Abstract
Background
Because systemic inflammation and endothelial dysfunction lead to heart failure with preserved ejection fraction, we characterized plasma levels of inflammatory and cardiac remodeling biomarkers in patients with Fabry disease (FD).
Methods and Results
Plasma biomarkers were studied in multicenter cohorts of patients with FD (n=68) and healthy controls (n=40). Plasma levels of the following markers of inflammation and cardiac remodeling were determined: tumor necrosis factor (TNF), TNF receptor 1 (TNFR1) and 2 (TNFR2), interleukin‐6, matrix metalloprotease‐2 (MMP‐2), MMP‐8, MMP‐9, galectin‐1, galectin‐3, B‐type natriuretic peptide (BNP), midregional pro–atrial natriuretic peptide (MR‐proANP), and globotriaosylsphingosine. Clinical profile, cardiac magnetic resonance imaging, and echocardiogram were reviewed and correlated with biomarkers. Patients with FD had elevated plasma levels of BNP, MR‐proANP, MMP‐2, MMP‐9, TNF, TNFR1, TNFR2, interleukin‐6, galectin‐1, globotriaosylsphingosine, and analogues. Plasma TNFR2, TNF, interleukin‐6, MMP‐2, and globotriaosylsphingosine were elevated in FD patients with left ventricular hypertrophy, whereas diastolic dysfunction correlated with higher BNP, MR‐proANP, and MMP‐2 levels. Patients with late gadolinium enhancement on cardiac magnetic resonance imaging had greater levels of BNP, MR‐proANP, TNFR1, TNFR2, and MMP‐2. Plasma BNP, MR‐proANP, MMP‐2, MMP‐8, TNF, TNFR1, TNFR2, galectin‐1, and galectin‐3 were elevated in patients with renal dysfunction. Patients undergoing enzyme replacement therapy who have more severe disease had higher MMP‐2, TNF, TNFR1, TNFR2, and globotriaosylsphingosine analogue levels.
Conclusions
Inflammatory and cardiac remodeling biomarkers are elevated in FD patients and correlate with disease progression. These features are consistent with a phenotype dominated by heart failure with preserved ejection fraction and suggest a key pathogenic role of systemic inflammation in FD.
Keywords: biomarkers, Fabry disease, heart failure with preserved ejection fraction, hypertrophy, inflammation
Subject Categories: Cardiomyopathy, Hypertrophy, Inflammatory Heart Disease, Biomarkers
Clinical Perspective
What Is New?
Inflammatory biomarkers are elevated in patients with Fabry disease.
Markers of disease progression, including kidney disease, left‐ventricular hypertrophy, and myocardial fibrosis, are associated with higher levels of inflammation and markers of adverse cardiac remodeling.
What Are the Clinical Implications?
Systemic inflammation likely plays a pathogenic role in Fabry disease and leads to a phenotype dominated by heart failure with preserved ejection fraction.
Targeting inflammatory pathways may have a therapeutic role in Fabry disease.
Fabry disease (FD, Online Mendelian Inheritance in Man [OMIM] #300644) is an X‐linked lysosomal storage disorder characterized by diminished or absent α‐galactosidase A (EC 3.2.1.22) enzyme activity,1 leading to the accumulation of the glycosphingolipid globotriaosylceramide (Gb3) in tissues.2 Recent neonatal screening data suggest that the actual prevalence is close to 1:3000.3, 4 Because Fabry disease is X‐linked, hemizygous males typically have much lower α‐galactosidase A activity than heterozygous females.5 However, the majority of female heterozygotes develop clinically significant disease, albeit with a milder disease course than hemizygous men, likely due to X‐chromosome inactivation.2, 6 Cardiac manifestations of FD include left ventricular hypertrophy (LVH), diastolic dysfunction, microvascular angina, valvular abnormalities, and conduction defects,7, 8 whereas proteinuria and progression to end‐stage renal disease are renal complications of FD.9 Patients suffering from the classic phenotype of FD typically have early‐onset symptoms with noticeable cardiovascular effects between 30 and 40 years of age,10 ultimately suffering from heart failure with preserved ejection fraction (HFpEF).11, 12
Cardiomyopathy with concentric hypertrophy and diastolic dysfunction is now the most common cause of death in patients with FD.13 FD variants are characterized by the presence of certain GLA gene mutations and are known as cardiac or renal variant phenotypes.2 Enzyme‐replacement therapy (ERT) slows the progression of disease including the development of LVH.7, 14 Plasma biomarkers are a rapidly growing area of research in FD and can provide prognostic value and insight into the pathophysiology of the disease.15, 16 These cytokines have a significant role in cardiac disease, particularly with respect to myocardial remodeling17 and chronic heart failure.18, 19 We report on the plasma levels of a variety of biomarkers in adults with FD compared with healthy controls to gain insight into the pathophysiology and burden of disease of this condition as well as its link to the HFpEF phenotype.
Methods
Ethics and Transparency Statement
All subjects gave written informed consent, and ethics approval was obtained from the University of Alberta (Edmonton, Alberta, Canada), University of Calgary (Calgary, Alberta, Canada), and QE II Health Sciences Centre (Halifax, Nova Scotia, Canada). Due to proprietary techniques used in certain portions of the data analysis section, analytic methods and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Clinicians and researchers are invited to contact the authors for the purposes of data replication or to share biomarkers samples as part of a collaborative effort.
Patient Population
Fabry disease patients (n=68) were recruited through Canadian metabolic clinics in Edmonton, Calgary, and Halifax. Healthy controls (n=40) with similar mean age and sex with no significant medical conditions were recruited through community outreach. Inclusion criteria for healthy controls included no history of cardiovascular disease, hypertension, diabetes mellitus, or renal disease; and no prescriptions for angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, β‐blockers, digoxin, mineralocorticoid‐receptor antagonists, thiazide diuretics, or loop diuretics. Enzyme‐replacement therapy with either agalsidase‐α (Replagal, Shire, Dublin, Ireland) or agalsidase‐β (Fabrazyme, Sanofi‐Genzyme, Cambridge, MA) in standard dose was given to patients who qualified under the Canadian Fabry Disease Initiative treatment guidelines.20
Baseline Analyses
Demographic information including date of birth, sex, height, and weight were collected. Clinical data including genetic mutation analysis, plasma leukocyte α‐galactosidase activity, duration of treatment, serum creatinine, cardiac imaging, and Mainz Severity Score Index (MSSI)21 data were also recorded for FD patients. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation.22 A cut‐off estimated glomerular filtration rate of 60 mL/(min·1.73 m2) was used for assigning kidney disease. LVH was defined using cardiac magnetic resonance imaging (MRI), as described below.
Cardiac Imaging
Transthoracic echocardiography was performed for the Fabry disease cohort as described previously.23, 24 Briefly, echocardiography was performed using standard techniques25 on commercial ultrasound equipment (M3 S Probe, Vivid 7; GE Vingmed Ultrasound AS, Horten, Norway). Chamber quantification and ejection fraction (EF) were assessed using the modified Simpson method.26 Preserved EF was defined as EF ≥50% as assessed by echocardiography. The presence of diastolic dysfunction was graded using the (peak mitral inflow during passive filling in early diastole)/(peak mitral inflow during active filling in atrial systole) ratio, peak mitral inflow velocity during passive filling in early diastole, (peak mitral inflow during passive filling in early diastole)/(mitral annular velocity during early diastole) ratio, and left atrial maximum volume index according to current guidelines,27 unless assessment was judged to be indeterminate or precluded by arrhythmia or patient factors.
Cardiac MRI was performed as previously described in a standardized fashion.24, 28, 29, 30 Cardiac MRI was performed on 1.5‐T Siemens Sonata or Avanto scanners (Siemens Medical Solutions, Erlangen, Germany). Typical imaging parameters using standard balanced steady‐state–free precession short‐axis and long‐axis cines were 1.24 milliseconds echo time, 2.89 milliseconds repetition time, 51° flip angle, 360×270‐mm field of view, 8‐mm slice thickness, and 2‐mm gap between short‐axis slices; 10 to 14 views per segment, reconstructed to 30 phases per cardiac cycle were used. Left‐ventricular mass index was measured in end‐diastole and was calculated using a modified method of disks31 measured from steady‐state free precession cines and analyzed using software (MATLAB 2010a; The MathWorks, Natick, MA) as previously described.31, 32 Assessment of LVH was performed as previously defined using cutoffs of left ventricular mass index ≥85 g/m2 in men and ≥81 g/m2 in women to denote LVH.33 Papillary muscles were included as part of the myocardium for left ventricular mass calculations but excluded for volume assessment. Conventional late gadolinium enhancement (LGE) imaging was performed 7 minutes after contrast injection using a phase‐sensitive inversion recovery sequence in the short‐axis, 2‐, 3‐, and 4‐chamber views to match the cine slice locations. LGE imaging was not performed in 3 patients due to advanced renal disease precluding the administration of gadolinium contrast.
Due to the specialized nature of the data acquisition and analysis, T1 cardiac resonance mapping and extracellular volume calculations were performed only in the Alberta cohort. T1 mapping used the Saturation‐Recovery Single‐Shot Acquisition pulse sequence as previously described.28, 30 T1 mapping was performed at baseline and 15 minutes after administration of 0.15 mmol/kg of gadopentetate dimeglumine (Magnevist; Bayer Inc, Toronto, Ontario, Canada) as previously described.28, 30 Endocardial and epicardial tracings were created for T1 analysis. Blood pool and myocardial T1 analysis were used based on a circular region of interest drawn in the left ventricular blood pool and a 2‐mm‐wide region of interest drawn over the interventricular septum, respectively. Normal left‐ventricular T1 values at our site are 1167±36 milliseconds (baseline) and 600±38 milliseconds (postcontrast) (n=30) in men and 1202±30 milliseconds (baseline) and 539±46 (postcontrast) (n=30) in women.28, 30 In each of the 18 segments the extracellular volume fraction, which is the volume in which gadolinium contrast agent is distributed, was estimated using the calculated concentrations of contrast agent in the blood and tissue.28, 30
Sample Collection and Processing
While patients were sitting and rested, whole blood for plasma analysis was collected into lithium‐heparin and EDTA tubes and stored immediately on ice. Subsequently, plasma fractionation was completed, and the samples were stored in liquid nitrogen at the Canadian BioSample Repository (Edmonton, Alberta, Canada).
Classical Plasma Biomarker Quantification
Enzyme‐linked immunosorbent assays were used to investigate plasma levels of tumor necrosis factor (TNF), NTF receptors (TNFR1, TNFR2), interleukin (IL)‐6, matrix metalloprotease (MMP)‐2, MMP‐8, MMP‐9, galectin‐1, and galectin‐3. Plasma B‐type natriuretic peptide (BNP) and midregional pro–atrial natriuretic peptide (MR‐proANP) levels were assessed as previously described using an Alere Triage reagent pack (Alere Inc, Ottawa, Ontario, Canada) and analyzed using an automated DxI 800 immunoanalyzer (Beckman‐Coulter, Fullerton, CA) at provincial health laboratories in the province of Alberta, Canada.34 Plasma α‐galactosidase activity was assessed as previously described.35 Plasma C‐reactive protein levels were measured using high‐sensitivity kits at provincial health laboratories in the province of Alberta, Canada. Commercial enzyme‐linked immunosorbent assays kits were used to assay plasma levels of TNF, TNFR1, TNFR2, and IL‐6 (catalogue no. HSTA00D, SRT100, SRT200, and HS600B respectively; R&D Systems, Minneapolis, MN) as previously described.36 The described kit protocol was used for enzyme‐linked immunosorbent assays for total MMP‐2, MMP‐8, MMP‐9, galectin‐1, and galectin‐3 (catalogue no. MMP200, DMP800, DMP900, DGAL10, and DGAL30, respectively; R&D Systems, Minneapolis, MN). Absorbance was measured using a SpectraMax M5 Plate Reader (Molecular Devices, San Jose, CA) at 450 nm for all assays with the wavelength correction set to 540 nm for TNFR1, TNFR2, MMP‐2, MMP‐8, MMP‐9, galectin‐1, and galectin‐3 and to 650 nm for TNF and IL‐6. Detection rates for the enzyme‐linked immunosorbent assays were 100% for all assays. The intra‐assay coefficients of variation were 3.7% (n=8), 5.2% (n=8), 3.5% (n=8), 3.6% (n=8), 11.4% (n=8), 13.4% (n=8), 4.6% (n=8), 7.9% (n=8), and 2.1% (n=8) for TNF, TNFR1, TNFR2, IL‐6, MMP‐2, MMP‐8, MMP‐9, galectin‐1, and galectin‐3 assays, respectively.
Analysis of Plasma Lyso‐Gb3 and Analogues
Plasma lysoglobotriaosylceramide (Lyso‐Gb3) and its 6 related analogues with modified sphingosine moieties (−C2H4; −H2; +O; +H2O; +H2O2, +H2O3) were analyzed in the plasma of Fabry patients with a method previously published by Boutin and Auray‐Blais37 (Figure 1). Briefly, 100 μL of plasma was spiked with in‐house–synthesized N‐glycinated Lyso‐Gb3 (Lyso‐Gb3‐Gly) as the internal standard and purified by solid‐phase extraction using mixed‐mode cation‐exchange cartridges (Oasis MCX, 30 mg, 60 μm; Waters Corp, Milford, MA). The samples were separated by ultraperformance liquid chromatography using an Acquity I‐Class (Waters) system equipped with a BEH C18 column (2.1×50 mm, particle diameter 1.7 μm; Waters). The analysis of Lyso‐Gb3 and its 6 analogues was performed by tandem mass spectrometry using the multiple reaction–monitoring mode on a Xevo TQ‐S mass spectrometer (Waters). Positive electrospray was used for the ionization. Plasma total Lyso‐Gb3 was reported as the sum of Lyso‐Gb3 and its 6 analogues.
Figure 1.

Examples of ion chromatograms for lysoglobotriaosylceramide (Lyso‐Gb3), its 6 analogues, and Lyso‐Gb3‐Gly (used as the internal standard) detected in plasma from a Fabry patient. The (+H2O2) analogue has 2 structural isomers with retention times of 3.29 and 4.57 minutes. The areas of these peaks were added together for computation results. cps indicates counts per second.
Statistical Analysis
Statistical analyses were carried out using IBM SPSS Statistics version 20 for Windows (SPSS Inc, Chicago, IL). Discrete variables are presented as count and/or percentage. Continuous variables with normal distributions are presented as mean±SD, whereas continuous variables with skewed distributions, including all biomarkers, are presented as median (first quartile, third quartile) unless otherwise indicated. A P‐value of <0.05 was considered statistically significant. Categorical data were compared using Pearson Chi‐squared tests or the Fisher Exact Test, where appropriate. Pairwise comparisons were evaluated using Mann‐Whitney U Tests or Kruskal‐Wallis Test with Mann‐Whitney U Tests, where appropriate. Analyses of continuous covariates were performed using linear regression with the MSSI versus biomarker levels and left‐atrial size versus MR‐proANP. Outliers identified by visual analysis were tested for potential impacts on the regression analysis. Receiver‐operator characteristic curve analysis was performed using a diagnosis of FD via α‐galactosidase levels and/or genetic testing as the gold standard.
Results
Clinical Characteristics
The mean age (±SD) of FD patients was 42±13 years (n=62) versus 46±12 years for the healthy controls (n=40), and there were equal numbers of women and men in the 2 cohorts (Table 1). The mean body‐mass index was 25.0±2.7 kg/m2 in the healthy controls versus 24.3±4.3 kg/m2 for the FD cohort. Of the 68 FD patients, 41 patients (60%) had LVH by cardiac MRI criteria, and 37 (54%) were receiving ERT. Among FD patients, median plasma α‐galactosidase A activity was 1.9 (0.63, 3.6) μmol/(h·g protein). By use of the MSSI,21 16 (24%) FD patients were classified as having mild disease (MSSI ≤20), 43 (63%) had moderate disease (MSSI 21‐40), and 9 (13%) had severe disease (MSSI ≥41). The mean score of the cardiac subset of MSSI (maximum of 20) was 5.5±4.9. The estimated glomerular filtration rate in FD patients was 83±33 mL/(min·1.73 m2). GLA mutation analysis, phenotype, medication use, and ERT status are reported in Table 2.
Table 1.
Demographic Data and Cardiac Imaging Parameters for the FD Cohort
| Healthy Controls (n=40) | Fabry Disease (n=68) | Fabry Male (n=34) | Fabry Female (n=34) | |
|---|---|---|---|---|
| Demographic and clinical information | ||||
| Age, y | 46±12 | 42±13 | 40±11 | 44±13 |
| Sex (% female) | 50 | 50 | 0 | 100 |
| BMI, kg/m2 | 25.0±2.7 | 24.3±4.3 | 24.3±3.9 | 24.4±4.8 |
| eGFR, mL/(min·1.73 m2) | ··· | 85.9±32.9 | 77.8±36.7 | 94.1±26.6 |
| Echocardiography parameters | ||||
| LVEF, % | ··· | 62.6±8.4 | 59.9±9.9 | 66.4±3.3 |
| End‐diastolic thickness, mm | ··· | 8.1±1.9 | 8.7±2.1 | 7.6±1.3 |
| End‐systolic thickness, mm | ··· | 11.9±2.5 | 12.7±2.9 | 11.1±1.6 |
| E‐wave velocity, m/s | ··· | 90.4±21.5 | 94.4±23.8 | 83.8±16.2 |
| A‐wave velocity, m/s | ··· | 71.3±22.4 | 71.9±23.0 | 70.4±22.8 |
| E/A ratio | ··· | 1.34±0.34 | 1.36±0.23 | 1.31±0.47 |
| e′ velocity, m/s | ··· | 0.089±0.026 | 0.093±0.030 | 0.084±0.022 |
| E/e′ ratio | ··· | 11.1±4.7 | 11.8±5.8 | 10.2±2.7 |
| LA volume index, mL/m2 | ··· | 29.3±9.7 | 30.9±10.4 | 27.0±8.4 |
| Diastolic dysfunction, % | ||||
| None | ··· | 60 | 56 | 69 |
| Grade I | ··· | 12 | 11 | 13 |
| Grade II | ··· | 28 | 33 | 19 |
| Grade III | ··· | 0 | 0 | 0 |
| Cardiac MRI parameters | ||||
| LVEDVi, mL/m2 | ··· | 81.6±16.4 | 92.1±16.0 | 71.6±9.1 |
| LVESVi, mL/m2 | ··· | 30.3±8.9 | 33.8±10.1 | 27.1±6.3 |
| LVEF, % | ··· | 63.6±6.7 | 64.0±8.5 | 63.2±4.8 |
| LVMI, g/m2 | ··· | 78.5±21.6 | 91.4±8.5 | 66.4±12.7 |
| T1 baseline, myo, ms (n=36) | ··· | 1068±39 | 1041±36 | 1085±45 |
| T1 postcontrast, myo, ms (n=33) | ··· | 536±32 | 551±36 | 519±41 |
| ECV, % (n=33) | ··· | 22.1±3.0 | 23.2±3.9 | 21.4±3.5 |
Demographic and imaging parameters are expressed as mean±SD. A indicates peak mitral inflow during active filling in atrial systole; BMI, body‐mass index; E, peak mitral inflow during passive filling in early diastole; e′, mitral annular velocity during early diastole; ECV, extracellular volume; eGFR, estimated glomerular filtration rate (using the Modification of Diet in Renal Disease equation); FD, Fabry disease; LA, left atrial; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index; MRI, magnetic resonance imaging; myo, myocardium.
Table 2.
List of Identified Mutations With Corresponding Phenotype and Selected Demographic and Clinical Information in FD Cohort (n=68)
| Mutation | Phenotype | Age, y (Sex) | ACE‐I or ARB | Statin | ASA | ERT |
|---|---|---|---|---|---|---|
| A143P (n=30) S345P (n=9) Y134S (n=4) E338K (n=3) N215S (n=3) R112H (n=3) R227Q (n=3) Other (n=13) | Classic 87% (n=59) Cardiac Variant 4.4% (n=3) Renal Variant 4.4% (n=3) Unclassified 4.4% (n=3) | 42.0±12.6 years (Mean±SD) Female 50% (n=34) | 74% (n=50) | 57% (n=39) | 68% (n=46) | 54% (n=37) |
| A143P | Classic38 | 15 (M) | Yes | No | No | No |
| A143P | Classic | 19 (F) | No | No | No | No |
| A143P | Classic | 26 (F) | No | No | No | No |
| A143P | Classic | 28 (M) | No | No | No | No |
| A143P | Classic | 29 (F) | Yes | Yes | Yes | No |
| A143P | Classic | 30 (M) | No | No | Yes | Yes |
| A143P | Classic | 33 (M) | No | No | Yes | Yes |
| A143P | Classic | 34 (F) | No | No | Yes | No |
| A143P | Classic | 34 (M) | Yes | No | Yes | Yes |
| A143P | Classic | 35 (M) | Yes | Yes | Yes | Yes |
| A143P | Classic | 35 (M) | Yes | Yes | Yes | Yes |
| A143P | Classic | 35 (M) | Yes | Yes | No | Yes |
| A143P | Classic | 36 (F) | Yes | Yes | Yes | Yes |
| A143P | Classic | 36 (F) | No | No | No | No |
| A143P | Classic | 39 (F) | No | No | Yes | No |
| A143P | Classic | 40 (M) | Yes | Yes | Yes | Yes |
| A143P | Classic | 45 (F) | No | No | No | No |
| A143P | Classic | 46 (F) | No | No | No | No |
| A143P | Classic | 47 (M) | Yes | No | No | Yes |
| A143P | Classic | 48 (M) | No | Yes | No | Yes |
| A143P | Classic | 48 (M) | Yes | No | Yes | Yes |
| A143P | Classic | 51 (F) | Yes | No | Yes | Yes |
| A143P | Classic | 51 (F) | No | Yes | No | No |
| A143P | Classic | 51 (M) | No | Yes | Yes | Yes |
| A143P | Classic | 52 (M) | Yes | Yes | No | Yes |
| A143P | Classic | 54 (F) | Yes | Yes | Yes | No |
| A143P | Classic | 55 (F) | Yes | Yes | No | Yes |
| A143P | Classic | 62 (M) | Yes | Yes | No | Yes |
| A143P | Classic | 66 (F) | Yes | No | Yes | Yes |
| A143P | Classic | 68 (F) | Yes | Yes | Yes | Yes |
| E338K | Classic39 | 34 (F) | Yes | No | Yes | No |
| E338K | Classic | 36 (F) | Yes | No | Yes | Yes |
| E338K | Classic | 62 (M) | Yes | Yes | Yes | Yes |
| G261V | Unclassified | 36 (F) | Yes | Yes | Yes | No |
| G43V | Classic40 | 35 (M) | Yes | Yes | Yes | Yes |
| intron2: c.369+5G>T | Unclassified | 41 (M) | Yes | Yes | Yes | No |
| N215S | Cardiac variant41 | 26 (F) | No | No | No | No |
| N215S | Cardiac variant | 41 (M) | Yes | Yes | Yes | No |
| N215S | Cardiac variant | 66 (F) | Yes | Yes | Yes | Yes |
| Q321E | Classic42 | 51 (F) | Yes | Yes | Yes | Yes |
| Q386X | Classic43 | 35 (M) | Yes | Yes | Yes | Yes |
| Q386X | Classic | 46 (F) | Yes | No | Yes | No |
| R112C | Classic42 | 51 (M) | No | Yes | Yes | Yes |
| R112C | Classic | 55 (F) | Yes | No | No | Yes |
| R112H | Renal variant44 | 25 (M) | Yes | No | No | No |
| R112H | Renal variant | 48 (F) | Yes | Yes | Yes | No |
| R112H | Renal variant | 50 (M) | Yes | Yes | Yes | No |
| R220X | Classic45 | 29 (F) | No | No | No | No |
| R220X | Classic | 55 (F) | Yes | Yes | Yes | Yes |
| R227Q | Classic45 | 19 (F) | No | No | No | No |
| R227Q | Classic | 40 (M) | Yes | Yes | Yes | Yes |
| R227Q | Classic | 55 (F) | Yes | Yes | Yes | No |
| R227X | Classic45 | 52 (M) | Yes | Yes | Yes | Yes |
| S345P | Classic45 | 15 (M) | No | No | No | No |
| S345P | Classic | 28 (M) | Yes | No | Yes | No |
| S345P | Classic | 33 (M) | Yes | Yes | Yes | Yes |
| S345P | Classic | 39 (F) | Yes | Yes | Yes | No |
| S345P | Classic | 45 (F) | Yes | Yes | Yes | No |
| S345P | Classic | 47 (M) | Yes | Yes | Yes | Yes |
| S345P | Classic | 50 (M) | Yes | Yes | Yes | Yes |
| S345P | Classic | 51 (F) | Yes | Yes | Yes | No |
| S345P | Classic | 54 (F) | Yes | Yes | Yes | No |
| V254Gfs | Unclassified | 50 (M) | Yes | Yes | No | Yes |
| W349X | Classic46 | 30 (M) | Yes | Yes | Yes | Yes |
| Y134S | Classic43 | 34 (M) | Yes | No | No | Yes |
| Y134S | Classic | 35 (M) | Yes | No | Yes | Yes |
| Y134S | Classic | 39 (F) | Yes | No | Yes | No |
| Y134S | Classic | 68 (F) | Yes | Yes | Yes | Yes |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; ERT, enzyme‐replacement therapy; FD, Fabry disease.
The cardiac phenotype of the Fabry cohort was characterized by hypertrophy, preserved EF, and diastolic dysfunction (Table 1).23, 28 The high median left ventricular mass index is consistent with the relatively high prevalence of males in our FD cohort (50%). Increased postcontrast myocardial T1 values suggest increased myocardial fibrosis, and low precontrast T1 values are consistent with a diagnosis of FD.28 The mean EF via echocardiography was 63±8%, and 2 male FD patients had reduced EF. Excluding patients who were unable to be assessed for diastolic dysfunction due to arrhythmia or who were judged to be indeterminate, 60% had none, 12% had grade I (mild), 28% had grade II (moderate), and no patients had grade III (severe) diastolic dysfunction. The mean average (peak mitral inflow during passive filling in early diastole)/(mitral annular velocity during early diastole) ratio was 11.1±4.7, and the mean left atrial maximum volume index was 29.3±9.7 mL/m2.
Differences in Plasma Biomarkers Between FD and Healthy Cohorts
Plasma BNP and MR‐proANP were elevated in patients with FD relative to healthy controls (P=0.006 and 0.013, respectively) (Figure 2, Table 3). Although there was no statistically significant difference between plasma MMP‐8 values in the FD and healthy control cohorts (P=0.079), there were significant differences in MMP‐2 and MMP‐9 (P=0.017 and P<0.001, respectively) (Figure 3). Patients with FD had significantly elevated plasma levels of inflammatory markers TNF, TNFR1, and TNFR2 relative to healthy controls (P=0.008, P=0.003, and P<0.001, respectively) (Figure 3). There was no difference in C‐reactive protein concentration between the FD patients and healthy control cohorts (P=0.839), but IL‐6 was significantly elevated in FD patients (P=0.021). Galectin‐1 was significantly elevated in the FD cohort when compared with healthy controls, whereas galectin‐3 was not statistically different (P<0.001 and P=0.533, respectively) (Figure 2). TNFR2 and galectin‐3 were found to have independent positive correlations with Lyso‐Gb3 (P=0.020 and 0.024, respectively).
Figure 2.

Plasma levels of cardiac remodeling biomarkers and Lyso‐Gb3 in cohorts of patients with FD (n=68) and healthy controls (n=40). Biomarkers BNP, MR‐proANP, galectin‐1, galectin‐3, and Lyso‐Gb3 are significantly elevated in the FD cohort relative to healthy controls. BNP indicates B‐type natriuretic peptide; FD, Fabry disease; HC, healthy controls; Lyso‐Gb3, lysoglobotriaosylceramide; MR‐proANP, midregional pro–atrial natriuretic peptide. *P<0.05; **P<0.01; ***P<0.001.
Table 3.
Biomarker Data for the Fabry Disease Cohort
| Healthy Controls (n=40) | Fabry Disease (n=68) | Fabry Male (n=34) | Fabry Female (n=34) | |
|---|---|---|---|---|
| Biomarkers | ||||
| BNP, pg/mL | 16.5 (11, 35.75) | 34.5 (15, 79.25) | 22 (10, 77.75) | 39.5 (20, 85.5) |
| MR‐proANP, pmol/L | 45.6 (33.0, 74.7) | 65.6 (41.3, 119) | 56.7 (39.5, 191) | 77.7 (43.9, 117) |
| TNFR1, pg/mL | 829 (686, 939) | 971 (696, 1726) | 1153 (914, 1992) | 890 (610, 1148) |
| TNFR2, pg/mL | 1350 (1217, 2124) | 2730 (1507, 4471) | 3608 (2014, 5492) | 2376 (1460, 3635) |
| TNF, pg/mL | 0.73 (0.49, 0.90) | 0.90 (0.62, 1.53) | 1.13 (0.71, 1.84) | 0.77 (0.55, 0.97) |
| IL‐6, pg/mL | 1.05 (0.66, 1.85) | 1.58 (0.97, 2.19) | 1.58 (0.99, 2.18) | 1.58 (0.96, 2.21) |
| MMP‐2, ng/mL | 204 (182, 229) | 232 (180, 295) | 256 (212, 348) | 199 (174, 262) |
| MMP‐8, ng/mL | 2.70 (0.91, 3.68) | 2.97 (2.44, 3.36) | 3.03 (2.45, 3.38) | 2.94 (2.09, 3.36) |
| MMP‐9, ng/mL | 34.1 (25.8, 55.3) | 58.7 (40.4, 78.0) | 64.1 (41.8, 83.6) | 55.4 (40.1, 74.1) |
| Galectin‐1, ng/mL | 16.7 (13.5, 21.7) | 27.2 (21.0, 35.8) | 25.5 (18.2, 38.8) | 28.9 (22.5, 35.0) |
| Galectin‐3, ng/mL | 4.48 (3.33, 5.88) | 4.08 (2.95, 5.85) | 3.65 (2.81, 6.61) | 4.38 (2.95, 5.85) |
| Lyso‐Gb3, nmol/L | 0.06 (0, 0.25) | 21.8 (10.3, 47.2) | 47.1 (31.3, 70.8) | 11.2 (8.7, 18.3) |
Biomarker data are reported as medians (25th percentile, 75th percentile). BNP indicates B‐type natriuretic peptide; IL, interleukin; Lyso‐Gb3, lysoglobotriaosylceramide; MMP, matrix metalloprotease; MR‐proANP, midregional pro–atrial natriuretic peptide; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor.
Figure 3.

Plasma levels of inflammatory biomarkers and selected matrix metalloproteases in cohorts of FD (n=68) and healthy controls (n=40). TNF, IL‐6, TNFR1, and TNFR2 are significantly elevated in the FD cohort relative to healthy controls, without significant elevation in CRP. In addition, MMP‐2 and MMP‐9 are also significantly elevated in the FD cohort relative to healthy controls, although no difference was observed for MMP‐8. CRP, C‐reactive peptide; FD, Fabry disease; HC, healthy controls; IL, interleukin; MMP, matrix metalloprotease; TNF, tumor necrosis factor; TNFR, TNF receptor. *P<0.05; **P<0.01; ***P<0.001.
Receiver‐operator characteristic curve analysis was performed for Lyso‐Gb3 which performed extremely well (area under the curve=0.998) (Figure 4). As a biomarker, plasma total Lyso‐Gb3 and analogues performed flawlessly for classic mutations (area under the curve=1.0 for both) but performed poorly for cardiac/renal variant mutations (area under the curve=0.41), although our sample size was low (n=6). In our cohort there was no additional utility to measuring Lyso‐Gb3 analogues (area under the curve=0.994) instead of Lyso‐Gb3 alone (Figure 4).
Figure 4.

Receiver operating characteristic (ROC) curve demonstrating the performance of Lyso‐Gb3 and Lyso‐Gb3 with analogues for the prediction of FD (n=40 healthy controls; n=68 FD patients). Lyso‐Gb3, the gold standard, had excellent performance (AUC=0.998), and Lyso‐Gb3 with analogues had an AUC=0.994. AUC indicates area under the curve; FD, Fabry disease; Lyso‐Gb3, lysoglobotriaosylceramide.
Cardiac and Renal Disease and Relationship With Plasma Biomarkers
In FD patients with LVH by cardiac MRI criteria (n=41, 60%), TNFR2, TNF, IL‐6, MMP‐2, and Lyso‐Gb3 were significantly elevated (P=0.045, 0.025, 0.001, 0.046, and 0.002, respectively) (Figure 5). Patients with late gadolinium enhancement on cardiac MRI had greater levels of BNP, MR‐proANP, TNFR1, TNFR2, and MMP‐2 (P=0.001, P<0.001, P=0.014, P=0.014, and P<0.001 respectively) (Figure 6). Patients with diastolic dysfunction had elevated BNP, MR‐proANP, and MMP‐2 levels (P=0.002, 0.01, and 0.003, respectively) (Figure 7A). Maximal left‐atrial size correlated with MR‐proANP (P<0.0001) (Figure 7B). Regression analysis revealed that the disease burden assessed by the MSSI was positively associated with increased plasma MMP‐9 (P=0.015), and the MSSI cardiac subset correlated with MMP‐2 (P=0.003). Fabry patients with renal dysfunction (n=18, 26%) had higher levels of BNP, MR‐proANP, TNF, TNFR1, TNFR2, MMP‐2, MMP‐8, galectin‐1, and galectin‐3 (P=0.001, P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, P=0.03, P<0.001, and P=0.004, respectively) (Figure 8).
Figure 5.

Plasma levels of biomarkers in FD patients (n=68) with (n=41) and without (n=27) LVH via imaging criteria. TNFR2, TNF, IL‐6, MMP‐2, and Lyso‐Gb3 are significantly higher in FD patients with LVH than in those without LVH. FD indicates Fabry disease; IL, interleukin; LVH, left ventricular hypertrophy (left ventricular mass index ≥85 g/m2 in male and ≥81 g/m2 in female patients); Lyso‐Gb3, lysoglobotriaosylceramide; MMP, matrix metalloprotease; TNF, tumor necrosis factor; TNFR, TNF receptor. *P<0.05; **P<0.01.
Figure 6.

Plasma levels of biomarkers in FD patients (n=65) with (n=30) and without (n=35) late gadolinium enhancement (LGE) on cardiac MRI. BNP, MR‐proANP, TNFR1, TNFR2, and MMP‐2 are elevated in FD patients with LGE. BNP indicates B‐type natriuretic peptide; FD, Fabry disease; LGE, late gadolinium enhancement; MMP, matrix metalloprotease; MRI, magnetic resonance imaging; MR‐proANP, midregional pro–atrial natriuretic peptide; TNFR, tumor necrosis factor receptor. **P<0.01; ***P<0.001.
Figure 7.

A, Plasma levels of biomarkers in FD patients (n=43) with (n=17) and without diastolic dysfunction (n=26) per echocardiography. BNP, MR‐proANP, and MMP‐2 are significantly higher in FD patients with diastolic dysfunction than in those without diastolic dysfunction. Diastolic dysfunction could not be assessed in some patients due to arrhythmia. B, Correlation plot of MR‐proANP vs maximum LA size index (R 2=0.44, P<0.001). MR‐proANP and maximum LA size index are positively correlated, as increasing LA size is known to cause atrial cardiomyocytes to release ANP. BNP indicates B‐type natriuretic peptide; DD, diastolic dysfunction; FD, Fabry disease; LA, left atrial; MMP, matrix metalloprotease; MR‐proANP, midregional pro–atrial natriuretic peptide. *P<0.05; **P<0.01.
Figure 8.

Plasma levels of biomarkers in FD patients (n=68) with (n=18) and without significant chronic kidney disease (n=50). BNP, MR‐proANP, TNF, TNFR1, TNFR2, MMP‐2, MMP‐8, galectin‐1, and galectin‐3 are significantly elevated in FD patients with CKD. BNP indicates B‐type natriuretic peptide; CKD, chronic kidney disease (estimated glomerular filtration rate <60 mL/[min·1.73 m2]); FD, Fabry disease; MMP, matrix metalloprotease; MR‐proANP, midregional pro–atrial natriuretic peptide; TNF, tumor necrosis factor; TNFR, TNF receptor. *P<0.05; **P<0.01; ***P<0.001.
Medical Therapy and Biomarkers
Fabry patients who qualify for and receive ERT (n=37, 54%) had greater plasma levels of TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3 (P=0.025, P=0.003, P<0.001, P<0.001, and P=0.001, respectively) (Figure 9). Among patients who were prescribed either an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (n=50, 74%), plasma MMP‐2 and MMP‐8 were elevated (P=0.027 and 0.015, respectively). Patients prescribed a statin (n=39, 57%) had significantly increased plasma levels of BNP, MR‐proANP, galectin‐1, and galectin‐3 (P=0.007, 0.001, 0.023, and 0.001, respectively), whereas patients prescribed aspirin (n=46, 68%) had significantly elevated plasma levels of BNP, MR‐proANP, and MMP‐8 (P=0.008, P=0.001, and P<0.001, respectively).
Figure 9.
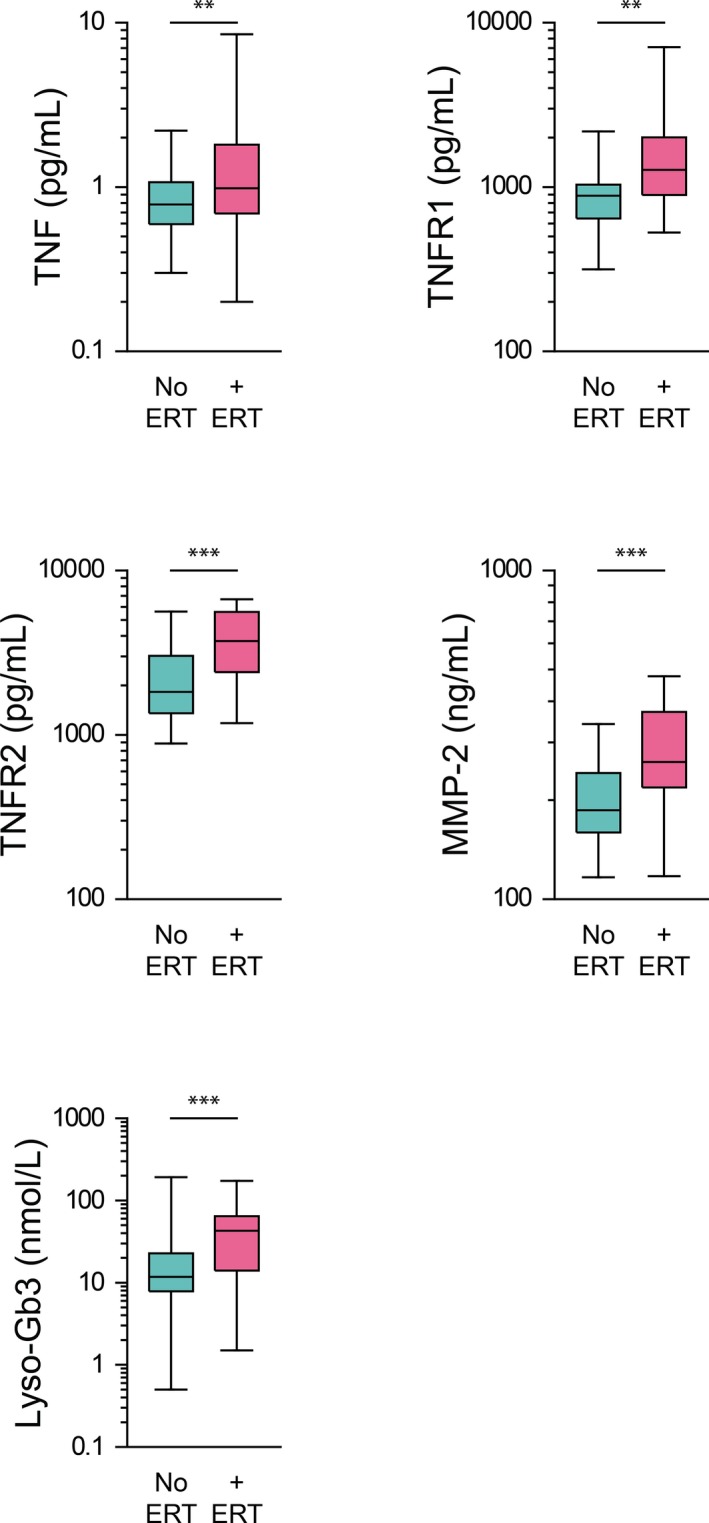
Plasma levels of biomarkers in FD patients (n=68), in cohorts of those not receiving ERT (n=31) and those who qualify for and are receiving ERT (n=37). TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3 are elevated in FD patients undergoing ERT relative to those not receiving ERT. ERT indicates enzyme replacement therapy; FD, Fabry disease; Lyso‐Gb3, lysoglobotriaosylceramide; MMP, matrix metalloprotease; TNF, tumor necrosis factor; TNFR, TNF receptor. **P<0.01; ***P<0.001.
Demographics and Biomarkers
Among patients with FD, TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3 were elevated in male (n=34, 50%) relative to female patients (P=0.003, P=0.002, P=0.035, P=0.012, and P<0.001, respectively) (Figure 10). Older FD patients (n=34, 50%) over the median age (45.5 years) had higher levels of BNP, MR‐proANP, MMP‐2, and galectin‐3, relative to younger patients with FD (P=0.002, 0.012, 0.038, and 0.011, respectively), but Lyso‐Gb3 levels were lower in these older patients (P=0.012). There were no significant differences in biomarkers between overweight or obese (n=36, 53%; body‐mass index ≥25.0 kg/m2) and normal–body‐mass index FD patients.
Figure 10.

Plasma levels of biomarkers in FD patients (n=68) of male (n=34) and female (n=34) sex. TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3 are elevated in male FD patients relative to female patients. F indicates female; FD, Fabry disease; Lyso‐Gb3, lysoglobotriaosylceramide; M, male; MMP, matrix metalloprotease; TNF, tumor necrosis factor; TNFR, TNF receptor. *P<0.05; **P<0.01; ***P<0.001.
Genotype and Phenotype and Plasma Biomarkers
Galectin‐3 concentrations differed significantly (P=0.03) between the type of mutation, missense (n=6, 9.1%) versus nonsense (n=60, 90.9%), but no other statistically significant differences were found for other biomarkers. Plasma concentrations of total Lyso‐Gb3, Lyso‐Gb3, and its analogues were significantly greater in patients with classic phenotypes (n=59, 90.8%; unclassified excluded) compared with those with cardiac/renal variant phenotypes (n=6, 9.2%), but no other significant differences were noted with other biomarkers (Table 4).
Table 4.
Plasma Concentration of Lyso‐Gb3 and its 6 Related Analogues With Modified Sphingosine Moieties (−H2O2; −H2; +O; +H2O; +H2O2, +H2O3) by Phenotype, Classic Versus Variant
| Biomarker | Classic (n=59), nmol/L | Variant (n=6), nmol/L | Significance P Value |
|---|---|---|---|
| Total Lyso‐Gb3 | 59±64 | 4.6±3.7 | <0.00001 |
| Lyso‐Gb3 (m/z 786) | 40±40 | 3.7±3.0 | <0.00001 |
| Lyso‐Gb3 analogue (m/z 758) | 0.71±1.6 | nd | 0.11 |
| Lyso‐Gb3 analogue (m/z 784) | 7.8±9.3 | 0.85±0.77 | <0.001 |
| Lyso‐Gb3 analogue (m/z 802) | 3.2±5.7 | 0.023±0.056 | 0.03 |
| Lyso‐Gb3 analogue (m/z 804) | 4.0±4.4 | nd | <0.0001 |
| Lyso‐Gb3 analogue (m/z 820) | 2.5±6.0 | nd | 0.04 |
| Lyso‐Gb3 analogue (m/z 836) | 0.44±1.4 | nd | 0.60 |
Unclassified mutations are excluded. Values are expressed as mean±SD. Lyso‐Gb3 indicates lysoglobotriaosylceramide; m/z, mass‐to‐charge ratio; nd, not detected.
Discussion
Proteomic biomarker discovery platforms have revealed several altered pathways in FD including vascular dysfunction, oxidative stress, and cytoskeletal remodeling.15, 16 Our study used a directed approach in which inflammatory and cardiac remodeling biomarkers were analyzed from the plasma of healthy controls and FD patients. The elevation of the inflammatory markers TNF, IL‐6, TNFR1, and TNFR2 in FD patients strongly implicates chronic inflammation as a major driver in the pathogenesis of FD. Mechanistically, glycolipids, including Lyso‐Gb3, bind to toll‐like receptor 4, activating nuclear factor κB and T lymphocytes and subsequent production of proinflammatory cytokines, leading to a chronic inflammatory state and associated vasculopathy.47, 48, 49 Both TNF and IL‐6 plasma levels are elevated in chronic heart failure and correlate with a decreasing functional status of these patients as well as all‐cause mortality.50, 51, 52 Furthermore, the positive correlation of inflammatory biomarkers in FD patients with higher disease burden based on MSSI scores, cardiac‐specific MSSI scores, LVH, LGE, and renal dysfunction suggests that systemic inflammation plays a central role in the morbidity and mortality associated with FD.53 These findings are further supported by the relatively high prevalence of diastolic dysfunction in our cohort. Vascular dysregulation in FD may also affect the coronary arteries,54 leading to microvascular angina7, 8 and subsequent HFpEF.55, 56 These findings support the evolving paradigm of inflammation and vascular dysfunction as key pathogenic processes in HFpEF.53, 56 Fabry patients develop significant renal dysfunction, and the strong association of elevated inflammatory markers and worsened renal function observed in our cohort is especially relevant given the connection between HFpEF and renal disease.57 Of particular interest to FD patients, biomarker panels may, in the future, help identify HFpEF phenotypes to guide appropriate phenotype‐specific therapy.58
Two different receptor subtypes of TNF, TNFR1 and TNFR2, mediate signaling pathways that have opposing effects on the heart.18, 59 TNFR1‐mediated signaling appears to be the primary cause of deleterious effects of TNF in the heart, including increased oxidative stress and cardiomyocyte apoptosis.18 In contrast, TNFR2‐mediated signaling appears to confer the cardioprotective benefits of TNF.59 The absence of effect of mutation type or phenotype suggests that TNFR1 and TNFR2 may be sensitive to a disease progression of FD that is independent of genetic makeup. The increases in both TNFR1 and TNFR2 suggest a strong systemic inflammatory component of FD. Novel chronic heart failure therapies targeting these receptors are currently being investigated and accordingly may eventually be found to play a role in the management of FD patients with HFpEF.60, 61 Importantly, TNFR1 and TNFR2 were associated with late gadolinium enhancement, which represents a prehypertrophic phenotype in FD.62 These biomarkers may also identify prehypertrophic stages of myocardial involvement in FD patients, triggering further investigations such as cardiac MRI.63
Fabry disease patients with LGE and/or diastolic dysfunction had significantly higher levels of BNP and MR‐proANP, suggesting the presence of significant long‐term pathological cardiac remodeling and possible eventual progression to heart failure.64 Elevated plasma troponins I and T provide further evidence for a cardiac‐specific involvement in FD12, 65 and, coupled with assessment of plasma natriuretic peptides, represent important diagnostic and prognostic tools in the evaluation of the cardiomyopathy associated with FD. MMP‐2 and MMP‐9 are implicated in remodeling of the extracellular matrix with increased MMP‐2 levels associated with the presence of HFpEF, whereas increased MMP‐9 levels predict LVH and adverse extracellular matrix modeling.66 Increased plasma levels of the gelatinases MMP‐2 and MMP‐9 in FD patients suggest that inflammation and extracellular matrix remodeling are significant components of heart disease in FD. These findings are further supported by the correlation of MMP‐2 with MSSI, LVH, LGE, and diastolic dysfunction. The elevation of MMP‐9 is consistent with previous work67 and, together with the elevated galectin‐1 levels, confirms a critical role of extracellular matrix remodeling in FD. Galectin‐3 correlated strongly positively with MSSI and left atrial maximum size index, which suggests that galectin‐3 may be a marker of advanced disease and the development of heart failure in patients with FD.68
ERT was associated with increased levels of TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3. These findings highlight the fact that patients who qualify for and receive ERT typically have more severe disease manifestations. Early detection of FD is especially critical as progression of the disease can be slowed by ERT. Importantly, TNFR1 and TNFR2 were associated with late gadolinium enhancement, which represents a prehypertrophic phenotype in female patients with FD.69 These biomarkers may also identify prehypertrophic stages of myocardial involvement in FD patients, triggering further investigations such as cardiac MRI.62 In contrast, patients with advanced FD continue to develop major adverse clinical events despite ERT,70 further underlying the need for early detection and appropriate intervention. Given that dysregulated inflammation persists in some patients despite ERT, monitoring these patients after initiation of ERT using inflammatory biomarkers may provide valuable information about disease control and long‐term prognosis, particularly if used in conjunction with other previously identified biomarkers.15
Lyso‐Gb3 is a glycosphingolipid that accumulates in FD and serves as a disease‐specific biomarker to identify clinically relevant mutations.2 Levels of plasma Lyso‐Gb3 and utility in the diagnosis of FD were similar to those reported in prior studies.2, 71, 72 Plasma Lyso‐Gb3 proved to be an ideal biomarker for the diagnosis of classic FD with both sensitivity and specificity of 100%. The use of a total plasma Lyso‐Gb3 level incorporating the levels of 6 analogues is novel but did not confer increased accuracy for diagnosis of FD in our cohort. Interestingly, the levels of plasma Lyso‐Gb3 for the 3 patients with unclassified mutations (G261V, intron2:c.369+5G>T, and V254Gfs) were not statistically different from those of classic mutations, suggesting that these 3 mutations may be classified as classic rather than cardiac/renal variants. In addition to its utility in diagnosis, Lyso‐Gb3 correlated strongly with left ventricular mass index, which suggests that it may be a marker of sphingolipid accumulation in the myocardium. This finding is consistent with prior work involving FD patients with cardiac‐specific variants.73 TNF, TNFR1, TNFR2, MMP‐2, and Lyso‐Gb3 were elevated in the plasma of male patients with FD compared with female patients, which is expected given that the gene for α‐galactosidase A follows an X‐linked inheritance pattern, and, accordingly, hemizygotes typically suffer from a more severe form of the disease.63, 69 Older patients with FD had elevated plasma biomarkers of remodeling, including BNP, MR‐proANP, and galectin‐3, without higher levels of inflammatory biomarkers. This finding could have therapeutic implications in that ERT may provide limited benefit in older patients. Indeed, in many patients with advanced FD, ERT does not prevent organ failure and death.70
Monitoring markers of cardiac remodeling and systemic markers of inflammation may confer increased sensitivity for early subclinical manifestations for disease, which may indicate the need for aggressive treatment including ERT to prevent progression to ERT‐refractory FD and its major associated complications.70 The presence of systemic inflammation in pediatric FD patients is of special interest because these patients may not display major cardiac or renal manifestations until significant irreversible progression of the disease has occurred. In this case, systemic inflammation may contribute to long‐term morbidity and mortality before these patients are symptomatic enough to qualify for ERT. Plasma biomarkers may also be valuable in patients with Fabry polymorphisms or mild mutations, particularly in female patients, where the diagnosis and therapy of choice may be less clear.74
Study Limitations
A limitation of this study is the sample size, primarily due to the rarity of diagnosis of FD. Although comparable to other studies involving plasma analysis in FD, the sample size may limit the generalizability of the results, particularly with respect to phenotype (classic versus cardiac/renal variant). In addition, the small sample size limits the quality of the statistical tests and may have resulted in underpowered tests. A large sample size would enable further exploration of the relationship between the clinical and imaging variables and biomarker levels. Another limitation is the multiple comparisons, but we attempted to account for these issues by only testing biologically plausible associations. However, many of the significances are strong enough that even the most stringent corrections would still result in rejection of the null hypothesis. Another, broader limitation of the study is the lack of clinical outcomes. Given the rarity of FD, multinational registries linked to phenotypic data would be valuable. Future studies linking these biomarkers to clinical outcomes are planned, which will help to further define their role in prognostication.
Conclusions
Plasma levels of inflammatory biomarkers, cardiac remodeling biomarkers, and Lyso‐Gb3 are elevated in patients with FD. Patients with more severe disease, assessed via MSSI and its cardiac subset, have higher levels of inflammatory and remodeling biomarker levels. Several inflammatory and cardiac remodeling biomarkers, as well as Lyso‐Gb3, were elevated in patients with LVH, whereas cardiac remodeling biomarkers were elevated in patients with diastolic dysfunction. Markers of cardiac remodeling, extracellular matrix turnover, and inflammation are significantly elevated in patients with renal dysfunction, suggesting that multisystem disease sequelae of FD are associated with greater states of inflammation. These features are consistent with a phenotype dominated by heart disease with preserved EF and renal disease and suggest a key pathogenic role of systemic inflammation. Exciting new advances in phenotype‐specific and targeted anti‐inflammatory therapy have the potential to revolutionize the management of FD.
Sources of Funding
Yogasundaram is supported by an Alberta Innovates Health Solutions (AIHS) Summer Studentship (Grant No. 201300994). Oudit is supported by operating grants from the Canadian Institute of Health Research (Grant No. 205413) and the Heart and Stroke Foundation of Canada (Grant No. 88453).
Disclosures
Khan is president of Metabolics and Genetics in Calgary (MAGIC) Clinic Ltd, is a member of the International Collaborate Gaucher Group (ICGG) and Pompe Registry (both sponsored by Sanofi‐Genzyme), is a recipient of a Revenue Distribution Agreement with the University Health Network related to Fabry Gene Therapy, and has received travel grants, speaker fees, research grants, and unrestricted educational funds from Sanofi‐Genzyme and Shire. Auray‐Blais has received research funding, honoraria, and travel funds from Amicus Therapeutics, BioMarin Pharmaceuticals Inc, Sanofi‐Genzyme, and Shire. West has received research funding, honoraria, and/or consultant fees from Alexion, Amicus Therapeutics, Excelsior Pharma, Idorsia, Sanofi‐Genzyme, Glaxo Smith Kline Inc, Shire Inc, and Sumitomo Pharma. Oudit has received speaker honoraria and research funding from Sanofi‐Genzyme and Shire Inc. The remaining authors have no disclosures to report.
Acknowledgments
The authors would like to recognize the physicians, nurses, and other allied healthcare workers who are responsible for the care of patients included in our study. The assistance of the Nova Scotia Fabry Disease research program coordinators and Kaye LeMoine, national CFDI coordinator, is gratefully acknowledged. We are also grateful to Waters Corporation for their continued scientific support and partnership in C. Auray‐Blais's laboratory.
(J Am Heart Assoc. 2018;7:e009098 DOI: 10.1161/JAHA.118.009098.)
References
- 1. Eng CM, Resnick‐Silverman LA, Niehaus DJ, Astrin KH, Desnick RJ. Nature and frequency of mutations in the alpha‐galactosidase A gene that cause Fabry disease. Am J Hum Genet. 1993;53:1186–1197. [PMC free article] [PubMed] [Google Scholar]
- 2. Niemann M, Rolfs A, Stork S, Bijnens B, Breunig F, Beer M, Ertl G, Wanner C, Weidemann F. Gene mutations versus clinically relevant phenotypes: lyso‐Gb3 defines Fabry disease. Circ Cardiovasc Genet. 2014;7:8–16. [DOI] [PubMed] [Google Scholar]
- 3. Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, Lin SJ, Chen CH, Chiang CC, Ho HJ, Lee PC, Kao CH, Cheng KH, Hsueh C, Niu DM. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–456. [DOI] [PubMed] [Google Scholar]
- 4. Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S. Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet. 2013;58:548–552. [DOI] [PubMed] [Google Scholar]
- 5. Gambarin FI, Disabella E, Narula J, Diegoli M, Grasso M, Serio A, Favalli V, Agozzino M, Tavazzi L, Fraser AG, Arbustini E. When should cardiologists suspect Anderson‐Fabry disease? Am J Cardiol. 2010;106:1492–1499. [DOI] [PubMed] [Google Scholar]
- 6. Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA, Maseri A, Frustaci A. Prevalence of Fabry disease in female patients with late‐onset hypertrophic cardiomyopathy. Circulation. 2004;110:1047–1053. [DOI] [PubMed] [Google Scholar]
- 7. Yogasundaram H, Kim D, Oudit O, Thompson RB, Weidemann F, Oudit GY. Clinical features, diagnosis, and management of patients with Anderson‐Fabry cardiomyopathy. Can J Cardiol. 2017;33:883–897. [DOI] [PubMed] [Google Scholar]
- 8. Frustaci A, Russo MA, Francone M, Chimenti C. Microvascular angina as prehypertrophic presentation of Fabry disease cardiomyopathy. Circulation. 2014;130:1530–1531. [DOI] [PubMed] [Google Scholar]
- 9. Grunfeld JP, Lidove O, Joly D, Barbey F. Renal disease in Fabry patients. J Inherit Metab Dis. 2001;24:71–74. [DOI] [PubMed] [Google Scholar]
- 10. Linhart A, Kampmann C, Zamorano JL, Sunder‐Plassmann G, Beck M, Mehta A, Elliott PM; European FOS Investigators . Cardiac manifestations of Anderson‐Fabry disease: results from the international Fabry Outcome Survey. Eur Heart J. 2007;28:1228–1235. [DOI] [PubMed] [Google Scholar]
- 11. Weidemann F, Linhart A, Monserrat L, Strotmann J. Cardiac challenges in patients with Fabry disease. Int J Cardiol. 2010;141:3–10. [DOI] [PubMed] [Google Scholar]
- 12. Seydelmann N, Liu D, Krämer J, Drechsler C, Hu K, Nordbeck P, Schneider A, Störk S, Bijnens B, Ertl G, Wanner C, Weidemann F. High‐sensitivity troponin: a clinical blood biomarker for staging cardiomyopathy in Fabry disease. J Am Heart Assoc. 2016;5:e002839 DOI: 10.1161/JAHA.115.002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, Sunder‐Plassmann G; Investigators FOS . Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet. 2009;46:548–552. [DOI] [PubMed] [Google Scholar]
- 14. Machann W, Breunig F, Weidemann F, Sandstede J, Hahn D, Köstler H, Neubauer S, Wanner C, Beer M. Cardiac energy metabolism is disturbed in Fabry disease and improves with enzyme replacement therapy using recombinant human galactosidase A. Eur J Heart Fail. 2011;13:278–283. [DOI] [PubMed] [Google Scholar]
- 15. Hollander Z, Dai DL, Putko BN, Yogasundaram H, Wilson‐McManus JE, Thompson RB, Khan A, West ML, McManus BM, Oudit GY. Gender‐specific plasma proteomic biomarkers in patients with Anderson‐Fabry disease. Eur J Heart Fail. 2015;17:291–300. [DOI] [PubMed] [Google Scholar]
- 16. Moore DF, Krokhin OV, Beavis RC, Ries M, Robinson C, Goldin E, Brady RO, Wilkins JA, Schiffmann R. Proteomics of specific treatment‐related alterations in Fabry disease: a strategy to identify biological abnormalities. Proc Natl Acad Sci USA. 2007;104:2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts—possible implication in left ventricular remodeling. Circulation. 1998;98:149–156. [DOI] [PubMed] [Google Scholar]
- 18. Defer N, Azroyan A, Pecker F, Pavoine C. TNFR1 and TNFR2 signaling interplay in cardiac myocytes. J Biol Chem. 2007;282:35564–35573. [DOI] [PubMed] [Google Scholar]
- 19. Cesari M, Penninx B, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding JZ, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events—results from the Health ABC Study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 20. Sirrs S, Bichet DG, Iwanochko RM, Khan A, Moore D, Oudit G, West ML. Canadian Fabry disease treatment guidelines 2016. Published May 18, 2016. Available at: http://www.garrod.ca/wp-content/uploads/Canadian-FD-Treatment-Guidelines-2016.pdf. Accessed September 1, 2018.
- 21. Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M. The Mainz Severity Score Index: a new instrument for quantifying the Anderson‐Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet. 2004;65:299–307. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 23. Shanks M, Thompson RB, Paterson ID, Putko B, Khan A, Chan A, Becher H, Oudit GY. Systolic and diastolic function assessment in Fabry disease patients using speckle‐tracking imaging and comparison with conventional echocardiographic measurements. J Am Soc Echocardiogr. 2013;26:1407–1414. [DOI] [PubMed] [Google Scholar]
- 24. Hazari H, Belenkie I, Kryski A, White JA, Oudit GY, Thompson R, Fung T, Dehar N, Khan A. Comparison of cardiac magnetic resonance imaging and echocardiography in assessment of left ventricular hypertrophy in Fabry disease. Can J Cardiol. 2018;34:1041–1047. [DOI] [PubMed] [Google Scholar]
- 25. Tajik AJ, Seward J, Hagler D, Mair D, Lie J. Two‐dimensional real‐time ultrasonic imaging of the heart and great vessels. Technique, image orientation, structure identification, and validation. Mayo Clin Proc. 1978;53:271–303. [PubMed] [Google Scholar]
- 26. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 27. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur J Echocardiogr. 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 28. Thompson RB, Chow K, Khan A, Chan A, Shanks M, Paterson I, Oudit GY. T(1) mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ Cardiovasc Imaging. 2013;6:637–645. [DOI] [PubMed] [Google Scholar]
- 29. Pagano JJ, Chow K, Khan A, Michelakis E, Paterson I, Oudit GY, Thompson RB. Reduced right ventricular native myocardial T1 in Anderson‐Fabry disease: comparison to pulmonary hypertension and healthy controls. PLoS One. 2016;11:e0157565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pagano JJ, Chow K, Paterson DI, Mikami Y, Schmidt A, Howarth A, White J, Friedrich MG, Oudit GY, Ezekowitz J, Dyck J, Thompson RB. Effects of age, gender, and risk‐factors for heart failure on native myocardial T1 and extracellular volume fraction using the SASHA sequence at 1.5T. J Magn Reson Imaging. 2018;48:1307–1317. [DOI] [PubMed] [Google Scholar]
- 31. Cheng‐Baron J, Chow K, Khoo NS, Esch BT, Scott JM, Haykowsky MJ, Tyberg JV, Thompson RB. Measurements of changes in left ventricular volume, strain, and twist during isovolumic relaxation using MRI. Am J Physiol Heart Circ Physiol. 2010;298:H1908–H1918. [DOI] [PubMed] [Google Scholar]
- 32. Cheng‐Baron J, Chow K, Pagano JJ, Punithakumar K, Paterson DI, Oudit GY, Thompson RB. Quantification of circumferential, longitudinal, and radial global fractional shortening using steady‐state free precession cines: a comparison with tissue‐tracking strain and application in Fabry disease. Magn Reson Med. 2015;73:586–596. [DOI] [PubMed] [Google Scholar]
- 33. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bionda C, Bergerot C, Ardail D, Rodriguez‐Lafrasse C, Rousson R. Plasma BNP and NT‐proBNP assays by automated immunoanalyzers: analytical and clinical study. Ann Clin Lab Sci. 2006;36:299–306. [PubMed] [Google Scholar]
- 35. Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W. Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes. Transl Res. 1973;81:157–171. [PubMed] [Google Scholar]
- 36. Valgimigli M, Ceconi C, Malagutti P, Merli E, Soukhomovskaia O, Francolini G, Cicchitelli G, Olivares A, Parrinello G, Percoco G, Guardigli G, Mele D, Pirani R, Ferrari R. Tumor necrosis factor‐α receptor 1 is a major predictor of mortality and new‐onset heart failure in patients with acute myocardial infarction: the Cytokine‐Activation and Long‐term Prognosis in Myocardial Infarction (C‐ALPHA) study. Circulation. 2005;111:863–870. [DOI] [PubMed] [Google Scholar]
- 37. Boutin M, Auray‐Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso‐Gb3‐related analogues in Fabry disease. Anal Chem. 2014;86:3476–3483. [DOI] [PubMed] [Google Scholar]
- 38. Eng CM, Desnick RJ. Molecular basis of fabry disease: Mutations and polymorphisms in the human alpha‐galactosidase a gene. Hum Mutat. 1994;3:103–111. [DOI] [PubMed] [Google Scholar]
- 39. Shabbeer J, Yasuda M, Benson SD, Desnick RJ. Fabry disease: Identification of 50 novel alpha‐galactosidase a mutations causing the classic phenotype and three‐dimensional structural analysis of 29 missense mutations. Human Genomics. 2006;2:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shabbeer J, Yasuda M, Luca E, Desnick RJ. Fabry disease: 45 novel mutations in the alpha‐galactosidase a gene causing the classical phenotype. Mol Genet Metab. 2002;76:23–30. [DOI] [PubMed] [Google Scholar]
- 41. Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, et al An atypical variant of fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. [DOI] [PubMed] [Google Scholar]
- 42. Topaloglu AK, Ashley GA, Tong B, Shabbeer J, Astrin KH, Eng CM, Desnick RJ. Twenty novel mutations in the alpha‐galactosidase a gene causing Fabry disease. Mol Med. 1999;5:806–811. [PMC free article] [PubMed] [Google Scholar]
- 43. Eng CM, Ashley GA, Burgert TS, Enriquez AL, D'Souza M, Desnick RJ. Fabry disease: Thirty‐five mutations in the alpha‐galactosidase a gene in patients with classic and variant phenotypes. Mol Med. 1997;3:174–182. [PMC free article] [PubMed] [Google Scholar]
- 44. Eng CM, Niehaus DJ, Enriquez AL, Burgert TS, Ludman MD, Desnick R. Fabry disease: Twenty‐three mutations including sense and antisense cpg alterations and identification of a deletional hot‐spot in the α‐galactosidase a gene. Hum Mol Genet. 1994;3:1795–1799. [DOI] [PubMed] [Google Scholar]
- 45. Lukas J, Giese AK, Markoff A, Grittner U, Kolodny E, Mascher H, Lackner KJ, Meyer W, Wree P, Saviouk V, Rolfs A. Functional characterisation of alpha‐galactosidase a mutations as a basis for a new classification system in Fabry disease. PLoS Genet. 2013;9:e1003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashley GA, Shabbeer J, Yasuda M, Eng CM, Desnick RJ. Fabry disease: Twenty novel alpha‐galactosidase a mutations causing the classical phenotype. J Hum Genet. 2001;46:192–196. [DOI] [PubMed] [Google Scholar]
- 47. De Francesco PN, Mucci JM, Ceci R, Fossati CA, Rozenfeld PA. Fabry disease peripheral blood immune cells release inflammatory cytokines: role of globotriaosylceramide. Mol Genet Metab. 2013;109:93–99. [DOI] [PubMed] [Google Scholar]
- 48. Pereira CS, Azevedo O, Maia ML, Dias AF, Sa‐Miranda C, Macedo MF. Invariant natural killer T cells are phenotypically and functionally altered in Fabry disease. Mol Genet Metab. 2013;108:241–248. [DOI] [PubMed] [Google Scholar]
- 49. Mauhin W, Lidove O, Masat E, Mingozzi F, Mariampillai K, Ziza JM, Benveniste O. Innate and adaptive immune response in Fabry disease. JIMD Rep. 2015;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJS, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. [DOI] [PubMed] [Google Scholar]
- 51. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PWF, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction—the Framingham Heart study. Circulation. 2003;107:1486–1491. [DOI] [PubMed] [Google Scholar]
- 52. Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. High levels of plasma brain natriuretic peptide and interleukin‐6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–1593. [DOI] [PubMed] [Google Scholar]
- 53. Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG; Coronary Vasomotion Disorders International Study Group (COVADIS) . The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2016;38:473–477. [DOI] [PubMed] [Google Scholar]
- 54. Tomberli B, Cecchi F, Sciagrà R, Berti V, Lisi F, Torricelli F, Morrone A, Castelli G, Yacoub MH, Olivotto I. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson‐Fabry disease. Eur J Heart Fail. 2013;15:1363–1373. [DOI] [PubMed] [Google Scholar]
- 55. Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, Escaned J, Koller A, Piek JJ, de Wit C. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the Working Group on Coronary Pathophysiology and Microcirculation. Eur Heart J. 2015;36:3134–3146. [DOI] [PubMed] [Google Scholar]
- 56. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 57. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. [DOI] [PubMed] [Google Scholar]
- 58. D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail. 2015;17:1231–1239. [DOI] [PubMed] [Google Scholar]
- 59. Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor‐α–induced cardiomyopathy. Circulation. 2004;109:1892–1897. [DOI] [PubMed] [Google Scholar]
- 60. Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36:261–270. [DOI] [PubMed] [Google Scholar]
- 61. Putko BN, Wang Z, Lo J, Anderson T, Becher H, Dyck JR, Kassiri Z, Oudit GY, Alberta HI. Circulating levels of tumor necrosis factor‐alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS One. 2014;9:e99495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nordin S, Kozor R, Baig S, Abdel‐Gadir A, Medina‐Menacho K, Rosmini S, Captur G, Tchan M, Geberhiwot T, Murphy E, Lachmann R, Ramaswami U, Edwards NC, Hughes D, Steeds RP, Moon JC. Cardiac phenotype of prehypertrophic Fabry disease. Circ Cardiovasc Imaging. 2018;11:e007168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nordin S, Kozor R, Medina‐Menacho K, Abdel‐Gadir A, Baig S, Sado DM, Lobascio I, Murphy E, Lachmann RH, Mehta A, Edwards NC, Ramaswami U, Steeds RP, Hughes D, Moon JC. Proposed stages of myocardial phenotype development in Fabry disease. JACC Cardiovasc Imaging. 2018. Available at: https://www.sciencedirect.com/science/article/pii/S1936878X18303073. Accessed October 26, 2018. [DOI] [PubMed] [Google Scholar]
- 64. Coats CJ, Parisi V, Ramos M, Janagarajan K, O'Mahony C, Dawnay A, Lachmann RH, Murphy E, Mehta A, Hughes D, Elliott PM. Role of serum N‐terminal pro‐brain natriuretic peptide measurement in diagnosis of cardiac involvement in patients with Anderson‐Fabry disease. Am J Cardiol. 2013;111:111–117. [DOI] [PubMed] [Google Scholar]
- 65. Tanislav C, Guenduez D, Liebetrau C, Giese AK, Eichler S, Sieweke N, Speth M, Bauer T, Hamm C, Rolfs A. Cardiac troponin I: a valuable biomarker indicating the cardiac involvement in Fabry disease. PLoS One. 2016;11:e0157640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zile MR, DeSantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, Mehurg SM, Spinale FG. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 2011;4:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shah JS, Hughes DA, Tayebjee MH, MacFadyen RJ, Mehta AB, Elliott PM. Extracellular matrix turnover and disease severity in Anderson‐Fabry disease. J Inherit Metab Dis. 2007;30:88–95. [DOI] [PubMed] [Google Scholar]
- 68. de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin‐3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. [DOI] [PubMed] [Google Scholar]
- 69. Niemann M, Herrmann S, Hu K, Breunig F, Strotmann J, Beer M, Machann W, Voelker W, Ertl G, Wanner C, Weidemann F. Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment. JACC Cardiovasc Imaging. 2011;4:592–601. [DOI] [PubMed] [Google Scholar]
- 70. Weidemann F, Niemann M, Stork S, Breunig F, Beer M, Sommer C, Herrmann S, Ertl G, Wanner C. Long‐term outcome of enzyme‐replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med. 2013;274:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aerts JM, Groener JE, Kuiper S, Donker‐Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci USA. 2008;105:2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maruyama H, Miyata K, Mikame M, Taguchi A, Guili C, Shimura M, Murayama K, Inoue T, Yamamoto S, Sugimura K. Effectiveness of plasma lyso‐Gb3 as a biomarker for selecting high‐risk patients with Fabry disease from multispecialty clinics for genetic analysis. Genet Med. 2018. Available at: https://www.nature.com/articles/gim201831. Accessed October 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Auray‐Blais C, Lavoie P, Boutin M, Ntwari A, Hsu T‐R, Huang C‐K, Niu D‐M. Biomarkers associated with clinical manifestations in Fabry disease patients with a late‐onset cardiac variant mutation. Clin Chim Acta. 2017;466:185–193. [DOI] [PubMed] [Google Scholar]
- 74. Weidemann F, Niemann M. Screening for Fabry disease using genetic testing. Eur J Heart Fail. 2010;12:530–531. [DOI] [PubMed] [Google Scholar]


