Abstract
Background
Recent studies have shown an increasing prevalence of vascular risk factors in young adults with ischemic stroke (IS). However, the strength of the association between all vascular risk factors and early‐onset IS has not been fully established.
Methods and Results
We compared 961 patients with a first‐ever IS at 25 to 49 years to 1403 frequency‐matched stroke‐free controls from a population‐based cohort study (FINRISK). Assessed risk factors included an active malignancy, atrial fibrillation, cardiovascular disease, current smoking status, a family history of stroke, high low‐density lipoprotein cholesterol, high triglycerides, low high‐density lipoprotein cholesterol, hypertension, and type 1 and type 2 diabetes mellitus. We performed subgroup analyses based on age, sex, and IS etiology. In a fully adjusted multivariable logistic regression analysis, significant risk factors for IS consisted of atrial fibrillation (odds ratio [OR], 10.43; 95% confidence interval [CI], 2.33–46.77], cardiovascular disease (OR, 8.01; 95% CI, 3.09–20.78), type 1 diabetes mellitus (OR, 6.72; 95% CI, 3.15–14.33), type 2 diabetes mellitus (OR, 2.31; 95% CI, 1.35–3.95), low high‐density lipoprotein cholesterol (OR, 1.81; 95% CI, 1.37–2.40), current smoking status (OR, 1.81; 95% CI, 1.50–2.17), hypertension (OR, 1.43; 95% CI, 1.17–1.75), and a family history of stroke (OR, 1.37; 95% CI, 1.04–1.82). High low‐density lipoprotein cholesterol exhibited an inverse association with IS. In the subgroup analyses, the most consistent associations appeared for current smoking status and type 1 diabetes mellitus.
Conclusions
Our study establishes the associations between 11 vascular risk factors and early‐onset IS, among which atrial fibrillation, cardiovascular disease, and both type 1 and 2 diabetes mellitus in particular showed strong associations.
Keywords: brain infarction, middle‐aged, risk factors, stroke, young adult
Subject Categories: Ischemic Stroke, Risk Factors
Clinical Perspective
What Is New?
Atrial fibrillation, cardiovascular disease, and both type 1 and type 2 diabetes mellitus emerge as strong risk factors for early‐onset IS.
Risk profiles among the age, sex, and etiological‐specific subgroups differ; however, smoking status and type 1 diabetes mellitus exhibit rather consistent associations.
What Are the Clinical Implications?
Thorough screening for cardiovascular risk factors and cessation of smoking appear to be important for the prevention of ischemic strokes in young population.
Ischemic stroke (IS) causes a remarkable loss of quality‐adjusted life years. The socioeconomic burden of IS among young adults is particularly severe because affected patients tend to expect a long life after becoming ill. The cutoff age most often used to define early‐onset IS or IS in young adults stands at less than 50 years.1
Recent studies have reported an increasing incidence of IS among young adults, contrary to decreasing IS incidence among older populations.2, 3, 4, 5, 6 This observation may result from vascular risk factors becoming more prevalent among young individuals in general and accumulating in certain high‐risk individuals who subsequently experience IS.4, 5, 6, 7, 8, 9 Determining the strength of the association between well‐documented vascular risk factors and early‐onset IS would justify more intensive risk factor screening among this age group and provide the tools for individually tailored primary prevention. Yet, thus far, only a few case‐control studies have assessed vascular risk factors in large, nonselected young IS patient populations, with a limited number of covariates and limited data on IS subtypes.10, 11, 12, 13, 14, 15
Therefore, we aimed to determine the strength of the association among 11 vascular risk factors and early‐onset IS for the Finnish population and stratify the analysis according to meaningful subgroups defined by age, sex, and stroke etiology.
Methods
We compared patients with first‐ever IS at age 25 to 49 years enrolled in the Helsinki Young Stroke Registry to frequency‐matched (by sex and 5‐year age bands) stroke‐free controls from a population‐based cohort study (the National FINRISK Study, hereafter FINRISK). The study was carried out in the Department of Neurology at the Helsinki University Hospital and approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. Written consent from the participants of this retrospective register‐based study was not required. It is not within our rights to make the data and study materials available to other researchers for purposes of reproducing the results or replicating the procedure.
Case Population
The detailed methods from Helsinki Young Stroke Registry were published previously.1 In short, the registry includes all consecutive patients aged 15 to 49 admitted to Helsinki University Hospital between January 1994 and May 2007 with a discharge diagnosis of first‐ever IS. All patients underwent a range of blood tests, a chest radiograph, an ECG, and brain imaging at admission. Stroke risk factors were obtained from medical, laboratory, and imaging records, a nationwide electronic hospital discharge register (Care Register for Health Care, hereafter hospital discharge register), and a register of reimbursed prescribed medications from the Social Insurance Institution of Finland.15, 16 Stroke subtypes were classified according to the modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria17, 18 and grouped as follows to allow for meaningful analysis: (1) large‐artery atherosclerosis, (2) cardioembolism from a high‐risk source (cardioembolism high‐risk), (3) small‐vessel occlusion, (4) dissections, (5) other determined etiologies, (6) embolic stroke of undetermined source (ESUS), and (7) undetermined non‐ESUS. Patients fulfilling ESUS criteria19 were identified among those originally classified as cryptogenic (TOAST 5b) or cardioembolism from a low‐risk source (cardioembolism low‐risk, TOAST 2).20 Remaining patients in TOAST categories 5a–c were classified as undetermined non‐ESUS.
Control Population
FINRISK is a large population‐based Finnish health examination survey on the risk factors of noncommunicable diseases coordinated by the National Institute for Health and Welfare.21 At present, surveys are carried out every 5 years using representative random population samples from different parts of Finland. Its target population includes individuals aged between 25 and 74, stratified to contain at least 250 subjects from each sex in 10‐year age groups (25–34, 35–44, 45–54, 55–64, and 65–74) from each geographic region.
For this study, a stroke‐free control population, frequency matched for age (5‐year bands) and sex, was randomly selected from the southern Finland FINRISK survey participants from years 1997, 2002, and 2007, that is, from the same time period and the same geographic region as the case population. Only one control per case for the oldest age groups (men 40–49 and women 45–49) was available, whereas in younger age groups we were able to include from 2 to 4 controls per case. The stroke risk factors among controls were collected from the FINRISK study questionnaires, a standardized health examination, blood samples, and electronic health registers (hospital discharge register, Drug Reimbursement Register, and a registry of prescribed medications from the Social Insurance Institution of Finland).15, 16
Risk Factors
We examined 11 well‐established vascular risk factors, for which we were able to create comparable covariates: active malignancy, atrial fibrillation (AF), cardiovascular disease (CVD), current smoking status, a family history of stroke, high low‐density lipoprotein cholesterol (LDL‐C), low high‐density lipoprotein cholesterol (HDL‐C), high triglycerides, hypertension, type 1 (T1D) and type 2 (T2D) diabetes mellitus. Detailed definitions of the risk factors appear in Data S1.
Statistical Analyses
Statistical analyses were performed using SPSS for Windows version 22.0 (Armonk, NY). We considered a 2‐sided P<0.05 as statistically significant.
First, we performed a comparison between groups using the chi‐square and Fisher's exact tests, and calculated univariate odds ratios (ORs) for the 11 dichotomized risk factors. Second, sex, age, lipid‐lowering treatment, and each risk factor with a significant association in the univariate comparisons were entered into a binary multivariable logistic regression model, for which the adjusted ORs and 95% confidence intervals were calculated. We used a backward stepwise logistic regression analysis with a statistical variable removal level of P<0.10. We calculated the population attributable risk percentages from the adjusted OR and 95% confidence interval values using the following formula: prevalence of exposed cases×[(OR–1)/OR]×100.22
Apart from the entire study population, we performed subgroup analyses by sex, age group (25–39 years and 40–49 years), and IS etiology. In the etiology‐specific analyses, the entire control group served as controls.
Additional description of statistical analyses is provided in Data S1.
Results
Among the 1008 patients in Helsinki Young Stroke Registry, 4 were excluded as stroke mimics during the follow‐up period, and another 43 were under 25 years old, for whom controls were not available. Thus, the study population in the univariate analysis consisted of 961 cases who fell between the age of 25 and 49 (358 women and 603 men) and 1403 controls (610 women and 793 men). The age distribution of cases and controls appears in Table S1, while Table S2 provides the distribution of IS etiologies. Due to missing data for dichotomous variables, 43 cases (4.5%) and 11 controls (0.8%) were excluded from the multivariable analyses. Hence, our fully adjusted multivariable analyses included data from 918 cases and 1392 controls. For a detailed description of the missing data and comparison of subjects with complete and incomplete data, please see the Data S1 and Tables S3 and S4.
Dichotomized Risk Factors
Among the entire study population, all studied risk factors were more prevalent among cases compared with controls, with the exception of high LDL‐C. In the multivariable logistic regression analysis, significant risk factors for IS beginning with the highest OR consisted of AF, CVD, T1D, T2D, low HDL‐C, current smoking status, hypertension, and a family history of stroke. High LDL‐C, however, was inversely associated with IS (Table 1).
Table 1.
Univariate Analysis and Multivariable Logistic Regression Analysis of Risk Factors for Early‐Onset Ischemic Stroke
| Prevalence | |||||
|---|---|---|---|---|---|
| Case (n=961) n/N (%) | Control (n=1403) n/N (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | PAR% (95% CI)a | |
| Atrial fibrillation | 23/961 (2.4) | 2/1403 (0.1) | 17.18 (4.04 to 73.03) | 10.43 (2.33 to 46.77) | 2.2% (1.4 to 2.3) |
| Cardiovascular diseaseb | 46/961 (4.8) | 5/1403 (0.4) | 14.06 (5.56 to 35.51) | 8.01 (3.09 to 20.78) | 4.2% (3.2 to 4.6) |
| Type 1 diabetes mellitus | 44/961 (4.6) | 9/1403 (0.6) | 7.43 (3.61 to 15.30) | 6.72 (3.15 to 14.33) | 3.9% (3.1 to 4.3) |
| Active malignancy | 15/961 (1.6) | 6/1396 (0.4) | 3.67 (1.42 to 9.50) | 2.73 (0.99 to 7.50) | 1.0% (0.0 to 1.4) |
| Type 2 diabetes mellitus | 44/961 (4.6) | 24/1403 (1.7) | 2.76 (1.67 to 4.57) | 2.31 (1.35 to 3.95) | 2.6% (1.2 to 3.4) |
| Low HDL‐Cc | 148/921 (16.1) | 114/1400 (8.1) | 2.16 (1.67 to 2.80) | 1.81 (1.37 to 2.40) | 7.2% (4.4 to 9.4) |
| Current smoking status | 427/961 (44.4) | 435/1403 (31.0) | 1.78 (1.50 to 2.11) | 1.81 (1.50 to 2.17) | 19.9% (14.8 to 23.9) |
| Hypertension | 391/961 (40.7) | 394/1402 (28.1) | 1.76 (1.48 to 2.09) | 1.43 (1.17 to 1.75) | 12.2% (5.9 to 17.4) |
| Family history of stroke | 126/961 (13.1) | 128/1403 (9.1) | 1.50 (1.16 to 1.95) | 1.37 (1.04 to 1.82) | 3.5% (0.5 to 5.9) |
| High triglyceridesc | 212/926 (22.9) | 217/1400 (15.5) | 1.62 (1.31 to 2.00) | 1.19 (0.94 to 1.53) | 3.7% (−1.5 to 7.9) |
| High LDL‐Cc | 473/921 (51.4) | 850/1400 (60.7) | 0.68 (0.58 to 0.81) | 0.51 (0.42 to 0.62) | −49.4% (−80.0 to −31.5) |
CI indicates confidence interval; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; n/N, number of subjects divided by total of subjects excluding those with missing data; OR, odds ratio; PAR%, population attributable risk percentage.
Age, sex, lipid‐lowering treatment, and each risk factor in the table were entered into the multivariate model. Multivariable analysis included 918 cases and 1392 controls.
The presence of coronary heart disease, heart failure, or peripheral arterial disease.
High LDL‐C ≥3.0 mmol/L (116 mg/dL), low HDL‐C <1.0 mmol/L (39 mg/dL), and high triglycerides ≥2.0 mmol/L (177 mg/dL).
Population Attributable Risks
For the entire young IS population, we found the highest population attributable risk percentages for current smoking status, followed by hypertension, low HDL‐C, CVD, T1D, a family history of stroke, T2D, and AF (Table 1).
Sex‐Specific Analysis
In the sex‐specific multivariable analysis (Table 2), T1D and current smoking status were significantly associated with IS in both sexes. However, CVD, low HDL‐C, T2D, a family history of stroke, and hypertension emerged as significant risk factors only among men, whereby CVD carried the strongest association. Active malignancy emerged as a strong risk factor only for women. Furthermore, high LDL‐C was inversely associated with IS among both sexes, although the association was stronger among women. We could not analyze the strength of the association between AF and IS among men because there were no male controls with AF.
Table 2.
Multivariable Logistic Regression Analysis of Risk Factors for Early‐Onset Ischemic Stroke by Sex
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Case (n=358) n/N (%) | Control (n=610) n/N (%) | Adjusted OR (95% CI)a | Case (n=603) n/N (%) | Control (n=793) n/N (%) | Adjusted OR (95% CI)a | |
| Atrial fibrillation | 3/358 (0.8) | 2/610 (0.3) | 1.36 (0.18–20.40) | 20/603 (3.3) | 0/793 (0.0) | NAb |
| Active malignancy | 8/358 (2.2) | 1/605 (0.2) | 10.88 (1.29–91.56) | 7/603 (1.2) | 5/791 (0.6) | 1.27 (0.36–4.48) |
| Cardiovascular diseasec | 11/358 (3.1) | 4/610 (0.7) | 2.08 (0.59–7.31) | 35/603 (5.8) | 1/793 (0.1) | 28.06 (3.77–209.05) |
| Current smoking status | 136/358 (38.0) | 192/610 (31.5) | 1.58 (1.17–2.13) | 291/603 (48.3) | 243/793 (30.6) | 1.95 (1.54–2.48) |
| Family history of stroke | 48/358 (13.4) | 55/610 (9.0) | 1.38 (0.88–2.16) | 78/603 (12.9) | 73/793 (9.2) | 1.49 (1.04–2.15) |
| High LDL‐Cd | 134/341 (39.3) | 346/593 (58.3) | 0.33 (0.24–0.44) | 339/580 (58.4) | 489/792 (61.7) | 0.67 (0.52–0.86) |
| High triglyceridesd | 55/341 (16.1) | 86/608 (14.1) | 1.09 (0.71–1.67) | 157/585 (26.8) | 131/792 (16.5) | 1.24 (0.91–1.67) |
| Hypertension | 116/358 (32.4) | 147/609 (24.1) | 1.34 (0.96–1.87) | 275/603 (45.6) | 247/793 (31.1) | 1.40 (1.08–1.80) |
| Low HDL‐Cd | 33/340 (9.7) | 54/608 (8.9) | 1.20 (0.73–1.96) | 115/581 (19.8) | 60/792 (7.6) | 2.36 (1.66–3.37) |
| Type 1 diabetes mellitus | 15/358 (4.2) | 3/610 (0.5) | 8.64 (2.35–31.75) | 29/603 (4.8) | 6/793 (0.8) | 5.83 (2.28–14.88) |
| Type 2 diabetes mellitus | 13/358 (3.6) | 11/610 (1.8) | 2.28 (0.96–5.41) | 31/603 (5.1) | 13/793 (1.6) | 2.19 (1.08–4.44) |
CI indicates confidence interval; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; n/N, number of subjects divided by total of subjects excluding those with missing data; NA, not applicable; OR, odds ratio.
Age, lipid‐lowering treatment, and each available risk factor in the table were entered into the model. Multivariable analyses included 339 female cases and 602 controls, and 579 male cases and 790 controls.
Atrial fibrillation could not be analyzed in men because of the lack of male controls with atrial fibrillation.
The presence of coronary heart disease, heart failure, or peripheral arterial disease.
High LDL‐C ≥3.0 mmol/L (116 mg/dL), low HDL‐C <1.0 mmol/L (39 mg/dL), and high triglycerides ≥2.0 mmol/L (177 mg/dL).
Age‐Specific Analysis
In the age‐specific analyses (Table 3), T1D, current smoking status, and low HDL‐C emerged as significant risk factors for IS among individuals aged 25 to 39 and 40 to 49 years. A family history of stroke was significantly associated with IS only in the younger group of individuals aged 25 to 39 years. In addition, AF, CVD, T2D, and hypertension emerged as significant risk factors among subjects aged ≥40 years only. High LDL‐C was inversely associated with IS among both age groups. We could not analyze the strength of the association between active malignancy and IS among the younger age group because there were no younger controls with active malignancy.
Table 3.
Multivariable Logistic Regression Analysis of Risk Factors for Early‐Onset Ischemic Stroke by Age Group
| Age 25–39 Years | Age 40–49 Years | |||||
|---|---|---|---|---|---|---|
| Case (n=267) n/N (%) | Control (n=620) n/N (%) | Adjusted OR (95% CI)a | Case (n=694) n/N (%) | Control (n=783) n/N (%) | Adjusted OR (95% CI)a | |
| Atrial fibrillation | 1/267 (0.4) | 1/620 (0.2) | 2.62 (0.15–46.34) | 22/694 (3.2) | 1/783 (0.1) | 16.01 (2.08–123.03) |
| Active malignancy | 1/267 (0.4) | 0/617 (0.0) | NAd | 14/694 (2.0) | 6/778 (0.4) | 2.48 (0.88–7.02) |
| Cardiovascular diseaseb | 4/267 (1.5) | 2/620 (0.3) | 2.57 (0.43–15.37) | 42/694 (6.1) | 3/783 (0.4) | 11.07 (3.34–36.71) |
| Current smoking status | 112/267 (41.9) | 187/620 (30.2) | 1.72 (1.26–2.35) | 315/694 (45.4) | 248/783 (31.3) | 1.86 (1.48–2.34) |
| Family history of stroke | 28/267 (10.5) | 32/620 (5.2) | 1.82 (1.03–3.24) | 98/694 (14.1) | 96/783 (12.3) | 1.27 (0.92–1.76) |
| High LDL‐Cc | 102/253 (40.3) | 311/617 (50.4) | 0.54 (0.39–0.74) | 371/668 (55.5) | 539/783 (68.8) | 0.48 (0.37–0.60) |
| High triglyceridesc | 42/255 (16.5) | 65/617 (10.5) | 1.50 (0.94–2.38) | 170/671 (25.3) | 152/783 (19.4) | 1.12 (0.84–1.49) |
| Hypertension | 52/267 (19.5) | 102/620 (16.5) | 0.98 (0.64–1.49) | 339/694 (37.3) | 292/782 (48.8) | 1.58 (1.26–1.99) |
| Low HDL‐Cc | 35/253 (13.8) | 40/617 (6.5) | 2.00 (1.20–3.33) | 113/668 (16.9) | 74/783 (9.5) | 1.66 (1.18–2.32) |
| Type 1 diabetes mellitus | 10/267 (3.7) | 3/620 (0.5) | 8.78 (2.30–33.58) | 34/694 (4.9) | 6/783 (0.8) | 6.45 (2.58–16.14) |
| Type 2 diabetes mellitus | 5/267 (1.9) | 4/620 (0.6) | 1.94 (0.48–7.78) | 39/694 (5.6) | 20/783 (2.6) | 2.38 (1.33–4.28) |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Age, sex, lipid‐lowering treatment, and each available risk factor in the table were entered into the model. Multivariable analyses included 253 cases and 615 controls between the ages of 25–39, and 665 cases and 777 controls between the ages of 40–49.
The presence of coronary heart disease, heart failure, or peripheral arterial disease.
High LDL‐C ≥3.0 mmol/L (116 mg/dL), low HDL‐C <1.0 mmol/L (39 mg/dL), and high triglycerides ≥2.0 mmol/L (177 mg/dL).
Active malignancy could not be analyzed in the younger age group because of the lack of controls with an active malignancy.
Etiological Subgroups
For large‐artery atherosclerosis, significant associations emerged for CVD, T1D, current smoking status, hypertension, T2D, and low HDL‐C. For cardioembolism high‐risk, AF, CVD, T1D, and low HDL‐C carried significant associations. For small‐vessel occlusion, T1D, CVD, T2D, hypertension, and current smoking status emerged as risk factors for IS. In these 3 subgroups, high LDL‐C showed no statistically significant association (Figure 1).
Figure 1.

Multivariable logistic regression analysis of the risk factors for early‐onset ischemic stroke in the following etiologies: large artery atherosclerosis (LAA), cardioembolism from high‐risk source, and small vessel occlusion (SVO). Age, sex, lipid‐lowering treatment, and each available risk factor were entered into the model. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
For dissection, T1D, low HDL‐C, and a family history of stroke emerged as significant. For other rare determined etiologies, active malignancy showed the strongest association, followed by current smoking status, a family history of stroke, and high triglycerides. For ESUS, only current smoking status exhibited a statistically significant association. For undetermined non‐ESUS, low HDL‐C and current smoking status emerged as risk factors for IS. In these latter 4 etiological groups, high LDL‐C was inversely associated with IS (Figure 2).
Figure 2.
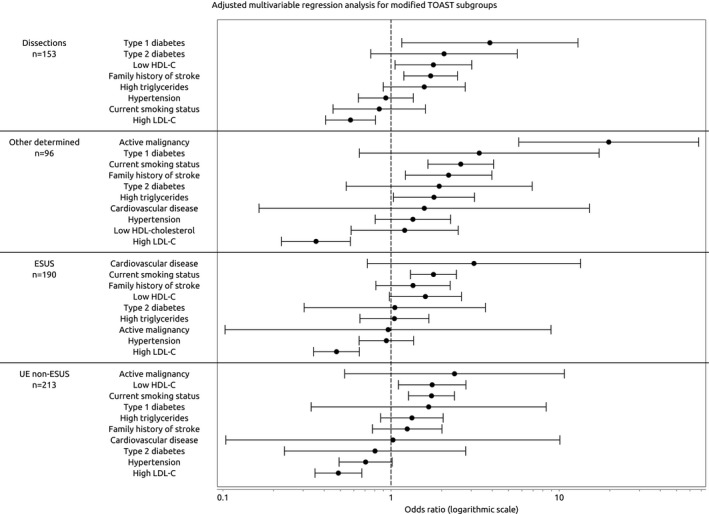
Multivariable logistic regression analysis of the risk factors for early‐onset ischemic stroke in the following etiologies: dissections, other determined etiologies, embolic stroke of an undetermined source (ESUS), and undetermined etiology non‐ESUS (undetermined non‐ESUS). Age, sex, lipid‐lowering treatment, and each available risk factor were entered into the model. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Because of the absence of risk factor–positive cases, AF could not be analyzed in subgroups other than cardioembolism high‐risk, active malignancy could not be analyzed in the small‐vessel occlusion or dissection subgroups, CVD could not be analyzed in the dissection subgroup, and T1D could not be analyzed in the ESUS subgroup.
Additional and Sensitivity Analyses
We performed additional analyses, specifically examining the alternative risk factor covariates, hospital discharge register, and the classification of high LDL‐C. The results from these sensitivity analyses did not contradict our main findings. For a detailed description, please see Data S1.
Discussion
Our study established the associations of 11 risk factors for early‐onset ischemic stroke. In particular, our results showed stronger associations than expected for AF, CVD, and both types of diabetes mellitus. Given these findings, a new hypothesis emerges suggesting that these comorbidities may represent more aggressive forms of disease predisposing individuals to a severe vascular end point early in life. This phenomenon has been characterized as “losing the relative protection of youth” in cases of early‐onset type 2 diabetes mellitus.23 As expected, we found marked differences in risk profiles among the age, sex, and etiological‐specific subgroups, which may help clinicians to establish personalized risk factor screening strategies and aid in focusing future research.
Compared with results among a young American white IS population,10 our study showed a similar population attributable risk percentage and association for current smoking status. Moreover, another 2 recent studies on European population11 and on young American men24 also showed a rather similar strengths of association for current smoking. Our study confirms that the association between current smoking and early‐onset ischemic stroke is present across both sexes, both age groups, and in most etiological subgroups. Various tobacco control policy measures have been implemented in Finland since the 1980s,25 and the rate of daily smoking has been decreasing, especially among men. Given that smoking is a modifiable risk factor, it appears essential to further support tobacco abstinence to prevent ischemic strokes among young adults.
The strength of the association with diabetes mellitus has varied widely across previous studies.10, 11, 12, 13, 14, 26, 27, 28 We analyzed T1D and T2D separately and found that both types of diabetes mellitus carried a high risk for IS at a young age, although T1D carried a stronger and more consistent association across demographic and etiological subgroups than did T2D.
Whether an association between dyslipidemia and young‐onset IS exists has remained unclear. Several studies found an association with low HDL‐C and early‐onset IS or transient ischemic attack26, 29, 30 but no association with LDL‐C.29, 30 Similar to prior studies,26, 29, 30 our study confirmed that low HDL‐C represents a risk factor for early‐onset IS. In addition to its reverse cholesterol transport action, HDL‐C is associated with antithrombotic and anti‐inflammatory actions.31, 32 Thus, it is reasonable to hypothesize that the entire spectrum of HDL‐C functions may play a more important role in young adult IS. Interestingly, high LDL‐C exhibited an inverse association with IS only in subgroups of etiologies that frequently occur among young individuals—that is, dissections, other determined causes, ESUS, and undetermined non‐ESUS—while the association with high LDL‐C was absent among older‐onset causes. Although our study cannot establish causality, at the very least this finding indicates that high LDL‐C does not play a role in the IS pathogenic mechanisms that occur more frequently among younger patients. Furthermore, low cholesterol could merely represent a confounder reflecting an underlying susceptibility factor for early‐onset IS.33
We demonstrated a family history of stroke as a significant risk factor for IS in the younger (aged 29–39) but not in the older (aged 40–49) age group. This finding emphasizes the hypothesis that genetic factors may have a stronger contribution to early‐onset than older‐onset IS.34 Furthermore, we observed a similar association with family history in those with dissection or other rare determined IS etiology. This suggests that inherited factors35 might be in the play in these etiological subgroups, although less frequent family history of young‐onset stroke also has been reported with dissection compared with strokes attributable to other causes.36
Accumulation of vascular risk factors with increasing age among young patients with IS was associated with increased risk of future atherothrombotic events.37 In accordance with this, we found that more of the vascular risk factors were significantly associated with first IS in those who were aged 40 to 49 compared with those who were under 40.
Furthermore, our study adds to the knowledge of the connection between new cancer38 and stroke in the young population, particularly in young women. Among our young female patients, 62.5% of the active malignancies were metastatic at stroke onset, and the most common primary locations were breast cancer (37.5%) and ovarian cancer (25%).
The major strength of our study is that our cases consist of a large, consecutive, and homogenous young patient population with IS. The Helsinki Young Stroke Registry can be considered a population‐based register given that it covers practically all hospitalized young patients with IS in the Helsinki University Hospital catchment area of 1.5 million people.1 Our register is free of consent bias and therefore able to include a truly nonselective group of young patients with IS. Furthermore, IS etiology among cases was classified according to the modified TOAST criteria, allowing us to meaningfully analyze IS risk factors by etiological subgroups. Our controls were drawn from a large population‐based cohort study from the same time period and geographic region. In addition, we analyzed 11 traditional vascular risk factors in a single study and were able to use a combination of reliable data sources including nationwide electronic register data. Hence, we could include risk factors rarely analyzed in early‐onset stroke, such as AF and CVD, and calculate specific risk estimates for both T1D and T2D.
The limitations of our study include its retrospective nature and several possible sources of biases inherent for any case‐control study. For instance, although we carefully harmonized risk factors, some residual bias may remain. We included an unequal ratio of younger and older controls, which affected the prevalence of risk factors and the unadjusted ORs. Still, the prevalence of AF, CVD, and active malignancy among our controls remained low, and we could not analyze AF and active malignancy across all subgroups. Overall, there were too few events, especially in the subgroups, to estimate associations precisely. Consequently, the estimated ORs for infrequent risk factors (AF, CVD, T1D, and active malignancy) should be interpreted with caution. Additionally, the sensitivity of older hospital discharge register data remains suboptimal in identifying all subjects with AF or CVD.16 As a limitation regarding current smoking status, we were unable to analyze the dose‐response relationship between cigarette smoking and early‐onset IS. Furthermore, we did not include any risk factors related to women's reproductive health or migraine with aura among our covariates. Finally, the inverse association for LDL‐C identified in our study could result from a reverse causality, as lipids among cases were measured 12 to 72 hours after IS.39, 40 While the shorter fasting time required for controls also may have minimally affected the lipid levels,41 several studies have established that plasma lipids change only modestly in response to habitual food intake.42
Conclusions
AF, CVD, both primary types of diabetes mellitus, low HDL‐C, current smoking status, hypertension, and a family history of stroke emerge as risk factors for IS among young patients. Additionally, current smoking status and T1D exhibited the most consistent associations across all demographic and etiological subgroups.
Sources of Funding
Dr Kivioja received research grants from the Helsinki University Hospital Research Fund and participates in the Doctoral Candidate Program funded by the Medical Faculty of the University of Helsinki. This study received funding from the Helsinki University Hospital Research Fund and the Academy of Finland (Dr Putaala). FINRISK has primarily received funding through the National Institute of Health and Welfare. Additional funding was obtained from the Academy of Finland and other domestic foundations. Dr Salomaa received support from the Finnish Foundation for Cardiovascular Research.
Disclosures
Dr Tatlisumak received funding from the Helsinki University Hospital Research Fund, Sahlgrenska University Hospital, and the University of Gothenburg for research in young patients with stroke (all significant). Dr Putaala received a speaker's honorarium from Bayer, BMS‐Pfizer, Boehringer‐Ingelheim, and Abbott (all modest). Dr Putaala also received compensation to serve on the following advisory boards; Bayer, BMS‐Pfizer, and Boehringer‐Ingelheim (all modest). The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental Methods.
Table S1. Frequencies of Cases and Controls According to Sex and Age Category
Table S2. Proportion of Cases According to Modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) Classification
Table S3. Comparison of Cases With Complete and Incomplete Data
Table S4. Comparison of Risk Factors in Subjects With Complete and Incomplete Dichotomous Data
Acknowledgments
We thank the staff of the Clinical Stroke Research Center at Meilahti Hospital in Helsinki, Finland, for their important contributions to the Helsinki Young Stroke Registry. We also thank Jani Pirinen, MD, for his constructive comments.
(J Am Heart Assoc. 2018;7:e009774 DOI: 10.1161/JAHA.118.009774.)
A preliminary version of this work was presented at the European Stroke Organisation Conference, May 10 to 12, 2016, in Barcelona, Spain.
References
- 1. Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first‐ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40:1195–1203. [DOI] [PubMed] [Google Scholar]
- 2. Rosengren A, Giang KW, Lappas G, Jern C, Torén K, Björck L. Twenty‐four‐year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44:2388–2393. [DOI] [PubMed] [Google Scholar]
- 3. Ramirez L, Kim‐Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ, Towfighi A. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc. 2016;11:e003233 DOI: 10.1161/JAHA.116.003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. [DOI] [PubMed] [Google Scholar]
- 6. Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, Huber R, Tanislav C, Lichy C, Demarin V, Basic‐Kes V, Ringelstein EB, Neumann‐Haefelin T, Enzinger C, Fazekas F, Rothwell PM, Dichgans M, Jungehulsing GJ, Heuschmann PU, Kaps M, Norrving B, Rolfs A, Kessler C, Tatlisumak T; sifap1 Investigators . Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the Stroke in Young Fabry Patients study. Stroke. 2013;44:119–125. [DOI] [PubMed] [Google Scholar]
- 8. Putaala J, Yesilot N, Waje‐Andreassen U, Pitkäniemi J, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, Kloss M, Ferrari J, Lemmens R, Coban O, Haapaniemi E, Maaijwee N, Rutten‐Jacobs L, Bersano A, Cereda C, Baron P, Borellini L, Valcarenghi C, Thomassen L, Grau AJ, Palm F, Urbanek C, Tuncay R, Durukan‐Tolvanen A, van Dijk EJ, de Leeuw FE, Thijs V, Greisenegger S, Vemmos K, Lichy C, Bereczki D, Csiba L, Michel P, Leys D, Spengos K, Naess H, Bahar SZ, Tatlisumak T. Demographic and geographic vascular risk factor differences in European young adults with ischemic stroke: the 15 cities young stroke study. Stroke. 2012;43:2624–2630. [DOI] [PubMed] [Google Scholar]
- 9. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. [DOI] [PubMed] [Google Scholar]
- 10. Rohr J, Kittner S, Feeser B, Hebel JR, Whyte MG, Weinstein A, Kanarak N, Buchholz D, Earley C, Johnson C, Macko R, Price T, Sloan M, Stern B, Wityk R, Wozniak M, Sherwin R. Traditional risk factors and ischemic stroke in young adults: the Baltimore‐Washington Cooperative Young Stroke Study. Arch Neurol. 1996;53:603–607. [DOI] [PubMed] [Google Scholar]
- 11. Aigner A, Grittner U, Rolfs A, Norrving B, Siegerink B, Busch MA. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017;48:1744–1751. [DOI] [PubMed] [Google Scholar]
- 12. Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM. Etiology of and risk factors for cerebral infarction in young adults in western Norway: a population‐based case‐control study. Eur J Neurol. 2004;11:25–30. [DOI] [PubMed] [Google Scholar]
- 13. You RX, McNeil JJ, O'Malley HM, Davis SM, Thrift AG, Donnan GA. Risk factors for stroke due to cerebral infarction in young adults. Stroke. 1997;28:1913–1918. [DOI] [PubMed] [Google Scholar]
- 14. Hillbom M, Numminen H, Juvela S. Recent heavy drinking of alcohol and embolic stroke. Stroke. 1999;30:2307–2312. [DOI] [PubMed] [Google Scholar]
- 15. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. [DOI] [PubMed] [Google Scholar]
- 16. Mähönen M, Jula A, Harald K, Antikainen R, Tuomilehto J, Zeller T, Blankenberg S, Salomaa V. The validity of heart failure diagnoses obtained from administrative registers. Eur J Prev Cardiol. 2013;20:254–259. [DOI] [PubMed] [Google Scholar]
- 17. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 18. Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence‐based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. [DOI] [PubMed] [Google Scholar]
- 19. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 20. Martinez‐Majander N, Aarnio K, Pirinen J, Lumikari T, Nieminen T, Lehto M, Sinisalo J, Kaste M, Tatlisumak T, Putaala J. Embolic strokes of undetermined source in young adults: baseline characteristics and long‐term outcome. Eur J Neurol. 2018;25:535–541. [DOI] [PubMed] [Google Scholar]
- 21. Borodulin K, Vartiainen E, Peltonen M, Jousilahti P, Juolevi A, Laatikainen T, Männistö S, Salomaa V, Sundvall J, Puska P. Forty‐year trends in cardiovascular risk factors in Finland. Eur J Public Health. 2015;25:539–546. [DOI] [PubMed] [Google Scholar]
- 22. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hillier TA, Pedula KL. Complications in young adults with early‐onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005. [DOI] [PubMed] [Google Scholar]
- 24. Markidan J, Cole JW, Cronin CA, Merino JG, Phipps MS, Wozniak MA, Kittner SJ. Smoking and risk of ischemic stroke in young men. Stroke. 2018;49:1276–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helakorpi SA, Martelin TP, Torppa JO, Patja KM, Kiiskinen UA, Vartiainen EA, Uutela AK. Did the tobacco control act amendment in 1995 affect daily smoking in Finland? Effects of a restrictive workplace smoking policy. J Public Health. 2008;30:407–414. [DOI] [PubMed] [Google Scholar]
- 26. Lipska K, Sylaja PN, Sarma PS, Thankappan KR, Kutty VR, Vasan RS, Radhakrishnan K. Risk factors for acute ischaemic stroke in young adults in South India. J Neurol Neurosurg Psychiatry. 2007;78:959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haapaniemi H, Hillbom M, Juvela S. Lifestyle‐associated risk factors for acute brain infarction among persons of working age. Stroke. 1997;28:26–30. [DOI] [PubMed] [Google Scholar]
- 28. Love BB, Biller J, Jones MP, Adams HP Jr, Bruno A. Cigarette smoking: a risk factor for cerebral infarction in young adults. Arch Neurol. 1990;47:693–698. [DOI] [PubMed] [Google Scholar]
- 29. Albucher JF, Ferrieres J, Ruidavets JB, Guiraud‐Chaumeil B, Perret BP, Chollet F. Serum lipids in young patients with ischaemic stroke: a case‐control study. J Neurol Neurosurg Psychiatry. 2000;69:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marini C, Carolei A, Roberts RS, Prencipe M, Gandolfo C, Inzitari D, Landi G, De Zanche L, Scoditti U, Fieschi C. Focal cerebral ischemia in young adults: a collaborative case‐control study. Neuroepidemiology. 1993;12:70–81. [DOI] [PubMed] [Google Scholar]
- 31. Van der Stoep M, Korporaal SJ, Van Eck M. High‐density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc Res. 2014;103:362–371. [DOI] [PubMed] [Google Scholar]
- 32. Rye Kerry‐Anne, Barter Philip J. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Debette S, Metso T, Pezzini A, Abboud S, Metso A, Leys D, Bersano A, Louillet F, Caso V, Lamy C, Medeiros E, Samson Y, Grond‐Ginsbach C, Engelter ST, Thijs V, Beretta S, Béjot Y, Sessa M, Lorenza Muiesan M, Amouyel P, Castellano M, Arveiler D, Tatlisumak T, Dallongeville J; Cervical Artery Dissection and Ischemic Stroke Patients (CADISP) Group . Association of vascular risk factors with cervical artery dissection and ischemic stroke in young adults. Circulation. 2011;123:1537–1544. [DOI] [PubMed] [Google Scholar]
- 34. MacClellan LR, Mitchell BD, Cole JW, Wozniak MA, Stern BJ, Giles WH, Brown DW, Sparks MJ, Kittner SJ. Familial aggregation of ischemic stroke in young women: the Stroke Prevention in Young Women Study. Genet Epidemiol. 2006;30:602–608. [DOI] [PubMed] [Google Scholar]
- 35. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40:e459–e466. [DOI] [PubMed] [Google Scholar]
- 36. Kloss M, Grond‐Ginsbach C, Pezzini A, Metso TM, Metso AJ, Debette S, Leys D, Dallongeville J, Caso V, Thijs V, Bersano A, Touzé E, Bonati LH, Tatlisumak T, Arnold ML, Lyrer PA, Engelter ST; Cervical Artery Dissection and Ischemic Stroke Patients (CADISP) Study Group . Stroke in first‐degree relatives of patients with cervical artery dissection. Eur J Neurol. 2014;21:1102–1107. [DOI] [PubMed] [Google Scholar]
- 37. Putaala J, Haapaniemi E, Kaste M, Tatlisumak T. How does number of risk factors affect prognosis in young patients with ischemic stroke? Stroke. 2012;43:356–361. [DOI] [PubMed] [Google Scholar]
- 38. Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, DeAngelis LM. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Butterworth RJ, Marshall WJ, Bath PMW. Changes in serum lipid measurements following acute ischaemic stroke. Cerebrovasc Dis. 1997;7:10–13. [Google Scholar]
- 40. Kargman DE, Tuck C, Berglund L, Lin IF, Mukherjee RS, Thompson EV, Jones J, Boden‐Albala B, Paik MC, Sacco RL. Lipid and lipoprotein levels remain stable in acute ischemic stroke: the Northern Manhattan Stroke Study. Atherosclerosis. 1998;139:391–399. [DOI] [PubMed] [Google Scholar]
- 41. Sundvall J, Laatikainen T, Hakala S, Leiviskä J, Alfthan G. Systematic error of serum triglyceride measurements during three decades and the effect of fasting on serum triglycerides in population studies. Clin Chim Acta. 2008;397:55–59. [DOI] [PubMed] [Google Scholar]
- 42. Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Borén J, Chapman MJ, Cobbaert C, Descamps OS, von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative . Fasting Is Not Routinely Required for Determination of a Lipid Profile: Clinical and Laboratory Implications Including Flagging at Desirable Concentration Cutpoints—A Joint Consensus Statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2016;62:930–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Frequencies of Cases and Controls According to Sex and Age Category
Table S2. Proportion of Cases According to Modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) Classification
Table S3. Comparison of Cases With Complete and Incomplete Data
Table S4. Comparison of Risk Factors in Subjects With Complete and Incomplete Dichotomous Data


