Abstract
Background:
Recent studies suggest that surgical lymph node evaluation may be omitted in select elderly breast cancer patients, as it may not influence adjuvant therapy decisions. To evaluate differences in adjuvant therapy receipt and overall survival (OS), we compared clinically node-negative (cN0) elderly patients who did and did not undergo axillary surgery.
Methods:
Patients ages ≥70 in the National Cancer Data Base (2004–2014) with cT1–3, cN0 breast cancer were divided into 2 cohorts – those with surgical lymph node evaluation (≥1 node removed) and those without (0 nodes removed). Propensity scores were used to match patients based on age, year of diagnosis, tumor grade, cT stage, estrogen receptor status, and Charlson/Deyo comorbidity score. A Cox proportional hazards model was used to estimate the effect of lymph node surgery on OS.
Results:
133,778 patients were matched. 102,247 patients (76.4%) underwent nodal surgery. Patients undergoing nodal surgery were more likely to receive chemotherapy (pN1–3: 22.2%, pN0: 5.8%, cN0-no nodal surgery: 2.8%, p<0.001), radiation (pN1–3: 49.7%, pN0: 47.5%, cN0-no nodal surgery: 26%, p<0.001), and endocrine therapy (pN1–3: 72%, pN0: 58.5%, cN0-no nodal surgery: 46.5%, p<0.001). After adjustment for known covariates, patients who did not undergo nodal surgery had a worse OS (HR 1.66, 95% CI 1.61–1.70).
Conclusions:
For elderly cN0 breast cancer patients, axillary surgery was associated with higher rates of adjuvant therapy and improved OS. A selective approach to omitting nodal surgery should be considered in elderly patients with cN0 breast cancer, as axillary staging may influence subsequent treatment decisions and long-term outcomes.
Keywords: sentinel lymph node, elderly, breast cancer, axillary lymph node dissection
INTRODUCTION
In 2016, the Society of Surgical Oncology Choosing Wisely Guidelines recommended against routine sentinel lymph node biopsy (SLNB) in women over the age of 70 with clinically node negative (cN0), hormone receptor positive (HR+), invasive breast cancer. This was based on studies evaluating recurrence and overall survival (OS) in a subset of elderly patients when axillary surgery was excluded.1,2 While less morbid than an axillary lymph node dissection, SLNB is still associated with a risk of sensory nerve injury and lymphedema.3,4 To mitigate these risks, omitting surgical procedures that do not impact adjuvant management decisions or survival is encouraged. However, the decision to omit SLNB is often made based on the physician’s assessment of a patient’s overall health, and in elderly patients with competing comorbidities, this assessment is especially important and potentially challenging.
In 2012, the International Society of Geriatric Oncology and European Society of Breast Cancer Specialists updated their recommendations regarding elderly breast cancer patients to include comprehensive geriatric assessment.5 In this functionally heterogeneous group, clinical management should be guided by numerous factors other than chronological age alone. However, in a study evaluating guideline concordant care among elderly breast cancer patients, increased age was associated with decreased guideline compliance, even after adjustment.6 Prior studies also demonstrate that older women are treated less aggressively, which may contribute to previously observed age-related disparities in breast cancer-specific survival (BCSS).7,8
Others have suggested that foregoing axillary staging in older women may compromise oncologic outcomes, and that adjuvant treatment decisions based on lymph node evaluation may impact OS.9,10 However, these findings may be related to selection bias, with healthier patients being more likely to undergo axillary nodal evaluation. For those with competing comorbidities, axillary staging may provide little benefit, due to predetermined omission of adjuvant chemotherapy or radiation, regardless of lymph node (LN) results. In light of these competing findings, we aimed to evaluate the potential differences in receipt of adjuvant therapy and subsequent impact on OS in cN0 elderly breast cancer patients using propensity score matching to compare patients who did and did not receive axillary surgery.
METHODS
Patients ≥70 years old diagnosed with cT1–3/cN0 breast cancer from 2004–2014 were selected from the National Cancer Database (NCDB).11 Patients with missing or unknown grade, estrogen receptor (ER) status, number of lymph nodes removed, surgery type, Charlson/Deyo comorbidity score, or survival data were also excluded. Patients with metastatic disease, those who underwent neoadjuvant treatment (chemotherapy, radiation therapy, or endocrine therapy), those who underwent a breast surgery other than lumpectomy or mastectomy, and those with less than 3 months of follow-up were excluded. Patients who did not undergo axillary surgery but had pathologically positive nodes (pN1–3) were also excluded (although not specifically indicated in the NCDB, these may have been diagnosed via core needle biopsy or fine needle aspiration). Per NCDB reporting requirements, survival information for patients diagnosed in the most recent year (2014) was not included; thus, all patients diagnosed in 2014 were excluded from the analyses. (Supplemental Figure 1)
Study groups were defined as those who underwent surgical lymph node evaluation (≥1 lymph node removed) and those who did not (0 lymph nodes removed). Patients were matched based on year of diagnosis (within 2 years), tumor grade, clinical T stage, age (within 5 years), ER status, and Charlson/Deyo comorbidity score using a GREEDY method.12 This method randomly selects a patient first in the untreated group (those who did not undergo lymph node surgery) and then selects a patient in the treated group who has the closest propensity score to the first patient. This process is repeated until all patients in the smaller group have been matched or until no reasonable matches remain.
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range) for continuous variables. Differences between study groups were tested using the Cochran-Mantel-Haenszel test or the stratified Wilcoxon Rank Sum (Van Elteren) test, as appropriate.13 Differences in treatment by lymph node surgery and pN stage were compared with chi-square tests.
OS was defined as the time from diagnosis to death or was censored at the date of last follow-up. Unadjusted median OS and 5- and 10-year survival rates were estimated using the Kaplan-Meier method. Differences between study groups were tested using the log-rank test. A Cox Proportional Hazards model was used to estimate the effect of lymph node surgery on OS after adjustment for known covariates. This included a robust sandwich covariance estimator to account for the correlation of patients treated at the same hospital and accounted for matched sets of patients using stratification. A sensitivity analysis of patients diagnosed from 2010–2013 was conducted to ensure that the results were maintained after adjustment for HER2 (human epidermal growth factor receptor 2) status, which is only reliably coded in the NCDB from 2010 onward. Similar to the population studied in CALGB (Cancer and Leukemia Group B) 9343, which compared lumpectomy plus tamoxifen with or without radiation2, a subgroup analysis was conducted for cT1/cN0/cM0, grade 1/2, ER+ patients.
No adjustments were made for multiple comparisons. Only patients with complete data for all covariates in a given model were included in each analysis. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.2. Due to use of de-identified data, our institutional review board granted the study exempt status.
RESULTS
Patient, Tumor, and Treatment Characteristics
133,778 matched patients met inclusion criteria (Supplemental Figure 1); 23.6% (n=31,531) did not undergo lymph node surgery. Median follow-up was 56.8 months (95% CI 56.4–57). Median age was 80 years (IQR 76–84), and 76.3% of patients had a comorbidity score of 0 (Table 1). 79.9% of tumors were grade 1/2, 89.2% were ER+, and median tumor size was 1.4 cm (IQR 0.9–2.1) (Table 2). In order to maintain the largest possible sample size, each patient who did not have nodal surgery was matched to at least one, but up to 4 patients who had nodal surgery. To achieve this, each untreated patient was first matched to one treated patient, and then the process was repeated up to 4 times to balance both groups of patients (Supplemental Table 1).
Table 1.
Patient and facility characteristics. IQR: interquartile range. LN: lymph node.
| All Patients (N=133778) |
LN Surgery (N=102247) |
No LN Surgery (N=31531) |
P-Value | |
|---|---|---|---|---|
| Age – Median (IQR) | 80 (76 – 84) | 80 (76 – 83) | 83 (78 – 87) | † |
| Gender | <0.001 | |||
| Female | 132222 (98.8%) | 100976 (98.8%) | 31246 (99.1%) | |
| Male | 1556 (1.2%) | 1271 (1.2%) | 285 (0.9%) | |
| Race | <0.001 | |||
| White | 121262 (90.6%) | 92718 (90.7%) | 28544 (90.5%) | |
| Black | 8835 (6.6%) | 6645 (6.5%) | 2190 (6.9%) | |
| Other | 2691 (2%) | 2144 (2.1%) | 547 (1.7%) | |
| Ethnicity | 0.01 | |||
| Hispanic | 3338 (2.5%) | 2645 (2.6%) | 693 (2.2%) | |
| Non-Hispanic | 122056 (91.2%) | 93300 (91.2%) | 28756 (91.2%) | |
| Income Level | <0.001 | |||
| <$35,000 | 34602 (25.9%) | 27098 (26.5%) | 7504 (23.8%) | |
| ≥$35,000 | 95162 (71.1%) | 72074 (70.5%) | 23088 (73.2%) | |
| Insurance Status | 0.44 | |||
| Private | 13331 (10%) | 10318 (10.1%) | 3013 (9.6%) | |
| Government | 118772 (88.8%) | 90654 (88.7%) | 28118 (89.2%) | |
| Not Insured | 331 (0.2%) | 256 (0.3%) | 75 (0.2%) | |
| Education Level | <0.001 | |||
| ≤80% High School Graduation Rate | 42133 (31.5%) | 32773 (32.1%) | 9360 (29.7%) | |
| >80% High School Graduation Rate | 87627 (65.5%) | 66396 (64.9%) | 21231 (67.3%) | |
| Charlson/Deyo Comorbidity Score | † | |||
| 0 | 102023 (76.3%) | 78079 (76.4%) | 23944 (75.9%) | |
| 1 | 24376 (18.2%) | 18640 (18.2%) | 5736 (18.2%) | |
| ≥2 | 7379 (5.5%) | 5528 (5.4%) | 1851 (5.9%) | |
| Facility Type | <0.001 | |||
| Academic | 32475 (24.3%) | 23728 (23.2%) | 8747 (27.7%) | |
| Integrated Network | 13247 (9.9%) | 10321 (10.1%) | 2926 (9.3%) | |
| Comprehensive | 71012 (53.1%) | 55058 (53.8%) | 15954 (50.6%) | |
| Community | 17044 (12.7%) | 13140 (12.9%) | 3904 (12.4%) | |
| Facility Location | <0.001 | |||
| Midwest | 36956 (27.6%) | 28570 (27.9%) | 8386 (26.6%) | |
| Northeast | 31180 (23.3%) | 21584 (21.1%) | 9596 (30.4%) | |
| South | 42273 (31.6%) | 33572 (32.8%) | 8701 (27.6%) | |
| West | 23369 (17.5%) | 18521 (18.1%) | 4848 (15.4%) |
Covariates used in matching.
Table 2.
Tumor and treatment characteristics by lymph node surgery and pathological node (pN) stage. ER: estrogen receptor. PR: progesterone receptor. HER2: human epidermal growth factor receptor 2. LN: lymph node.
| cN0 without LN Surgery (N=31531) |
cN0 + LN Surgery → pN0 (N=85474) |
cN0 + LN Surgery → pN1–3 (N=14290) |
P-Value | |
|---|---|---|---|---|
| ER Status | † | |||
| ER+ | 28222 (89.5%) | 76199 (89.1%) | 12674 (88.7%) | |
| ER- | 3309 (10.5%) | 9275 (10.9%) | 1616 (11.3%) | |
| PR Status | 0.002 | |||
| PR+ | 24209 (76.8%) | 65663 (76.8%) | 10881 (76.1%) | |
| PR- | 7096 (22.5%) | 19314 (22.6%) | 3335 (23.3%) | |
| HER2 Status* | <0.001 | |||
| HER2+ | 1085 (3.4%) | 3641 (4.3%) | 805 (5.6%) | |
| HER2- | 14324 (45.4%) | 40968 (47.9%) | 6984 (48.9%) | |
| Grade | † | |||
| 1 | 10613 (33.7%) | 29734 (34.8%) | 2971 (20.8%) | |
| 2 | 14687 (46.6%) | 39580 (46.3%) | 7281 (51%) | |
| 3 | 6231 (19.8%) | 16160 (18.9%) | 4038 (28.3%) | |
| Clinical T-Stage | † | |||
| 1 | 24196 (76.7%) | 69681 (81.5%) | 8413 (58.9%) | |
| 2 | 6744 (21.4%) | 14776 (17.3%) | 5185 (36.3%) | |
| 3 | 591 (1.9%) | 1017 (1.2%) | 692 (4.8%) | |
| Pathologic N-Stage | - | |||
| 0 | 8748 (27.7%) | 85474 (100%) | 0 (0%) | |
| 1 | 0 (0%) | 0 (0%) | 11810 (82.6%) | |
| 2 | 0 (0%) | 0 (0%) | 1787 (12.5%) | |
| 3 | 0 (0%) | 0 (0%) | 693 (4.8%) | |
| X | 20509 (65%) | 0 (0%) | 0 (0%) | |
| Treatment with Chemotherapy | 883 (2.8%) | 4989 (5.8%) | 3165 (22.2%) | <0.001 |
| Treatment with Radiation Therapy | 8187 (26%) | 40604 (47.5%) | 7104 (49.7%) | <0.001 |
| Treatment with Endocrine Therapy | ||||
| Among All Patients | 14646 (46.5%) | 49966 (58.5%) | 10286 (72%) | <0.001 |
| Among ER+ or PR+ Patients | 14499 (51.1%) | 49627 (64.7%) | 10225 (80.1%) | <0.001 |
| Surgery Type | ||||
| Lumpectomy | 24738 (78.5%) | 56549 (66.2%) | 6391 (44.7%) | |
| Mastectomy | 6793 (21.5%) | 28925 (33.8%) | 7899 (55.3%) | |
| Surgery + Radiation | <0.001 | |||
| Mastectomy + Radiation | 252 (0.8%) | 1226 (1.4%) | 2068 (14.5%) | |
| Lumpectomy + Radiation | 7935 (25.2%) | 39378 (46.1%) | 5036 (35.2%) | |
| Mastectomy + No Radiation | 6438 (20.4%) | 27250 (31.9%) | 5733 (40.1%) | |
| Lumpectomy + No Radiation | 16351 (51.9%) | 16538 (19.3%) | 1303 (9.1%) | |
| Radiation duration– Median (IQR)** | 43 (26 – 49) | 44 (23 – 49) | 46 (41 – 50) | <0.001 |
Covariates used in matching.
HER2 status has high missingness due to unreliable coding prior to 2010.
Reported in days
Nodal surgery was associated with higher rates of all adjuvant treatments when pN1–3 (chemotherapy-22%, radiation-49.7%, endocrine-72%) and pN0 (chemotherapy-5.8%, radiation-47.5%, endocrine-58.5%). Patients without nodal surgery had the lowest rates of adjuvant therapy (chemotherapy-2.8%, radiation-26%, endocrine-46.5%) all values p<0.001 (Table 2). In cN0, pN0, and pN1–3 patients, duration of radiation in days was (43 vs 44 vs 46, p<0.001).
Overall Survival Analyses
The unadjusted median OS was higher for patients undergoing axillary surgery than those who did not (111 months vs 74.5 months, log-rank p<0.001; Supplemental Figure 2). When stratified by lymph node surgery receipt and pN status, the unadjusted median OS remained higher for those who underwent axillary surgery (cN0>pN0: 114.8 months, vs cN0>pN1–3: 89.3 months, vs cN0-no LN surgery: 74.5 months; log-rank p<0.001; Figure 1). Of all node-positive patients (pN1–3), there was a survival benefit for patients who underwent chemotherapy, radiation, or endocrine therapy (Supplemental Table 2).
Figure 1.

Kaplan-Meier curve for unadjusted overall survival of all patients by receipt of axillary lymph node surgery and pN stage (N=131295). LN: lymph node.
After adjustment, patients who did not undergo nodal surgery had a worse OS (HR 1.66, 95% CI 1.61–1.70; Table 3). Factors associated with improved OS included receipt of chemotherapy (HR 0.86, 95% CI 0.81–0.91), radiation (HR 0.70, 95% CI 0.68–0.72), and endocrine therapy (HR 0.75, 95% CI 0.73–0.78). The sensitivity analysis of patients diagnosed from 2010–2013 resulted in similar associations (Supplemental Table 3). After adjustment for covariates, including HER2 status, patients who did not undergo nodal surgery still had a worse OS (HR 1.59, 95% CI 1.51–1.67).
Table 3.
Adjusted overall survival (N=118705). Groups were propensity matched based on year of diagnosis (within 2 years), grade, clinical T stage, age (within 5 years), ER status, and Charlson/Deyo comorbidity score. HR: hazard ratio. CI: confidence interval. PR: progesterone receptor.
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Study Group | <0.001 | ||
| Lymph Node Surgery | REF | <0.001 | |
| No Lymph Node Surgery | 1.657 (1.611–1.704) | ||
| Gender | <0.001 | ||
| Female | REF | ||
| Male | 1.307 (1.177–1.450) | <0.001 | |
| Race | <0.001 | ||
| White | REF | ||
| Black | 1.034 (0.980–1.091) | 0.22 | |
| Other | 0.785 (0.704–0.875) | <0.001 | |
| Income Level | 0.07 | ||
| <$35,000 | REF | ||
| ≥$35,000 | 0.969 (0.937–1.003) | 0.07 | |
| Insurance | 0.07 | ||
| Government | REF | ||
| Private | 0.950 (0.911–0.992) | 0.02 | |
| Not Insured | 1.006 (0.763–1.327) | 0.97 | |
| Education Level | <0.001 | ||
| >80% High School Graduation Rate | REF | ||
| ≤80% High School Graduation Rate | 1.058 (1.026–1.092) | <0.001 | |
| Facility Type | <0.001 | ||
| Academic | REF | ||
| Integrated Network | 1.098 (1.040–1.159) | 0.001 | |
| Comprehensive | 1.135 (1.092–1.180) | <0.001 | |
| Community | 1.183 (1.129–1.241) | <0.001 | |
| Facility Location | <0.001 | ||
| South | REF | ||
| Midwest | 1.089 (1.049–1.131) | <0.001 | |
| Northeast | 0.966 (0.925–1.010) | 0.13 | |
| West | 0.900 (0.859–0.943) | <0.001 | |
| PR Status | <0.001 | ||
| PR+ | REF | ||
| PR- | 1.093 (1.054–1.133) | <0.001 | |
| Treatment with Chemotherapy | <0.001 | ||
| No | REF | ||
| Yes | 0.861 (0.814–0.912) | <0.001 | |
| Treatment with Radiation | <0.001 | ||
| No | REF | ||
| Yes | 0.701 (0.679–0.723) | <0.001 | |
| Treatment with Endocrine Therapy | <0.001 | ||
| No | REF | ||
| Yes | 0.753 (0.731–0.776) | <0.001 | |
| Breast Surgery Type | 0.06 | ||
| Lumpectomy | REF | ||
| Mastectomy | 0.969 (0.938–1.001) | 0.06 |
In the subgroup analysis of patients with cT1, grade 1/2, ER+ disease, the unadjusted median OS was significantly higher for patients undergoing nodal surgery compared to those not (121.5 months vs 85.7 months, log-rank p<0.001; data not shown). When stratified by lymph node surgery receipt and pN status, the unadjusted median OS remained higher for those who underwent axillary surgery (cN0>pN0: 122.3 months, vs cN0>pN1–3: 109.9 months, vs cN0-no nodal surgery: 85.7 months; log-rank p<0.001; Supplemental Figure 3). When stratified by receipt of endocrine therapy (ET), the unadjusted median OS was also significantly higher for all patients undergoing nodal surgery compared to those not (Nodal surgery + ET: 128.2 months; Nodal surgery + No ET: 109.2 months; No nodal surgery + ET: 93.6 months; No nodal surgery + No ET: 76.3 months; log-rank p<0.001; Figure 2).
Figure 2.
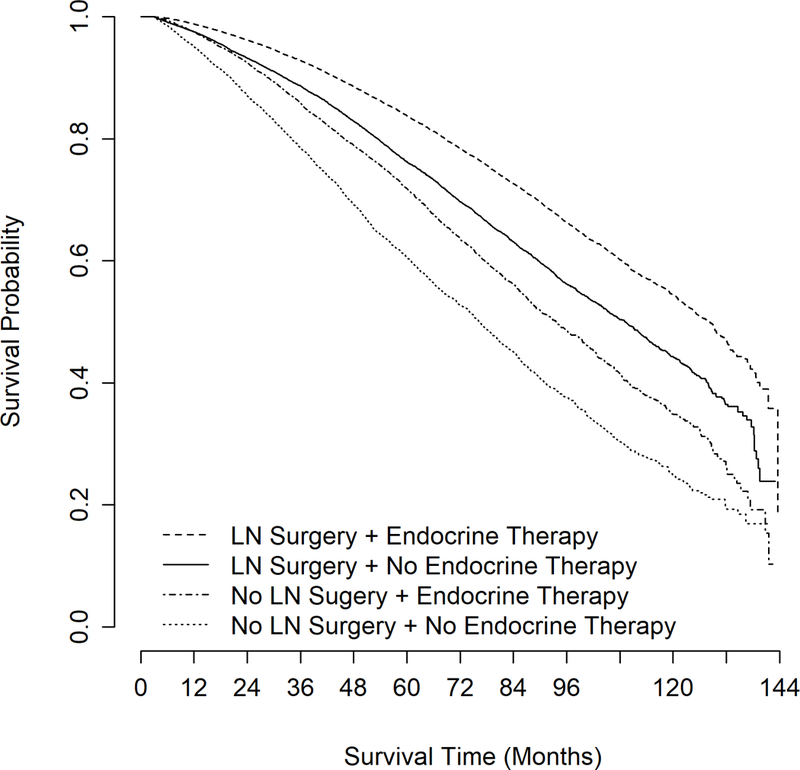
Kaplan-Meier curve for unadjusted overall survival of patients with cT1/cN0/cM0, grade 1–2, ER+ breast cancer by receipt of axillary lymph node surgery and endocrine therapy receipt (N=80445). ER: estrogen receptor. LN: lymph node.
DISCUSSION
The risk of breast cancer increases with age, and over 40% of all breast cancers are diagnosed in women ages ≥ 65.14 Several studies have evaluated the utility of SLNB in elderly patients.15–19 Based on these studies, the Choosing Wisely Guidelines recommended omission of SLNB in select elderly patients (ages ≥70 years) with early stage, cN0, HR+ breast cancer, as results from axillary staging may not impact subsequent treatment decisions.
In contrast, we demonstrated that 22.2% of node positive patients undergoing nodal surgery received chemotherapy, compared to 5.8% of those who underwent nodal surgery but were node negative, suggesting that nodal surgery may still be important for some adjuvant therapy decisions. Similarly, higher rates of endocrine therapy receipt were noted for pN1–3 patients than for those who underwent nodal surgery but were pN0. Although chemotherapy and endocrine therapy decisions appeared to be associated with the SLNB results, receipt of radiation was not, as rates of radiation receipt were similar for those who were pN0 and pN1–3. While the specific type of chemotherapy administered may influence outcomes, the NCDB does not delineate between chemotherapy regimens and dosing. However, our data demonstrated that differences in duration of radiation based on surgical axillary staging were not clinically meaningful (difference of 2 days). Regardless of the treatments received, undergoing surgical nodal evaluation remained significantly associated with an improved OS, even after adjusting for known covariates, likely suggesting additional unmeasured variables.
However, OS may not parallel BCSS in elderly patients specifically. In a study of NCDB and SEER (Surveillance, Epidemiology, and End Results) populations, improved BCSS and OS were observed in elderly patients who underwent lymph node evaluation, compared to those who did not.10 These findings suggest that many patients tolerate standard management strategies, similar to their younger counterparts, which may prolong survival. However, the authors also comment that patient selection and other unaccounted variables almost certainly affected survival outcomes. To address the issue of patient selection bias, we used propensity score matching to compare outcomes of elderly breast cancer patients who did and did not undergo surgical nodal evaluation, and the results were similar overall.
Others have attempted to identify low risk elderly populations that may benefit least from axillary nodal evaluation. Welsh et al. demonstrated that utilizing “low-risk criteria” for HR+ elderly breast cancer patients, which included grade 1, cT1mi-T1c (≤2.0 cm), or grade 2, cT1mi-T1b (≤1.0 cm), yielded a pN+ rate that was nearly one third of that observed in those who did not meet criteria (7.8% vs 22.8%).9 Similarly, we also demonstrated that surgical nodal evaluation was associated with an improved OS for patients with early stage (cT1/cN0/cM0), grade 1/2, ER+ breast cancer, suggesting that even select low risk patients may benefit from standard axillary staging.
Routine omission of axillary staging based on age and tumor subtype alone has the potential to lead to under-treatment of elderly breast cancer patients. In an analysis of stage I breast cancer patients in the SEER database, older patients were less likely to receive standard of care (radiation and LN sampling), which was associated with improved OS and BCSS rates when received.20 Comparably, we also noted lower rates of radiation receipt than would be expected for those undergoing breast conserving surgery (25.2–46.1%), while radiation therapy was associated with improved survival, even after adjustment. Therefore, our findings confirm that some elderly breast cancer patients may indeed be under-treated, and this may vary by region. In our univariate analysis, the Northeast region had the highest rates of omission of axillary surgery compared to other regions across the US at 30.4%. In the adjusted analysis, this finding was not associated with survival (HR 0.97; 95% CI 0.86–0.94, p=0.13). This observed trend may be due to presence of several tertiary care centers in the Northeast, and elderly patients with complex management questions may be referred to cancer referral networks in this area.
For otherwise healthy older women, a diagnosis of breast cancer may be the leading threat to their survival. In addition to stratification by tumor type, treatment risk stratification among elderly patients by comorbidities is essential to mitigating risks and complications of treatments. Although it is clear for elderly patients with limited survival from competing comorbidities that omission of SLNB is reasonable, our study demonstrated that the majority of patients (94.5%) were classified as having a comorbidity score of 0 or 1. As such, it is particularly important to balance the risk of over-treatment against the potential benefit of adjuvant therapy (based on SLNB results). Our data suggest that physicians are already choosing wisely and surgically staging patients whom they consider clinically healthy enough to tolerate surgery and adjuvant treatments, and they are avoiding surgery in those patients likely to die from something else.
Furthermore, there is a growing body of evidence regarding the integration of geriatric care into the multidisciplinary oncology setting to ensure appropriate surgical and adjuvant treatment.5 In a study by Okonji et al, elderly patients with stages I-III breast cancer underwent Comprehensive Geriatric Assessment (CGA) to determine “fitness” for treatment.21 While all “fit” women ages ≥70 underwent primary surgery, only 51% of those with high-risk disease received adjuvant chemotherapy, suggesting potential under-treatment in this population.21 Interestingly, we found that among patients who underwent lymph node surgery, those with pN0 disease still had higher rates of adjuvant treatments compared to those without nodal surgery: chemotherapy (5.8% vs 2.8%), radiation (47.5% vs 26.0%), and endocrine therapy (58.5% vs 46.5%). These findings suggest that the SLNB results are not the only drivers of adjuvant therapy decisions. Such variation in practice highlights the importance of geriatric assessment and consensus in a multidisciplinary setting for elderly patients with breast cancer.5 We suggest a selective approach to sentinel lymph node biopsy that includes a geriatric assessment using validated scales weighed against the risk of local recurrence or distant disease in a multidisciplinary setting. In addition, use of tumor genomic analysis such as Onctoype Dx can delineate patients at high risk for recurrence and assist clinicians in patient selection.
There were several limitations to our study that need to be acknowledged, including those inherent to large database analyses, such as variations in data entry and coding. Although most patients with zero lymph nodes removed likely had no axillary surgery, it is possible that some attempts at sentinel node identification were unsuccessful (e.g. dye/tracer did not map to the axilla, etc). In addition, the NCDB does not provide BCSS, and OS may not reflect BCSS in elderly breast cancer patients. However, OS is often used as the primary outcome measure in elderly patients to evaluate efficacy of breast cancer treatments in the context of competing comorbidities. We also had limited data on Oncotype Dx Recurrence Scores, and it is possible that some adjuvant treatment decisions were based on Oncotype scores in select HR+ patients that obviated the need for axillary nodal evaluation. Although we attempted to minimize patient selection bias (by using the propensity matching for our analysis), it is likely that additional unmeasured variables contributed to our findings to an unknown degree. Furthermore, patients that were selected to undergo axillary surgery may have been more likely to undergo other treatments as well (treatment bias).
CONCLUSION
In clinically node-negative breast cancer patients ≥70 years old, positive nodes were identified in 14% of those undergoing axillary surgery, and treatment patterns in this population were highly variable, which may be reflective of the lack of clear guidelines and/or patient heterogeneity. Regardless, it should not be routinely assumed that the results from a SLNB will not alter treatment decisions. In an overall healthy elderly population, surgical axillary staging was associated with higher rates of adjuvant therapy and improved OS, demonstrating that it remains an important component of surgical therapy. A selective approach to the omission of nodal surgery should be considered in elderly patients with node negative breast cancer, as the surgical outcome may influence subsequent treatment decisions and long-term outcomes. Future studies should evaluate whether similar findings may be reflected in BCSS rates.
Supplementary Material
Patient flow diagram of inclusion and exclusion criteria.
Kaplan-Meier curve for unadjusted overall survival of all patients (N=133778).
Kaplan-Meier curve for unadjusted overall survival of patients with cT1/cN0/cM0, grade 1/2, ER+ breast cancer by receipt of axillary lymph node surgery and pN stage (N=83797). ER: estrogen receptor. LN: lymph node.
SYNOPSIS.
In elderly cN0 breast cancer patients, axillary surgery was associated with higher rates of adjuvant therapy and improved survival. A selective approach to omitting nodal surgery should be considered in elderly patients with cN0 breast cancer.
Acknowledgements:
Portions of this manuscript are to be presented at the Annual Meeting of the American Society of Breast Surgeons (ASBrS). The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding: Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 5KL2TR001115 (PI: Boulware). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author Disclosures: None
REFERENCES
- 1.Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(19):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg JI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy: relationship between number of excised lymph nodes and patient perceptions of lymphedema. Ann Surg Oncol. 2011;18(10):2866–2872. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–160. [DOI] [PubMed] [Google Scholar]
- 6.Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23(4):783–791. [DOI] [PubMed] [Google Scholar]
- 7.Owusu C, Lash TL, Silliman RA. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102(2):227–236. [DOI] [PubMed] [Google Scholar]
- 8.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. Jama. 2005;293(9):1073–1081. [DOI] [PubMed] [Google Scholar]
- 9.Welsh JL, Hoskin TL, Day CN, Habermann EB, Goetz MP, Boughey JC. Predicting Nodal Positivity in Women 70 Years of Age and Older with Hormone Receptor-Positive Breast Cancer to Aid Incorporation of a Society of Surgical Oncology Choosing Wisely Guideline into Clinical Practice. Ann Surg Oncol. 2017;24(10):2881–2888. [DOI] [PubMed] [Google Scholar]
- 10.Chagpar AB, Hatzis C, Pusztai L, et al. Association of LN Evaluation with Survival in Women Aged 70 Years or Older With Clinically Node-Negative Hormone Receptor Positive Breast Cancer. Ann Surg Oncol. 2017;24(10):3073–3081. [DOI] [PubMed] [Google Scholar]
- 11.Surgeons A.C.o. American College of Surgeons: National Cancer Data Base [cited 2015 June 19th]; Available from: https://http://www.facs.org/quality- programs/cancer/ncdb. Accessed August 1, 2016. In.
- 12.Mandrekar JN MS. An Introduction to Matching and its Application using SAS. Paper 208–209, SESUG 29 http://www2.sas.com/proceedings/sugi29/208-29.pdf. Accessed 4/23/2018, 2018. [Google Scholar]
- 13.Breslow NE DN. Statistics Methods in Cancer Research: Volume I – The analysis of case-control studies. Vol 1: International Agency for Research on Cancer; 1980. [PubMed] [Google Scholar]
- 14.American Cancer Society: Breast Cancer Facts & Figures 2017–2018. 2017; https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Accessed 4/23/2018.
- 15.Chagpar AB, McMasters KM, Edwards MJ, Trial NAFTA. Can sentinel node biopsy be avoided in some elderly breast cancer patients? Ann Surg. 2009;249(3):455–460. [DOI] [PubMed] [Google Scholar]
- 16.Caywood J, Gray RJ, Hentz J, Pockaj BA. Older age independently predicts a lower risk of sentinel lymph node metastasis in breast cancer. Ann Surg Oncol. 2005;12(12):1061–1065. [DOI] [PubMed] [Google Scholar]
- 17.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast. 2012;21(5):678–681. [DOI] [PubMed] [Google Scholar]
- 18.Mendenhall NP. Age-related variations in the use of axillary dissection. Int J Radiat Oncol Biol Phys. 2002;54(3):637–639. [DOI] [PubMed] [Google Scholar]
- 19.Wildiers H, Van Calster B, van de Poll-Franse LV, et al. Relationship between age and axillary lymph node involvement in women with breast cancer. J Clin Oncol. 2009;27(18):2931–2937. [DOI] [PubMed] [Google Scholar]
- 20.Sun SX, Hollenbeak CS, Leung AM. Deviation from the Standard of Care for Early Breast Cancer in the Elderly: What are the Consequences? Ann Surg Oncol. 2015;22(8):2492–2499. [DOI] [PubMed] [Google Scholar]
- 21.Okonji DO, Sinha R, Phillips I, Fatz D, Ring A. Comprehensive geriatric assessment in 326 older women with early breast cancer. British journal of cancer. 2017;117(7):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient flow diagram of inclusion and exclusion criteria.
Kaplan-Meier curve for unadjusted overall survival of all patients (N=133778).
Kaplan-Meier curve for unadjusted overall survival of patients with cT1/cN0/cM0, grade 1/2, ER+ breast cancer by receipt of axillary lymph node surgery and pN stage (N=83797). ER: estrogen receptor. LN: lymph node.


