Abstract
Background
The debate on the relative benefits of off‐pump and on‐pump coronary artery bypass surgery (OPCABG and ONCABG) is still open. We aimed to provide an updated and complete summary of the evidence on the differences between OPCABG and ONCABG and to explore whether the length of the follow‐up and the surgeons’ experience in OPCABG modify the comparative results.
Methods and Results
All randomized clinical trials comparing OPCABG and ONCABG were included. Primary outcome was follow‐up mortality. Secondary outcomes were operative mortality, perioperative stroke, perioperative myocardial infarction, and late repeated revascularization. Subgroup analyses were performed based on the length of the follow‐up and the percentage of crossover from the OPCABG group (used as a surrogate of surgeon experience with OPCABG). One hundred four trials were included (20 627 patients, OPCABG: 10 288; ONCABG: 10 339). Weighted mean follow‐up time was 3.7 years (range 1–7.5 years). OPCABG was associated with a higher risk of follow‐up mortality (incidence rate ratio 1.11, 95% confidence interval 1.00–1.23, P=0.05). The difference was significant only for trials with mean follow‐up of ≥3 years and for studies with a crossover rate of ≥10%. There was a trend toward lower risk of perioperative stroke and higher need for late repeated revascularization in the OPCABG arm.
Conclusions
OPCABG is associated with a higher incidence of incomplete revascularization, an increased need for repeated revascularization, and decreased midterm survival compared with ONCABG. Surgeon inexperience in OPCABG is associated with late mortality.
Keywords: coronary artery bypass, coronary artery bypass grafting, myocardial revascularization, off‐pump coronary artery bypass grafting, off‐pump surgery, revascularization
Subject Categories: Cardiovascular Surgery, Revascularization
Short abstract
See Editorial by Lazar
Clinical Perspective
What Is New?
-
☐
Off‐pump coronary artery bypass surgery is associated with a higher incidence of incomplete revascularization, an increased need for repeated revascularization, and decreased midterm survival compared with on‐pump coronary artery bypass surgery.
-
☐
Surgeon inexperience in off‐pump coronary artery bypass surgery is associated with late mortality.
What Are the Clinical Implications?
-
☐
Surgeon's experience in off‐pump coronary artery bypass surgery may be associated with late mortality.
-
☐
We hypothesize that off‐pump coronary artery bypass surgery can be comparable to on‐pump coronary artery bypass surgery only in the hands of experienced operators.
Introduction
Coronary artery disease is the most common cause of death worldwide, and coronary artery bypass grafting (CABG) remains the most effective mode of revascularization for those with severe and extensive disease.1 Concerns for perioperative neurological events from aortic cross‐clamping and the detrimental effects of cardiopulmonary bypass has spurred much research into techniques to improve the safety and efficacy of CABG. Off‐pump CABG (OPCABG) has emerged as a potential solution to address these concerns. However, despite nearly 2 decades of debate, 115 randomized clinical trials (RCTs), and 62 meta‐analyses, the controversy regarding the benefits and risks of OPCABG compared with on‐pump CABG (ONCABG) continues.2 Recently, 2 large RCTs comparing on to off‐pump CABG, the ROOBY (Randomized On/Off Bypass) and CORONARY (CABG Off or On Pump Revascularization Study) trials, published their 5‐year results, eliciting further controversy.3, 4 In fact, ROOBY showed reduced survival in OPCABG, whereas CORONARY showed lack of difference in long‐term outcome between the 2 arms.
The ROOBY trial, however, has been criticized for having less strict case requirements for surgeons performing OPCABG (the mean and median number of cases of OPCABG performed by surgeons in other large RCTs were higher than that of ROOBY).
The publication of these 2 important and large clinical trials warrants an updated meta‐analysis of all the current available RCT evidence to determine the potential risk and benefits to OPCABG to ONCABG. Furthermore, technical and biological explanations for the divergence in outcomes seen in these recent clinical trials have not yet been fully elucidated.
The objectives of this analysis were as follows:
to provide the most complete and rigorous evaluation of the differences in early and late outcomes between ONCABG and OPCABG.
to explore whether the length of the follow‐up, the surgeons’ experience in OPCABG, and the degree of incomplete revascularization in the OPCABG arm impact the comparative results between the 2 techniques.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Search Strategy and Study Selection
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.5
PubMed and OVID's version of MEDLINE archives were searched from inception to August 2017 for publications comparing ONCABG versus OPCABG surgeries. The following keywords were combined with the Boolean operator “or:” “Coronary Artery Bypass,” “CABG,” “aorto coronary bypass,” “aorto coronary anastomosis,” “aorto coronary,” “bypass or graft,” “coronary,” “revascularization or revascularisation or cardiopulmonary bypass,” “on pump or on‐pump,” “off‐Pump,” “off pump or off‐pump or OPCAB,” “beating heart.” The full search strategy can be found in Data S1. All citations were screened for study inclusion independently by 2 investigators (AA and JL). Any disagreements were discussed and resolved by consensus with a third investigator (MG). In addition, the bibliography of all studies and meta‐analyses were searched to identify further publications.
Inclusion criteria were the following:
RCTs comparing OPCABG and ONCABG in patients with multivessel coronary disease reporting any outcome and with any follow‐up duration.
Full‐text articles written in the English language.
Studies reporting different outcomes from the same trial were classified together.
Risk of bias among included trials were appraised by 2 independent investigators (UB and ADF) based on the “risk of bias assessment tool” provided by the Cochrane collaboration,6 in which 7 domains were assessed for each RCT: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and presence of other bias. The presence of a possible source of bias in each domain was assessed, and a final judgment of low, unclear, or high risk of bias was assigned.
Data Abstraction
Two investigators (AA and MG) independently abstracted the following: study demographics (study period, country, centers involved, sample size), patient characteristics (age, sex, dyslipidemia, hypertension, diabetes mellitus, ejection fraction, urgency, prior cerebrovascular accident, prior percutaneous intervention, previous myocardial infarction (MI), and peripheral vascular disease), mean follow‐up, operative characteristics (diseased vessels, left main disease, number of grafts per patient, mean number of arterial grafts per patient, distal anastomosis, completeness of revascularization, crossover rates in OPCABG).
The definitions used by the authors in the original articles were used for all the abstracted variables.
Continuous variables were expressed as median (interquartile range) or as mean±SD. Categorical variables were reported as frequency (%).
Risk difference (RD) was used in preference to odds ratio for the short‐term outcomes because of the high number of studies with zero events in both comparison arms. The analysis using odds ratios was used as sensitivity analysis and the results are given in Data S1.
The incidence rate ratio (IRR) was used for the long‐term outcomes.
Outcome Analyses
The primary outcome was all‐cause mortality at the longest reported follow‐up.
The secondary outcomes were operative mortality, perioperative stroke, perioperative MI, and late repeated revascularization.
To assess the hypotheses that the length of the follow‐up, the surgeons’ experience with OPCABG, and the completeness of revascularization in the OPCABG arm could influence the results of the comparison between the 2 techniques, we performed the following subgroup analyses for the primary outcome:
Length of the follow‐up
Studies with a mean follow‐up <3 years were compared with studies with a mean follow‐up ≥3 years.
Surgeons’ experience
The proportion of patients who crossed over from OPCABG to ONCABG was used as a surrogate for surgeons’ experience with OPCABG. We divided the included studies into 3 groups according to the percentage of OPCABG crossover: ≤3%, >3, and <10%, ≥10%.
Relative difference in the rate of incomplete revascularization
We calculated the relative rate of incomplete revascularization by dividing the rate of incomplete revascularization in the OPCABG arm by the rate of incomplete revascularization in the ONCABG arm and compared studies with a relative rate of incomplete revascularization >2 to studies with a relative rate of incomplete revascularization ≤2.
All boundaries for subgroup analysis were selected based on the authors’ opinion and experience. For all subgroup analyses, P value for subgroups differences was reported.
Statistical Analysis
Relative effect estimates were calculated as risk differences and log IRRs with 95% confidence intervals (95% CIs) for short‐term and long‐term outcomes, respectively. We pooled late outcomes as the natural logarithm of the IRR to account for potentially different follow‐up durations between different treatments. We estimated the IRR through different means depending on the available study data. When hazard ratios were reported, we took the natural logarithm of the hazard ratio with the standard error calculated from the 95% CI or log rank P value. When Kaplan‐Meier curves were available, event rates were estimated from the curves using GetData Graph Digitizer software 2.26 (http://getdata-graph-digitizer.com/) and in case Kaplan‐Meier curves were not available, we used the reported event rates to calculate the IRR.7
IRRs were pooled using random effect model with generic inverse variance (DerSimonian and Laird method);8 however, we also reported fixed‐effect model results in Data S1. Heterogeneity was reported as low (I2=0–25%), moderate (I2=26–50%), and high (I2 >50%).9 In all comparisons, the ONCABG group was used as a reference.
For the primary outcome, leave‐1‐out analysis and funnel plot with trim‐and‐fill method to assess for publication bias were performed. Egger's test together with visual inspection were used to assess for funnel asymmetry.9 Univariate meta‐regression was used to assess the relationship between the proportion of patients who crossed over from OPCABG to ONCABG and the completeness of revascularization. Restricted cubic spline analysis10 was used to assess the effect of crossover on long‐term mortality.
Meta and metaphor packages in R (version 3.3.3 R Project for Statistical Computing) using Rstudio were used for analyses. Review Manager (RevMan) v. 5.3 was used for risk of bias assessment.
Results
Selected Studies
From 1906 titles, 160 pertinent studies were included for full‐text review. We excluded 56 studies that did not meet the inclusion criteria. Further details of the study flow are shown in Figure S1. A total of 104 RCTs were selected for the quantitative analysis (individual references are given in Data S1).
The selected trials reported on 20 627 patients (OPCABG: 10 288; ONCABG: 10 339). The studies were published from 1995 to 2017, and the sample size ranged from 9 to 2375 patients.
Ten studies (3591 patients) had a mean follow‐up <3 years and 9 (8532 patients) had a mean follow‐up ≥3 years. The percentage of crossover was ≤3% in 6 studies, >3 to <10% in 8, and ≥10% in 4 studies, representing 33.3%, 44.4%, and 22.2% of all studies and 10.0%, 67.9%, and 22.1% of all patients included in the subgroup analysis on long‐term mortality based on the crossover rate from OPCABG group, respectively. The relative rate of incomplete revascularization was ≤2% in 5 studies and >2% in 5 trials.
Overall, the OPCABG and ONCABG groups had similar preoperative risk factor distributions: the mean age ranged from 47.2 to 78.6 years in the OPCAB group versus 49.4 to 78.4 years in the ONCAB. The rates of the most relevant preoperative characteristics in the 2 groups were as follows: male sex, 79.25% OPCABG versus 79.9% in the ONCABG; hypertension 73.1% OPCABG versus 66.9% ONCABG, diabetes mellitus 24.5% OPCABG versus 24.17% ONCABG, previous stroke 8.45% OPCABG versus 7.99% ONCABG, previous MI 21.2% OPCABG versus 20.1% ONCABG (Table S1; details of operative data are reported in Table S2).
Primary Outcome
The weighted mean follow‐up time across the 19 studies that reported long‐term mortality was 3.7 years (range 1–7.5 years). The overall mortality rate at the end of follow‐up was 12.3% in the OPCABG versus 11.1% in the ONCABG series.
There was a borderline statistically significant excess risk of mortality at follow‐up in the OPCABG group (IRR 1.11, 95% CI 1.00–1.23, P=0.05, Figure 1).3, 4, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Figure 1.
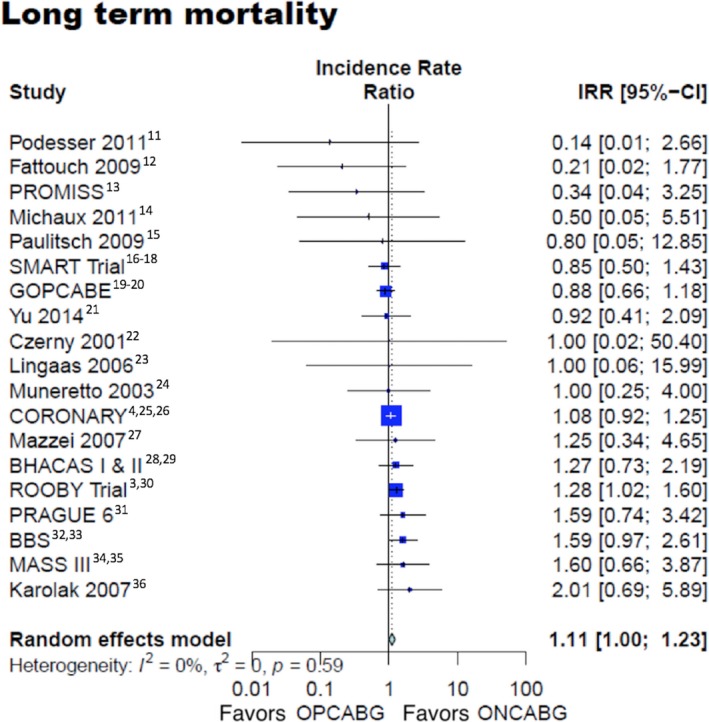
Long‐term mortality. 95% CI indicates 95% confidence interval; IRR, incidence rate ratio; ONCABG, on‐pump coronary artery bypass surgery; OPCABG, off‐pump coronary artery bypass surgery.
Leave‐1‐out analysis and funnel plot with trim‐and‐fill method for the primary outcome are shown in Figure S2.
In the subgroup analysis based on the length of the mean follow‐up, late mortality was higher in the OPCABG group (IRR 1.16, 95% CI 1.01–1.34) in studies with ≥3‐year follow‐up (Figure 2)3, 4, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 and no different in studies with <3 years of follow‐up (IRR 0.93, 95% CI 0.72–1.20). The test for subgroup difference was not significant (Table S3, interaction P=0.13).
Figure 2.
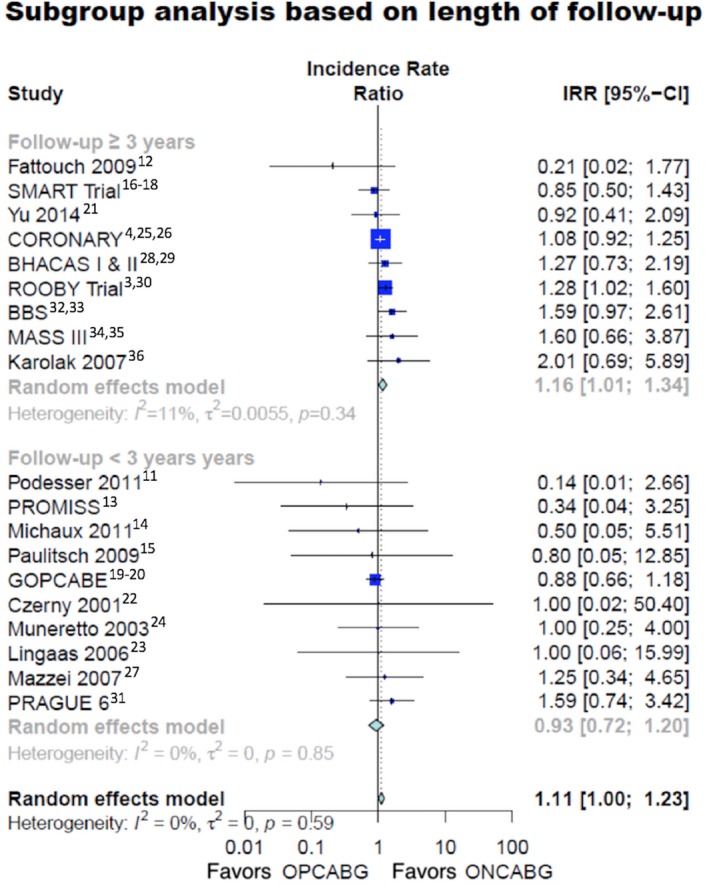
Long‐term mortality in different subgroups based on the length of follow‐up. 95% CI indicates 95% confidence interval; IRR, incidence rate ratio; ONCABG, on‐pump coronary artery bypass surgery; OPCABG, off‐pump coronary artery bypass surgery.
In the subgroup analysis based on the percentage of crossover from the OPBAG group, there was no difference in late mortality for studies with a crossover rate of ≤3% (IRR 0.96, 95% CI 0.60–1.42) or those with a crossover rate between 3% and 10% (IRR 1.07, 95% CI 0.94–1.21). However, there was a statistically significant excess risk of late mortality in the OPCABG group for studies with a crossover rate ≥10% (Figure 3—IRR 1.30, 95% CI 1.04–1.62).3, 4, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Figure 3.
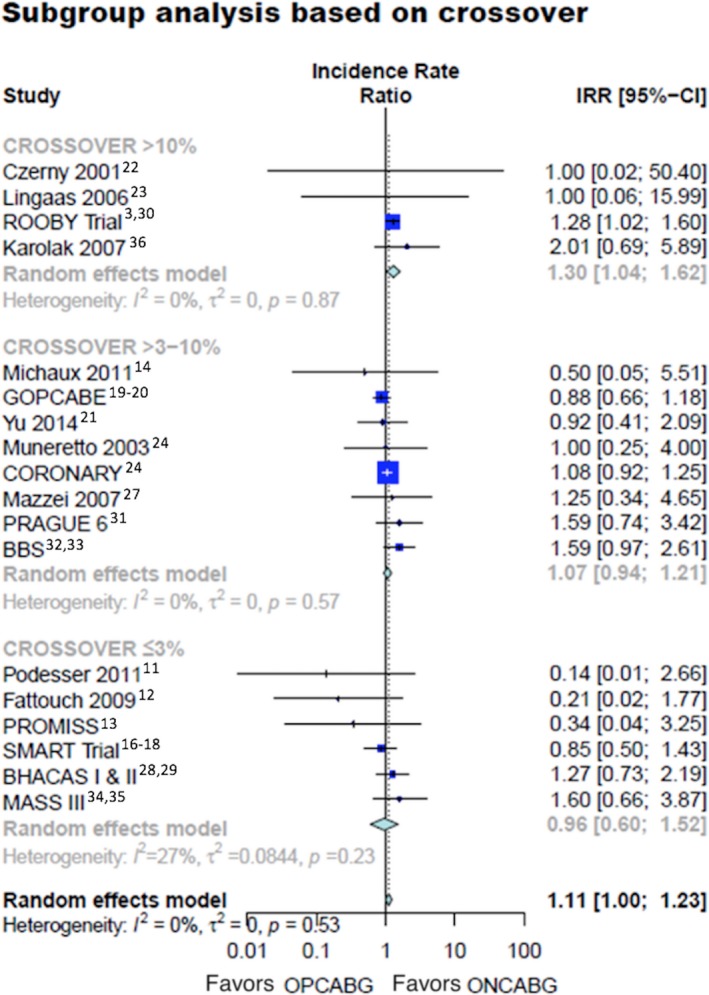
Long‐term mortality in different subgroups based on the crossover rate from OPCABG group. 95% CI indicates 95% confidence interval; IRR, incidence rate ratio; ONCABG, on‐pump coronary artery bypass surgery; OPCABG, off‐pump coronary artery bypass surgery.
The test for subgroup differences was not significant (Table S3—Interaction P=0.27) Assessment by restricted cubic spline showed that the inflection point was around 9.5% (β coefficient=5.68, 95% CI 0.42–10.94, P=0.03) (Figure S3).
In the subgroup analysis based on completeness of revascularization, there was no difference in mortality for those with a high degree of incomplete revascularization (Figure 4—IRR 1.16, 95% CI 0.91–1.48)* and those with a lower degree of incomplete revascularization (IRR 0.81, 95% CI 0.44–1.48). Test for subgroup differences was not significant (interaction P=0.28).
Figure 4.
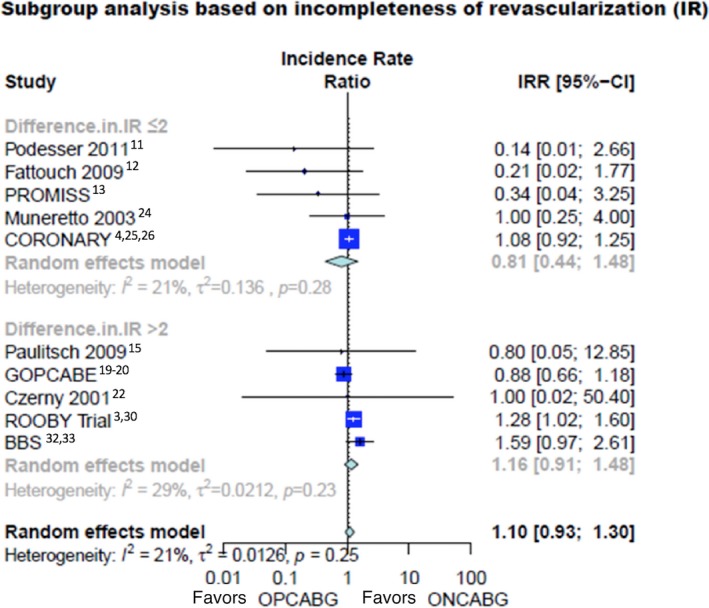
Long‐term mortality in different subgroups based on the relative rate of incomplete revascularization. 95% CI indicates 95% confidence interval; IR, incomplete revascularization; IRR, incidence rate ratio; ONCABG, on‐pump coronary artery bypass surgery; OPCABG, off‐pump coronary artery bypass surgery.
At meta‐regression, there was a correlation between the proportion of patients who crossed over from OPCABG to ONCABG and the rate of incomplete revascularization in the OPCABG arm (β=0.0019, P=0.03).
Secondary Outcomes
Operative mortality was 1.6% in the OPCABG group and 2.0% in the ONCABG series. No significant differences in operative mortality (RD −0.17, 95% CI −0.53 to 0.19, P=0.37) and perioperative MI (RD −0.4, 95% CI −0.95 to 0.15], P=0.15) were found between the 2 groups. A nonsignificant trend toward lower perioperative stroke in the OPCABG group was evident (RD −0.34, 95% CI −0.68 to 0.00, P=0.05). The difference in stroke became significant when using odds ratio instead of RD (Figures S4 through S9, Table S3).
The incidence of late repeated revascularization was not statistically different between OPCABG and ONCABG, although it was numerically higher in the OPCABG patients (IRR 1.17, 95% CI 0.98–1.38) (Figure S10).
Discussion
This analysis was undertaken to address 3 important clinical questions: the effect of the length of follow‐up, the surgeons’ experience in OPCABG, and the completeness of revascularization in the OPCABG arm in determining the results of the comparison between the 2 techniques.
We found that:
The use of OPCABG was not associated with a decrease in operative mortality. The perioperative outcome of the 2 groups was similar, although OPCABG was associated with a reduced perioperative stroke rate. At follow‐up, however, there was a significant increase in the risk of mortality for patients who underwent OPCABG. A trend toward higher risk of late repeated revascularization was also noted in the off‐pump series, supporting the hypothesis of less effective coronary revascularization as the cause of the difference in mortality.
Our findings of higher late mortality with OPCABG may have been driven by the length of study follow‐up. While late survival was similar for studies with a mean follow‐up of <3 years, there was a significant difference in mortality in favor of ONCABG when the study follow‐up was >3 years.
We used the proportion of patients who crossed over from OPCABG to ONCABG as a surrogate for surgeons’ experience. At subgroup analysis we found no difference in late mortality in studies with low crossover (0%–10%). However, in studies with 10% or higher crossover, survival was significantly reduced in the off‐pump group.
Although our subgroup analysis examining the degree of incomplete revascularization found no statistical difference between OPCABG and ONCABG, visually, it appeared that those with a higher degree of incomplete revascularization had worst survival compared with those with less incomplete revascularization.
As the percentage of crossover from the OPCABG group increased, the rate of incomplete revascularization in the off‐pump arm also increased.
In the past 3 years, 3 meta‐analyses comparing OPCABG and ONCABG have been published. Deppe and colleagues examined 51 RCTs and combined data of 16 904 patients.37 They found similar incidence of major adverse cardiac and cerebrovascular events in the off‐ and on pump series at 30 days and long‐term follow‐up. Of note, the authors used odds ratios as estimators of risk even for long‐term outcomes.
Kowalewski and associates pooled data from 100 RCTs and 19 192 patients to compare short‐term outcomes between the 2 techniques.38 The authors reported significantly lower risk of stroke in the OPCABG arm and no differences in mortality and MI between groups.
Filardo et al, in a meta‐analysis of 42 RCTs and 31 risk‐adjusted observational studies, found lower short‐term mortality but an excess of 10% in 5‐year mortality for OPCABG.39
Our analysis includes 2 large recently published trials comparing OPCABG to ONCABG3, 4 for a total of 104 RCTs and 20 627 patients.
Contrary to previous analyses, we have used IRR for time‐to‐event outcomes in order to account for the difference in follow‐up among the different trials. Because of the demonstrated variation of the results of the comparison between OPCABG and ONCABG with the time, adjusting for the length of follow‐up seems particularly important in this type of analysis. Also, we focused on RCTs only, to avoid unmatched confounder and bias intrinsic to observational studies comparing different surgical techniques40 and provide a synthesis of the best possible evidence.
Another novel aspect of this meta‐analysis includes the examination of the relationship between surgical confidence with OPCABG and long‐term survival. OPCABG is technically more challenging than ONCABG, and it has been shown that the volume–outcome effect is much steeper for off‐pump procedures.41 We used crossover from the OPCABG arm as a surrogate of surgeons’ experience and we postulated an inverse relationship between crossover and surgeons’ confidence with OPCABG. The results support this hypothesis because the mortality benefit of ONCABG was greater in the higher crossover studies than in the studies with a low conversion rate.
While some crossover of patients who were initially assigned to OPCABG is inevitable because of patient anatomy, a high crossover rate suggests that surgeon experience may be lacking in expertly performing off‐pump surgery or predicting which patients will best tolerate off‐pump surgery. In a retrospective review of the Nationwide Inpatient Sample of over 2 million patients undergoing CABG, we previously showed that multivessel OPCABG performed at low OPCABG volume centers or by low volume surgeons is associated with increased mortality.41
Completeness of revascularization is likely related to the surgeon experience and comfort with the more complex off‐pump technique. In our analysis, we found a trend toward better OPCABG results for studies with higher rate of complete revascularization in the off‐pump group. Of note, there was a statistically significant correlation between the percentage of crossover from the OPCABG group and the completeness of revascularization in the off‐pump series, suggesting that both may be consequences of surgeon experience.
Taken together, those findings suggest that surgeon's comfort with OPCABG plays an important role in determining the results of the technique and the comparison with ONCABG. More experienced OPCABG teams (including cardiac surgeons, cardiac anesthesiologists, scrub nurses, and technicians) have lower crossover rate, achieve higher rate of complete revascularization, and have results similar to those of ONCABG.
A purported benefit of OPCABG includes a reduction of neurological events or stroke as a result of minimal or no aortic manipulation. In our meta‐analysis, a trend toward reduced perioperative stroke in the off‐pump arm was noted, but this difference did not reach statistical significance. Wide heterogeneity in off‐pump practices may explain this finding—techniques range from complete anaortic to the use of a side‐biting clamp for construction of the proximal anastomoses. A meta‐analysis of mostly observational studies that compared anaortic OPCABG to OPCABG that involved aortic manipulation with a side‐biting clamp showed a 61% risk reduction in stroke with the anaortic technique.42 However, all RCTs that compare OPCABG to ONCABG allowed for variation in the procedural conduct of CABG; thus, the separation of anaortic versus the use of a partial clamp is not possible in a meta‐analysis of RCTs. Furthermore, the use of an anaortic technique may increase the arterial graft utilization (namely, a bilateral internal thoracic artery grafting strategy), which may bias results towards OPCABG. Of note, improved neurological outcomes with ONCABG have been described with the use of the single cross‐clamp technique.43
This study must be interpreted in the context of some important limitations. Intrinsic to any meta‐analysis is a concern for publication bias. However, our funnel plot analysis for the primary outcome suggests that there was no large publication bias. The enrolled trials did not standardize the details of the surgical technique. There was wide variation in how both the OPCABG and ONCABG was performed, in particular with respect to aortic manipulation and the number and type of arterial grafts used. Furthermore, heterogeneity in our study was low, suggesting that these differences may have been minimal.
We used subgroup analyses to explore the potential effect of length of the follow‐up, cross‐over, and completeness of revascularization on the primary outcome. We acknowledge that post hoc subgroup analyses are only hypothesis generating and that the number of studies in each subgroup was limited (despite having a large total sample size). Also, we established the boundaries between subgroups mainly based on our clinical judgment and expertise and not using a purely statistical method. However, we did conduct a cubic spline analysis, which suggested that a 10% crossover rate may be the threshold for differences in late mortality. Because this analysis was meant to answer a clinically relevant question based on surgical considerations, we decided to privilege a clinical rather than statistical approach, but this is open to critique. The use of crossover as a marker of surgeons’ experience has intrinsic limitations and may also be criticized. Furthermore, we recognize that by performing multiple subgroup analyses without correction, there is an increased risk for type 1 error (ie, false‐positive results) in our observed patterns.
In addition, we could not specify the cause of follow‐up death as well as the reasons for crossing over, and we had no information on the use of multiple arterial grafts (a potential effect modifier for long‐term outcomes) and on the details of the surgical technique used, in particular regarding the degree of aortic manipulation (a potential modifier for short‐term outcomes, especially perioperative stroke). Also, we could not capture the reason for crossover. This is particularly relevant because the reason for conversion to ONCABG itself might influence patients’ outcomes. In an analysis of the Society of Thoracic Surgeons Adult Cardiac Database,44 patients converted for hemodynamic instability or bleeding (representing 37.2% of all conversions) had an in‐hospital mortality rate of 6.4% (observed‐to‐expected ratio, 2.38), whereas patients converted for poor visualization (representing 13.2% of all conversions) had an in‐hospital mortality of 2.4% (observed‐to‐expected ratio, 1.33).
Finally, while we showed that the proportion of crossover may have an impact on the primary outcome, we do not have enough information to explain the mechanism—whether it could be related to surgeon's experience or a dilution of the true treatment benefit with OPCABG because of intention‐to‐treat analysis in the RCT.
In summary, this meta‐analysis represents a comprehensive summary of RCTs comparing OPCABG to ONCABG. Our results showed that OPCABG was associated with no reduction in operative risk, an excess mortality at follow‐up ≥3 years, and a trend toward higher risk of repeated revascularization.
We hypothesize that a surgeon's experience in OPCABG may be associated with late mortality and that OPCABG can be comparable to ONCABG only in the hands of experienced operators.
Disclosures
None.
Supporting information
Data S1. Full Search Strategy.
Table S1. Patients’ Characteristics
Table S2. Operative Data
Table S3. Results of the Analysis of the Long‐Term Mortality (overall and in different subgroups based on follow‐up period, crossover, and difference in incomplete revascularization) Using Incidence Rate Ratio and of the Short‐Term Outcomes Using Odds Ratio
Figure S1. PRISMA flowchart.
Figure S2. A, Leave 1‐out‐analysis and (B) funnel plot with trim and fill for the primary outcome.
Figure S3. Results of meta‐regression for the effect of crossover on long‐term mortality using restricted cubic spline (B coefficient=5.6814, CI [0.42–10.94], P=0.03).
Figure S4. Operative mortality (as assessed using Risk Difference).
Figure S5. Perioperative myocardial infarction (as assessed using Risk Difference).
Figure S6. Perioperative stroke (as assessed using Risk Difference).
Figure S7. Operative mortality (as assessed using Odds Ratios).
Figure S8. Perioperative myocardial infarction (as assessed using Odds Ratios).
Figure S9. Perioperative stroke (as assessed using Odds Ratios).
Figure S10. Late repeated revascularization.
(J Am Heart Assoc. 2018;7:e010034 DOI: 10.1161/JAHA.118.010034.)
Note
References
- 1. Vilahur G, Badimon J, Bugiardini R, Badimon L. Perspectives: the burden of cardiovascular risk factors and coronary heart disease in Europe and worldwide. Eur Heart J Suppl. 2014;16:A7–A11. [Google Scholar]
- 2. Tam DY, Fremes SE. Can we settle the on‐pump or off‐pump debate with more than a million patients? J Thorac Cardiovasc Surg. 2018;155:180–181. [DOI] [PubMed] [Google Scholar]
- 3. Shroyer AL, Hattler B, Wagner TH, Collins JF, Baltz JH, Quin JA, Almassi GH, Kozora E, Bakaeen F, Cleveland JC, Bishawi M, Grover FL; Veterans Affairs ROOBY‐FS Group . Five‐year outcomes after on‐pump and off‐pump coronary‐artery bypass. N Engl J Med. 2017;377:623–632. [DOI] [PubMed] [Google Scholar]
- 4. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Straka Z, Piegas LS, Avezum A, Akar AR, Lanas Cas F, Jain AR, Noiseux N, Padmanabhan C, Bahamondes J‐C, Novick RJ, Tao L, Olavegogeascoechea PA, Airan B, Sulling T‐A, Whitlock RP, Ou Y, Gao P, Pettit S, Yusuf S; CORONARY Investigators . Five‐year outcomes after off‐pump or on‐pump coronary‐artery bypass grafting. N Engl J Med. 2016;375:2359–2368. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC; Cochrane Bias Methods Group, Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 9. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harre FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. [DOI] [PubMed] [Google Scholar]
- 11. Podesser B, Holzinger C, Binder K, Schor I, Haumberger W, Valicek G, Wendler R, Ameri L, Dietl W, Trescher K, Kassal H. Off‐pump multi‐vessel revascularization in patients with poor left ventricular function. Eur Surg. 2011;42:103–109. [Google Scholar]
- 12. Fattouch K, Guccione F, Dioguardi P, Sampognaro R, Corrado E, Caruso M, Ruvolo G. Off‐pump versus on‐pump myocardial revascularization in patients with ST‐segment elevation myocardial infarction: a randomized trial. J Thorac Cardiovasc Surg. 2009;137:650–657. [DOI] [PubMed] [Google Scholar]
- 13. Sousa Uva M, Cavaco S, Oliveira AG, Matias F, Silva C, Mesquita A, Aguiar P, Bau J, Pedro A, Magalhães MP. Early graft patency after off‐pump and on‐pump coronary bypass surgery: a prospective randomized study. Eur Heart J. 2010;31:2492–2499. [DOI] [PubMed] [Google Scholar]
- 14. Michaux I, Filipovic M, Skarvan K, Bolliger D, Schumann R, Bernet F, Seeberger M. A randomized comparison of right ventricular function after on‐pump versus off‐pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011;141:361–367. [DOI] [PubMed] [Google Scholar]
- 15. Paulitsch FS, Schneider D, Sobel BE, Rached R, Ramires J, Jatene F, Stolf N, Hueb W, Lopes NH. Hemostatic changes and clinical sequelae after on‐pump compared with off‐pump coronary artery bypass surgery: a prospective randomized study. Coron Artery Dis. 2009;20:100–105. [DOI] [PubMed] [Google Scholar]
- 16. Puskas JD, Williams WH, Duke PG, Staples JR, Glas KE, Marshall JJ, Leimbach M, Huber P, Garas S, Sammons BH, McCall SA, Petersen RJ, Bailey DE, Chu H, Mahoney EM, Weintraub WS, Guyton RA. Off‐pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off‐pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. [DOI] [PubMed] [Google Scholar]
- 17. Puskas JD, Williams WH, Mahoney EM, Huber PR, Block PC, Duke PG, Staples JR, Glas KE, Marshall JJ, Leimbach ME, McCall SA, Petersen RJ, Bailey DE, Weintraub WS, Guyton RA. Off‐pump vs conventional coronary artery bypass grafting: early and 1‐year graft patency, cost, and quality‐of‐life outcomes: a randomized trial. JAMA. 2004;291:1841–1849. [DOI] [PubMed] [Google Scholar]
- 18. Puskas JD, Williams WH, O'Donnell R, Patterson RE, Sigman SR, Smith AS, Baio KT, Kilgo PD, Guyton RA. Off‐pump and on‐pump coronary artery bypass grafting are associated with similar graft patency, myocardial ischemia, and freedom from reintervention: long‐term follow‐up of a randomized trial. Ann Thorac Surg. 2011;91:1836–1843. [DOI] [PubMed] [Google Scholar]
- 19. Faerber G, Zacher M, Reents W, Boergermann J, Kappert U, Boening A, Diegeler A, Doenst T. Female sex is not a risk factor for post procedural mortality in coronary bypass surgery in the elderly: a secondary analysis of the GOPCABE trial. PLoS One. 2017;12:e0184038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diegeler A, Börgermann J, Kappert U, Breuer M, Böning A, Ursulescu A, Rastan A, Holzhey D, Treede H, Rieß F‐C, Veeckmann P, Asfoor A, Reents W, Zacher M, Hilker M; GOPCABE Study Group . Off‐pump versus on‐pump coronary‐artery bypass grafting in elderly patients. N Engl J Med. 2013;368:1189–1198. [DOI] [PubMed] [Google Scholar]
- 21. Yu L, Gu T, Shi E, Wang C, Fang Q, Yu Y, Zhao X, Qian C. Off‐pump versus on‐pump coronary artery bypass surgery in patients with triple‐vessel disease and enlarged ventricles. Ann Saudi Med. 2014;34:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Czerny M, Baumer H, Kilo J, Zuckermann A, Grubhofer G, Chevtchik O, Wolner E, Grimm M. Complete revascularization in coronary artery bypass grafting with and without cardiopulmonary bypass. Ann Thorac Surg. 2001;71:165–169. [DOI] [PubMed] [Google Scholar]
- 23. Lingaas PS, Hol PK, Lundblad R, Rein KA, Mathisen L, Smith H‐J, Andersen R, Thaulow E, Tønnesen TI, Svennevig JL, Hauge SN, Fredriksen PM, Andersen M, Fosse E. Clinical and radiologic outcome of off‐pump coronary surgery at 12 months follow‐up: a prospective randomized trial. Ann Thorac Surg. 2006;81:2089–2095. [DOI] [PubMed] [Google Scholar]
- 24. Muneretto C, Bisleri G, Negri A, Manfredi J, Metra M, Nodari S, Dei Cas L. Off‐pump coronary artery bypass surgery technique for total arterial myocardial revascularization: a prospective randomized study. Ann Thorac Surg. 2003;76:778–783. [DOI] [PubMed] [Google Scholar]
- 25. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes J‐C, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling T‐A, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S; CORONARY Investigators . Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. [DOI] [PubMed] [Google Scholar]
- 26. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes J‐C, Novick RJ, Vaijyanath P, Reddy SK, Tao L, Olavegogeascoechea PA, Airan B, Sulling T‐A, Whitlock RP, Ou Y, Pogue J, Chrolavicius S, Yusuf S; CORONARY Investigators . Effects of off‐pump and on‐pump coronary‐artery bypass grafting at 1 year. N Engl J Med. 2013;368:1179–1188. [DOI] [PubMed] [Google Scholar]
- 27. Mazzei V, Nasso G, Salamone G, Castorino F, Tommasini A, Anselmi A. Prospective randomized comparison of coronary bypass grafting with Minimal Extracorporeal Circulation System (MECC) versus off‐pump coronary surgery. Circulation. 2007;116:1761–1767. [DOI] [PubMed] [Google Scholar]
- 28. Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off‐pump and on‐pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194–1199. [DOI] [PubMed] [Google Scholar]
- 29. Angelini GD, Culliford L, Smith DK, Hamilton MCK, Murphy GJ, Ascione R, Baumbach A, Reeves BC. Effects of on‐ and off‐pump coronary artery surgery on graft patency, survival, and health‐related quality of life: long‐term follow‐up of 2 randomized controlled trials. J Thorac Cardiovasc Surg. 2009;137:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D; Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group . On‐pump versus off‐pump coronary‐artery bypass surgery. N Engl J Med. 2009;361:1827–1837.19890125 [Google Scholar]
- 31. Hlavicka J, Straka Z, Jelinek S, Budera P, Vanek T, Maly M, Widimsky P. Off‐pump versus on‐pump coronary artery bypass grafting surgery in high‐risk patients: PRAGUE‐6 trial at 30 days and 1 year. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:263–270. [DOI] [PubMed] [Google Scholar]
- 32. Moller CH, Perko MJ, Lund JT, Andersen LW, Kelbaek H, Madsen JK, Winkel P, Gluud C, Steinbruchel DA. No major differences in 30‐day outcomes in high‐risk patients randomized to off‐pump versus on‐pump coronary bypass surgery: the best bypass surgery trial. Circulation. 2010;121:498–504. [DOI] [PubMed] [Google Scholar]
- 33. Møller CH, Perko MJ, Lund JT, Andersen LW, Kelbæk H, Madsen JK, Winkel P, Gluud C, Steinbrüchel DA. Three‐year follow‐up in a subset of high‐risk patients randomly assigned to off‐pump versus on‐pump coronary artery bypass surgery: the Best Bypass Surgery Trial. Heart. 2011;97:907–913. [DOI] [PubMed] [Google Scholar]
- 34. de Melo V, Morel R, Hueb W, Rezende PC, da Costa A, Menezes L, Oikawa FTC, Lima EG, Hueb AC, Scudeler TL, Kalil Filho R. Comparison between off‐pump and on‐pump coronary artery bypass grafting in patients with severe lesions at the circumflex artery territory: 5‐year follow‐up of the MASS III trial. Eur J Cardiothorac Surg. 2015;47:455–458. [DOI] [PubMed] [Google Scholar]
- 35. Hueb W, Lopes NH, Pereira AC, Hueb AC, Soares PR, Favarato D, Vieira RD, Lima EG, Garzillo CL, Paulitch F da S, César LA, Gersh BJ, Ramires JA. Five‐year follow‐up of a randomized comparison between off‐pump and on‐pump stable multivessel coronary artery bypass grafting. The MASS III trial. Circulation. 2010;122:S48–S52. [DOI] [PubMed] [Google Scholar]
- 36. Karolak W, Hirsch G, Buth K, Légaré J‐F. Medium‐term outcomes of coronary artery bypass graft surgery on pump versus off pump: Results from a randomized controlled trial. Am Heart J. 2007;153:689–695. [DOI] [PubMed] [Google Scholar]
- 37. Deppe A‐C, Arbash W, Kuhn EW, Slottosch I, Scherner M, Liakopoulos OJ, Choi Y‐H, Wahlers T. Current evidence of coronary artery bypass grafting off‐pump versus on‐pump: a systematic review with meta‐analysis of over 16,900 patients investigated in randomized controlled trials. Eur J Cardiothorac Surg. 2016;49:1031–1041; discussion 1041. [DOI] [PubMed] [Google Scholar]
- 38. Kowalewski M, Pawliszak W, Malvindi PG, Bokszanski MP, Perlinski D, Raffa GM, Kowalkowska ME, Zaborowska K, Navarese EP, Kolodziejczak M, Kowalewski J, Tarelli G, Taggart DP, Anisimowicz L. Off‐pump coronary artery bypass grafting improves short‐term outcomes in high‐risk patients compared with on‐pump coronary artery bypass grafting: meta‐analysis. J Thorac Cardiovasc Surg. 2016;151:60–77. e1‐58. [DOI] [PubMed] [Google Scholar]
- 39. Filardo G, Hamman BL, da Graca B, Sass DM, Machala NJ, Ismail S, Pollock BD, Collinsworth AW, Grayburn PA. Efficacy and effectiveness of on‐ versus off‐pump coronary artery bypass grafting: A meta‐analysis of mortality and survival. J Thorac Cardiovasc Surg. 2018;155:172–179.e5. [DOI] [PubMed] [Google Scholar]
- 40. Gaudino M, Di Franco A, Rahouma M, Tam DY, Iannaccone M, Deb S, D'Ascenzo F, Abouarab AA, Girardi LN, Taggart DP, Fremes SE. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta‐analysis. J Am Heart Assoc. 2018;7:e008010 DOI: 10.1161/JAHA.117.008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benedetto U, Lau C, Caputo M, Kim L, Feldman DN, Ohmes LB, Di Franco A, Soletti G, Angelini GD, Girardi LN, Gaudino M. Comparison of outcomes for off‐pump versus on‐pump coronary artery bypass grafting in low‐volume and high‐volume centers and by low‐volume and high‐volume surgeons. Am J Cardiol. 2018;121:552–557. [DOI] [PubMed] [Google Scholar]
- 42. Zhao DF, Edelman JJ, Seco M, Bannon PG, Wilson MK, Byrom MJ, Thourani V, Lamy A, Taggart DP, Puskas JD, Vallely MP. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta‐analysis. J Am Coll Cardiol. 2017;69:924–936. [DOI] [PubMed] [Google Scholar]
- 43. Hammon JW, Stump DA, Butterworth JF, Moody DM, Rorie K, Deal DD, Kincaid EH, Oaks TE, Kon ND. Coronary artery bypass grafting with single cross‐clamp results in fewer persistent neuropsychological deficits than multiple clamp or off‐pump coronary artery bypass grafting. Ann Thorac Surg. 2007;84:1174–1178; discussion 1178–1179. [DOI] [PubMed] [Google Scholar]
- 44. Keeling B, Thourani V, Aliawadi G, Kim S, Cyr D, Badhwar V, Jacobs JP, Brennan JM, Meza J, Matsouaka R, Halkos ME. Conversion from off‐pump coronary artery bypass grafting to on‐pump coronary artery bypass grafting. Ann Thorac Surg. 2017;104:1267–1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Full Search Strategy.
Table S1. Patients’ Characteristics
Table S2. Operative Data
Table S3. Results of the Analysis of the Long‐Term Mortality (overall and in different subgroups based on follow‐up period, crossover, and difference in incomplete revascularization) Using Incidence Rate Ratio and of the Short‐Term Outcomes Using Odds Ratio
Figure S1. PRISMA flowchart.
Figure S2. A, Leave 1‐out‐analysis and (B) funnel plot with trim and fill for the primary outcome.
Figure S3. Results of meta‐regression for the effect of crossover on long‐term mortality using restricted cubic spline (B coefficient=5.6814, CI [0.42–10.94], P=0.03).
Figure S4. Operative mortality (as assessed using Risk Difference).
Figure S5. Perioperative myocardial infarction (as assessed using Risk Difference).
Figure S6. Perioperative stroke (as assessed using Risk Difference).
Figure S7. Operative mortality (as assessed using Odds Ratios).
Figure S8. Perioperative myocardial infarction (as assessed using Odds Ratios).
Figure S9. Perioperative stroke (as assessed using Odds Ratios).
Figure S10. Late repeated revascularization.


