Abstract
Angiotensin II preconditioning (APC) involves an angiotensin II type 1 receptor (AT1-R)-dependent translocation of PKCε and survival kinases to the mitochondria leading to cardioprotection after ischemia-reperfusion (IR). However, the role that mitochondrial AT1-Rs and angiotensin II type 2 receptors (AT2-Rs) play in APC is unknown. We investigated whether pretreatment of Langendorff-perfused rat hearts with losartan (L, AT1-R blocker), PD 123,319 (PD, AT2-R blocker) or their combination (L+PD), affects mitochondrial AT1-R, AT2-R, PKCε, PKCδ, Akt, PKG-1, MAPKs (ERK1/2, JNK, p38), mitochondrial respiration, cardiac function, and infarct size (IS). The results indicate that the expression of mitochondrial AT1-Rs and AT2-Rs were enhanced by APC 1.91-fold and 2.32-fold, respectively. Expression of AT2-R was abolished by PD but not by L, whereas the AT1-R levels were abrogated by both blockers. The AT1-R response profile to L and PD was also shared by PKCε, Akt, MAPKs, and PKG-1, but not by PKCδ. A marked increase in state 3 (1.84-fold) and respiratory control index (1.86-fold) of mitochondria was observed with PD regardless of L treatment. PD also enhanced the post-ischemic recovery of the rate pressure product (RPP) by 74% (p<0.05) compared with APC alone. Losartan, however, inhibited the RPP by 44% (p<0.05) before IR and reduced the APC-induced increase of post-ischemic cardiac recovery by 73% (p<0.05). Finally, L enhanced the reduction of IS by APC through a PD-sensitive mechanism. These findings suggest that APC upregulates angiotensin II receptors in mitochondria and that AT2-Rs are cardioprotective through their permissive action on AT1-R signaling and the suppression of cardiac function.
Keywords: ischemia-reperfusion, cardioprotection, angiotensin II preconditioning, angiotensin II receptors, mitochondria, protein kinases
1. Introduction
Angiotensin II (Ang II) is a multifunctional hormone that affects a myriad of cellular processes and organs, in particular, the kidneys, the heart, and the vascular system [33]. It acts mainly through two G-protein coupled receptors: Ang II type 1 (AT1-Rs) and type 2 (AT2-Rs) receptors. The AT1-R mediates most of the well-known effects of the hormone (i.e., vasoconstriction, aldosterone secretion, cardiac and vascular hypertrophy, and ROS generation, among others) whereas the AT2-R reportedly antagonizes the AT1-R and exerts protective effects on cells, tissues, and organs [14, 27].
The renin-angiotensin system (RAS) of which Ang II is a critical component, was initially described as a circulatory, endocrine system for the control of blood pressure, through renal, vascular and central mechanisms. However, a local RAS system is present in different organs (i.e., brain, kidney, vasculature and the heart) with distinct functional properties (i.e., control of fibroblast homeostasis, inflammation, cell-matrix deposition, regulation of glomerular filtration and ion transport in the kidney). More recently, an intracellular/intracrine RAS system has been proposed [22] although its role is largely unknown. Evidence is available for intracellular co-localization of Ang II and renin in various cell types [10,16,37]. Besides, intracellular AT1-R-dependent Ca2+ mobilization in vascular smooth muscle cells [3], and the regulation of RNA synthesis, cell proliferation, and collagen secretion are regulated by intracellular Ang II through nuclear receptors in cardiac fibroblasts [46]. AT1-Rs and AT2-Rs in the nuclear membrane of cardiac fibroblasts appear to be involved in the latter effects [19]. Also, intracellular injection of Ang II in cardiomyocytes reportedly affects cell volume and communication, and ion-channel activity [11]. These intracellular actions of Ang II are insensitive to extracellular Ang II receptor blockers (ARBs) indicating that the effects of intracellular Ang II are independent of plasma membrane receptors [46]. These intracellular receptors are affected by Ang II originating either from cellular or internalized Ang II [22].
Although it is well established that Ang II is necessary for normal cell function, growth, and development, chronic exposure to the hormone through RAS upregulation in animals and patients is associated with pathologies such as hypertension, hypertrophy of the vascular wall, inflammation, and renal disease, among others [33]. Indeed, treatment with RAS inhibitors [ARBs, and angiotensin-converting enzyme (ACE) inhibitors] ameliorates the pathophysiological conditions associated with RAS upregulation. However, recent reports [13,30,35,36,44] indicate that brief and repetitive exposure of rat hearts to Ang II, known as Ang II preconditioning (APC), exerts cardioprotective effects against ischemia-reperfusion (IR) injury. The cardioprotective effect of APC is characterized by improved cardiac function, reduced mitochondrial permeability transition pore (mPTP) opening, improved respiratory control index (RCI), and reduced infarct size (IS) and LDH release [35]. APC also increased the PKCε/PKCδ ratio and promoted a chelerythrine-sensitive translocation of Akt, and MAPKs (ERK1/2, p38, JNK) to the mitochondria [36]. The AT1-R is required for APC because blockade of the receptor by losartan abolished the APC-induced post-ischemic recovery of cardiac function as well as PKCε-dependent signaling in heart mitochondria. Also, the reduction of IS by APC can be prevented by pretreatment with chelerythrine (pan-PKC inhibitor) or its infusion at reperfusion [36]. These studies indicated that the effects of APC include an AT1-R-mediated mechanism that promotes cardioprotection through the activation/translocation of PKCε-dependent survival kinases to the mitochondria and the inhibition of mPTP. However, the relationship of APC with recently described mitochondrial AT1-Rs and AT2-Rs [1,47] remains unknown.
In the present study, we investigated whether APC is associated with changes in AT1-Rs and AT2-Rs in the mitochondria and their relationship with the expression/translocation of PKCε-dependent survival kinases to these organelles. We also evaluated the interaction of these receptors with mitochondrial respiration and cardiac function and assess their role in tissue recovery by measuring IS. Therefore, Langendorff perfused rat hearts were subjected to APC followed by IR with or without pretreatment with losartan, PD 123,319 (PD) or its combination. The results indicate that APC increases the expression of Ang II receptors (AT1-Rs and AT2-Rs) in mitochondria. Interestingly, AT2-Rs have a permissive effect on AT1-R-dependent signaling and suppress mitochondrial respiration and cardiac function. AT2-Rs appear to play a central role in the cardioprotective effects of APC because losartan enhanced the APC-induced reduction of IS through a PD-sensitive mechanism. These findings support a pivotal role of mitochondrial AT2-Rs in the control of cardiac function in APC.
2. Materials & Methods
Animals: Male Sprague-Dawley rats weighing 140–160 g were purchased from Charles River (Wilmington, MA, USA). All experiments were performed according to protocols approved by the University Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996).
2.1. Langendorff-mode perfusion
Hearts were isolated and perfused in the Langendorff mode as described previously [35]. Briefly, rats were anesthetized intraperitoneally with pentobarbital sodium (35 mg/kg body weight). The hearts were rapidly excised and immediately arrested in ice-cold buffered Krebs-Henseleit solution (KHS) containing (in mM): 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.25 CaCl2, 1.2 MgSO4, 11 glucose, and 25 NaHCO3 equilibrated at pH 7.4 with 5% CO2/95% O2. The isolated hearts were mounted on a Langendorff’s perfusion apparatus and perfused at constant flow (10–12 mL/g heart weight per min) with KHS at 37⁰C. A water-filled latex balloon was inserted into the left ventricle and connected to a pressure transducer for continuous monitoring of left ventricular pressure. Balloon volume was set to give an initial left ventricular end diastolic pressure (LVEDP) of 4–6 mmHg. Data acquisition to determine left ventricular developed pressure (LVDP), heart rate (HR), and coronary perfusion pressure (CPP) was performed with the Labscribe2 Data Acquisition Software (iWorx 308T, Dover, NH). Rate pressure product (RPP) was determined using LVDP and HR and expressed as mmHg/min. CPP was registered with a pressure transducer connected to a side arm of the aortic perfusion cannula. Coronary resistance (CR) was determined using CPP and perfusate flow during the first (5 min) cycle of Ang II preconditioning. Data of CR (mmHg × min/mL) is reported at 1 and 5 min after the onset of Ang II action.
2.2. Animal groups
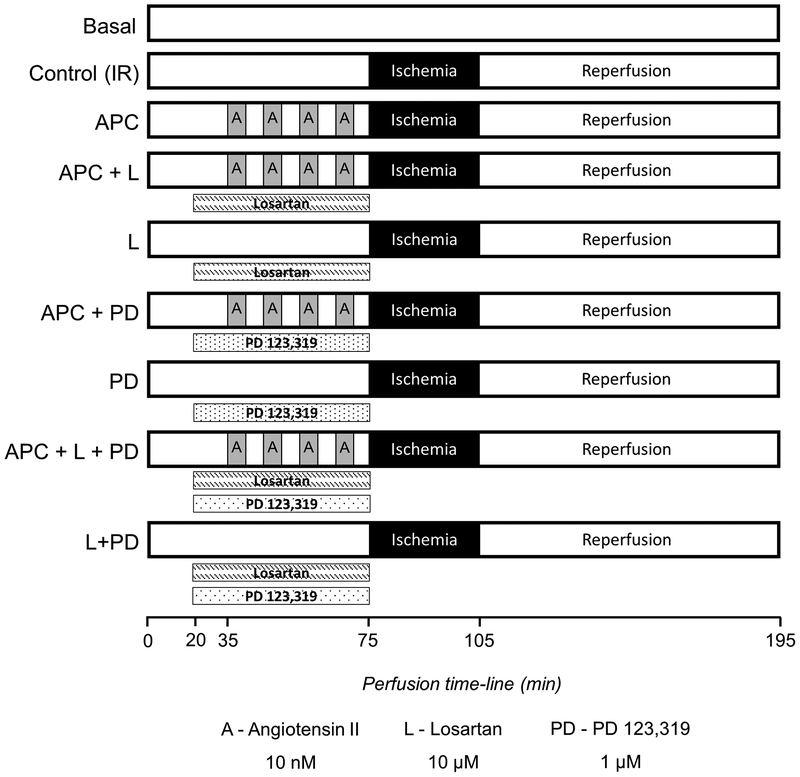
Isolated buffer-perfused rat hearts were stabilized for at least 20 min and randomized into the following six groups (i) Basal group, hearts were perfused for 195 min with KHB, (ii) Control (IR) group, hearts were perfused for 75 min without any intervention followed by 30 min of global ischemia and 90 min of reperfusion, (iii) APC group, hearts underwent four cycles of 5 min perfusion with Ang II (10 nM) and 5 min washout for a total of 40 min prior to the 30 min global ischemia and 90 min reperfusion, (iv) APC+losartan group, hearts were infused with losartan (AT1-R blocker, 10 μM) for 15 min prior and during APC protocol followed by the 30 min global ischemia and 90 min reperfusion, (v) losartan group, hearts were infused with losartan for 55 min during pre-ischemia, followed by the 30 min global ischemia and 90 min reperfusion, (vi) APC+PD group, hearts were infused with PD (AT2-R blocker, 1 μM) for 15 min prior and during APC protocol followed by the 30 min global ischemia and 90 min reperfusion, (vii) PD group, hearts were infused with PD for 55 min during pre-ischemia, followed by the 30 min global ischemia and 90 min reperfusion, (viii) APC+losartan+PD group, hearts were infused with losartan and PD for 15 min prior and during APC protocol followed by the 30 min global ischemia and 90 min reperfusion, and (ix) losartan+PD group, hearts were infused with losartan and PD for 55 min during pre-ischemia, followed by the 30 min global ischemia and 90 min reperfusion. Global ischemia was induced by switching off the pump. During ischemia, hearts were immersed in KHS buffer maintained at 37°C. The concentrations of losartan and PD used in this study were chosen from previous work with cultured cells [15], vasculature [5], and isolated hearts [17,30,41,45]. A diagram of perfusion protocols is illustrated in Figure 1.
Fig. 1.
Schematic representation of the perfusion protocols. See “Methods” for details
2.3. Infarct size assessment
A subset of hearts (n = 4–7 per group) underwent the corresponding perfusion protocols for each group to determine infarct area as described previously by Liu et al. [30]. Briefly, at the end of reperfusion, the ventricles were isolated, weighed and frozen for 90 min at −20°C. The ventricles were then cut into 1.5-mm-thick slices and stained with 1% triphenyltetrazolium chloride (TTC) in phosphate buffer (pH=7.4) at 37°C followed by fixation with 10% formalin at room temperature. The slices were then mounted on glass plates of 1.5 mm thickness and digitally photographed. The digital images were analyzed for the infarcted area (TTC negative) using the NIH ImageJ software. Results were expressed as infarcted area/total area × 100.
2.4. Isolation of mitochondria
At the end of reperfusion, mitochondria were isolated as described previously [35]. Briefly, the ventricles were cut, weighed, and homogenized with a Polytron homogenizer in 3 mL of ice-cold sucrose buffer containing 300 mM sucrose, 10 mM Tris-HCl, and 2 mM EGTA; pH 7.4. Mitochondria were isolated from homogenate by centrifugation at 2,000g for 3 min, followed by centrifugation of the supernatant at 10,000g for 6 min. The pellet was then resuspended and washed two times by centrifugation at 10,000g for 6 min. The final pellet containing mitochondria was resuspended and used for analysis of protein expression and determination of mitochondrial respiration rates. The purity of the mitochondrial preparation was evaluated using the mitochondrial marker, voltage-dependent anion channel (VDAC) and the cytosolic marker GAPDH.
2.4. Determination of mitochondrial respiration rates
Mitochondrial respiration rates [35] were determined as previously described using a YSI Oxygraph (Yellow Springs, OH, USA) model 5300 and a Clark-type oxygen electrode. Mitochondria were suspended in a buffer containing (in mM): 125 KCl, 20 MOPS, 10 Tris, 0.5 EGTA and 2 KH2PO4 supplemented with the substrates for complex I of the electron transfer chain, 2.5 mM 2-oxoglutarate and 1 mM L-malate. Oxygen consumption rates (nmol/mg protein × min) were measured at 30°C in the absence (state 2) and presence of 1 mM ADP (state 3), and normalized to mg of mitochondrial protein. Since state 2 was measured in the presence of substrates without the addition of ADP, the measurement provided values similar to those of state 4, which occurs after ADP depletion by the end of state 3. The respiratory control index (RCI) by Lardy was calculated as the ratio of state 3 to state 2 [26].
2.5. Western blot analysis
Immunoblotting was performed as described previously [4]. Protein concentrations in mitochondria and homogenates were determined using a Bradford assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of mitochondrial and homogenate proteins from the different experimental groups were resolved on 10% SDS-PAGE and transferred onto Amersham Hybond ECL nitrocellulose membranes (GE Healthcare Bio-Sciences Corp., Pittsburgh, PA). Membranes were blocked with 5% BSA in TBS. The antibodies used in this study were against total forms of PKCɛ (1:1000), PKCδ (1:200), AT1-R (1:200), AT2-R (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA), Akt (1:1000), ERK1/2 (1:1000), JNK (1:1000), p38 (1:1000), PKG-1 (1:1000), VDAC (1:1000) (Cell Signaling Technology, Danvers, MA), and GAPDH (1:10000) (Sigma-Aldrich, MO, USA). The signals were visualized by Odyssey CLx Quantitative Fluorescent Imaging System with the secondary infrared antibodies IRDye 800CW goat anti-rabbit (1:25000) and 800CW donkey anti-goat (1:25000). Results were analyzed with Image Studio Lite Software (LI-COR Biotechnology, Lincoln, NE).
2.6. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) of 4–14 experiments per group. Statistical comparisons were performed with One-way analysis of variance (ANOVA). A p value of <0.05 was considered significant. When a significant overall effect was present, intergroup comparisons were performed using a Tukey-Kramer correction for multiple comparisons.
3. Results
3.1. Effects of losartan and PD on coronary resistance and cardiac work in control and APC hearts
3.1.1. Validation for the use of losartan and PD as blockers of AT1 and AT2 receptors: Coronary resistance
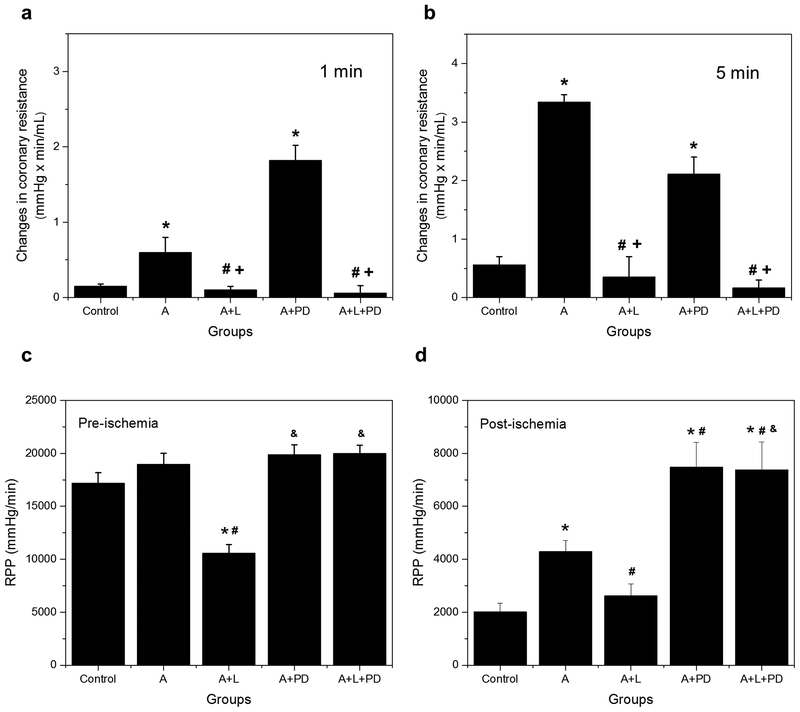
Losartan and PD are two angiotensin receptor blockers (ARBs) commonly used to define AT1-R and AT2-R actions. To validate their use in this study, the effects of losartan (10 μM) and PD (1 μM) on coronary resistance (CR) were tested in non-ischemic perfused hearts after Ang II infusion. These determinations were performed at 1 and 5 min (Fig. 2a and 2b, respectively) to assess the initial and secondary effects of AT1-R and AT2-R blockers on this parameter. Ang II increased CR 4–6-fold over the basal value at both time points (p<0.05 for both), and this effect was abolished by losartan indicating the involvement of AT1-Rs. By contrast, AT2-R blockade with PD markedly increased CR by 3-fold (p<0.05) in the presence of Ang II at 1 min but reduced it by 37% (p=0.16) at 5 min. These data confirm previous findings [5,43] indicating that Ang II increases vasomotor tone through AT1-Rs and that AT2-R blockade potentiates this action during short-term exposure to the hormone.
Fig. 2.
Effect of losartan and PD on coronary resistance and cardiac work in APC hearts. (a) Changes in coronary resistance were determined during 1 and 5 min after the onset of angiotensin II (10 nM) in Langendorff-perfused hearts. The values shown are means ± of 5–14 experiments per groups. *p<0.05 vs Control, #p<0.05 vs AII, +p<0.05 vs AII+PD. AII = angiotensin II, L = losartan, PD = PD 123,319. (b) Rate pressure product (RPP) was determined 5 minutes before global ischemia and at 60 min of reperfusion. RPP values at 90 min were not significantly different from those at 60 min. The values shown are means ± SEM of 5–16 experiments per group. *p<0.05 vs Control; #p<0.05 vs A; &p<0.05 vs A+L. A = APC; A+L = APC+losartan; A+PD = APC+PD; A+L+PD = APC+losartan+PD
3.1.2. Stimulatory effect of APC on cardiac work: Inhibition by Losartan and stimulation by PD
During pre-ischemia (Fig. 2c), the RPP, which is used as an index of cardiac work, was not affected by APC with or without PD. Losartan, however, inhibited this parameter in APC hearts by 44% (p<0.05) indicating an AT1-R-dependent contribution to cardiac function under basal conditions. This effect was not observed in the presence of PD. During the post-ischemic phase (Fig. 2d), APC improved cardiac recovery by 2.1-fold (p<0.05) and this effect was abrogated by losartan as previously reported [35,36]. However, cardiac recovery significantly enhanced compared with the control or APC in the presence of PD with or without losartan (APC+PD versus Control: 3.7-fold, p<0.05; APC+L+PD versus APC: 72%, p<0.05). These data demonstrate a significant cardioprotective effect of AT2-R suppression on the post-ischemic recovery of cardiac function that is independent of AT1-R stimulation.
3.2. APC restores the normal abundance of mitochondrial AT1-Rs and AT2-Rs following ischemia-reperfusion.
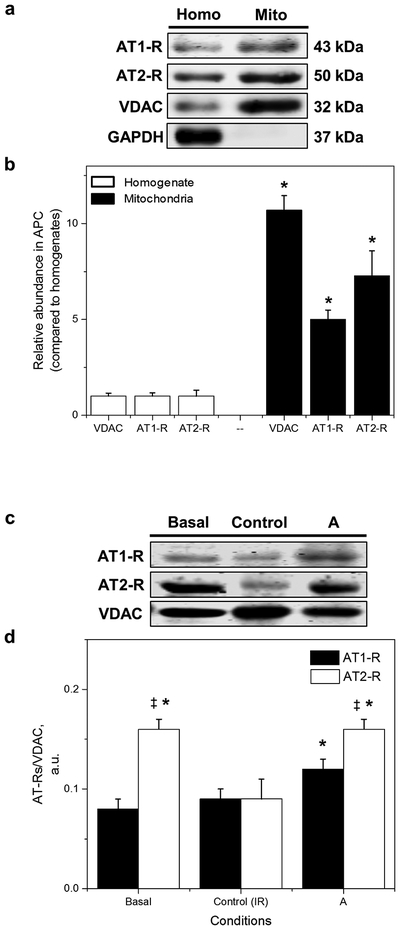
To evaluate the effects of APC on the expression of Ang II receptors, their relative abundance was determined in cardiac mitochondria and homogenates. Figure 3a shows that the protein levels of the mitochondrial marker, voltage-dependent anion channel (VDAC) were significantly higher (10-fold, p<0.05) in mitochondrial samples compared with homogenates. High levels of VDAC and the absence of the cytosol marker GAPDH in the mitochondria, indicate that the current isolation procedure yields a highly enriched mitochondrial suspension. Notably, AT1-R and AT2-R levels in cardiac mitochondria were significantly higher (5-fold and 7-fold, respectively, p<0.05 for both) compared with heart homogenates. These data indicate the presence of both receptors in mitochondria in agreement with previous observations [38,47].
Fig. 3.
Protein expression of AT1-Rs and AT2-Rs in mitochondria isolated from basal, control, and APC hearts. (a) Relative abundance of mitochondrial AT1-Rs and AT2-Rs in APC hearts. Mitochondrial proteins were normalized to their respective protein in homogenate. Note that GAPDH, a cytosol marker, was absent in the mitochondrial preparation. The values are shown as means ± SEM of five experiments per group. *p<0.05 vs. homogenate. (b) Proteins were calculated as the ratio of total protein to VDAC (mitochondrial housekeeping protein). The values are shown as means ± SEM of 5 experiments per group. *p<0.05 vs Control; ‡p<0.05 vs AT1-R. A = APC
Figure 3b shows the protein levels of AT1-Rs and AT2-Rs in mitochondria isolated from normal or IR hearts with and without APC. Under basal conditions (no IR), protein levels of AT2-Rs were 2-fold higher (p<0.05) than those of AT1-Rs. However, AT2-R levels were reduced to values similar to those of AT1-Rs by the end of IR. Of note, APC restored the basal profile of AT2-R and AT1-R levels in IR supporting a role for AT2-Rs in APC signaling in mitochondria.
3.3. Increased protein levels of AT1-R and AT2-R in mitochondria from APC hearts: Permissive effect of AT2-Rs on AT1-Rs.
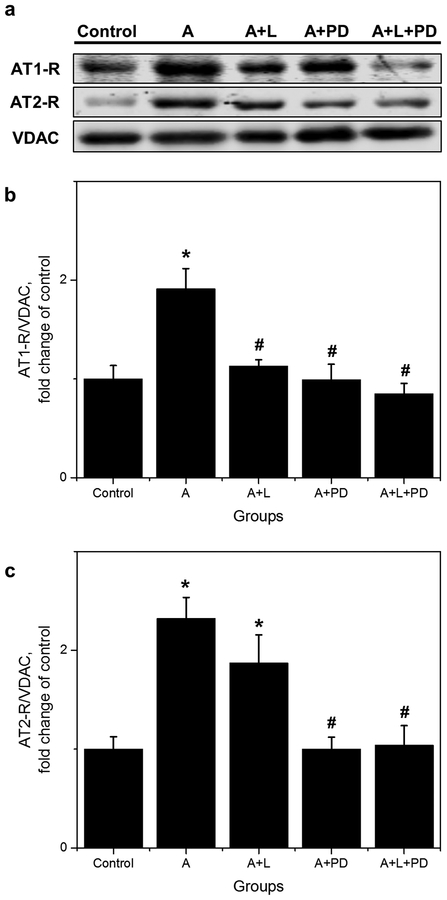
As shown in Figure 4b, AT1-R levels were increased (1.9-fold, p<0.05) by APC compared to controls, and this effect was abolished by losartan. Likewise, PD also prevented the APC-induced up-regulation of AT1-Rs (Fig. 4b). Protein levels of AT2-Rs were increased by APC (2.3-fold, p<0.05) in a PD-sensitive manner but, as expected, were not affected by losartan (Fig. 4c). These data suggest that mitochondrial AT2-Rs play a permissive role in APC-induced upregulation of AT1-Rs.
Fig. 4.
Effect of losartan and PD in protein levels of AT1-R and AT2-R in isolated mitochondria. (a) Representative Western Blots and quantitative data of (b), AT1-R; (c), AT2-R. Proteins were calculated as the ratio of total protein to VDAC and normalized to control for each receptor. The values are shown as means ± SEM of five experiments per group, except A+L+PD (n=4). *p<0.05 vs Control; #p<0.05 vs A. A = APC; A+L = APC+losartan, A+PD = APC+PD; A+L+PD = APC+losartan+PD
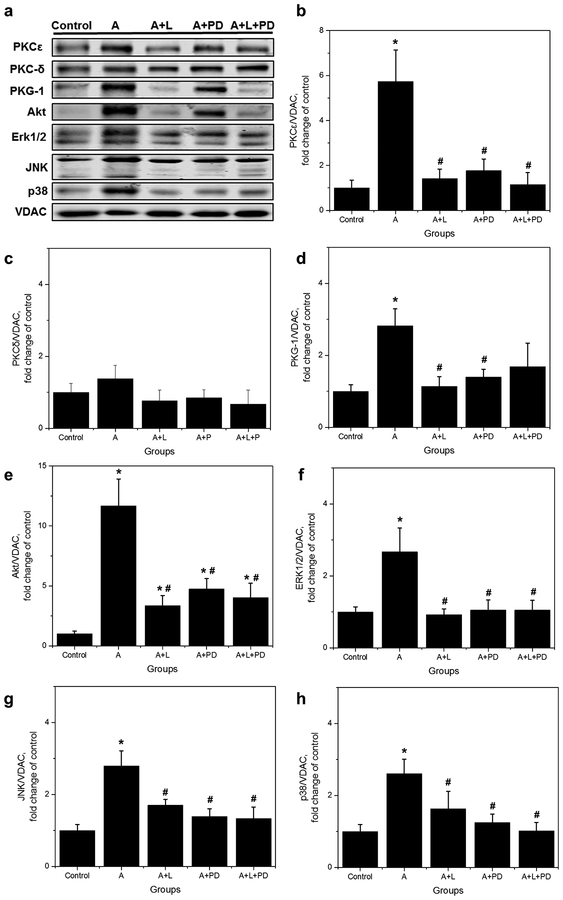
3.4. APC stimulates an AT1-R mediated, PKCε signaling in mitochondria that is AT2-R dependent.
We previously reported [36] that APC operates through a chelerythrine-sensitive, PKCε-dependent signaling in the mitochondria. For this reason, we then evaluated the effects of AT1-Rs and AT2-Rs blockade on the levels of protein kinases (PKCε, PKCδ, Akt, MAPKs, and PKG-1) involved in the APC-mediated signaling pathway in mitochondria from control and APC hearts. As shown in Figure 5, protein levels of all kinases, except PKCδ, demonstrated a profile response similar to that observed for AT1-R. The levels of PKCɛ, Akt, MAPKs, and PKG-1 were upregulated by APC in a losartan, and PD-sensitive manner since both ARBs prevented Ang II-induced upregulation of the protein kinases. PKCδ was unaffected by APC or ARBs. Marked increases in protein levels by APC were observed, however, for PKCε (5.7-fold, p<0.05) and Akt (11.7-fold, p<0.05). These data support previous findings [36] indicating that APC is an AT1-R-mediated event requiring PKCε–dependent signaling, including PKG-1 in mitochondria.
Fig. 5.
Effect of losartan and PD in protein levels of PKCε, PKCδ, PKG-1, Akt, ERK1/2, JNK, and p38 in isolated mitochondria. (a), Representative Western blots and quantitative data of total (b), PKCɛ; (c), PKCδ; (d), PKG-1; (e), Akt; (f), ERK1/2; (g), JNK; (h), p38. Proteins were calculated as the ratio of total protein to VDAC and normalized to control for each kinase. The values are shown as means ± SEM of five experiments per group, except A+L+PD (n=4). *p<0.05 vs Control; #p<0.05 vs A. A = APC, A+L = APC+losartan; A+PD = APC+PD; A+L+PD = APC+losartan+PD
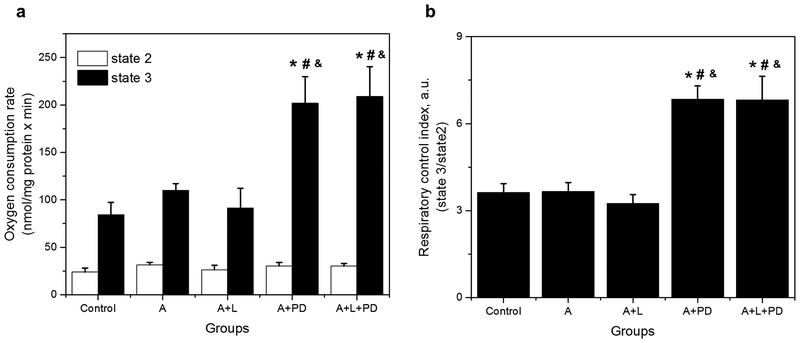
3.5. PD markedly increases state 3 respiration of mitochondria in APC hearts.
To assess the effect of AT1-Rs and AT2-Rs on mitochondrial function, the effects of losartan and PD on mitochondrial respiration were determined in control and APC hearts. Figure 6a shows that respiratory function of mitochondria (state 2 and state 3) was not significantly affected by APC or APC+losartan. However, PD induced a 1.8-fold increase (from 110 ± 7 in APC to 202 ± 28 nmol/mg protein × min in APC+PD, p<0.05) the state 3 respiration rate. Similar effects on state 3 were observed when APC hearts were treated with PD in combination with losartan (1.9-fold increase from 110 ± 7 in APC to 209 ± 31 nmol/mg protein × min in APC+PD+losartan, p<0.05). State 2 of respiration rate was not affected by APC in the presence or absence of ARBs. Analysis of the respiratory control index (RCI: state3/state2, Fig. 6b) also revealed significant increases (96%, p<0.05) by PD or PD+losartan in APC hearts. These findings suggest that inhibition of AT2-Rs, but not AT1-Rs, improves mitochondrial respiratory function in APC hearts.
Fig. 6.
Effect of losartan and PD on mitochondrial respiration in APC hearts. (a), State 2 and state 3; (b), Respiratory control index (RCI). Oxygen consumption rates are given in nmol/mg protein × minutes. The values shown are the mean ± SEM of 4–14 experiments per group. *p<0.05 vs Control; #p<0.05 vs A; &p<0.05 vs A+L. A = APC, A+L = APC+losartan, A+PD = APC+PD; A+L+PD = APC+losartan+PD
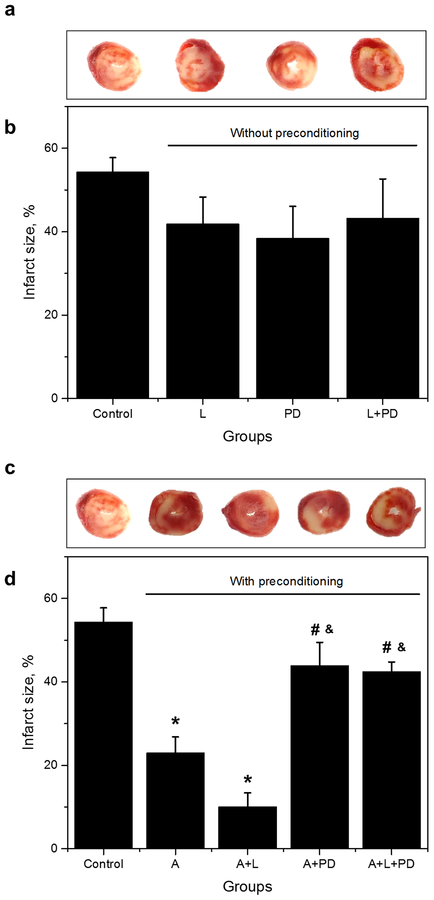
3.6. PD prevents the reduction of infarct size by APC and APC+L.
Our data on the enhancement of state 3 mitochondrial respiration (Fig. 6a) and cardiac work (Fig. 2d) by PD, prompted us to evaluate the status of tissue damage under these conditions. To accomplish this task, infarct size was determined using the TTC method in hearts without preconditioning (Fig. 7a,b) and with preconditioning (APC, APC+L, APC+PD, and APC+L+PD; Fig 7c,d). Figure 7a,b shows that neither losartan nor PD affects infarct size in hearts without APC. However, both APC and losartan reduced IS by 54% and 81% (p<0.05), respectively, compared with controls (Fig. 7c,d). Notably, PD prevented the inhibition of IS by both APC and APC+L. These results support the idea that AT1-R blockade with losartan, promotes a PD-sensitive, AT2-R activity that reduces cardiac tissue damage.
Fig. 7.
Effect of losartan and PD on infarct size in preconditioned and non-preconditioned rat hearts. Representative cross-sections of rat hearts (a) without preconditioning and (c) with preconditioning stained with TTC for infarct (white) and viable tissue (red). Quantitative data of infarct size expressed in percent of total area (whole heart) in hearts treated (b) without preconditioning and (d) with preconditioning. The values are shown as means ± SEM of 4–7 per group. *p<0.05 vs Control; #p<0.05 vs A; &p<0.05 vs A+L. L = losartan; PD = PD 123319; L+PD = losartan+PD 123,319; A = APC, A+L = A+losartan, A+PD = APC+PD, A+L+PD = APC+losartan+PD
4. Discussion
The results of the present work indicate that APC increases the protein levels of Ang II receptors in mitochondria, and blockade of AT2-Rs with PD reduced protein levels of AT1-Rs and the subsequent PKCε-dependent signaling mechanisms in mitochondria of rat hearts subjected to ex-vivo IR injury. By contrast, blockade of AT1-Rs by losartan did not affect AT2-R protein levels. The effects of AT2-R inhibition are associated with increased mitochondrial respiration and coupling, and stimulation of cardiac function, suggesting that AT2-Rs suppress mitochondrial respiration and coupling, resulting in inhibition of cardiac function. These findings suggest that modulation of mitochondrial AT1-Rs and AT2-Rs by their plasma membrane counterparts during APC plays a critical role in the regulation of both mitochondrial and cardiac function.
The inhibition of mitochondrial AT1-Rs by PD is at variance with the selective inhibition of AT2-Rs by PD in other model systems [6,31,50]. For this reason, the effect of PD on AT1-R levels was unexpected. Besides, our data indicate that while losartan abolished the Ang II-induced CR, the AT2-R antagonist markedly increased it 1 min after the onset of Ang II infusion. These data support the well-known vasodilator effect of AT2-Rs that attenuates AT1-R-induced contraction through both direct (i.e., stimulation of NOS and the bradykinin-NO/cGMP cascade) and indirect (cross-inhibition of AT1-R activity) mechanisms [28,42,51]. Although at 5 min, PD reduces the Ang II-induced CR by 37%, this effect is probably secondary to an indirect increase in CR by α-adrenergic vasoconstriction and endothelin release [41,43]. Indeed, CR in the presence of the AT2-R antagonist did not vary between 1 min and 5 min supporting an indirect increase in contractility. Besides, the heart was pretreated with these inhibitors for 15 min prior to Ang II infusion, making it unlikely that the reduction of CR by PD observed at 5 min is due to the lengthier drug exposure. For these reasons, we consider that the inhibitory effect of PD on AT1-R does not represent a direct inhibition of the receptor but rather a permissive effect of mitochondrial AT2-R. In other words, the stimulation of mitochondrial AT1-R requires AT2-R activation. This crosstalk is at variance with the well-established counterbalance of AT1-R action by AT2-R activation under physiological or pathological conditions such as ischemic injury and hypertension [7,42,48]. Whether the permissive effect reported here requires changes in transcriptional levels [53] or protein-protein interactions as reported for G-protein-coupled receptors (GPCRs) [20,23,34] remains to be established. To the best of our knowledge, this is the first report of such interaction of mitochondrial AT2-Rs with AT1-Rs.
Our results indicate that the PKCε-dependent signaling pathways associated with AT1-Rs [36] show the same profile of ARB responses observed for AT1-Rs protein levels. Thus, these kinase pathways were stimulated by APC through losartan- and PD-sensitive pathways. Except for PKCδ that was unaffected by APC or ARBs, other protein kinases (PKCε, Akt, ERK1/2, JNK, and p38) were upregulated by APC and inhibited by losartan and PD. These data extend our observations on AT1-R-mediated PKCε–dependent signaling in mitochondria and suggest that AT2-Rs exert positive control over AT1-R-dependent signaling pathways. Of note, plasma membrane AT2-Rs working through tyrosine and serine/threonine phosphatases, modulate protein kinases involved in AT1-R-dependent growth and proliferation [51]. Besides, AT2-Rs could target AT1-Rs to the mitochondria through AT1-R/AT-2R heterodimers as shown in the endoplasmic reticulum of LCC-PK1 cells [15]. The mechanisms involved in AT1-R modulation by AT2-R in mitochondria could be similar but acting on the transcription or synthesis of AT1-Rs and their translocation or targeting to the mitochondria. Further experiments are necessary to clarify this issue.
Recent reports by Abadir and colleagues [1] identified and characterized a functional mitochondrial angiotensin system. The authors reported that AT2-Rs present in the inner mitochondrial membrane co-localize with Ang II and operate to inhibit respiration through the NO/cGMP pathway. Thus, PD and L-NG-nitroarginine methyl ester (L-NAME, a nitric oxide synthase inhibitor) blocked the stimulation of NO production by AT2-R stimulation and CGP41140 in isolated mitochondria. Similar results have been published by Valenzuela et al. [47], in mitochondria from dopaminergic neurons that express both AT1-Rs and AT2-Rs. To correlate the effects of AT2-R blockade with NO-dependent signaling, we determined the protein levels of mitochondrial PKG-1, a NO-downstream signaling target. However, this kinase was also inhibited by losartan and PD suggesting that its activation by APC follows a similar AT1-R-mediated PKCε-dependent mechanism. It appears, therefore, that the activation of PKG-1 in the mitochondria takes place through both AT2-R-dependent and AT2-R-independent pathways. Of note, an AT1-R-dependent, NO-cGMP pathway has been reported in the rat carotid artery [9] and endothelial cells [39,40]. In neuroblastoma cells and H9c2 rat cardiomyocytes [21], both AT1-Rs and AT2-Rs appear to modulate this pathway [54]. Our data with mitochondrial receptors are consistent with the latter studies.
Recently, data presented by Valenzuela et al. [47] indicate that in contrast with AT2-Rs, mitochondrial AT1-Rs stimulate respiration. Indeed, these studies showed that activation of AT1-Rs with Ang II in the presence of PD, increased oxidative phosphorylation and the maximum respiratory rate. Besides, mice lacking AT1-Rs showed a decrease in respiratory rate compared with wild-type mice. Our data on state 3 respiration rate supports this finding, as there is a tendency of APC to increase respiration that disappears in the presence of losartan. However, in the presence of PD, a marked stimulation of state 3 respiration (2.4-fold) was observed even with losartan treatment. The stimulatory effect of the AT2-R antagonist, resulted in a pronounced increase in RCI, suggesting an increased coupling of the mitochondrial respiratory chain. These actions of PD support the idea that AT2-Rs maintain a tonic suppression of mitochondrial respiration and coupling independent of possible AT1-R-mediated effects. It is worth noting that the post-ischemic cardiac function also increased with the AT2-R blockade. This observation is in contrast with the lack of effect of PD on cardiac function during the preconditioning period and suggests that IR promotes AT2-R suppression of both mitochondrial and cardiac function. These findings are significant because following IR mitochondrial AT2-R could operate to attenuate myocardial damage. It could be hypothesized that under these conditions, inhibition of mitochondrial respiration and cardiac function by AT2-Rs, decrease the energy and oxygen demands of the tissue promoting cell survival through signaling pathways involving AT1-R-dependent PKCε-stimulation or through the NO/cGMP pathway. Indeed, the AT1-R blockade in APC with losartan that supports selective AT2-R activation reduces infarct size to a larger extent than APC alone. This process is prevented by PD, as is the protection conferred by APC. By contrast, these drugs did not affect IS in hearts without APC. These observations indicate that AT2-R activity in APC is responsible for the beneficial effects of AT1-R blockade on IS. The potent inhibitory effect of AT2-R on IS probably obscures an otherwise expected increase of IS by the inhibitory effect of losartan on APC cardioprotection [35]. Concerning the stimulatory effect of PD on mitochondrial oxygen consumption and cardiac function mentioned earlier, the observed increase of IS by AT2-R blockade strongly supports the idea that suppression of these functional parameters is critical for the reduction of IS. Besides, we have previously reported [36] that chelerythrine (panPKC inhibitor) abolishes the IS reduction, but not the stimulation of cardiac function by APC. This indicates that PKCε-dependent signaling is essential for the decrease of IS by APC. For these reasons, it is reasonable to postulate that AT2-R activity requires PKCε-dependent signaling (i.e., Akt, MAPKs) to limit IS, but not to stimulate cardiac function and respiration in APC. Although the precise mechanisms by which AT2-Rs mediate these actions remain to be established, it is likely that they involve direct (NO/PKG-cGMP) or indirect (AT1-R-dependent) mechanisms because losartan inhibits PKCε-dependent signaling. However, additional studies are needed to settle these important points.
AT1-Rs and to a less extent, AT2-Rs are expressed in the adult heart. However, AT2-Rs appear to be upregulated in pathophysiological conditions such as hypertension, heart failure, and hypertrophy [24,51]. However, there are reports indicating downregulation of AT2-Rs following post-infarct remodeling [25] and heart failure [32]. Similarly, in the isolated working rat heart, ischemia and reperfusion induce a reduction in mRNA and protein levels of AT2-Rs [52]. Consistent with these findings, our data on mitochondrial Ang II receptor expression indicate that mitochondrial AT2-Rs are also downregulated after IR. It should be noted, however, that APC restored the normal ratio of AT2-Rs to AT1-Rs, suggesting that a balance between mitochondrial AT1-R and AT2-R levels and action is critical to Ang II-preconditioning. A high proportion of AT2-Rs to AT1-Rs has also been reported in mitochondria from young (20 week-old) mice [1]. These findings are in line with the data presented in this work using relatively young rats (7-week old). Such a high AT2-R/AT1-R ratio in mitochondria is at variance with the low expression of AT2-Rs to AT1-Rs in coronary arteries, cardiomyocytes and ventricular myocardium of adult animals [42]. Although APC is an AT1-R-dependent process [35,36], the restoration of mitochondrial AT2-R by preconditioning and its permissive role on AT1-R, support the protective role of AT2-Rs in the myocardium following IR. Our results on the effect of PD on IS in APC lends strong support for this action of AT2-Rs.
Ang II action is closely related to alterations of cellular calcium homeostasis. Indeed, the pathogenesis of cardiovascular diseases such as HF, hypertension, arrhythmias, and cardiac hypertrophy has been associated with Ang II-induced alterations in calcium homeostasis in cells [18,33]. Data indicate that voltage-gated L-type Ca2+ channels, a major pathway for Ca2+ entry in ventricular cardiomyocytes, could play a crucial role in excitation-contraction coupling [12,29,49]. Ang II and other mediators modulate these pathways in various cell models through Gβγ-sensitive phosphoinositide 3-kinase (PI3K) and protein kinase activation [2]. However, whether APC involves L-Type Ca2+ channels in the heart has not been defined. Also, information is lacking on the role of mitochondrial Ca2+ signaling and handling, in this process. The latter is important because mitochondrial Ca2+ homeostasis could be relevant to maintain cell viability [8] following APC. Indeed, we have reported that the mitochondrial permeability transition pore, a Ca2+ -activated pore that induces the apoptotic cascade and cell death, is attenuated by ischemic preconditioning and APC [35]. This suggests that Ca2+ handling by mitochondria is affected by IR and this process is responsive to modulation by preconditioning protocols. However, the routes for mitochondrial Ca2+ entry and control in the setting of APC has not been evaluated.
In summary, our findings demonstrate regulation of mitochondrial AT1-Rs and AT2-Rs by their membrane counterparts during APC. This regulation includes a permissive action of AT2-Rs on AT1-R-mediated, PKCε-dependent signaling in mitochondria suggesting that AT2-Rs exert critical control over AT1-R signaling pathways. Furthermore, our data indicate that AT2-Rs tonically suppress mitochondrial respiration and cardiac function following IR in APC hearts. These processes appear to be relevant to promote APC-mediated cardioprotection because AT2-R inhibition augments IS while AT1-R blockade reduces it. Although more studies are necessary to elucidate the role of mitochondrial receptors in the modulation of cardiac function, the characterization of such a system opens new possibilities for the understanding of the mitochondrial function and its impact in cardiac pathophysiology.
5. Limitations of the Study
Some limitations must be considered in the analysis of the current work. This study employed a long perfusion protocol to evaluate the effects of angiotensin II receptor blockers on APC. Although long perfusion could affect cardioprotection secondary to glycogen store depletion, no effect was observed in control hearts after IR in the present study. Also, we measured protein levels of the total but not phosphorylated PKCɛ, Erk1/2, Akt, PKCδ, JNK, p38 MAPK and PKG-1 in mitochondrial fractions, to assess whether the translocation of these kinases was associated with the preconditioning protocols. The high levels of total PKCɛ, Erk1/2, Akt, JNK, and PKG-1, but not PKCδ in mitochondria indicates an increased translocation of the kinases to the mitochondria subsequently to their apparent activation in the cytoplasm. Besides, lack of knowledge on the mechanisms of translocation and regulation of AT1-Rs and AT2-Rs in mitochondria obscures understanding the role of mitochondrial angiotensin II system in mediating APC signaling. Although it has been postulated [1] that AT1-Rs and AT2-Rs are synthetized as cytosolic precursors, the process by which they are translocated to the mitochondria has not been defined. Finally, it is not clear if the increased protein levels of AT1-R and AT2-R in the mitochondria reported here are the result of enhanced synthesis, changes in mRNA, and/or protein turnover.
Funding information
This study was supported by the National Institutes of Health Research Centers in Minority Institutions (RCMI) Program grant G12M007600, National Institute of General Medical Sciences grant SC1GM128210 (S.J.), and the University of Puerto Rico.
Footnotes
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD (2011) Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108:14849–14854. doi: 10.1073/pnas.1101507108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvin Z, Laurence GG, Coleman BR, Zhao A, Hajj-Moussa M, Haddad GE (2011) Regulation of L-type inward calcium activity by captopril and angiotensin II via the phosphatidyl inositol 3-kinase pathway in cardiomyocytes from volume-overload hypertrophied rat hearts. Can J Physiol Pharmacol 89(3):206–125. doi: 10.1139/Y11-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R (2004) Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120:5–13. doi: 10.1016/j.regpep.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Barreto-Torres G, Parodi-Rullan R, Javadov S (2012) The role of PPARalpha in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int J Mol Sci 13:7694–7709. doi: 10.3390/ijms13067694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH (2004) Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation 109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57 [DOI] [PubMed] [Google Scholar]
- 6.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE (2011) Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121:297–303. doi: 10.1042/CS20110036 [DOI] [PubMed] [Google Scholar]
- 7.Brouwers S, Smolders I, Massie A, Dupont AG (2013) Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension 62:920–926. doi: 10.1161/HYPERTENSIONAHA.112.00762 [DOI] [PubMed] [Google Scholar]
- 8.Cano-Abad MF, Villarroya M, Garcia AG, Gabilan NH, Lopez MG (2001) Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J Biol Chem 276(43):39695–39704 [DOI] [PubMed] [Google Scholar]
- 9.Caputo L, Benessiano J, Boulanger CM, Levy BI (1995) Angiotensin II increases cGMP content via endothelial angiotensin II AT1 subtype receptors in the rat carotid artery. Arterioscler Thromb Vasc Biol 15:1646–1651 [DOI] [PubMed] [Google Scholar]
- 10.Celio MR, Inagami T (1981) Angiotensin II immunoreactivity coexists with renin in the juxtaglomerular granular cells of the kidney. Proc Natl Acad Sci USA 78:3897–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Mello WC (2014) Beyond the circulating Renin-Angiotensin aldosterone system. Front Endocrinol (Lausanne) 5:104. doi: 10.3389/fendo.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Mello WC, Monterrubio J (2004) Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension 44(3):360–364 [DOI] [PubMed] [Google Scholar]
- 13.Diaz RJ, Wilson GJ (1997) Selective blockade of AT1 angiotensin II receptors abolishes ischemic preconditioning in isolated rabbit hearts. J Mol Cell Cardio 29:129–139. doi: 10.1006/jmcc.1996.0258 [DOI] [PubMed] [Google Scholar]
- 14.Dikalov SI, Nazarewicz RR (2013) Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 19:1085–1094. doi: 10.1089/ars.2012.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrao FM, Cardoso LHD, Drummond HA, Li XC, Zhuo JL, Gomes DS, Lara LS, Vieyra A, Lowe J (2017) Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells. Am J Physiol Renal Physiol 313:F440–F449. doi: 10.1152/ajprenal.00261.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishman MC, Zimmerman EA, Slater EE (1981) Renin and angiotensin: the complete system within the neuroblastoma × glioma cell. Science 214:921–923 [DOI] [PubMed] [Google Scholar]
- 17.Flynn JD, Akers WS (2003) Effects of the angiotensin II subtype 1 receptor antagonist losartan on functional recovery of isolated rat hearts undergoing global myocardial ischemia-reperfusion. Pharmacotherapy 23(11):1401–1410 [DOI] [PubMed] [Google Scholar]
- 18.Freichel M, Berlin M, Schürger A, Mathar I, Bacmeister L, Medert R, Frede W, Marx A, Segin S, Londoño JEC (2017) TRP Channels in the Heart In: Emir TLR (ed) Neurobiology of TRP Channels. 2nd edn. CRC Press/Taylor & Francis, Boca Raton (FL), Chapter 9 [PubMed] [Google Scholar]
- 19.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC (2009) Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296:F1484–1493. doi: 10.1152/ajprenal.90766.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert TE, Loisel TP, Adam L, Ethier N, Onge SS, Bouvier M (1998) Functional rescue of a constitutively desensitized beta2AR through receptor dimerization. Biochemi J 330 (Pt 1):287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S (2014) Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 18:709–720. doi: 10.1111/jcmm.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inagami T (2011) Mitochondrial angiotensin receptors and aging. Circ Res 109:1323–1324. doi: 10.1161/RES.0b013e31823f05e0 [DOI] [PubMed] [Google Scholar]
- 23.Jordan BA, Devi LA (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399:697–700. doi: 10.1038/21441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaschina E, Namsolleck P, Unger T (2017) AT2 receptors in cardiovascular and renal diseases. Pharmacol Res 125 (Pt A):39–47. doi: 10.1016/j.phrs.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 25.Lax CJ, Domenighetti AA, Pavia JM, Di Nicolantonio R, Curl CL, Morris MJ, Delbridge LM (2004) Transitory reduction in angiotensin AT2 receptor expression levels in postinfarct remodelling in rat myocardium. Clin Exp Pharmacol Physiol 31:512–517. doi: 10.1111/j.1440-1681.2004.04034.x [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Doliba N, Osbakken M, Oz M, Mancini D (1998) Improvement of myocardial mitochondrial function after hemodynamic support with left assist devices in patients with heart failure. J Thorac Cardiovasc Surg 116(2):344–349 [DOI] [PubMed] [Google Scholar]
- 27.Lemarie CA, Schiffrin EL (2010) The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst 11:19–31. doi: 10.1177/1470320309347785 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Li XH, Yuan H (2012) Angiotensin II type-2 receptor-specific effects on the cardiovascular system. C Diagn Ther 2:56–62. doi: 10.3978/j.issn.2223-3652.2012.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang W, Oudit GY, Patel MM, Shah AM, Woodgett JR, Tsushima RG, Ward ME, Backx PH (2010) Role of phosphoinositide 3-kinase {alpha}, protein kinase C, and L-type Ca2+ channels in mediating the complex actions of angiotensin II on mouse cardiac contractility. Hypertension 56(3):422–429 doi: 10.1161/HYPERTENSIONAHA.109.149344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Tsuchida A, Cohen MV, Downey JM (1995) Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol 27:883–892 [DOI] [PubMed] [Google Scholar]
- 31.MaassenVanDenBrink A, de Vries R, Saxena PR, Schalekamp MA, Danser AH (1999) Vasoconstriction by in situ formed angiotensin II: role of ACE and chymase. Cardiovasc Res 44:407–415 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Ozono R, Oshima T, Matsuura H, Sueda T, Kajiyama G, Kambe M (2000) Type 2 angiotensin II receptor is downregulated in cardiomyocytes of patients with heart failure. Cardiovasc Res 46:73–81 [DOI] [PubMed] [Google Scholar]
- 33.Mehta PK, Griendling KK (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am Journal Physiol Cell Physiol 292:C82–97. doi: 10.1152/ajpcell.00287.2006 [DOI] [PubMed] [Google Scholar]
- 34.Ng GY, O’Dowd BF, Lee SP, Chung HT, Brann MR, Seeman P, George SR (1996) Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem Biophys Res Commun 227:200–204. doi: 10.1006/bbrc.1996.1489 [DOI] [PubMed] [Google Scholar]
- 35.Nuñez RE, Castro M, Javadov S, Escobales N (2014) Angiotensin II and ischemic preconditioning synergize to improve mitochondrial function while showing additive effects on ventricular postischemic recovery. J Cardiovasc Pharmacol 64:172–179. doi: 10.1097/FJC.0000000000000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuñez RE, Javadov S, Escobales N (2017) Angiotensin II-preconditioning is associated with increased PKCepsilon/PKCdelta ratio and prosurvival kinases in mitochondria. Clin Exp Pharmacol Physio 44:1201–1212. doi: 10.1111/1440-1681.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamura T, Clemens DL, Inagami T (1981) Renin, angiotensins, and angiotensin-converting enzyme in neuroblastoma cells: evidence for intracellular formation of angiotensins. Proc Natl Acad Sci USA 78:6940–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S (2012) Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem 29:841–850. doi: 10.1159/000178526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pueyo ME, Arnal JF, Rami J, Michel JB (1998) Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol 274 (1 Pt 1):C214–220 [DOI] [PubMed] [Google Scholar]
- 40.Saito S, Hirata Y, Emori T, Imai T, Marumo F (1996) Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens Res 19:201–206 [DOI] [PubMed] [Google Scholar]
- 41.Sasaoka T, Egi Y, Tawa M, Yamamoto A, Ohkita M, Takaoka M, Maruyama T, Akira T, Matsumara Y (2008) Angiotensin II type 2 receptor-mediated inhibition of norepinehrine release in isolated rat hearts. J Cardiovasc Pharmacol 52(2):176–183 [DOI] [PubMed] [Google Scholar]
- 42.Savoia C, Volpe M (2015) AT1R-AT2R Cross Talk In: Unger T, Steckelings UM, dos Santos RAS (eds) The Protective Arm of the Renin Angiotensin System: Functional Aspects and Therapeutic Implications. 1st edn. Elsevier, United Kingdom, pp 35–39 [Google Scholar]
- 43.Schmermund A, Lerman LO, Ritman EL, Rumberger JA (1999) Cardiac production of angiotensin II and its pharmacologic inhibition: effects on the coronary circulation. Mayo Clin Proc 74:503–513. doi: 10.4065/74.5.503 [DOI] [PubMed] [Google Scholar]
- 44.Sharma A, Singh M (1999) Role of angiotensin in cardioprotective effect of ischemic preconditioning. J Cardiovasc Pharmacol 33:772–778 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Maehara K, Yaoita H, Maruyama Y (2004) Altered effects of angiotensin II type 1 and type 2 receptor blockers on cardiac norepinephrine release and inotropic responses during cardiac sympathetic nerve stimulation in aorto-caval shunt rats, Circ J 68(7):683–690 [DOI] [PubMed] [Google Scholar]
- 46.Tadevosyan A, Xiao J, Surinkaew S, Naud P, Merlen C, Harada M, Qi X, Chatenet D, Fournier A, Allen BG, Nattel S (2017) Intracellular angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J Am Heart Assoc 6 (4). doi: 10.1161/JAHA.116.004965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valenzuela R, Costa-Besada MA, Iglesias-Gonzalez J, Perez-Costas E, Villar-Cheda B, Garrido-Gil P, Melendez-Ferro M, Soto-Otero R, Lanciego JL, Henrion D, Franco R, Labandeira-Garcia JL (2016) Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. doi: 10.1038/cddis.2016.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez E, Coronel I, Bautista R, Romo E, Villalon CM, Avila-Casado MC, Soto V, Escalante B (2005) Angiotensin II-dependent induction of AT(2) receptor expression after renal ablation. Am J Physiol Renal Physiol 288:F207–213. doi: 10.1152/ajprenal.00216.2004 [DOI] [PubMed] [Google Scholar]
- 49.Volk T, Nguyen TH, Schultz JH, Ehmke H (1999) Relationship between transient outward K+ current and Ca2+ influx in rat cardiac myocytes of endo- and epicardial origin. J Physiol 519 Pt 3:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wexler RR, Greenlee WJ, Irvin JD, Goldberg MR, Prendergast K, Smith RD, Timmermans PB (1996) Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy. J Med Chem 39:625–656. doi: 10.1021/jm9504722 [DOI] [PubMed] [Google Scholar]
- 51.Widdop RE, Jones ES, Hannan RE, Gaspari TA (2003) Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol 140:809–824. doi: 10.1038/sj.bjp.0705448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Clanachan AS, Jugdutt BI (2000) Enhanced expression of angiotensin II type 2 receptor, inositol 1,4, 5-trisphosphate receptor, and protein kinase C epsilon during cardioprotection induced by angiotensin II type 2 receptor blockade. Hypertension 36:506–510 [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C (2012) Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens 30:1176–1184. doi: 10.1097/HJH.0b013e3283532099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarahn ED, Ye X, Ades AM, Reagan LP, Fluharty SJ (1992) Angiotensin-induced cyclic GMP production is mediated by multiple receptor subtypes and nitric oxide in N1E-115 neuroblastoma cells. J Neurochem 58:1960–1963 [DOI] [PubMed] [Google Scholar]