Abstract
Background
Oral anticoagulants (OACs) in patients with atrial fibrillation (AF), in addition to reducing stroke risk, could also prevent adverse cognitive outcomes. The purpose of this study was to compare the risk of dementia incidence across patients with AF initiating different OACs.
Methods and Results
We identified patients with nonvalvular AF initiating OACs in 2 US healthcare claim databases, MarketScan (2007–2015) and Optum Clinformatics (2009–2015). Dementia, comorbidities, and use of medications were defined on the basis of inpatient and outpatient claims. We performed head‐to‐head comparisons of warfarin, dabigatran, rivaroxaban, and apixaban in propensity score–matched cohorts. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) of incident dementia for each propensity score–matched cohort and meta‐analyzed database‐specific results. We analyzed 307 099 patients with AF from the MarketScan database and 161 346 from the Optum database, of which 6572 and 4391, respectively, had a diagnosis of incident dementia. The mean follow‐up of each cohort ranged between 0.7 and 2.2 years. Patients initiating direct OACs experienced lower rates of dementia than those initiating warfarin (dabigatran: HR, 0.85; 95% CI, 0.71–1.01; rivaroxaban: HR, 0.85; 95% CI, 0.76–0.94; apixaban: HR, 0.80; 95% CI, 0.65–0.97). There were no differences in rates of dementia comparing direct OAC user groups (dabigatran versus rivaroxaban: HR, 1.02; 95% CI, 0.79–1.32; dabigatran versus apixaban: HR, 0.92; 95% CI, 0.63–1.36; apixaban versus rivaroxaban: HR, 1.01; 95% CI, 0.86–1.19).
Conclusions
Patients with AF initiating direct OACs experienced lower rates of incident dementia than warfarin users. No obvious benefit was observed for any particular direct OAC in relation to dementia rates.
Keywords: atrial fibrillation, dementia, direct oral anticoagulant, warfarin
Subject Categories: Atrial Fibrillation
Clinical Perspective
What Is New?
This study evaluated the association between type of oral anticoagulant and incidence of dementia in a large population of anticoagulated patients with atrial fibrillation.
We found that rates of dementia were lower among patients initiating direct oral anticoagulants than among those initiating warfarin, without differences between specific direct oral anticoagulants.
The associations were consistent across age, sex, and baseline stroke risk groups.
The possibility of confounding by indication was addressed by propensity score matching and sensitivity analyses; nonetheless, the results should be interpreted with caution because of the nonrandomized study design.
What Are the Clinical Implications?
The study results provide evidence that can be used to inform decisions about the type of oral anticoagulant prescription in patients with atrial fibrillation in whom cognitive outcomes are of particular concern (eg, elderly patients and those with mild cognitive impairment).
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia in clinical practice.1 Because of its high prevalence and the high risk of associated complications, such as stroke,2 AF is a major contributor to the burden of cardiovascular diseases in both the United States and worldwide.3 In addition, growing evidence points to cognitive decline and dementia as additional outcomes associated with AF. An elevated stroke risk could partly mediate this association. Other mechanisms, such as repetitive cerebral injury attributable to lacunar infarcts or microbleeds and brain hypoperfusion, are likely to play a role, but are not well characterized.4, 5 Research on potential therapeutic targets to lower dementia risk is required to address this issue.
Oral anticoagulants (OACs) are recommended for stroke prevention in patients with nonvalvular AF at moderate or high stroke risk.6 This has been commonly achieved with vitamin K antagonists (ie, warfarin in the United States). Since October 2010, several direct OACs (DOACs), including dabigatran, rivaroxaban, apixaban, and edoxaban, have been approved by the US Food and Drug Administration for the prevention of stroke and systemic embolism among patients with nonvalvular AF on the basis of the results of large phase 3 randomized trials.7 Observational studies have generated results similar to randomized trials, showing noninferiority of DOACs versus warfarin for stroke prevention, and have provided evidence of DOAC effectiveness in usual clinical practice.8, 9, 10, 11 Limited additional evidence suggests an association between risk of dementia and type of OAC (DOACs versus warfarin) in patients with AF. A previous study in 5254 anticoagulated patients, managed by the Intermountain Healthcare Clinical Pharmacist Anticoagulation Service in Utah, documented a lower rate of dementia in patients taking a DOAC compared with warfarin.12 However, a recent study with a larger sample size using data from Swedish registers found no difference of dementia risk between DOAC and warfarin users after adjusting for multiple baseline characteristics.13 Neither of these studies, however, evaluated the risk of dementia comparing warfarin with individual DOACs, or between individual DOACs.
On the basis of this previous suggestive but inconclusive evidence, we analyzed data from 2 large US healthcare use databases to evaluate whether the risk of dementia incidence among patients with AF differs between warfarin users and DOAC users, as well as across DOAC user groups.
Methods
Study Population
The study population was identified from 2 databases (MarketScan and Optum Clinformatics). The Truven Health MarketScan Commercial Claims and Encounter Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics Inc, Ann Arbor, MI) included data from January 1, 2007, to September 30, 2015. The MarketScan databases contain claims data and linked patient enrollment information from insured employees and their dependents for active employees as well as Medicare‐eligible retirees with employer‐provided Medicare Supplemental plans. Similarly, Optum Clinformatics Data Mart included data from January 1, 2009, to September 30, 2015. The Optum database contains health insurance medical and pharmacy claims as well as linked patient enrollment data from privately insured and Medicare Advantage enrollees throughout the United States. The authors cannot make data and study materials available to other investigators for purposes of reproducing the results because of licensing restrictions. Interested parties, however, could obtain and license the data by contacting Truven Health Analytics Inc and Optum.
We restricted the analysis to patients with nonvalvular AF with a prescription for an OAC. Enrollees were included if they had at least 1 inpatient claim or 2 consecutive outpatient claims with an AF diagnosis separated by at least 7 days but <1 year, defined by an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code 427.31 or 427.32 in any position, without history of mitral stenosis (ICD‐9‐CM code 394.0) or mitral valve disorder (ICD‐9‐CM code 424.0).14 At least 1 prescription for warfarin or one of the DOACs (dabigatran, rivaroxaban, or apixaban) was required, restricting the data to 678 683 and 362 357 patients in MarketScan and Optum, respectively. A systematic review of validation studies identifying AF from administrative databases suggested that similar algorithms had a median positive predictive value of 89% and a median sensitivity of 79%.15 Because our analysis is restricted to patients initiating OACs, we expect the positive predictive value to be even higher. We excluded enrollees with <90 days of enrollment before the first OAC prescription (328 300 in MarketScan and 178 656 in Optum) to enhance the ability to identify comorbidities and other medications before OAC initiation. Those who took OACs 90 days before (or earlier) their AF diagnosis were excluded, because they may have been using OACs for other indications. Enrollees with a dementia diagnosis before or at the time of their first OAC prescription (4458 in MarketScan and 3674 in Optum) were excluded. The inclusion procedure is shown in the Figure.
Figure 1.
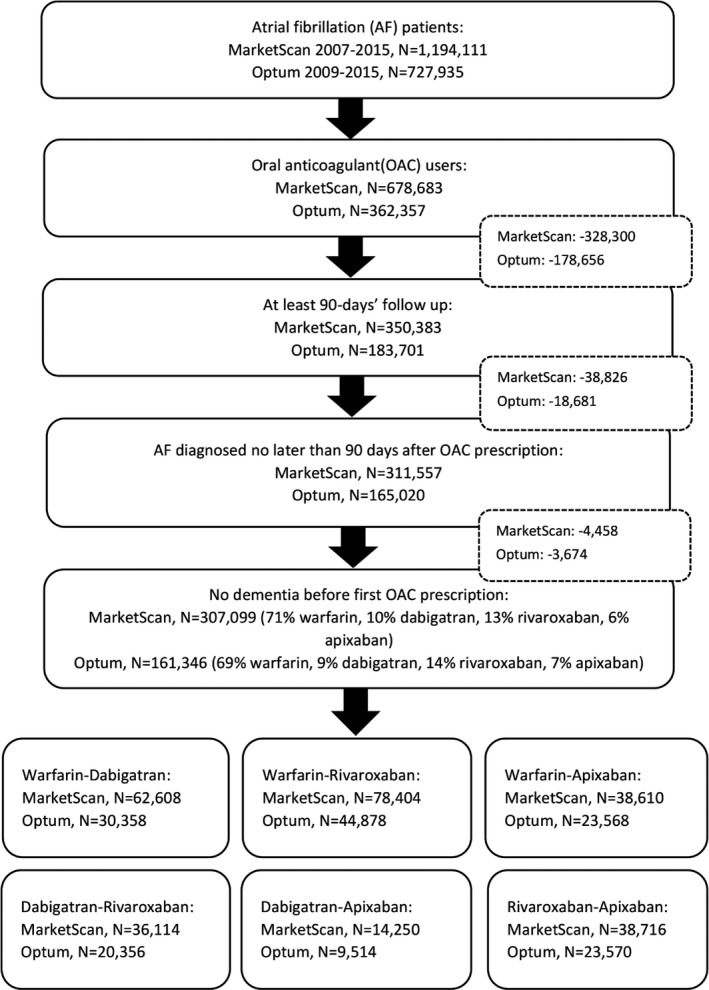
Flow chart of enrollees’ selection to final analysis sample. Inclusion criteria were applied to the MarketScan and Optum databases, and all the eligible enrollees were matched 1:1 on propensity score to generate 6 final head‐to‐head oral anticoagulant (OAC) comparison cohorts. AF indicates atrial fibrillation.
OAC Use
Outpatient pharmaceutical claim data include, among other variables, the National Drug Code and prescription fill date. Enrollees were classified in exclusive categories according to their first filled OAC prescription: warfarin initiators, dabigatran initiators, rivaroxaban initiators, or apixaban initiators. We did not consider edoxaban use, because it was approved by the Food and Drug Administration in January 2015 and few patients in our population received this medication.
Outcome
The primary outcome of interest was a diagnosis code for dementia on an inpatient claim, defined with the following ICD‐9‐CM codes in any position: 290.xx (dementia); 294.xx (persistent mental disorders attributable to conditions classified elsewhere); and 331.0 (Alzheimer disease). Positive predictive values for these codes have been shown to be >80% in a previous study.16 In a sensitivity analysis, we defined dementia with an inpatient or an outpatient claim, using the diagnosis codes in any position.
Covariates
Covariates included demographic characteristics, as well as cognitive impairment, comorbidities, and use of medications at baseline. Baseline demographic information included in our study were age and sex, as well as (in the Optum database only) race, education, and household income. Approximately 30% of the race/ethnicity data in Optum were collected from public records (eg, driver's license records), and the remaining were imputed by the E‐Tech commercial software using individuals’ names and zip codes. This validated imputation method has 97% specificity and 48% sensitivity for estimating the race of black individuals.17 Enrollees with missing values were categorized into the unknown group for race, education, and household income in Optum (8%, 5%, and 13%, respectively).
We included the following baseline comorbidities that might affect both OAC type and dementia: heart failure, hypertension, diabetes mellitus, myocardial infarction, peripheral artery disease, kidney disease, ischemic stroke, gastrointestinal tract bleeding, cerebral bleeding, other bleeding, anemia, coagulopathy, cancer, and mood disorders. We also included use of the following medications: antiplatelet therapy, diuretics, antiarrhythmic drugs, digoxin, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β blockers, calcium channel blockers, and lipid‐lowering medications. Cognitive impairment, comorbidities, and prescription fills of medications were defined on the basis of inpatient and outpatient claims and outpatient pharmacy claims before first OAC prescription since their enrollment in the database (ie, using all available data and not restricted to fixed look‐back time windows). The ICD‐9‐CM codes to define cognitive impairment and comorbidities have been used in previous analyses and are provided in Table S1.14 Stroke and bleeding risk stratification scores of CHA2DS2‐VASc and HAS‐BLED were calculated for everyone.18, 19
Statistical Analysis
For each database, we performed all pairwise comparisons of different OACs (warfarin versus dabigatran, warfarin versus rivaroxaban, warfarin versus apixaban, dabigatran versus rivaroxaban, dabigatran versus apixaban, and rivaroxaban versus apixaban). For each analysis, we restricted the initial cohort to enrollees initiating OAC after the date when both anticoagulants were available (October 19, 2010, for dabigatran; November 4, 2011, for rivaroxaban; and December 28, 2013, for apixaban). We calculated propensity scores for treatment with a particular anticoagulant at the time of OAC initiation for each comparison in each cohort, using logistic regression models that included all the comorbidities and medications previously described. Finally, we matched enrollees 1:1 on propensity score with calipers of 0.2 of SD of the propensity scores, using a greedy matching algorithm20 implemented with the gmatch SAS macro. Any patient without a match was excluded from the analysis.
We assessed the association between anticoagulant type and incidence of dementia using Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Time to dementia was calculated from time of first anticoagulant prescription (index date) to September 30, 2015, database disenrollment, or dementia diagnosis, whichever occurred earlier. Information on the reason of database disenrollment (eg, death or change of insurance plan) was not available. In model 1, we adjusted for age, sex, and cognitive impairment, as well as (only in the Optum database) race, education, and household income. In model 2, we additionally adjusted for comorbidities and medications listed in Table S2 and CHA2DS2‐VASc and HAS‐BLED scores. In a supplementary analysis, we added incident ischemic stroke to our model as model 3. We tested the proportional hazards assumption by introducing an interaction term of OAC group and logarithmic scale of time in the model. No violation of the proportionality assumption was detected in both MarketScan and Optum databases across the comparisons. We meta‐analyzed the database‐specific results using a random‐effects model. Homogeneity of results between the databases was tested. We performed a sensitivity analysis by defining dementia incidence using both inpatient and outpatient claims to evaluate the robustness of our primary results to changes in end point definition. In addition, to evaluate the impact of discontinuation of initial OAC or switching to a different anticoagulant, we conducted an additional analysis censoring patients at the time of discontinuation or switching. We defined discontinuation as no additional refill for at least 60 days since the end of days’ supply for a prescription.
Effect measure modification by age (≤75 and >75 years), sex (male and female), and CHA2DS2‐VASc score (<2 as low risk and ≥2 as moderate/high risk) between OAC therapy and risk of dementia was assessed, after adjusting for other covariates in model 2 in each database. The significance of effect measure modifications is reported as P values.
We conducted an additional analysis to address the concern that patients prescribed warfarin may have inherently different cognitive status at baseline from those prescribed DOACs in ways that our adjustment cannot control (confounding by indication). Specifically, we included all eligible enrollees from our primary analysis, plus patients previously excluded because of a dementia diagnosis before or at the time of their first OAC prescription (311 557 in MarketScan and 165 020 in Optum). We calculated, at the time of anticoagulant initiation, the odds ratios of being prescribed a DOAC (versus warfarin) in patients with prevalent dementia (defined by an inpatient claim) or cognitive impairment (defined by an inpatient or outpatient claim) compared with cognitively normal patients. Logistic regression was used, adjusting for all the other covariates previously mentioned, to determine if underlying cognitive status likely influenced the type of prescribed anticoagulant. The Institutional Review Board of Emory University (Atlanta, Ga) approved the present study, which provided a waiver of informed consent for the analysis of these deidentified data.
Results
The MarketScan database included 1 194 111 patients with nonvalvular AF. After excluding ineligible patients using the criteria previously described, 307 099 enrollees remained. The Optum database included 727 935 identified patients with nonvalvular AF, and 161 346 remained in the cohort after excluding enrollees who did not meet the study criteria. The inclusion procedure is shown as a flow chart in the Figure. In both cohorts, most enrollees took warfarin as their first OAC (71% and 69%, respectively), whereas few of them were apixaban initiators (6% and 7%, respectively). We performed propensity score matching separately in MarketScan and Optum, obtaining 6 cohorts each as our final analytical data sets. Overall, there were 62 608 MarketScan and 30 358 Optum enrollees in the warfarin‐dabigatran matched cohorts, 78 404 MarketScan and 44 878 Optum enrollees in the warfarin‐rivaroxaban matched cohorts, 38 610 MarketScan and 23 568 Optum enrollees in the warfarin‐apixaban matched cohorts, 36 114 MarketScan and 20 356 Optum enrollees in the dabigatran‐rivaroxaban matched cohorts, 14 250 MarketScan and 9514 Optum enrollees in the dabigatran‐apixaban matched cohorts, and 38 716 MarketScan and 23 570 Optum enrollees in the rivaroxaban‐apixaban matched cohorts (Figure).
Baseline characteristics in MarketScan before propensity score matching by prescribed first OAC are presented in Table S2. In general, dabigatran and rivaroxaban initiators had similar demographic, health, and medication use profiles. Warfarin and apixaban initiators were slightly older, were more likely to be women, and had higher CHA2DS2‐VASc and HAS‐BLED scores compared with dabigatran and rivaroxaban initiators. The prevalence of comorbidities was generally higher among warfarin users compared with DOAC users. Similar results were observed in Optum, which are shown in Table S3. Characteristics at baseline after propensity score matching were similar between OAC treatment groups in each cohort. Across the cohorts, the average age ranged between 67 years in MarketScan and 73 years in Optum (SD, ≈12 years), percentage of female enrollees ranged between 35% in MarketScan and 45% in Optum, and the average CHA2DS2‐VASc score ranged between 2.9 in MarketScan and 4.3 in Optum (SD, ≈2.0). In addition, race was distributed similarly across the Optum cohorts, with ≈77% white enrollees. Patients in Optum were generally older and had higher predicted risk of stroke and bleeding than those in MarketScan. Distribution of age, sex, race (in the Optum database only), and CHA2DS2‐VASc and HAS‐BLED scores in each study after propensity score matching are shown in Tables 1 and 2. The proportion of patients receiving a standard and reduced dose of each DOAC is presented in Table S4, stratified by sex. Overall, the proportion receiving a reduced dose was higher in women than in men and among those receiving rivaroxaban compared with other DOACs.
Table 1.
Baseline Characteristics of Patients With AF, According to First Prescribed OAC After Propensity Score Matching: MarketScan, 2010–2015
| Characteristics | Comparison of DOACs With Warfarin | Comparison Among Dabigatran, Rivaroxaban, and Apixaban | ||
|---|---|---|---|---|
| Warfarin | Dabigatran | Dabigatran | Rivaroxaban | |
| N | 31 304 | 31 304 | 18 057 | 18 057 |
| Age, mean (SD), y | 67 (13) | 67 (13) | 67 (12) | 66 (13) |
| Women, % | 35 | 35 | 35 | 34 |
| CHA2DS2‐VASc score, mean (SD) | 3.0 (2.0) | 3.1 (2.0) | 3.0 (2.0) | 2.9 (1.9) |
| HAS‐BLED score, mean (SD) | 1.8 (1.1) | 1.8 (1.2) | 1.7 (1.1) | 1.7 (1.1) |
| Warfarin | Rivaroxaban | Dabigatran | Apixaban | |
| N | 39 202 | 39 202 | 7125 | 7125 |
| Age, mean (SD), y | 68 (13) | 67 (13) | 67 (12) | 67 (13) |
| Women, % | 39 | 38 | 35 | 36 |
| CHA2DS2‐VASc score, mean (SD) | 3.1 (1.9) | 3.1 (1.9) | 3.0 (1.9) | 2.9 (1.9) |
| HAS‐BLED score, mean (SD) | 1.8 (1.1) | 1.8 (1.1) | 1.7 (1.1) | 1.7 (1.1) |
| Warfarin | Apixaban | Rivaroxaban | Apixaban | |
| N | 19 305 | 19 305 | 19 358 | 19 358 |
| Age, mean (SD), y | 69 (13) | 69 (13) | 69 (12) | 69 (13) |
| Women, % | 40 | 40 | 40 | 40 |
| CHA2DS2‐VASc score, mean (SD) | 3.4 (1.9) | 3.4 (2.0) | 3.3 (2.0) | 3.4 (2.0) |
| HAS‐BLED score, mean (SD) | 1.9 (1.1) | 1.9 (1.2) | 1.9 (1.2) | 1.9 (1.2) |
Comparison groups are matched 1:1. AF indicates atrial fibrillation; DOAC, direct oral anticoagulant.
Table 2.
Baseline Characteristics of Patients With AF, According to First Prescribed OAC After Propensity Score Matching: Optum, 2010–2015
| Characteristics | Comparison of DOACs With Warfarin | Comparison Among Dabigatran, Rivaroxaban, and Apixaban | ||
|---|---|---|---|---|
| Warfarin | Dabigatran | Dabigatran | Rivaroxaban | |
| N | 15 179 | 15 179 | 10 178 | 10 178 |
| Age, mean (SD), y | 69 (12) | 69 (12) | 69 (12) | 69 (12) |
| Women, % | 37 | 37 | 37 | 37 |
| Race, % white | 79 | 79 | 78 | 78 |
| CHA2DS2‐VASc score, mean (SD) | 3.6 (2.0) | 3.6 (2.0) | 3.7 (2.0) | 3.7 (2.1) |
| HAS‐BLED score, mean (SD) | 2.3 (1.3) | 2.3 (1.3) | 2.3 (1.3) | 2.3 (1.3) |
| Warfarin | Rivaroxaban | Dabigatran | Apixaban | |
| N | 22 439 | 22 439 | 4757 | 4757 |
| Age, mean (SD), y | 71 (11) | 70 (12) | 70 (11) | 70 (12) |
| Women, % | 40 | 40 | 38 | 38 |
| Race, % white | 77 | 78 | 76 | 76 |
| CHA2DS2‐VASc score, mean (SD) | 3.8 (2.0) | 3.8 (2.1) | 3.8 (2.0) | 3.8 (2.1) |
| HAS‐BLED score, mean (SD) | 2.5 (1.3) | 2.5 (1.3) | 2.4 (1.3) | 2.4 (1.3) |
| Warfarin | Apixaban | Rivaroxaban | Apixaban | |
| N | 11 784 | 11 784 | 11 785 | 11 785 |
| Age, mean (SD), y | 73 (10) | 73 (11) | 72 (11) | 73 (11) |
| Women, % | 45 | 45 | 45 | 45 |
| Race, % white | 77 | 77 | 77 | 77 |
| CHA2DS2‐VASc score, mean (SD) | 4.3 (2.0) | 4.2 (2.1) | 4.2 (2.1) | 4.2 (2.1) |
| HAS‐BLED score, mean (SD) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.3) |
Comparison groups are matched 1:1. AF indicates atrial fibrillation; DOAC, direct oral anticoagulant.
Comparison of DOACs With Warfarin
Table 3 shows the meta‐analyzed HRs and 95% CIs of dementia comparing DOAC initiators with warfarin initiators. Mean follow‐up ranged between 0.7 years for analyses comparing apixaban with warfarin in MarketScan to 2.2 years in analyses comparing dabigatran with warfarin in Optum. Total numbers of 1463, 1592, and 751 dementia cases were identified in warfarin‐dabigatran matched cohorts, warfarin‐rivaroxaban matched cohorts, and warfarin‐apixaban matched cohorts, respectively. The rate of dementia was lower among dabigatran users compared with warfarin users (model 1: HR, 0.85; 95% CI, 0.74–0.97) after adjusting for demographic characteristics and prior cognitive impairment. Results were similar after additional adjustment for comorbidities, nonanticoagulant medication use, and CHA2DS2‐VASc and HAS‐BLED scores (model 2: HR, 0.85; 95% CI, 0.71–1.01). Similar results also were observed for rivaroxaban and apixaban when compared with warfarin users, with HRs (95% CIs) of 0.85 (0.76–0.94) for rivaroxaban users and 0.80 (0.65–0.97) for apixaban users after full adjustment (Table 3). There was no evidence of heterogeneity across databases. Database‐specific results are presented in Tables S5 and S6. In a supplementary analysis, after additional adjustment for incident ischemic stroke, results remained similar to what we observed from model 2 (Table S7).
Table 3.
Meta‐Analyzed HRs and 95% CIs of Incident Dementia in OAC Comparison Cohorts: MarketScan and Optum, 2010–2015
| Variable | Warfarin | Dabigatran | Warfarin | Rivaroxaban | Warfarin | Apixaban |
|---|---|---|---|---|---|---|
| N | 46 483 | 46 483 | 61 641 | 61 641 | 31 089 | 31 089 |
| Dementia, N | 739 | 724 | 944 | 648 | 474 | 277 |
| Follow‐up, mean, y | 1.7 | 1.9 | 1.4 | 1.2 | 1.2 | 0.8 |
| Incidence rate (per 1000 person‐years) | 9.2 | 8.0 | 10.5 | 8.7 | 12.9 | 11.0 |
| HRs (95% CIs) | ||||||
| Model 1a | 1 | 0.85 (0.74–0.97) | 1 | 0.85 (0.77–0.94) | 1 | 0.80 (0.63–1.03) |
| Model 2b | 1 | 0.85 (0.71–1.01) | 1 | 0.85 (0.76–0.94) | 1 | 0.80 (0.65–0.97) |
| Rivaroxaban | Dabigatran | Apixaban | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|---|---|
| N | 28 235 | 28 235 | 11 882 | 11 882 | 31 143 | 31 143 |
| Dementia, N | 290 | 399 | 87 | 119 | 360 | 279 |
| Follow‐up, mean, y | 1.2 | 1.7 | 0.8 | 1.3 | 1.1 | 0.8 |
| Incidence rate (per 1000 person‐years) | 8.5 | 8.2 | 8.8 | 7.3 | 10.6 | 11.0 |
| HRs (95% CIs) | ||||||
| Model 1a | 1 | 1.03 (0.85–1.24) | 1 | 0.95 (0.65–1.38) | 1 | 0.99 (0.85–1.16) |
| Model 2b | 1 | 1.02 (0.79–1.32) | 1 | 0.92 (0.63–1.36) | 1 | 1.01 (0.86–1.19) |
CI indicates confidence interval; HR, hazard ratio; OAC, oral anticoagulant.
Model 1 was adjusted for age, sex, and prevalent cognitive impairment in the study from MarketScan and for age, sex, race, education level, household income level, and prevalent cognitive impairment in the study from Optum.
Model 2 was additionally adjusted for comorbidities, medications, and CHA2DS2‐VASc and HAS‐BLED scores.
Comparisons Among Dabigatran, Rivaroxaban, and Apixaban
Meta‐analyzed results from database‐specific analysis showed comparable hazards of dementia across different DOAC groups (Table 3, model 2, dabigatran versus rivaroxaban: HR, 1.02; 95% CI, 0.79–1.32; dabigatran versus apixaban: HR, 0.92; 95% CI, 0.63–1.36; apixaban versus rivaroxaban: HR, 1.01; 95% CI, 0.86–1.19). There was no evidence of significant heterogeneity across database‐specific results (Tables S8 and S9).
Sensitivity and Subgroup Analyses
We identified >2 times the number of dementia cases when ascertaining dementia diagnoses using both inpatient and outpatient ICD‐9 codes instead of only using inpatient claims, with the number of enrollees comparable to the number in primary analyses in each comparison cohort (Tables S10 through S13). Results were similar to the primary analyses. DOACs were associated with a lower incidence of dementia compared with warfarin initiation, with HRs (95% CIs) of 0.79 (0.63–0.88), 0.79 (0.63–0.99), and 0.73 (0.52–1.02) in the comparisons of dabigatran, rivaroxaban, and apixaban with warfarin, respectively (Table 4). Results were essentially unchanged when baseline cognitive impairment was included as a covariate differentiating outpatient and inpatient diagnosis (Table S14) and when we censored patients at the time of OAC discontinuation or switching (Table S15). The incidence of dementia was similar in head‐to‐head comparisons of the different DOACs. However, some of these comparisons showed evidence of heterogeneity across databases, most notably for the comparison of dabigatran with other DOACs, which had the opposite direction of association in each database (Tables S12 and S13).
Table 4.
Meta‐Analyzed HRs and 95% CIs of Incident Dementia in OAC Comparison Cohorts: MarketScan and Optum, 2010–2015. Dementia was defined on the basis of inpatient and outpatient diagnoses
| Variable | Warfarin | Dabigatran | Warfarin | Rivaroxaban | Warfarin | Apixaban |
|---|---|---|---|---|---|---|
| N | 45 439 | 45 439 | 60 178 | 60 178 | 30 218 | 30 218 |
| Dementia, N | 1877 | 1709 | 2352 | 1587 | 1143 | 660 |
| Follow‐up, mean, y | 1.7 | 1.9 | 1.4 | 1.2 | 1.2 | 0.8 |
| Incidence rate (per 1000 person‐years) | 24.5 | 19.6 | 27.3 | 22.2 | 32.6 | 27.5 |
| HRs (95% CIs) | ||||||
| Model 1a | 1 | 0.79 (0.74–0.84) | 1 | 0.80 (0.62–1.04)b | 1 | 0.75 (0.52–1.06)b |
| Model 2c | 1 | 0.79 (0.71–0.88) | 1 | 0.79 (0.63–0.99)b | 1 | 0.73 (0.52–1.02)b |
| Rivaroxaban | Dabigatran | Apixaban | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|---|---|
| N | 27 596 | 27 596 | 11 582 | 11 582 | 30 271 | 30 271 |
| Dementia, N | 690 | 900 | 186 | 321 | 869 | 660 |
| Follow‐up, mean, y | 1.2 | 1.7 | 0.8 | 1.4 | 1.1 | 0.8 |
| Incidence rate (per 1000 person‐years) | 20.9 | 19.1 | 19.9 | 20.7 | 26.9 | 27.4 |
| HRs (95% CIs) | ||||||
| Model 1a | 1 | 1.01 (0.72–1.41)b | 1 | 1.22 (0.77–1.92)b | 1 | 0.94 (0.85–1.04) |
| Model 2c | 1 | 1.00 (0.71–1.42)b | 1 | 1.19 (0.74–1.92)b | 1 | 0.96 (0.86–1.06) |
CI indicates confidence interval; HR, hazard ratio; OAC, oral anticoagulant.
Model 1 was adjusted for age, sex, and prevalent cognitive impairment in the study from MarketScan and age, sex, race, education level, household income level, and prevalent cognitive impairment in the study from Optum.
Heterogeneous between studies.
Model 2 was additionally adjusted for comorbidities, medications, and CHA2DS2‐VASc and HAS‐BLED scores.
Results of subgroup analyses from each database are shown in Tables S16 and S17. No multiplicative interactions of OAC treatment with age, sex, and CHA2DS2‐VASc score were detected. In the Optum database, we observed that dabigatran initiation was associated with a lower hazard of dementia than warfarin initiation among younger patients, ≤75 years (HR, 0.61; 95% CI, 0.41–0.89), but not among patients >75 years (HR, 1.04; 95% CI, 0.87–1.25; P for interaction=0.01). Rivaroxaban was associated with lower dementia hazard compared with warfarin among female enrollees (HR, 0.70; 95% CI, 0.56–0.87) but not among male enrollees (HR, 0.96; 95% CI, 0.76–1.21; P for interaction=0.04). For the dabigatran‐rivaroxaban comparison, we observed a lower hazard of dementia for dabigatran among younger patients (HR, 0.54; 95% CI, 0.32–0.91) and a higher hazard of dementia for dabigatran among older patients (HR, 1.41; 95% CI, 1.09–1.84), with a P for interaction of 0.002. However, despite identifying several significant interactions in the Optum database, additional confirmatory evidence is required because of the inconsistency between the 2 databases, the limited number of cases in subgroups from each cohort, and multiple comparisons. Finally, HRs and 95% CIs were not calculated for CHA2DS2‐VASc–categorized subgroups in the apixaban‐dabigatran cohort from MarketScan, and all 6 cohorts from Optum database, because few dementia occurrences were identified among enrollees with a CHA2DS2‐VASc score of 0 or 1.
Assessment of Confounding by Indication
In the analysis that included patients with AF with prevalent dementia or cognitive impairment at the time of anticoagulant initiation, the odds of receiving DOACs versus warfarin were similar regardless of baseline cognitive status. In MarketScan, the odds ratio (95% CI) of DOAC initiation was 0.99 (0.90–1.10) comparing those with dementia/cognitive impairment with cognitively normal individuals, whereas the corresponding odds ratio (95% CI) in Optum was 0.91 (0.81–1.03), for a combined odds ratio (95% CI) of 0.96 (0.88–1.04) (Table S18).
Discussion
In this study, which used data from 2 independent large healthcare claims databases, we found that patients with nonvalvular AF initiating DOACs (dabigatran, rivaroxaban, and apixaban) had consistently lower rates of dementia compared with warfarin initiators. In head‐to‐head DOAC comparisons, however, we found that the hazard of dementia did not vary according to DOAC prescribed. Our findings suggest the following: (1) DOACs may be superior to warfarin with respect to outcome of dementia, which is considered as an important adverse outcome of AF; and (2) future risk of dementia does not appear to be influenced by choice of DOAC. Therefore, DOAC choice should be driven by other efficacy, safety, and preference considerations.21, 22 However, misclassification and confounding may be partly responsible for these findings.
Growing evidence indicates that cognitive decline and dementia are frequent adverse outcomes in patients with AF.4 Although preventing dementia is not the primary focus of antithrombotic treatment in patients with AF, concerns exist about higher risk of microbleeds in patients receiving suboptimal management of anticoagulation with warfarin, because of either underanticoagulation or overanticoagulation. These microbleeds could cause chronic cerebral injury and finally lead to dementia.23, 24 On the other hand, the role that DOACs can play in the prevention of dementia is of considerable interest. Through prevention of ischemic stroke, both warfarin and DOACs can reduce the risk of vascular dementia. Moreover, DOAC users experience lower risk of intracranial bleeding compared with warfarin users. As shown in pivotal clinical trials, dabigatran, rivaroxaban, and apixaban were all reported noninferior in preventing ischemic stroke or systemic embolism, and they had lower rates of intracranial hemorrhage compared with warfarin.25, 26, 27 Last, DOACs provide steady therapeutic levels without the fluctuations that are common among warfarin users, and they may be a promising approach to reduce the risk of dementia among patients with AF.5 To have a better understanding of potential mechanisms that underlie the association between DOACs versus warfarin and incidence of dementia in this study, we further adjusted for incidence of ischemic stroke in the multivariate regression analysis; we detected no change compared with the primary results. Although the number of incident stroke events in each cohort is limited, it might indicate that a mechanism other than the reduction in risk of clinically recognized stroke, and possibly reduction of intracranial bleeding, is the primary factor underlying the observed beneficial association of DOACs versus warfarin in dementia risk.
Results from our analysis were consistent with the prior study using healthcare clinical data in Utah, where a lower rate of dementia was observed in patients taking a DOAC compared with warfarin users.12 In contrast, no difference between warfarin and DOACs was observed in a study using registry data from Sweden, in which patients treated with warfarin had a mean time in therapeutic range well above 70%. The mean time spent in therapeutic range in the United States is much lower, at ≈54%.28 This inconsistency might indicate that DOACs offer better protection than warfarin in the United States compared with Sweden, possibly because of the difference in the time in therapeutic range for warfarin users.13
We did not observe differences in the hazard of dementia among users of different DOACs, although there was some between‐database heterogeneity in the comparisons of dabigatran with rivaroxaban and apixaban. Several previous analyses, including both direct and indirect comparisons between DOACs, found a more favorable profile of effectiveness and safety for dabigatran and apixaban users over rivaroxaban users, with no differences in risk of stroke or systemic embolism and lower risk of intracranial bleeding.10, 21 Comparisons including apixaban, however, need to be interpreted with caution given the shorter follow‐up period and the limited number of events in this group because of the later Food and Drug Administration approval date. In addition, we have the fewest enrollees in the dabigatran‐apixaban cohort because of the limited number of dabigatran users since the approval of apixaban. Future analyses comparing dementia risk in apixaban users versus dabigatran and rivaroxaban users need to be based on a larger sample size and longer follow‐up to generate a better understanding of dementia incidence across DOAC groups.
We did not identify consistent effect measure modification in the association of OAC use with rates of dementia by age, sex, or CHA2DS2‐VASc score. Rates of dementia incidence were lower in DOAC users among both younger and older patients, men and women, and low‐ and high‐risk patients.
Study Strengths and Limitations
Our study has some important limitations. First, we relied on claims data to define AF, dementia, and baseline characteristics, which may lack clinical fidelity compared with using clinical criteria, complete medical records, and detailed evaluation of cognitive function. In our primary analysis, we used inpatient claims to define dementia diagnosis, likely leading to an underestimate of dementia rates, because cases of dementia that were less severe were not captured by hospitalization records. Although we assumed a low sensitivity in this analysis, the specificity was expected to be high, because ICD‐9‐CM codes of high validity were used to define dementia diagnosis.16 We also repeated the analysis using an alternative definition of incident dementia, using dementia codes in either inpatient or outpatient records, which did not have a major impact on the results. Second, in addition to outcome misclassification, we categorized OAC user groups on the basis of their first prescription of OACs, regardless of whether they stopped taking them or switched to other OACs later. Third, patterns of discontinuation and switching may not be random across OACs (eg, patients prescribed warfarin were more likely to switch to other OAC therapies or to stop taking their medication). As a result, using initiation of prescription to represent OAC use in this study would have limitations. However, associations remained essentially the same when we censored patients at the time of OAC discontinuation or switching. Fourth, misclassifications of covariates, including race, were also possible.
Confounding by indication is a concern in this analysis. Individuals who were prescribed DOACs may be inherently different from those who were prescribed warfarin, and there may be uncontrolled confounding, because of unmeasured information at the time of prescription. Of particular concern is baseline cognitive status if the likelihood of being prescribed a DOAC or warfarin is affected by perceived cognitive function. To address this limitation, we conducted an analysis evaluating type of OAC prescribed in patients with AF and dementia or cognitive impairment codes compared with patients without dementia or cognitive impairment codes. This analysis showed that baseline cognition status had only a weak association with the OAC prescribed (ie, a DOAC or warfarin). In addition, in the main analysis, we adjusted for an extensive list of potential confounders by conducting propensity score matching and including the confounders in the Cox model; this makes the problem of confounding by indication less of a concern, although it cannot be ruled out completely.
Other limitations of the present analysis include the potential lack of generalizability, because the population was restricted to individuals with commercial insurance, Medicare Supplemental insurance (MarketScan), or Medicare Advantage (Optum). Restricting analyses to patients matched on propensity score could be an additional source of restricted generalizability, especially for those initiating warfarin and those initiating dabigatran in their comparison with other DOACs. Finally, the limited follow‐up led to a small number of events, particularly for some comparisons, and to uncertainties about long‐term effects of anticoagulation. Because dementia‐associated diseases can begin decades before they are clinically obvious, it will be important to assess the long‐term effect of OACs on risk of dementia in future analyses.
Despite the limitations discussed, our analysis has considerable strengths. We were able to assess a relatively rare event in 2 independent, large populations using administrative claims data. The large number of enrollees in each group of anticoagulant users enabled us to perform head‐to‐head comparisons between different anticoagulants and use propensity score matching to generate exchangeable cohorts with practice‐based claims data. The results were robust, because they were comparable in the MarketScan and Optum databases, even after adjustment for several markers of social economic status, and were consistent across diverse definitions of the outcome.
Conclusions
In this analysis comparing dementia rates by type of OAC in 2 large, independent retrospective healthcare use databases, we observed lower hazards of dementia among patients with AF initiating DOACs compared with warfarin initiators and similar hazards of dementia across different DOAC user groups. Future long‐term studies assessing dementia risk in patients with AF initiating OACs are needed.
Sources of Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health awards F32HL134290 (O'Neal), R01HL122200, and R01HL131579; the National Institute on Aging of the National Institutes of Health award R21AG058445; and the American Heart Association award 16EIA26410001 (Alonso).
Disclosures
Bengtson is an employee of Optum. The remaining authors have no disclosures to report.
Supporting information
Table S1. ICD‐9‐CM Codes Used to Define Comorbidities
Table S2. Baseline Characteristics of Patients With Atrial Fibrillation According to First Prescribed Oral Anticoagulant Before Propensity Score Matching: MarketScan, 2007–2015
Table S3. Baseline Characteristics of Patients With Atrial Fibrillation According to First Prescribed Oral Anticoagulant Before Propensity Score Matching: Optum 2009–2015
Table S4. Distribution of Patients by Sex and Anticoagulant Dosage
Table S5. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: MarketScan, 2010–2015
Table S6. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: Optum, 2010–2015
Table S7. Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts With Further Adjustment of Incident Ischemic Stroke: MarketScan and Optum, 2010–2015
Table S8. Comparisons of incidence of dementia (defined based on inpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: MarketScan, 2010–2015.
Table S9. Comparisons of incidence of dementia (defined based on inpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: Optum, 2010–2015.
Table S10. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: MarketScan, 2010–2015
Table S11. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: Optum, 2010–2015
Table S12. Comparisons of incidence of dementia (defined based on inpatient and outpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: MarketScan, 2010–2015
Table S13. Comparisons of incidence of dementia (defined based on inpatient and outpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: Optum, 2010–2015
Table S14. Database‐Specific and Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts Using Refined Baseline Cognitive Impairment Adjustment: MarketScan and Optum, 2010–2015
Table S15. Database‐Specific and Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts Censoring Patients at the Time of Oral Anticoagulant Discontinuation or Switching: MarketScan and Optum, 2010–2015
Table S16. Adjusted Hazard Ratios (HRs) and 95% Confidence Intervals (CIs)* of Dementia in Matched Cohort Stratified by Age, Sex, and CHA2DS2‐VASc Score: MarketScan, 2010–2015
Table S17. Adjusted Hazard Ratios (HRs) and 95% Confidence Intervals (CIs)* of Dementia in Matched Cohort Stratified by Age and Sex: Optum, 2010–2015
Table S18. Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of Having DOAC Prescription (Versus Warfarin) Among Atrial Fibrillation Patients With Dementia and Cognitive Impairment Before OAC Initiation Compared With Cognitively Normal Patients: MarketScan, 2007–2015, and Optum, 2009–2015
(J Am Heart Assoc. 2018;7:e009561 DOI: 10.1161/JAHA.118.009561.)
References
- 1. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 4. Alonso A, Arenas de Larriva AP. Atrial fibrillation, cognitive decline and dementia. Eur Cardiol. 2016;11:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs V, Graves KG, Bunch TJ. Anticoagulant use in atrial fibrillation and risk of dementia: review of contemporary knowledge. Expert Rev Cardiovasc Ther. 2017;15:897–903. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayala N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 8. Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Alonso A. Comparative effectiveness of dabigatran and rivaroxaban versus warfarin for the treatment of non‐valvular atrial fibrillation. J Cardiol. 2017;69:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725 DOI: 10.1161/JAHA.118.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norby FL, Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Chamberlain AM, Rapson IR, Alonso A. Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non‐valvular atrial fibrillation. BMC Cardiovasc Disord. 2017;17:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs V, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD, Mallender C, Osborn JS, Stevens SM, Weiss JP, Woller SC, Bunch TJ. Long‐term population‐based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long‐term anticoagulated patients for atrial fibrillation. Am J Cardiol. 2016;118:210–214. [DOI] [PubMed] [Google Scholar]
- 13. Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39:453–460. [DOI] [PubMed] [Google Scholar]
- 14. Alonso A, Maclehose RF, Chen LY, Bengtson LGS, Chamberlain AM, Norby FL, Lutsey PL. Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillation. Heart. 2017;103:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiyoshi A, Jacobs DR Jr, Alonso A, Luchsinger JA, Rapp SR, Duprez DA. Validity of death certificate and hospital discharge ICD codes for dementia diagnosis: the Multi‐Ethnic Study of Atherosclerosis. Alzheimer Dis Assoc Disord. 2017;31:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeFrank JT, Bowling JM, Rimer BK, Gierisch JM, Skinner CS. Triangulating differential nonresponse by race in a telephone survey. Prev Chronic Dis. 2007;4:A60. [PMC free article] [PubMed] [Google Scholar]
- 18. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 20. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150:1302–1312. [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, Batson S. Relative efficacy and safety of non‐vitamin K oral anticoagulants for non‐valvular atrial fibrillation: network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs V, Woller SC, Stevens S, May HT, Bair TL, Anderson JL, Crandall BG, Day JD, Johanning K, Long Y, Mallender C, Olson JL, Osborn JS, Weiss JP, Bunch TJ. Time outside of therapeutic range in atrial fibrillation patients is associated with long‐term risk of dementia. Heart Rhythm. 2014;11:2206–2213. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs V, Woller SC, Stevens SM, May HT, Bair TL, Crandall BG, Cutler M, Day JD, Weiss JP, Osborn JS, Mallender C, Anderson JL, Bunch TJ. Percent time with a supratherapeutic INR in atrial fibrillation patients also using an antiplatelet agent is associated with long‐term risk of dementia. J Cardiovasc Electrophysiol. 2015;26:1180–1186. [DOI] [PubMed] [Google Scholar]
- 25. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H‐C, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 26. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 27. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 28. Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9‐CM Codes Used to Define Comorbidities
Table S2. Baseline Characteristics of Patients With Atrial Fibrillation According to First Prescribed Oral Anticoagulant Before Propensity Score Matching: MarketScan, 2007–2015
Table S3. Baseline Characteristics of Patients With Atrial Fibrillation According to First Prescribed Oral Anticoagulant Before Propensity Score Matching: Optum 2009–2015
Table S4. Distribution of Patients by Sex and Anticoagulant Dosage
Table S5. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: MarketScan, 2010–2015
Table S6. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: Optum, 2010–2015
Table S7. Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts With Further Adjustment of Incident Ischemic Stroke: MarketScan and Optum, 2010–2015
Table S8. Comparisons of incidence of dementia (defined based on inpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: MarketScan, 2010–2015.
Table S9. Comparisons of incidence of dementia (defined based on inpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: Optum, 2010–2015.
Table S10. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: MarketScan, 2010–2015
Table S11. Incidence of Dementia Among Patients With Atrial Fibrillation Initially Treated With Dabigatran, Rivaroxaban, or Apixaban Versus Warfarin: Optum, 2010–2015
Table S12. Comparisons of incidence of dementia (defined based on inpatient and outpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: MarketScan, 2010–2015
Table S13. Comparisons of incidence of dementia (defined based on inpatient and outpatient diagnoses) among patients with atrial fibrillation initially treated with dabigatran, rivaroxaban, or apixaban: Optum, 2010–2015
Table S14. Database‐Specific and Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts Using Refined Baseline Cognitive Impairment Adjustment: MarketScan and Optum, 2010–2015
Table S15. Database‐Specific and Meta‐Analyzed Hazard Ratios and 95% Confidence Intervals of Incident Dementia in Oral Anticoagulant Comparison Cohorts Censoring Patients at the Time of Oral Anticoagulant Discontinuation or Switching: MarketScan and Optum, 2010–2015
Table S16. Adjusted Hazard Ratios (HRs) and 95% Confidence Intervals (CIs)* of Dementia in Matched Cohort Stratified by Age, Sex, and CHA2DS2‐VASc Score: MarketScan, 2010–2015
Table S17. Adjusted Hazard Ratios (HRs) and 95% Confidence Intervals (CIs)* of Dementia in Matched Cohort Stratified by Age and Sex: Optum, 2010–2015
Table S18. Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of Having DOAC Prescription (Versus Warfarin) Among Atrial Fibrillation Patients With Dementia and Cognitive Impairment Before OAC Initiation Compared With Cognitively Normal Patients: MarketScan, 2007–2015, and Optum, 2009–2015


