27 (12) | 25 (12) | 27 (13) | 26 (14) | 0.2 | 23.4 (10.2) | 26.0 (13.1) | 26.1 (12.1) | 29.7 (14.9) | <0.0001 |
| BNP, pg/mL (N=656) | 506 (591) | 368 (359) | 344 (319) | 372 (369) | 0.01 | 130 (16.6) | 208 (29) | 350 (59) | 898 (596) | <0.0001 |
| NT‐ProBNP, pg/mL (N=341) | 2263 (2341) | 1444 (1387) | 1215 (1031) | 1853 (2455) | 0.03 | 461 (66) | 801 (124) | 1453 (275) | 4050 (2539) | <0.0001 |
| Afib (%) | 44.0 | 48.4 | 51.0 | 42.6 | 0.91 | 18.1 | 26.1 | 26.3 | 29.5 | <0.0001 |
| BB (%) | 76.8 | 80.7 | 84.3 | 82.3 | 0.07 | 79.2 | 77.2 | 80 | 87.6 | 0.01 |
| Diuretic (%) | 82.8 | 86.7 | 90.8 | 94 | <0.0001 | 82.4 | 88 | 92.8 | 91.2 | 0.001 |
| ACEi/ARB (%) | 69.6 | 72.3 | 79.9 | 84.3 | <0.0001 | 73.6 | 77.2 | 77.6 | 78 | 0.25 |
| Warfarin (%) | 33.2 | 40.2 | 39.8 | 35.3 | 0.66 | 28.4 | 38 | 41.6 | 40 | 0.005 |
ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; Afib, atrial fibrillation; BB, Beta‐blockers; BMI, body mass index; BNP, brain natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; Cr, creatinine; HR, heart rate; NP, natriuretic peptide; NT‐ProBNP, N‐terminal brain natriuretic peptide; SBP, systolic blood pressure.
Table 3 compares the echocardiographic characteristics of the subset of study participants with available baseline echocardiographic data (n=365). Left ventricular (LV) end diastolic volume was higher in the higher BMI categories irrespective of the NP strata and the LA volume was higher in the higher NP categories irrespective of the BMI strata. The measures of LV mass, diastolic function, and systolic function were significantly different across the BMI/NP categories. Participants in the high BMI/high NP group had the highest median values for LV mass and E/e’. Furthermore, median EF and global longitudinal strain were lowest in the high BMI/high NP group.
Table 3.
Echocardiographic Characteristics of the Study Participants Stratified by BMI and NP Z‐Score
| Low BMI/Low BNP (N=82) | Low BMI/High BNP (N=95) | High BMI/Low BNP (N=91) | High BMI/High BNP (N=97) | P Value | |
|---|---|---|---|---|---|
| LV end diastolic dimension, cm | 4.59 (4.26–5.01) | 4.55 (4.11–5.06) | 4.97 (4.53–5.28) | 5.04 (4.69–5.25) | 0.0001 |
| LVEDV, mL | 80.9 (64.2–100.7) | 82.9 (67.9–101.7) | 100.2 (74.9–122.5) | 103.9 (83.2–125.4) | 0.0001 |
| LVEDV indexed | 35.6 (27.6–41.9) | 34.0 (27.8–41.6) | 40.2 (33.4–50.5) | 44.2 (34.7–52.2) | 0.0001 |
| LV mass, g | 189 (149–230) | 193 (159–240) | 226 (186–279) | 248 (201–284) | 0.0001 |
| LV mass indexed | 77.0 (65.5–92.4) | 80.0 (63.3–97.4) | 91.4 (78.4–114.0) | 101.4 (84.3–114.3) | 0.0001 |
| LA volume, mL | 54.0 (42.8–70.5) | 63.1 (43.9–80.1) | 56.3 (45.6–82.0) | 64.1 (52.4–81.7) | 0.06 |
| LA volume indexed | 23.0 (17.8–32.5) | 25.9 (18.2–32.8) | 24.0 (18.6–32.3) | 25.5 (21.9–32.7) | 0.09 |
| E/a lateral | 1.15 (0.88–1.58) | 1.23 (0.77–2.21) | 1.10 (0.92–1.54) | 1.39 (1.16–1.98) | 0.006 |
| Ejection fraction (%) | 61 (56–65) | 60 (56–64) | 62 (58–67) | 58 (54–63) | 0.001 |
| e/e′ lateral | 11.4 (8.5–14.1) | 11.0 (7.4–15.0) | 10.6 (8.4–15.9) | 13.1 (9.8–17.0) | 0.13 |
| Relative wall thickness | 0.46 (0.42–0.51) | 0.49 (0.44–0.57) | 0.48 (0.42–0.54) | 0.49 (0.45–0.54) | 0.02 |
| GLS, s−1 | −15.4 (−18.5 to 13.3) | −15.3 (−18.0 to 12.3) | −17.4 (−18.6 to 13.6) | −14.8 (−16.5 to 13.2) | 0.14 |
| RV end diastolic area, cm2 | 18.3 (15.9–21.2) | 18.3 (15.6–22.4) | 20.7 (17.8–26.7) | 22.3 (17.8–27.2) | 0.0001 |
| PVR (wood units) | 1.7 (1.4–2.2) | 2.1 (1.6–2.4) | 1.7 (1.3–1.9) | 1.9 (1.5–2.1) | 0.02 |
| Deceleration Time, s | 195 (165–250) | 188 (140–240) | 197 (163–230) | 197 (150–220) | 0.33 |
Study groups are defined based on the median cutoffs for BMI and NP Z‐score (median BMI: 31.95 kg/m2, median NP Z‐score: −0.16): low BMI low NP (BMI and NP Z‐score below median); low BMI‐high NP (BMI below median and NP Z‐score above median); high BMI‐low NP (BMI above median and NP Z‐score below median); and high BMI‐high NP (BMI and NP Z‐score above median). BMI indicates body mass index; BNP, brain natriuretic peptide; GLS, global longitudinal strain; LA, left atrium; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; PVR, pulmonary vascular resistance; RV, right ventricle.
There were 199 primary end point events, 161 HF hospitalization events, and 111 all‐cause mortality events during the 2‐year follow‐up period. There was a nonlinear relationship between continuous measures of BMI and risk of adverse clinical outcomes; therefore, a linear spline with a knot at 25 kg/m2 was used to model BMI (Figure 2, Table 4). At levels below 25 kg/m2, BMI was not associated with the primary composite end point, but a significant association was noted between higher BMI and lower risk of all‐cause mortality. In contrast, at levels of 25 kg/m2 or above, higher BMI was associated with a higher risk of primary composite end point largely driven by a significantly increased risk of HF hospitalization but not all‐cause mortality (Table 4). For NP levels, there was a linear association between increasing NP Z‐score values and risk of primary composite and secondary clinical end points. One SD higher NP Z‐score was associated with 28% higher risk of primary composite end point, 29% higher risk of HF hospitalization, and 43% higher risk of all‐cause mortality (Table 4, Figure S2). The association of BMI and NP levels with the risk of primary composite event in sensitivity analysis including participants from both Americas and Europe was consistent with that observed in the primary analysis (Table S1). In Cox models substituting continuous measures of BMI with WC, a nonlinear relationship was observed between continuous measures of WC and the risk of adverse clinical outcomes (Figure S3). In adjusted analysis, WC was not associated with risk of primary composite event or HF hospitalization. However, a significant association was noted between higher WC, above the threshold of 100 cm, and risk of all‐cause death (hazard ratio [95% confidence interval] per 10‐cm higher WC: 1.27 [1.107–1.50]).
Figure 2.

Fractional polynomial plot showing continuous association between body mass index and risk of primary composite end point (A), heart failure hospitalization (B), and all‐cause mortality (C).
Table 4.
Adjusted Association of Continuous Measures of BMI and NP Z‐Score With Outcomes
| Primary End Point (N=199) | HF Hospitalization (N=161) | All‐Cause Mortality (N=111) | ||||
|---|---|---|---|---|---|---|
| HRa (95% CI) | P Value | HRa (95% CI) | P Value | HRa (95% CI) | P Value | |
| Per 1 SD higher log NP Z‐score | 1.28 (1.10–1.49) | 0.001 | 1.29 (1.10–1.52) | 0.002 | 1.43 (1.15–1.77) | 0.001 |
| Per 5‐unit higher BMI (below 25 kg/m2)a | 0.68 (0.31–1.48) | 0.33 | 0.87 (0.34–2.23) | 0.77 | 0.36 (0.16–0.79) | 0.01 |
| Per 5‐unit higher BMI (≥25 kg/m2) | 1.15 (1.03–1.29) | 0.01 | 1.25 (1.12–1.40) | 0.005 | 1.12 (0.95–1.33) | 0.20 |
Adjusted models includes age, sex, blood urea nitrogen, systolic blood pressure (linear spline with knot at 140 mm Hg), New York Heart Association class, sodium level, history of atrial fibrillation, history of diabetes mellitus, BMI (linear spline with knot at 25 kg/m2), and log NP Z‐score. BMI indicates body mass index; CI, confidence interval; HF, heart failure; HR, hazard ratio; NP, natriuretic peptide.
HR are reported per 5‐unit higher measure of BMI levels above and below the spline knot of 25 kg/m2.
Cumulative incidence estimates for each time‐to‐event end point are shown in Figure 3. The high BMI/high NP category had the highest cumulative incidence of the primary end point (Figure 3A) and HF hospitalization (Figure 3B). In adjusted analyses, the high BMI/high NP category had a >2‐fold increased risk of both the primary end point and HF hospitalization in comparison to the low BMI/low NP category (Table 5). The 2 other categories, however, were not associated with a higher risk of primary composite end point or HF hospitalization. For all‐cause mortality, both the low BMI/high NP and high BMI/high NP categories had a higher cumulative incidence than the other 2 BMI/NP categories (Figure 3C). In adjusted analyses, the low BMI/high NP and high BMI/high NP categories were associated with ≈3‐fold increased risk of all‐cause mortality (Table 5). Among other clinical factors, male sex and systolic blood pressure were significantly associated with risk of all‐cause mortality (Table S2).
Figure 3.
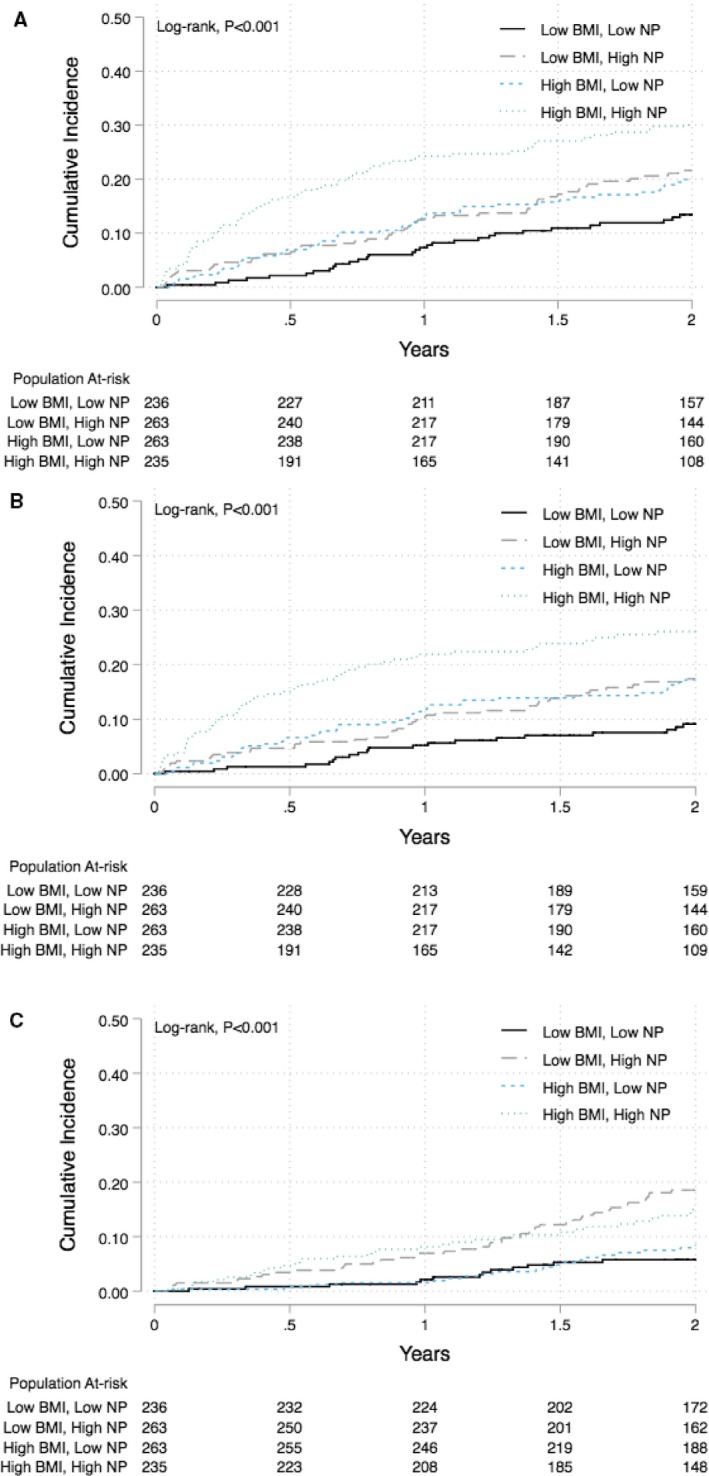
Cumulative incidence curves comparing the risk of primary outcome events (A), heart failure hospitalization (B), and all‐cause mortality (C) across different BMI/NP groups based on their median cutoffs (median BMI: 31.95 kg/m2, median NP Z‐score: −0.16): low BMI low NP (BMI and NP Z‐score below median [average BMI: 27.3 kg/m2, average BNP/NT‐ProBNP levels: 156/636 pg/mL]); low BMI‐high NP (BMI below median and NP Z‐score above median [average BMI: 26.3 kg/m2, average BNP/NT‐ProBNP: 494/2258 pg/mL]); high BMI‐low NP (BMI above median and NP Z‐score below median [average BMI: 38.9 kg/m2 pg/mL, average BNP/NT‐ProBNP: 156/618 pg/mL]); and high BMI‐high NP (BMI and NP Z‐score above median [average BMI: 39.2 kg/m2, average NP Z‐score Q 3,4 [(median BNP/NT‐ProBNP: 425/1937 pg/mL]). BMI indicates body mass index; NP, natriuretic peptide; NT‐ProBNP, N‐terminal pro‐brain natriuretic peptide.
Table 5.
Adjusted Risk of Clinical Outcomes Associated With Different BMI and NP Z‐Score‐Based Groups
| Primary End Point | HF Hospitalization | All‐Cause Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | HR (95% CI) | P Value | No. of Events | HR (95% CI) | P Value | No. of Events | HR (95% CI) | P Value | |
| Low BMI/low NP (N=234) | 30 | Ref. | 20 | Ref. | 13 | Ref. | |||
| Low BMI/high NP (N=263) | 52 | 1.38 (0.81–2.33) | 0.23 | 41 | 1.35 (0.74–2.46) | 0.32 | 45 | 2.81 (1.33–5.96) | 0.007 |
| High BMI/low NP (N=263) | 49 | 1.33 (0.77–2.29) | 0.30 | 42 | 1.48 (0.83–2.64) | 0.17 | 20 | 1.95 (0.84–4.54) | 0.12 |
| High BMI/high NP (N=235) | 68 | 2.29 (1.36–3.84) | 0.002 | 58 | 2.41 (1.37–4.24) | 0.002 | 33 | 3.07 (1.40–6.79) | 0.005 |
Separate Cox model was constructed for each outcome and includes age, sex, blood urea nitrogen, systolic blood pressure (linear spline with knot at 140 mm Hg), New York Heart Association class, sodium level, history of atrial fibrillation, history of diabetes mellitus, BMI, Z‐log NP. Study groups are defined based on the median cutoffs for BMI and NP Z‐score (median BMI: 31.95 kg/m2, median NP Z‐score: −0.16): low BMI low NP (BMI and NP Z‐score below median); low BMI‐high NP (BMI below median and NP Z‐score above median); high BMI‐low NP (BMI above median and NP Z‐score below median); and high BMI‐high NP (BMI and NP Z‐score above median). BMI indicates body mass index; CI, confidence interval; HF, heart failure; HR, hazard ratio; NP, natriuretic peptide.
There was no statistically significant interaction between BMI and NP levels for the risk of adverse clinical outcomes (P‐interaction >0.1). Furthermore, no significant interaction was observed between BMI/NP study groups and treatment with spironolactone for the risk of adverse clinical outcomes (P‐interaction >0.1 for all). In sensitivity analyses, using tertile‐based cutoffs to define high and low BMI/NP levels (tertile 1 as low and tertile 3 as high for both BMI and NP), the risk of primary composite events and mortality across the study groups was similar to that observed in the primary analysis (Figure S4).
Discussion
We observed several findings that are key to understanding the relationship between body habitus and natriuretic peptide and their impact on clinical outcomes in HFpEF. First, in a cohort of chronic stable patients with HFpEF, there was a U‐shaped association between BMI and NP levels, with elevated NP levels noted at the extremes of BMI distribution. Second, there was a U‐shaped relationship between BMI and the risk of adverse clinical outcomes with the highest risk at both the low and high BMI extremes. At the lower end of BMI distribution (BMI <25 kg/m2), increasing BMI was associated with lower risk of all‐cause mortality. In contrast, at the higher end of the BMI distribution (BMI ≥25 kg/m2), increasing BMI was associated with a significantly higher risk of HF hospitalization risk. Third, high NP levels were associated with a significantly higher risk of mortality across both BMI strata. Finally, among patients with higher BMI, elevated NP levels may identify more definitive abnormalities in cardiac structure and function and thus, are associated with the highest risk of adverse clinical outcomes, with significantly higher mortality as well as HF hospitalization risk. Whether phenotype‐specific treatment benefits this group is unknown and should be the subject of further investigation.
The present study is the first to our knowledge to evaluate the steady‐state relationship between BMI and NP levels among stable, chronic outpatients with HFpEF. Prior studies have evaluated the relationship between BMI and NP levels in patients with acute decompensated HFpEF and demonstrated a consistent inverse association between BMI and BNP, even at extremely high BMI levels.8, 13 Unlike these prior studies, however, we observed a U‐shaped distribution of NP levels across BMI categories among stable outpatients with HFpEF with higher NP levels noted at extremes of BMI. This difference in the observed relationship between BMI and NP levels in our study as compared with those reported previously may be related to the cohort‐specific differences. In contrast to those prior studies with heterogeneous cohorts of patients with decompensated HF, the TOPCAT study included patients with stable, chronic HFpEF patients with elevated NP levels at baseline. This is particularly relevant since NP levels may fluctuate in the short term with acute HF treatments, therefore not capturing what may be a more chronically perturbed hemodynamic state.
We also evaluated the interrelationship between NP levels, BMI, and risk of adverse clinical outcomes. Several prior studies have demonstrated an obesity paradox in patients with HFpEF such that higher BMI is associated with lower risk of mortality.7, 9, 23 However, the impact of BMI on nonfatal outcomes such as HF hospitalization is not well studied. Like these prior studies, the findings of the present study do suggest the presence of an obesity paradox for all‐cause mortality.24, 25 In contrast, however, the obesity paradox may not be relevant to nonfatal outcomes such as HF hospitalization. Specifically, higher BMI above the normal range (≥25 kg/m2) was associated with a higher risk of HF hospitalization, which was independent of NPs. Furthermore, consistent with prior findings, we failed to observe obesity paradox for measures of central adiposity such as WC with a significantly higher risk of mortality noted at higher levels of WC.23 Therefore, these findings support the hypothesis that obesity may contribute to higher risk of adverse clinical outcomes in patients with HFpEF.25, 26
We also observed that higher NP levels were consistently associated with an increased risk of all‐cause mortality independent of BMI. In contrast, higher BMI, above the normal range, was not associated with the risk of mortality independent of the NP levels and other risk factors. These findings highlight the primacy of NP over BMI levels for prognosis in HFpEF. Furthermore, among patients with higher BMI, the risk of adverse clinical outcomes, both mortality and HF hospitalization, was significantly increased only among those with elevated NP levels. In contrast, among high BMI patients with lower NP levels, the risk of adverse clinical outcomes was not different from the low BMI/low NP group. Taken together, among HFpEF patients with high BMI, elevated NP levels may identify a relatively higher‐risk subset of patients with increased risk of both fatal and nonfatal events over time. Phenotypically, this group has more echocardiographic evidence of perturbed cardiac function: more abnormal diastolic function, lower ejection fraction, and lower myocardial strain than the other BMI/NP strata. These findings are complementary to a recent study by Obakata et al,5 which demonstrated that obese HFpEF patients may have more severe hemodynamic abnormalities than their nonobese counterparts including increased plasma volume and greater LV and right ventricular filling pressure with exercise. It is therefore plausible that obese patients with elevated NP levels represent a unique subset of HFpEF patients with more severe hemodynamic impairments and thus, have worse long‐term outcomes.
These findings may have important clinical implications. Our study findings highlight the usefulness of elevated NP levels as an inclusion criterion to identify higher‐risk obese HFpEF patients for future clinical trials. This is particularly relevant considering the high burden of obesity among HFpEF patients. A recent study by Kitzman et al27 demonstrated that intentional weight loss and exercise training is associated with a synergistic improvement in exercise capacity and quality of life among patients with HFpEF. However, considering the resource‐intensive nature of these interventions, it may not be financially feasible to implement these in all obese patients with HFpEF. Because patients with higher BMI and NP are at a heightened risk of adverse clinical outcomes, these results may identify the highest‐risk HFpEF patients who are most likely to benefit from such interventions. However, whether such interventions translate into fewer HF hospitalizations and reduce other adverse clinical outcomes is unknown and should be the subject of future study.28
There are several limitations to our study. First, the NP levels used in the study were measured at clinical site laboratories, and we cannot exclude the possibility that there were issues related to site‐specific variability in its measurement. However, this would bias the study towards the null. Second, this analysis was limited to a subset of the TOPCAT participants from US/South America centers in our analysis and this may have introduced a selection bias, particularly since the majority of the study participants at these sites were enrolled based on elevated NP levels at baseline. Third, we do not have follow‐up BMI or BNP data available to determine how changes in these variables over time may modify long‐term outcomes. Fourth, there may be regional and racial/ethnic variability in the diagnostic thresholds and care‐seeking patterns for HF hospitalization that may have influenced the observed associations between BMI/NP levels and risk of HF hospitalizations. However, owing to the clinical trial setting, the definitions and criteria used for HF hospitalization were standardized and clinically adjudicated. Thus, the likelihood that regional or racial/ethnic variability in patterns of HF may have influenced the study outcomes substantially is low. Finally, considering the observational nature of this analysis, there is a potential for residual confounding in the observed associations.
In conclusion, among patients with chronic stable HFpEF, there is a U‐shaped association between BMI and NP levels with higher NP levels at both extremes of BMI distribution. Although there was evidence for an obesity paradox for all‐cause mortality, there was none for HF hospitalization because higher BMI levels were at higher risk. In contrast, high NP levels are consistently associated with increased risk of adverse cardiovascular events, particularly all‐cause mortality. Among patients with higher BMI, higher NP levels may identify a higher‐risk subset with more perturbed LV diastolic function, strain impairment, and an increased incidence of mortality and HF hospitalization.
Disclosures
Dr Pandey and Dr Grodin are funded by the Texas Health Resources research scholarship. Other authors have no disclosures relevant to this work.
Supporting information
Table S1. Association Among Body Mass Index, NP Z‐Score, and Risk of Primary Composite Outcome in the Study Cohort With Versus Without Participants From Europe
Table S2. Association of Baseline Demographic and Risk Factors With Risk of Mortality in the Study Cohort
Figure S1. Association between BMI and NT‐ProBNP (A) and BNP (B) levels in the study population.
Figure S2. Fractional polynomial plot showing continuous association between NP Z‐score and risk of primary composite end point.
Figure S3. Fractional polynomial plot showing continuous association between waist circumference and risk of primary composite end point, heart failure hospitalization, and all‐cause mortality.
Figure S4. Kaplan–Meier plot comparing the cumulative incidence of primary composite end point across different BMI/NP Z‐score based groups.
(J Am Heart Assoc. 2018;7:e009664 DOI: 10.1161/JAHA.118.009664.)
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 2. Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O'Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2014;2:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kitzman DW, Lam CSP. Obese heart failure with preserved ejection fraction phenotype: from pariah to central player. Circulation. 2017;136:20–23. [DOI] [PubMed] [Google Scholar]
- 5. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I‐PRESERVE) trial. Circ Heart Fail. 2011;4:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, Jneid H, Agarwal S, Chang PP, Loehr L, Basra SS, Rosamond W, Ballantyne CM, Deswal A. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: the Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol. 2017;233:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padwal R, McAlister FA, McMurray JJ, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C, Earle N, Whalley G, Poppe KK, Doughty RN; Bayes‐Genis A and Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta‐analysis of individual patient data. Int J Obes (Lond). 2014;38:1110–1114. [DOI] [PubMed] [Google Scholar]
- 10. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe S, Shite J, Takaoka H, Shinke T, Imuro Y, Ozawa T, Otake H, Matsumoto D, Ogasawara D, Paredes OL, Yokoyama M. Myocardial stiffness is an important determinant of the plasma brain natriuretic peptide concentration in patients with both diastolic and systolic heart failure. Eur Heart J. 2006;27:832–838. [DOI] [PubMed] [Google Scholar]
- 12. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. [DOI] [PubMed] [Google Scholar]
- 13. Stavrakis S, Pakala A, Thomas J, Chaudhry MA, Thadani U. Obesity, brain natriuretic peptide levels and mortality in patients hospitalized with heart failure and preserved left ventricular systolic function. Am J Med Sci. 2013;345:211–217. [DOI] [PubMed] [Google Scholar]
- 14. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972.e10. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; Investigators T . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 16. de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med. 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 18. Rossignol P, Zannad F. Regional differences in heart failure with preserved ejection fraction trials: when nephrology meets cardiology but east does not meet west. Circulation. 2015;131:7–10. [DOI] [PubMed] [Google Scholar]
- 19. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O'Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT Trial. JACC Heart Fail. 2017;5:241–252. [DOI] [PubMed] [Google Scholar]
- 20. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hegde SM, Claggett B, Shah AM, Lewis EF, Anand I, Shah SJ, Sweitzer NK, Fang JC, Pitt B, Pfeffer MA, Solomon SD. Physical activity and prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation. 2017;136:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc: 1999;94:496–509. [Google Scholar]
- 23. Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all‐cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–2749. [DOI] [PubMed] [Google Scholar]
- 24. Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez‐Jimenez F, Milani RV, Ventura HO. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400. [DOI] [PubMed] [Google Scholar]
- 25. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez‐Jimenez F, Arbab‐Zadeh A, Mukherjee D, Lazar JM. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 26. Lavie CJ, Milani RV, Ventura HO. Adipose composition and heart failure prognosis: paradox or not? J Am Coll Cardiol. 2017;70:2750–2751. [DOI] [PubMed] [Google Scholar]
- 27. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horwich TB, Broderick S, Chen L, McCullough PA, Strzelczyk T, Kitzman DW, Fletcher G, Safford RE, Ewald G, Fine LJ, Ellis SJ, Fonarow GC. Relation among body mass index, exercise training, and outcomes in chronic systolic heart failure. Am J Cardiol. 2011;108:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association Among Body Mass Index, NP Z‐Score, and Risk of Primary Composite Outcome in the Study Cohort With Versus Without Participants From Europe
Table S2. Association of Baseline Demographic and Risk Factors With Risk of Mortality in the Study Cohort
Figure S1. Association between BMI and NT‐ProBNP (A) and BNP (B) levels in the study population.
Figure S2. Fractional polynomial plot showing continuous association between NP Z‐score and risk of primary composite end point.
Figure S3. Fractional polynomial plot showing continuous association between waist circumference and risk of primary composite end point, heart failure hospitalization, and all‐cause mortality.
Figure S4. Kaplan–Meier plot comparing the cumulative incidence of primary composite end point across different BMI/NP Z‐score based groups.


