Abstract
Novel genotypes evolve under selection through mutations in pre-existing genes. However, mutations have pleiotropic phenotypic effects that influence the fitness of emerging genotypes in complex ways. The evolution of antimicrobial resistance is mediated by selection of mutations in genes coding for antibiotic-target proteins. Drug-resistance is commonly associated with a fitness cost due to the impact of resistance-conferring mutations on protein function and/or stability. These costs are expected to prohibit the selection of drug-resistant mutations at low drug pressures. Using laboratory evolution of rifampicin resistance in Escherichia coli, we show that when exposed intermittently to low concentration (0.1 × minimal inhibitory concentration) of rifampicin, the evolution of canonical drug resistance was indeed unfavorable. Instead, these bacterial populations adapted by evolving into small-colony variants that displayed enhanced pellicle-forming ability. This shift in lifestyle from planktonic to pellicle-like was necessary for enhanced fitness at low drug pressures, and was mediated by the genetic activation of the fim operon promoter, which allowed expression of type I fimbriae. Upon continued low drug exposure, these bacteria evolved exclusively into high-level drug-resistant strains through mutations at a limited set of loci within the rifampicin-resistance determining region of the rpoB gene. We show that our results are explained by mutation-specific epistasis, resulting in differential impact of lifestyle switching on the competitive fitness of different rpoB mutations. Thus, lifestyle-alterations that are selected at low selection pressures have the potential to modify the fitness effects of mutations, change the genetic structure, and affect the ultimate fate of evolving populations.
Keywords: fitness, mutations, selection, antimicrobial resistance, lifestyle adaptation, rifampicin
THE relationship between genotype, phenotype, and fitness predicts the fate of populations evolving under selection (Pigliucci 2010). However, such predictions are nontrivial to make due to confounding factors that shape the fitness landscape of evolving genotypes, such as interactions among genes, i.e., epistasis, and between genes and the environment, e.g., phenotypic plasticity and pleiotropy (Wagner and Zhang 2011). Hence, a thorough understanding of how the fitness of individual genotypes is altered by intrinsic and extrinsic factors is crucial in scenarios where more that one kind of genotype may be selected. The evolution of antimicrobial resistance in bacteria represents one such scenario in which environmental drug pressure selects from among pre-existing genetic variants.
Drug resistance in bacteria is acquired through mutations that confer a selective advantage by preventing drug-binding to the target, by enhancing drug-efflux or by drug-inactivation (Munita and Arias 2016). The pleiotropic impact of resistance-conferring mutations on the activity and/or stability of target proteins is well documented. As a result, resistance to antibiotics is commonly associated with a fitness cost, i.e., compromised fitness of drug-resistant strains in the absence of drug (Björkman and Andersson 2000; Hughes and Andersson 2015; Melnyk et al. 2015). For instance, in the case of rifampicin—an inhibitor of bacterial transcription—the fitness cost of drug resistance is associated with lower RNA polymerase activity (Reynolds 2000; Hall et al. 2011; Qi et al. 2016). In some cases, such as rifampicin-resistant Staphylococcus aureus, in vitro fitness also correlates with epidemiological fitness (O’Neill et al. 2006), which warrants a better understanding of how fitness costs affect the emergence and spread of drug-resistant bacteria.
Recent studies have shown that antibiotic-resistant bacteria can be selected at far lower concentrations of the drug than the minimum inhibitory concentration (MIC) (Gullberg et al. 2011; Andersson and Hughes 2012, 2014; Sandegren 2014). Sublethal drug concentrations are encountered by bacteria in natural environments due to antibiotic-producing fungi/bacteria as well as to human activity. In addition, sublethal drug concentrations may also be present in the bodies of humans and livestock due to poor drug-pharmacokinetics or lack of patient compliance (Andersson and Hughes 2014). It is predicted that selection environments with low drug concentrations strongly select against costly resistance mutations (Andersson and Hughes 2012; Hughes and Andersson 2015), though experimental tests of this prediction are limited. Further, sublethal antibiotic doses facilitate a number of other adaptations in bacteria such as biofilm formation (Nguyen et al. 2014; Aka and Haji 2015; Oliveira et al. 2015), altered metabolic signatures (Wu et al. 2014; Molina-Quiroz et al. 2015), or transcriptional deregulation (Hesketh et al. 2011). These adaptations are likely to alter the fitness effects of drug-resistant mutations. Hence they may influence, both qualitatively and quantitatively, how resistant bacteria are selected at low antibiotic pressure. However, this possibility remains relatively unexplored.
In natural environments, additionally, antibiotic exposure is likely to be discontinuous (Olofsson and Cars 2007; Ambrose et al. 2010). Temporal variability in the environment has the potential to alter evolutionary outcomes of selection significantly. Constant environmental conditions select “specialists” that maximize fitness in a single growth condition. Fluctuating environments, on the other hand, appear to favor the evolution of “generalists” that have high net fitness under all encountered environmental conditions (Cooper and Lenski 2010; Condon et al. 2014; de Vos et al. 2015; Karve et al. 2015; Melbinger and Vergassola 2015). In the context of antibiotic resistance, the effects of temporal variability on the outcomes of selection for resistance have been explored in a few studies (Fridman et al. 2014; Karve et al. 2015; Levin-Reisman et al. 2017).
In this study, we investigated how the evolutionary trajectories of bacterial populations are impacted by temporal variability in drug exposure at different drug pressures. For this, we have chosen rifampicin resistance in Escherichia coli as our system of study. In E. coli, rifampicin resistance maps primarily to the rpoB locus (Campbell et al. 2001; Garibyan et al. 2003), which codes for the β-subunit of the bacterial RNA polymerase. This system is a well-established experimental paradigm for studying the fitness costs of resistance (Reynolds 2000). Since rifampicin resistance is costly under laboratory conditions, it was expected that, under relaxed selection for resistance (i.e., at low drug concentrations or when drug exposure is discontinuous), only low-cost resistance would be permissible. Alternatively, other adaptations that circumvent the need for drug resistance would be selected. We show that the evolutionary outcomes of discontinuous drug exposure are contingent on the drug concentrations used. At high drug pressures, E. coli populations experiencing discontinuous drug exposure evolved bona fide drug resistance, i.e., a higher MIC of the drug. However, populations that were exposed discontinuously to low concentration of rifampicin remained drug-sensitive. Instead, these populations switched to a pellicle-forming lifestyle that was mediated by a genetic induction of type I fimbriation. This lifestyle switch allowed better survival than the ancestor under low drug pressure without the need for drug resistance-conferring mutations. We also found that, by differentially modifying the fitness effects of rpoB mutations, this lifestyle change altered the fitness landscape of drug-resistant bacteria that emerged subsequently. This, in turn, resulted in different mutational spectra among drug-resistant bacteria isolated under sustained or intermittent rifampicin exposures.
Materials and Methods
Strains and culture conditions
E. coli K-12 MG1655—a kind gift from Sutirth Dey (Indian Institute of Science Education and Research, Pune)—was used as the ancestral strain for all selection experiments. E. coli ΔlacZ was constructed inhouse by insertional inactivation using a chloramphenicol resistance cassette from the pKD3 plasmid by the Lambda Red Recombineering system (Datsenko and Wanner 2000). Strains were maintained in Luria-Bertani (LB) broth at 37° with shaking at 150–200 rpm. Rifampicin (Merck-Millipore) stocks (10 mg/ml) were made in 50% DMSO and added to media prior to inoculation of bacteria at the required concentration.
MIC for rifampicin
For determination of MIC, frozen stocks were revived in LB broth for 8–10 hr, and 5 µl of the culture was added to wells of a 96-well plate containing 100 µl of LB broth or LB broth supplemented with serially diluted rifampicin (final concentrations ranging from 2 mg/ml to 3 µg/ml). The plates were incubated at 37° overnight and the optical density of each well was measured at 600 nm on a microplate reader (Varioskan LUX multimode reader; Thermo Scientific). MIC was defined as the lowest concentration of rifampicin at which no growth was visible. Inhibitory concentration–50 (or IC50, i.e., the concentration of antibiotic that inhibited 50% of bacterial growth) was determined from the above measurements by fitting experimental data from two to three independent observations to a variable slope dose-response inhibition curve using GraphPad Prism software (version 6.07).
Scheme for selection of rifampicin-resistant E. coli
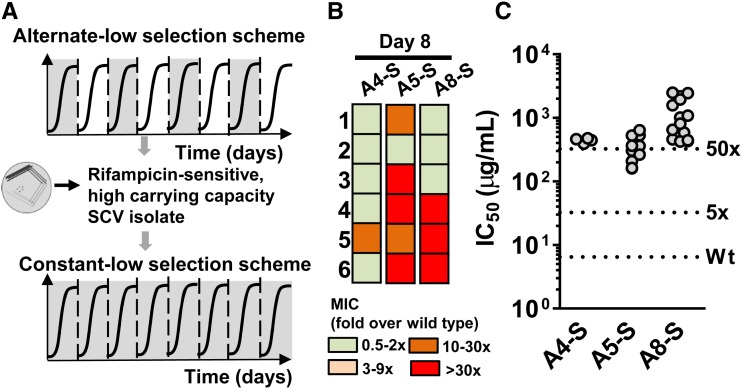
E. coli K-12 MG1655 was streaked out onto LB agar plates, and 10 random colonies were used as the ancestral populations for all selection experiments (henceforth wild type). The MIC of rifampicin for each ancestor was determined prior to selection. All selections were performed in LB broth in 96-well plates, each well containing a total volume of 150 µl. Wells on the periphery of the plate were filled with deionized water to prevent dehydration of the cultures during growth. On the 1st day of selection, 15 µl (∼7.5 × 107 bacteria) of an overnight-grown saturated culture of each ancestor was inoculated into LB supplemented with rifampicin at 3 µg/ml (0.1 × MIC; low selection pressure) or 15 µg/ml (0.5 × MIC; high selection pressure) (Figure 1a). This established 40 evolving populations that experienced low or high antibiotic concentrations during every growth cycle (designated constant-low and constant-high, respectively) or during every alternate growth cycle (designated alternate-low and alternate-high, respectively) (Figure 1b). After 20–24 hr of growth (i.e., after ∼3.3 generations; final population size ∼7.5 × 108 bacteria), 10% of the culture (∼7.5 × 107 bacteria) was passaged into fresh LB medium without rifampicin (for alternate lines) or containing rifampicin at appropriate concentration (for constant lines). Ten control lines were also maintained in rifampicin-free medium resulting in a total of 50 evolving lineages (40 lines under selection for resistance and 10 controls). Every 2 days, 100 µl of culture from each of the lines was frozen in an equal volume of sterile 50% glycerol and stored at −80°.
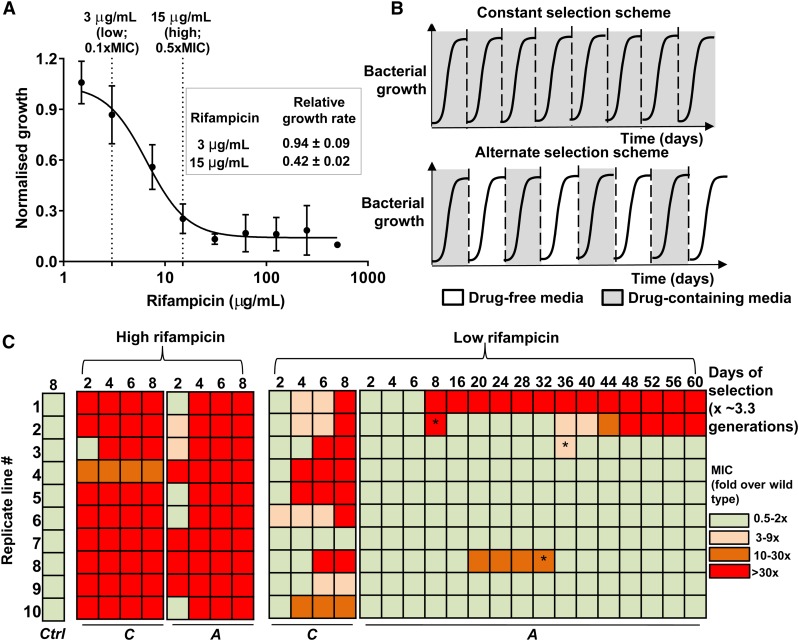
Figure 1.
Selection of de novo rifampicin resistant E. coli. (a) Growth of wild type E. coli (carrying capacity) at various rifampicin concentrations normalized to growth in the absence of rifampicin. Mean ± SEM from three biological replicates are shown. “Low” and “high” rifampicin concentrations that were used for selection of resistant bacteria are indicated, and the maximum growth rate of wild type E. coli at each of these concentrations, normalized to growth rate in drug-free medium, is shown in the inset. (b) Selections schemes used for evolving rifampicin resistance in E. coli. Multiple replicate populations of drug-sensitive E. coli were serially passaged through media supplemented with rifampicin. Bacterial populations were exposed to rifampicin either during every growth cycle (constant selection scheme) or every alternate growth cycle (alternate selection scheme). Each growth cycle lasted for 1 day and corresponded to roughly 3.3 generations. Between growth cycles 10% of the culture was passaged to fresh media. (c) Heat map of fold change in MIC of evolving populations relative to the ancestor. C: Continuous rifampicin exposure; A: rifampicin exposure during every alternate growth cycle; Ctrl: control populations that did not experience rifampicin; * reversion to ancestral MIC in the alternate-low lines.
The MIC of each of the evolving lines was monitored every 2 days (i.e., after ∼6.6 generations) and an increase in MIC by more than twofold over wild type was considered indicative of rifampicin resistance. This cut-off was based on the assumption that control lineages, which are not under direct selection for resistance, should not have altered MIC for rifampicin. Any deviation in the values of MIC of control lineages, therefore, would represent the extent of variation to be expected in the absence of selection. We found that control lineage MIC values typically varied between 0.5- and 2-fold of wild type. Thus, an increase beyond twofold was considered as evidence for evolution of resistance due to selection by the drug. Selection for resistance was carried out for eight growth cycles (each growth cycle lasting for roughly 1 day, ∼27 generations) for constant-high, constant-low, and alternate-high lines, by which time a majority of lines showed dramatic increases in MIC. For alternate-low lines, selection was carried out over a period of 60 days (∼200 generations) since most lineages failed to show a change in MIC over shorter durations of selection. From each line that showed a change in MIC, rifampicin-resistant clones were isolated after appropriate number of days of selection by streaking out frozen stocks directly onto LB agar supplemented with 50 µg/ml rifampicin. This concentration of rifampicin inhibited growth of wild type E. coli. Five random single colonies from each lineage were selected for further analysis, resulting in a total of 165 rifampicin-resistant isolates; five each from nine constant-low, four alternate-low, 10 alternate-high, and 10-constant high lineages.
Growth analysis
Growth rates and carrying capacities of E. coli strains were determined in LB broth or LB broth supplemented with rifampicin at appropriate concentrations. Saturated cultures were diluted 1:100 in 150 µl of growth medium in a 96-well plate and then incubated at 37° with shaking. Bacterial growth was monitored every 10 min by measuring optical density at 600 nm in a microplate reader (Varioskan LUX multimode reader; Thermo Scientific) for a total of 6 hr, by which time the cultures were saturated. The highest value of the slope of the ln(OD600) vs. time plot was determined to be the maximum growth rate. Optical density reached at the end of 6 hr of growth (at saturation) was used as a proxy for carrying capacity. For each strain, measurements from two to three biological replicates (each in triplicate) were averaged. Growth rates and carrying capacities were normalized to the wild type ancestor unless otherwise specified.
Characterization of colony size
For characterization of colony size, saturated cultures of appropriate bacterial strain/isolate were serially diluted, and 100 µl of 106-fold diluted cultures were spread onto LB agar plates. This typically resulted in 30–50 colonies per plate. Plates were incubated overnight at 37°, photographed on a gel documentation system (G:Box F3; Syngene) and the diameters of 20–30 randomly picked colonies per plate were measured in ImageJ. At least three replicate cultures were used for each strain.
Relative fitness measurements
In order to assess the fitness of various strains isolated in this study, a competitive growth paradigm was used. For fitness of small colony variants (SCVs), saturated cultures of each strain were mixed 1:1 with an E. coli strain harboring a deletion in lacZ (ΔlacZ). For comparison between rifampicin-resistant and sensitive strains, saturated cultures of the appropriate strains were mixed volumetrically in a 10:1 ratio (resistant strains in minority). Mixtures were inoculated into the wells of a 96-well microtiter plate at an initial density of ∼5 × 108 CFU/ml. Initial mixing ratios were ascertained by plating serial dilutions of the cultures on LB agar supplemented with IPTG (1 mM) and X-Gal (40 µg/ml) for SCV/ΔlacZ or with and without rifampicin (50 µg/ml) for rpoB mutants strains. After 24 hr at 37° with shaking at 150–200 rpm (∼3.3 generations), the relative numbers of competing bacteria were estimated once again. For alternate-low environment, cultures were grown for 24 hr in rifampicin (3 µg/ml)-containing medium, following which 10% of the culture was transferred to drug-free medium and allowed to grow for another 24 hr period (∼6.6 generations) to mimic the selection condition. This constituted one cycle of alternate-low growth, after which the numbers of competing bacteria were estimated. Relative fitness of the strains was calculated as selection rate (r) as given by Travisano and Lenski (1996).
In the above expressions, Af, Bf, Ai, and Bi represent the final (f) and initial (i) population densities of the test (A) and reference (B) strains, calculated as CFU/ml.
Data availability
Whole genome sequencing data has been deposited to GenBank under the project ID: PRJNA494985 (BioSample accessions: SAMN10187669, SAMN10187670, SAMN10187671). Supplemental figures have been submitted to Figshare. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7553237.
Results
Discontinuous exposure to low levels of rifampicin limits the evolution of resistance
In order to assess the impact of selection conditions on the evolution of drug resistance, we serially passaged replicate populations of E. coli K-12 MG1655 in media containing either high (0.5 × MIC, 15 µg/ml) or low (0.1 × MIC, 3 µg/ml) rifampicin concentrations (Figure 1a, detailed selection scheme in Materials and Methods). These concentrations of rifampicin represent two different selection pressures within the sublethal drug regime as seen from the extents of growth inhibition they impose on wild type E. coli (Figure 1a). At each concentration of rifampicin, bacterial populations were exposed to drug either during every growth cycle (“constant” drug exposure) or every alternate growth cycle (“alternate” day drug exposure) (Figure 1b). This resulted in four kinds of lineages, which we designated as constant-high, constant-low, alternate-high, and alternate-low.
Constant exposure to rifampicin led to rapid enrichment of rifampicin-resistant E. coli regardless of drug concentration (Figure 1c). Indeed, by eight cycles of growth in the presence of rifampicin (∼27 generations), all 10 constant-high lines, and 9 of the 10 constant-low lines, had evolved rifampicin resistance (Figure 1c). Not surprisingly, constant-high lines evolved resistance faster than constant-low lines (Figure 1c). In contrast, while all 10 alternate-high lines evolved resistance, only 2 of the 10 alternate-low lines evolved rifampicin-resistance at equivalent number of generations under selection (Figure 1c). Even upon extending the alternate-low selection scheme for 60 days (∼200 generations), we found that a majority of the alternate-low lines failed to show a detectable increase in MIC (Figure 1c). It is noteworthy, however, that, in a few instances, rifampicin resistance did evolve, and, in two of these cases, rifampicin-resistant bacteria formed ≥10% of the population, which was comparable to most of the constant-low lineages (Supplemental Material, Figure S1). At least two instances of “loss” of resistance were also observed during this period, evidenced by a reversion of the MIC of the population back to wild type levels (Figure 1c). In both the alternate-low lineages that lost rifampicin resistance, the fraction of the population that was drug resistant was <0.0001% of the total (Figure S1). Thus, we concluded that constant exposure to rifampicin selected for canonical drug resistance (i.e., an increase in MIC) even at low drug pressure. However, discontinuous exposure to a low concentration of rifampicin limited the evolution of drug resistance under the selection conditions used by us.
Enrichment of SCVs in alternate-low lineages
Though not conventionally “resistant” to rifampicin (i.e., unaltered MIC), the alternate-low lineages may have adapted to tolerate low concentrations of rifampicin. To test this possibility, we analyzed the growth of all 10 alternate-low lineages from day 6 of selection (∼20 generations) in the presence of low rifampicin (3 µg/ml). Day 6 populations were used for these analyses as all the alternate-low populations had similar MIC as wild type at this time point. All 10 alternate-low day 6 populations grew to higher densities (i.e., had higher carrying capacities) than wild type in the presence of low rifampicin, with no discernible difference in growth rates (Figure 2a). For five of these populations, carrying capacities were also significantly higher than control populations that had been passaged for an equivalent number of generations in drug-free medium (Figure 2a). Thus, despite unchanged MIC for rifampicin, alternate-low lineages grew better than the ancestor in low-rifampicin conditions. Interestingly, these alternate-low populations had higher carrying capacity in the absence of rifampicin as well (Figure 2a).
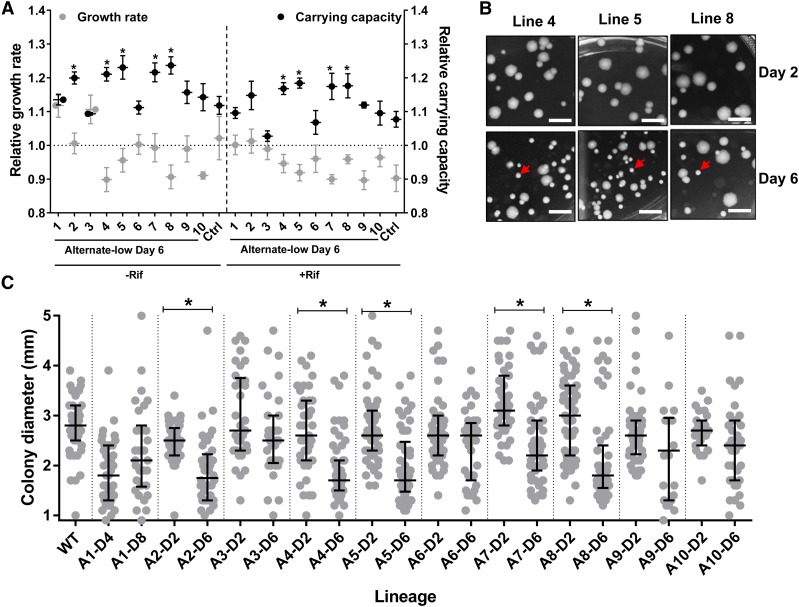
Figure 2.
Enrichment of a small colony phenotype in alternate-low lineages. (a) Maximum growth rates and carrying capacities of 10 day 6 populations from the alternate-low selection scheme (1–10) estimated in the presence or absence of rifampicin (3 µg/ml) and normalized to the wild type ancestor. For alternate-low lineages, mean ± SEM from three independent measurements, each in triplicate, are plotted. For control populations (Ctrl) the mean ± SEM of all 10 day 6 populations are plotted as a single data point. Statistical significance was tested between values of carrying capacity and growth rate of alternate-low selected lineages and control lineages using Student’s t-test. *P <0.05. (b and c) Alternate-low lineages show a progressive decrease in median colony size resulting in the enrichment of small colony variants (SCVs). Colony morphology for day 2 and day 6 of alternate-low lineages 4, 5, and 8 are shown in (b) and the SCVs are indicated by an arrow. Quantitation of colony diameters of all 10 alternate-low lineages from day 2 and day 6 of selection are plotted as a scatter in (c). Median and interquartile range is indicated. Statistical significant differences between median colony diameters from day 2 and day 6 was tested using a nonparametric t-test; *P <0.05. Colony diameter of wild type colonies are provided as a reference.
We observed that those alternate-low lineages that had evolved higher carrying capacities also tended to form smaller colonies than the ancestral wild type (Figure 2, b and c). Further, the small-colony phenotype was enriched over passages (Figure 2, b and c) and undetectable in control lineages (Figure S2). Formation of SCVs is triggered in bacteria by environmental stresses, including antibiotics (Wei et al. 2011; Frenzel et al. 2015; Ramiro et al. 2016; Tuchscherr et al. 2016). Hence, we hypothesized that SCV formation in the alternate-low lineages may allow adaptation to low-drug pressure without acquisition of costly drug-resistance mutations. To test this, we first isolated SCVs from three independent alternate-low lineages (henceforth A4-S, A5-S, and A8-S), randomly picked from the five lineages that had enhanced carrying capacity. These isolates were then replated to ensure that the small-colony phenotype was stable (Figure 3a). This was indeed the case and the small-colony phenotype of these isolates was stable even after ∼100 generations of growth in drug-free medium (Figure S3). Like the lineages that they were isolated from, the A4-S, A5-S, and A8-S isolates grew to higher densities than wild type in the presence of low rifampicin (Figure 3b), but did not show a change in the MIC or IC50 for rifampicin (Figure 3c).
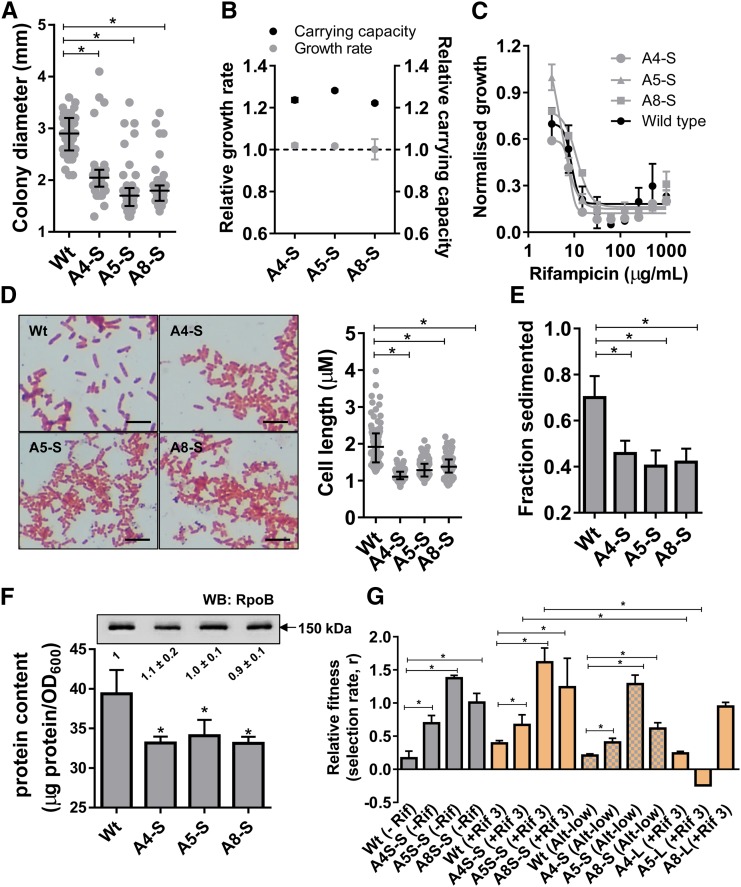
Figure 3.
Small colony phenotype is associated with an increase in competitive fitness without conferring rifampicin resistance. (a) Colony diameters of wild type (Wt) or small colony isolates from alternate-low lineages 4, 5, and 8 (A4-S, A5-S, and A8-S) after replating. Individual colony diameters are plotted as a scatter and median and interquartile range is indicated. Statistical significance was tested between median colony diameters of SCV isolates and wild type using a nonparametric t-test; * P <0.05. (b) Maximum growth rates and carrying capacities of A4-S, A5-S, and A8-S isolates estimated in the presence of rifampicin (3 µg/ml) normalized to wild type. Mean ± SEM from three independent measurements, each in triplicate, are plotted. (c) Growth (carrying capacity) of wild type E. coli or A4-S, A5-S, and A8-S SCVs at various rifampicin concentrations normalized to growth in the absence of rifampicin. Mean ± SEM from three biological replicates are shown. (d) Microscopic evaluation of cultures of Wt, A4-S, A5-S, and A8-S E. coli stained with safranin and viewed using a 100× oil immersion. Bacterial lengths were quantitated for at least 100 bacteria from three independent cultures per strain. Individual bacterial lengths are plotted as a scatter and median and interquartile range in indicated. Statistical significance was tested using a nonparametric t-test; *P <0.05. (e) Sedimentation of late-log phase cultures of Wt, A4-S, A5-S, and A8-S E. coli under low centrifugal force. Fraction of bacteria that were sedimented were calculated from three independent cultures and plotted as mean ± SD. Statistical significance was tested using a nonparametric t-test; *P <0.05. (f) Protein content calculated as protein amount in bacterial pellets from 6 ml of exponentially growing cultures normalized to optical density at 600 nm for wild type (Wt) or A4-S, A5-S, and A8-S E. coli. Mean ± SEM from three biological replicates is shown. Statistical significance was tested using a Student’s t-test; *P <0.05. The inset shows an immunoblot assaying RpoB protein levels in lysates of wild type (Wt) or A4-S, A5-S, and A8-S E. coli. A representative image from experiments repeated thrice is shown. Levels of RpoB were estimated densitometrically and expression in the wild type was set to “1.” Mean ± SEM from three biological replicates in shown. (g) Relative fitness of A4-S, A5-S, and A8-S isolates in the presence and absence of rifampicin (3 µg/ml), or under alternate-low selection conditions (alt-low). A strain harboring a deletion in lacZ was used as the reference strain. Deletion of lacZ had only a marginal effect on fitness as seen by competition with wild type. Strains were mixed at a starting ratio of 1:1 and allowed to compete for 24 hr in appropriate medium. Fitness is expressed as selection rate (r). Competition between reference strain and large colony isolates from alternate-low lineage 4, 5, and 8 (A4-L, A5-L, and A8-L) in the presence of rifampicin was also performed. Mean ± SD from at least three biological replicates are plotted. Statistical significance was tested using a Student’s t-test; * P <0.05.
E. coli SCVs are reported to have low growth rates, which allow them to resist killing by antibiotics (Ramiro et al. 2016; Santos and Hirshfield 2016). In this case, however, there was no appreciable change in the growth rates of the SCVs relative to the wild type (Figure 3b and Figure S4). Instead, microscopic examination showed that the A4-S, A5-S, and A8-S isolates tended to form smaller cells than wild type (Figure 3d). Reduction in bacterial cell size of the SCV isolates was corroborated by less efficient sedimentation at low relative centrifugal forces (Figure 3e) and lower protein content (Figure 3f). Importantly, the relative level of RpoB protein, relative to total cellular protein, was not detectably altered in these bacteria (Figure 3f), which was in line with unaltered MIC for rifampicin (Figure 3c).
We next calculated the relative fitness of these SCV isolates by allowing them to compete with a derivative of the ancestor that was marked by a deletion in the lacZ gene. Deletion of lacZ had only a marginal effect on the relative fitness of E. coli under these experimental conditions (Figure 3g). All three SCV isolates had enhanced relative fitness, both in the presence of low rifampicin and under no-drug growth conditions (Figure 3g). The fitness of the SCV isolates was also higher than wild type under the alternate-low selection conditions (Figure 3g). As an additional control, we tested the fitness of large colony isolates from the same lineages as the A4-S, A5-S, and A8-S SCVs. In two of the three cases, we found that the large colony isolates did not show as big an increase in fitness over the ancestor as the SCVs, negating the possibility that the higher fitness of A4-S, A5-S, and A8-S bacteria was due merely to acclimatization to the experimental growth conditions (Figure 3g). These data proved that the small colony phenotype represented an adaptation to discontinuous drug exposure without the need for resistance-conferring mutations.
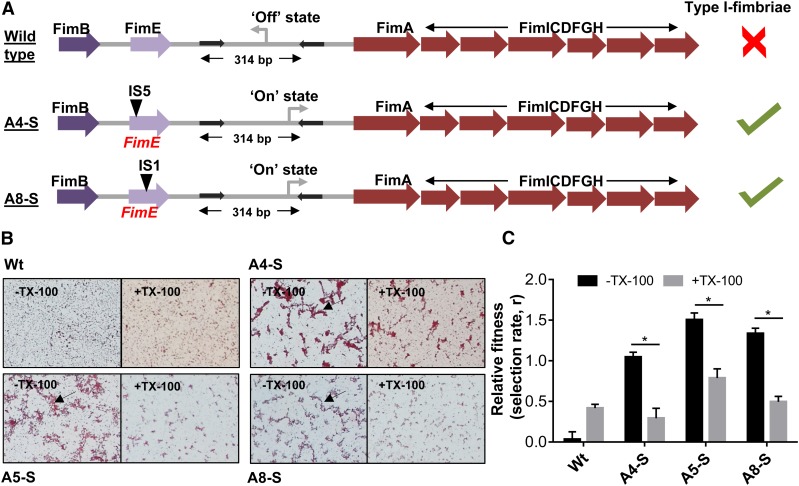
Was the small colony adaptation associated with genetic changes? In order to answer this, we sequenced the genomes of two of the three SCVs (A8-S and A4-S) isolated by us, as well as the ancestor, to identify possible genetic alterations that may have accumulated during selection. Only two genetic changes were identified in each of the SCVs with respect to the ancestor. The first of these, present in both the sequenced SCVs, was an inversion of a 314-bp fragment in the promoter of the fim operon. This operon codes for Type I fimbrial proteins in E. coli (Figure 4a and Table 1). The inversion of the fim promoter resulted in switching “on” of the fim operon in the SCVs, which was in the “off” state in the ancestor. Inversion of the fim promoter is responsible for phase variation in E. coli and related enterobacteria, i.e., stochastic switching between fimbriated (i.e., promoter “on”) and unfimbriated (i.e., promoter “off”) phenotypes. Phase variation allows E. coli to alternate between these phenotypic states at a frequency that can be as high as 10−3 per cell per generation, and is thought to be an evolutionary bet-hedging strategy (Abraham et al. 1985; Gally et al. 1993; Stentebjerg-Olesen et al. 2000). Importantly, production of Type I fimbriae is associated with a reduced colony size in E. coli (Orndorff and Falkow 1984; Hasman et al. 2000), explaining why A4-S and A8-S bacteria formed smaller colonies than the ancestral wild type despite similar growth rate. The second genetic alteration in both the small colony isolates was the disruption of the fimE integrase gene by the insertion of either the IS5 or IS1 transposable elements (Figure 4a and Table 1). The FimE enzyme is a site-specific integrase responsible for “on” to “off” state conversion of the fim promoter (Klemm 1986). Its disruption in the SCV isolates, thus, ensured that the phenotype of the SCVs would be clamped in the “on,” i.e., fimbriated state (Stentebjerg-Olesen et al. 2000). Thus, fimbriation appeared to be strongly selected in the alternate-low selection scheme, and explained the appearance of SCVs in these lineages.
Figure 4.
Fimbriation and pellicle formation in A4-S, A5-S, and A8-S isolates contribute to enhanced fitness at low rifampicin concentrations. (a) Schematic representation of genomic alterations at the fim locus resulting in switching “on” of the fim operon in A4-S and A8-S isolates, as inferred from whole genome sequencing data (Table 1). The schematic is not drawn to scale. (b) Microscopic examination of cultures of safranin-stained Wt, A4-S, A5-S, and A8-S E. coli, with or without treatment with 0.01% Triton X-100 (TX-100) for 15 min under 10× magnification. Pellicle formation (indicated by an arrow) was seen in A4-S, A5-S, and A8-S strains, but not in Wt, and was significantly reduced upon detergent treatment. Representative images from three independent experiments are shown. (c) Relative fitness, expressed as selection rate (r), of A4-S, A5-S, and A8-S E. coli calculated in the presence of rifampicin (3 µg/ml) with or without the addition of 0.01% Triton X-100 (TX-100) in the growth media. Mean ± SD from at least three biological replicates are plotted. Statistical significance was tested using a Student’s t-test; *P <0.05.
Table 1. Genomic rearrangements in A4-S and A8-S isolates as determined by whole genome sequencing.
| Position (WT E. coli-K12 MG1655) | Mutation | Frequency of reads (%) | Annotation | Gene | Description | |
|---|---|---|---|---|---|---|
| A4-S | ||||||
| 1 | 4544075 | IS5 | 97.50 | IS5 insertion within CDS (+124) | fimE | IS5 mediated disruption of fimE ORF |
| 2 | 4544078 | IS5 | 99.10 | IS5 insertion within CDS (+131) | fimE | IS5 mediated disruption of fimE ORF |
| 3 | 4544596–4544910 | Inversion | 80.08 | Inversion of intergenic region | — | Inversion of fimA promoter from “off” to “on” state |
| 4 | 4544604–4544900 | Inversion | 79.60 | Inversion of intergenic region | — | Inversion of fimA promoter from “off” to “on” state |
| A8-S | ||||||
| 1 | 4544402 | IS1 | 66.70 | IS5 insertion within CDS (+451) | fimE | IS5 mediated disruption of fimE ORF |
| 2 | 4544409 | IS1 | 60.40 | IS5 insertion within CDS (+458) | fimE | IS5 mediated disruption of fimE ORF |
| 3 | 4544596–4544910 | Inversion | 92.70 | Inversion of intergenic region | — | Inversion of fimA promoter from “off” to “on” state |
| 4 | 4544604–4544900 | Inversion | 95.50 | Inversion of intergenic region | — | Inversion of fimA promoter from “off” to “on” state |
We next asked why fimbriation may enhance the fitness of E. coli in the alternate-low selection environment. Fimbriation, which allows greater bacterial adhesion, has been associated with pellicle formation in broth cultures (Stentebjerg-Olesen et al. 2000; Avalos Vizcarra et al. 2016). In line with this, microscopic examination of A4-S, A5-S, and A8-S cultures at high densities showed greater pellicle formation than wild type (Figure 4b). These pellicles could be disrupted by mild detergent treatment (0.01% Triton X-100 for 15 min with gentle agitation) and hence represented loosely adhered bacteria (Figure 4b). Pellicles, structurally similar to biofilms, are known to afford greater drug tolerance to bacteria (Van Acker et al. 2014). To directly interrogate the importance of pellicle formation for enhancing the fitness of small colony isolates, we compared the relative fitness of the A4-S, A5-S, and A8-S bacteria in the presence of detergent. The fitness advantage of all three SCVs over the ancestor at low drug concentrations was substantially compromised by the presence of detergent in the growth media (Figure 4c). Thus, pellicle formation due to activation of the fim operon was causally linked to the higher fitness of the A4-S, A5-S, and A8-S isolates from alternate-low lineages. These data unambiguously showed that a genetically triggered alteration in lifestyle from planktonic to pellicle-like, rather than acquisition of drug resistance, was the preferred adaptive strategy under low drug exposure conditions.
Lifestyle switching mediates the selection of exclusively high-level rifampicin resistant bacteria at low drug pressures
Did switching to a pellicle lifestyle impact the drug-resistant phenotypes that subsequently evolved in the alternate-low lineages? In order to answer this, we characterized the level of resistance displayed by rifampicin-resistant isolates from each lineage (five resistant isolates per lineage) that showed an increase in MIC across the four selection schemes. All the characterized rifampicin-resistant isolates from a single evolving lineage had indistinguishable values of IC50 for rifampicin (Figure S5), which suggested that they may be clonal in origin. This observation is not surprising given the population size (∼7.5 × 108 bacteria/population), bottleneck between passages (10%) and frequency of rpoB mutants in E. coli (∼1 in 108) that confer rifampicin resistance. We confirmed this suggestion by sequencing the rifampicin resistance-determining region (RRDR) of the rpoB gene of all five isolates from two different lineages, and found that they did indeed harbor the same mutation at this locus. For all other lines, therefore, we sequenced the RRDR from one to two isolates in order to identify rifampicin resistance conferring mutations. All sequenced isolates harbored single nonsynonymous substitutions in the RRDR that have previously been associated with rifampicin resistance (Table 2) (Garibyan et al. 2003).
Table 2. Summary of mutations identified in the rifampicin resistance determining region (RRDR) of isolates from alternate-low, constant-low, and constant-high selection schemes, and their IC50 values (mean ± SEM) for rifampicin.
| Mutation in RRDR | IC50 (µg/ml) | |
|---|---|---|
| Alternate-low | ||
| Line 1 | H526Y | 1097 ± 25 |
| Line 2 | S531F | 1153 ± 36 |
| Line 2 (Day 44) | H526Qa | 422 ± 50a |
| Line 8 (Day 20) | D516G | 789 ± 64 |
| Constant-low | ||
| Line 1 | I572L | 195 ± 17 |
| Line 2 | Q148L | 105 ± 19 |
| Line 3 | D516A | 52 ± 8 |
| Line 4 | P564L | 1569 ± 82 |
| Line 5 | S531F | 1656 ± 46 |
| Line 6 | I572L | 180 ± 35 |
| Line 8 | Q148L | 190 ± 21 |
| Line 9 | N518D | 118 ± 8 |
| Line 10 | H526Qa | 119 ± 25a |
| Constant-high | ||
| Line 1 | I572S | 110 ± 33 |
| Line 2 | H526D | 1710 ± 19 |
| Line 3 | Q148L | 226 ± 33 |
| Line 4 | Q148L | 95 ± 21 |
| Line 5 | L533P | 1271 ± 158 |
| Line 6 | D516G | 691 ± 64 |
| Line 7 | S574F | 477 ± 54 |
| Line 8 | H526L | 1696 ± 156 |
| Line 9 | L511R | 316 ± 22 |
| Line 10 | Q148L | 300 ± 38 |
Strains harboring the His526Gln mutation were isolated from alternate-low and constant-low selection schemes but showed very different values of IC50 for rifampicin.
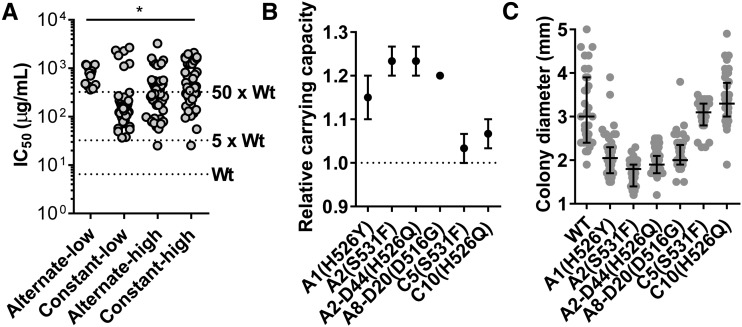
The rifampicin IC50 values of resistant isolates spanned two orders of magnitude ranging from ∼30 µg/ml (5 × wild type) to ∼2 mg/ml (333 × wild type), though isolates with low-level resistance (5–50 × wild type) occurred more frequently (Figure 5a and Figure S6). Curiously, while this bias toward low-level rifampicin resistance was observed in the constant-low, constant-high, and alternate-high lineages, all isolates from the alternate-low lineages had high IC50 (≥50 × wild type) for rifampicin (Figure 5a, Figure S6, and Table 2). In addition, isolates from the alternate-low lineages, but not from constant-low lineages that harbored the same rpoB mutation, had enhanced carrying capacities (Figure 5b) and formed smaller colonies (Figure 5c) than wild type. Thus, we concluded that, in the alternate-low lineages, rifampicin resistance-conferring mutations occurred in the SCV background.
Figure 5.
Rifampicin-resistant isolates from alternate-low lineages are highly drug resistant and retain small-colony phenotypes. (a) Scatter of IC50 for rifampicin observed for 165 isolates from constant or alternate lineages evolved at high or low antibiotic pressure. Each data point represents the mean of two to three independent measurements. The IC50 of the wild type (Wt) and 5× or 50× Wt are indicated for reference. Statistical significance was tested using a two-way ANOVA; * P <0.01. (b) Carrying capacities of rifampicin-resistant isolates from the alternate-low lineages (A-) relative to the wild type. Isolates from the constant-low lineages (C-) harboring same rpoB mutations as alternate-low isolates are also shown for comparison. If the isolates were not isolated from day 8 populations, then the days of selection are indicated (for instance D20: day 20). Mutations in rpoB are indicated in parentheses. (c) Colony diameters of rifampicin-resistant isolates from the alternate-low lineages (A-) and isolates from the constant-low lineages (C-) harboring same rpoB mutations as alternate-low isolates are shown as scatters. Median and interquartile range are indicated. Mutations in rpoB are indicated in parentheses.
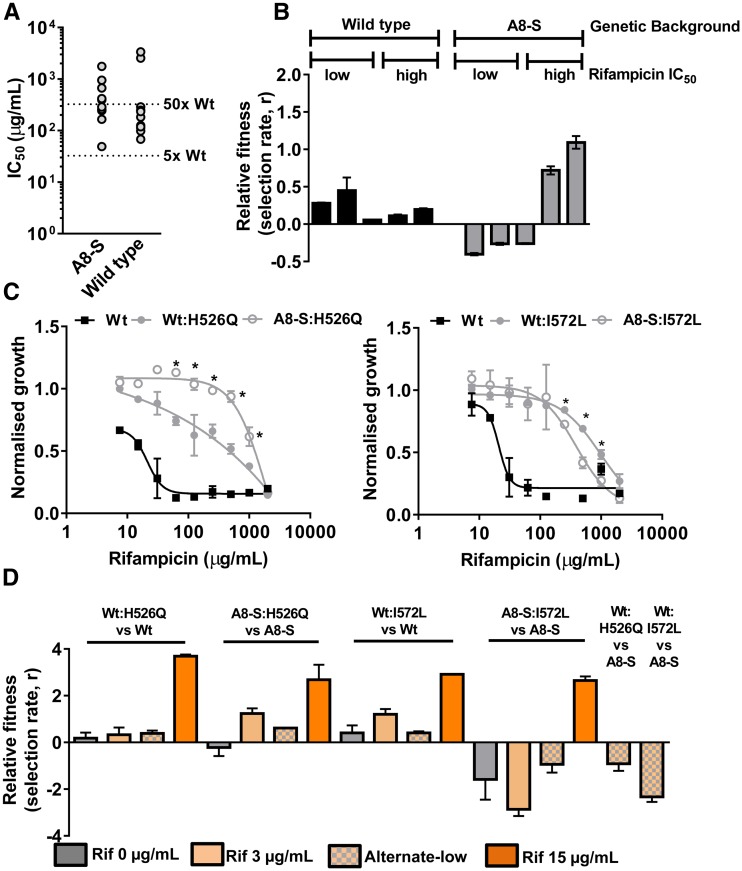
The apparent enrichment of high-level rifampicin resistant E. coli in alternate-low lineages could be an artifact of the relatively low number of resistant strains isolated by us from this selection scheme. To address this issue, we subjected replicate populations of the A4-S, A5-S, and A8-S isolates to constant-low selection (six replicate populations established from each of the three SCVs; total of 18 evolving populations) (Figure 6a). At the end of 8 days of selection (∼24 generations), we analyzed the MIC of the populations as well as characterized the dose-response profiles of rifampicin-resistant bacteria isolated from these experiments. We argued that, if the SCVs were biased toward evolving only high-level rifampicin resistance, then even in the constant-low selection scheme, they would continue to evolve only high-IC50 phenotypes. However, if the enrichment of high-level rifampicin resistant strains in the alternate-low scheme were merely a sampling artifact, then the SCVs subjected to constant-low selection would evolve both low and high-IC50 drug-resistant phenotypes. Interestingly, the A4-S, A5-S, and A8-S isolates from alternate-low lines continued to show features of the alternate-low lineages even in the constant-low selection scheme, i.e., lower frequency of rifampicin resistance (i.e., number of replicate populations showing a change in MIC after 8 days of selection) and a bias toward evolving high-level rifampicin resistant phenotypes (Figure 6, b and c). This strong historical contingency demonstrated that adaptation to low rifampicin by switching to an SCV phenotype promoted the selective evolution of high-level rifampicin-resistant bacteria.
Figure 6.
SCVs show bias toward evolution of high-level rifampicin resistance at low drug pressure. (a) A4-S, A5-S, and A8-S isolates were subjected to constant-low selection for 8 days to allow the evolution of rifampicin-resistance in these backgrounds. (b) Heat map of fold change in MIC relative to the wild type of 18 evolving replicate populations derived from the A4-S, A5-S, and A8-S ancestors after 8 days of constant-low selection. (c) Scatter of IC50 for rifampicin observed for 55 resistant-isolates from constant-low lineages derived from A4-S, A5-S, and A8-S ancestors. Each point represents the mean of two independent measurements.
Altered fitness effects of rpoB mutations due to lifestyle switching
In order to explain the above pattern of resistance-evolution in alternate low lineages, we first tested whether SCVs from alternate-low lineages were altogether incapable of evolving low-level resistance. We found that this was not the case, as spontaneous rifampicin-resistant mutants isolated from stationary phase cultures of A8-S or wild type E. coli using a fluctuation test showed high as well as low IC50 values (Figure 7a). Thus, while SCVs were capable of evolving both kinds of rifampicin resistance, at low drug pressure, only high-level resistance was selected. Next, we tested the fitness of spontaneous high and low resistant mutants in wild type or A8-S backgrounds isolated from the fluctuation test in the alternate-low environment (Figure 7b). Interestingly, while low and high-IC50 mutants in the wild type background were both slightly fitter than the wild type under alternate-low conditions, there was a dramatic difference in the fitness effects of high and low-IC50 mutants in the A8-S background. High-IC50 mutants in the A8-S background had enhanced relative fitness under alternate-low conditions. However, low-IC50 mutants in the A8-S background had compromised fitness (Figure 7b). Further, we noticed that mutations at Ile572 and Gln148 positions, though frequently isolated from constant-low and constant-high lineages, were missing entirely from the alternate-low isolates (Table 2). Mutations at these positions confer low-level resistance and are the most frequently isolated mutations within the RRDR of laboratory-evolved rifampicin-resistant E. coli (Garibyan et al. 2003). Taken together, these observations indicated that both low and high-IC50 mutants could occur in the small colony background. However, the SCV background modified the fitness effects of low and high-IC50 mutants, differentially leading to the selective enrichment of high-IC50 mutants under alternate-low selection.
Figure 7.
Modified fitness effects of rpoB mutations in the SCV background. (a) Isolation of high and low-level rifampicin resistant E. coli from A8-S and wild type ancestor using a fluctuation test. Approximately 109 bacteria from stationary phase cultures of A8-S or Wt strains were plated onto medium containing rifampicin (50 µg/ml). Distribution of IC50 values of 10 random rifampicin-resistant clones derived from the two ancestors was similar. (b) Relative fitness of low-IC50 and high-IC50 mutants in the wild type or A8-S background under alternate-low conditions, expressed as selection rate, r. Mean ± SD from three biological replicates are plotted. (c) Growth (carrying capacity) of wild type (Wt) E. coli strains in which the rpoB:His526Q or rpoB:Ile572Leu allele was engineered in the genome of the wild type or A8-S strains (Wt:H526Q and A8-S:H526Q or Wt:I572L and A8-S:I572L, respectively) in the presence of varying concentrations of rifampicin. Mean ± SD from three measurements are shown. Statistically significance between growth of these strains was tested using Student’s t-test; *P < 0.05. (d) Fitness effects of the His526Gln and Ile572Leu mutations in wild type (Wt) or A8-S backgrounds in the presence or absence of indicated concentration of rifampicin and under alternate-low conditions (alt-low). Rifampicin resistant and sensitive strains (indicated) were mixed in a 10:1 ratio (resistant strain in minority) and allowed to compete for 24 hr in the indicated growth conditions, following which the fraction of resistant bacteria in the population was estimated. Relative fitness was calculated as selection rate (r). Data shown represent mean ± SEM of three independent experiments.
In order to directly test the above hypotheses, we introduced either the Ile572Leu mutation (low-IC50) or the His526Gln mutation (high-IC50, which was present in the isolates from constant-low as well as alternate-low lineages, Table 2) in the genomic copy of rpoB in the ancestral (i.e., wild type) or the A8-S backgrounds. While the Ile572Leu mutation conferred similar drug dose-response in both genetic backgrounds, the His526Gln mutation conferred higher level of rifampicin-resistance in the A8-S SCV background than in the ancestral wild type background (Figure 7c). This was in agreement with the different IC50 values of resistant isolates harboring the His526Gln mutation from constant-low and alternate-low lineages (Figure S8 and Table 2). We next tested whether the fitness effects of these two mutations differed between the two backgrounds by competing each of the mutants with their respective drug-sensitive ancestors (Figure 7d). The relative fitness of the His526Gln mutation was similar in both genetic backgrounds. However, the Ile572Leu mutation was far costlier in the A8-S SCV background than in the wild type. Further, the His526Gln mutation was enriched over the drug-sensitive ancestor in the wild type and the A8-S backgrounds at low rifampicin (3 µg/ml). In contrast, the Ile572Leu mutation conferred a selective advantage only in the wild type background but not the A8-S SCV at low rifampicin. Similar observations were also made in in the alternate-low growth conditions (Figure 7d), which explained why the Ile572Leu mutation did not evolve in the alternate-low lineages. These differences in fitness effects were not seen at high rifampicin concentrations (Figure 7d), which explained why both low-IC50 and high-IC50 mutants were permissible at high drug pressure. Interestingly, both mutations in the wild type background performed poorly against the drug-sensitive A8-S strain in alternate-low conditions (Figure 7d). Thus, once the SCV phenotype had been selected, resistant mutants in the wild type background would be out-competed under alternate-low conditions.
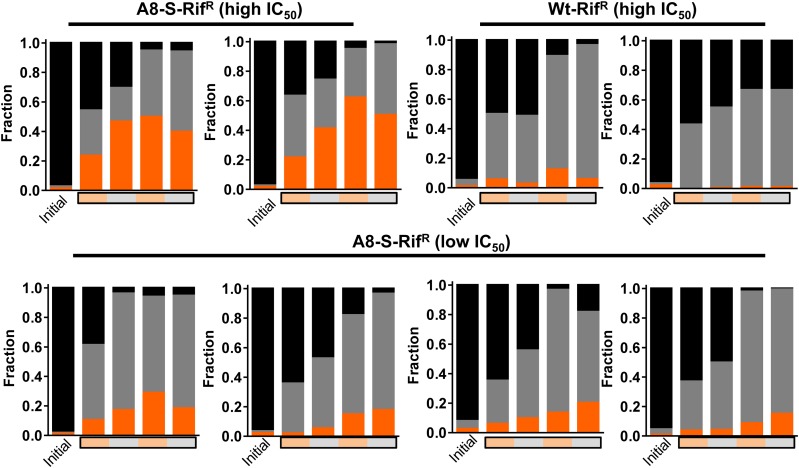
Based on the above results, we attempted to recapitulate the results of the alternate-low selection scheme. We mixed the drug sensitive wild type ancestor (marked with a lacZ deletion), the drug-sensitive A8-S small colony isolate and spontaneous rifampicin-resistant mutants (98:1:1) and allowed these mixed populations to evolve under alternate-low conditions. The relative proportions of each of the strains were estimated over two cycles of alternate-low selection conditions (4 days, ∼14 generations). Wild type, though initially the majority constituent of the population, was rapidly outcompeted by the A8-S SCV in all tested mixtures (Figure 8). In line with our model, all four low-IC50 mutants in the A8-S background that were tested remained in a minority over the course of the experiment. High-IC50 mutants in the A8-S background, on the other hand, either equalled or exceeded the A8-S SCV over the course of the experiment. Importantly, high-IC50 mutants without the SCV phenotype were outcompeted by the A8-S SCV (Figure 8). These data demonstrated that, under alternate-low selection conditions, the drug-sensitive SCV was fitter than canonical drug-resistant strains. Further, once the SCV was the majority constituent in the population, only high-IC50 conferring mutations in the SCV background were permissible as low-IC50 mutations in the SCV background incurred high fitness costs. These data, therefore, validated out model for why evolution of the SCV mediated the subsequent enrichment of high-IC50 mutants under alternate-low selection conditions.
Figure 8.
Recapitulation of alternate-low selection using three-strain competition. Wild type ancestor, marked with a deletion in lacZ (black), A8-S SCV (gray), and spontaneous rifampicin resistant isolates in the wild type or A8-S backgrounds (orange) were mixed (98:1:1) and allowed to compete under alternate-low conditions. The relative proportion of each of the strains was estimated initially and over the course of alternate-low growth. Presence of rifampicin during growth is indicated by an orange bar, while its absence is indicated by a gray bar. One of two biological replicates for each mixture is shown.
Discussion
It is now well-established that very low antibiotic levels are sufficient to select clinically relevant drug resistance-conferring mutations in bacteria (Gullberg et al. 2011; Gutierrez et al. 2013). It has also been shown that, in the case of antibiotics like ciprofloxacin, low and high drug pressures select mutations at different loci (Zhou et al. 2000). These studies suggest that low and high drug environments are likely to have different impacts on the selection of drug resistant bacteria, both qualitatively and quantitatively. In the present study, we have shown that, in low antibiotic environments, periods of growth in drug-free conditions significantly alter the evolutionary trajectories and ultimate fates of bacterial populations. We find that a genetically driven switch in bacterial lifestyle from planktonic to pellicle-like can enhance the fitness of bacteria that are challenged with discontinuous drug exposure at low drug pressure without the acquisition of canonical drug-resistance. These evolutionary intermediates then redirect evolutionary trajectories by modifying the fitness of resistant mutants, which can facilitate the eventual evolution of drug resistant strains without significant fitness costs. The interaction between these two adaptive strategies thus alters the resultant mutational spectrum and selection properties of drug-resistant bacteria.
In our experimental model, genetic activation of the fim operon represented an advantageous strategy for E. coli under discontinuous low-concentration antibiotic exposure. Fitness measurements and phenotypic assays showed that this adaptation allowed better survival at low antibiotic by driving pellicle formation. Activation of the fim operon through a conserved inversion in the promoter, referred to as phase variation, is a commonly encountered bet-hedging strategy among enterobacteria and has been documented to provide an adaptive advantage in low oxygen conditions (Stentebjerg-Olesen et al. 2000). This study is the first report of fimbriation leading to higher fitness under low-level antibiotic exposure. This is particularly significant, since the pathogenesis of several bacteria, including uropathogenic E. coli strains, is dependent on their ability to adhere to surfaces, as well as each other, using fimbriae (Lim et al. 1998). The second important adaptation in response to low drug pressure, most likely as a consequence of fimbriation, was the adoption of a smaller bacterial size and a small colony phenotype (Orndorff and Falkow 1984; Hasman et al. 2000). SCV formation is also one among several generic strategies that bacteria use to counter stressful environments. SCVs of bacteria such as S. aureus (von Eiff 2008), E. coli (Negishi et al. 2018) and Pseudomonas aeruginosa (Malone 2015) have also been isolated from patient samples, and, hence, represent a clinically relevant phenotype of bacterial pathogens. SCV formation has typically been associated with mutations that lower metabolic activity in bacteria conferring resistance to antibiotics as a consequence (Pränting and Andersson 2011; Proctor et al. 2014; Ramiro et al. 2016; Santos and Hirshfield 2016). In this study, however, two features of the SCVs isolated from alternate-low lineages set them apart from earlier reports of SCVs. First, the SCV phenotype did not significantly alter growth rate under the conditions of selection. Indeed, SCVs isolated by us grew to higher density than wild type, which indicates that respiratory pathways, which are normally implicated in SCV formation (Pränting and Andersson 2011; Proctor et al. 2014; Ramiro et al. 2016; Santos and Hirshfield 2016), were unaffected. Second, genome sequencing did not reveal any of the commonly encountered mutations in respiratory enzymes found in SCVs documented thus far (Ramiro et al. 2016; Santos and Hirshfield 2016). Thus, our study reiterates the relatively less-appreciated impact of fimbriation on SCV formation in E. coli.
Transition from planktonic to pellicle/biofilm-like lifestyle has been repeatedly observed in laboratory and clinical E. coli populations under various environmental conditions (Jefferson 2004; Hadjifrangiskou et al. 2012; Rossi et al. 2018). Though in our study a genetic rearrangement mediated this lifestyle shift, bacterial populations also demonstrate purely phenotypic changes in lifestyle that represent additional mechanisms of adaptation that may be potentially accessible under low drug environments. Pellicle formation and biofilm induction (which are thought to be mechanistically similar) are medically important alternate lifestyles of bacteria. For instance, bacterial pathogens that are commonly associated with nosocomial infections, such as Acinetobacter baumannii and P. aeruginosa, exist as biofilms/pellicles, which enhances their virulence (Pour et al. 2011; Mulcahy et al. 2014; Percival et al. 2015). In addition to their contribution in pathogenesis, bacterial biofilms also enhance survival upon antibiotic challenge (Hall and Mah 2017). Importantly, our study demonstrates that lifestyle changes could differentially alter the fitness effects of rpoB mutations that conferred rifampicin-resistance. Indeed, rifampicin resistance-conferring mutations are known to be highly pleiotropic, and, even though most rifampicin resistance conferring mutations map to a relatively small region of the RNA polymerase beta-subunit (Campbell et al. 2001), the phenotypes associated with different mutations in the rifampicin-binding pocket are nonoverlapping. These range from enhancing thermo-tolerance (mutations at Ile572) (Rodríguez-Verdugo et al. 2013) to altered stringent response under nutrient starvation (mutations at Ser531, Pro564) (Jin and Gross 1989; Zhou and Jin 1998; Bergman et al. 2014). The inherently different pleiotropy of rpoB mutations may explain why these mutations interacted differently with the fimbriation phenotype. In the future, examining the mechanistic basis of this interaction at the systems level would no doubt be instructive.
One of the main consequences of the above genetic interaction was an altered spectrum of rpoB mutations that was accessible to low-drug adapted bacterial populations under sustained selection. This altered mutational spectrum also led to a higher level of drug-resistance in the emerging strains, despite there having been no deliberate selection for high-level resistance. A similar observation was recently made by Wistrand-Yuen et al. (2018), who showed that high-level streptomycin resistance could evolve in Salmonella under sub-MIC selection conditions due to the collaborative effects of several low-benefit mutations. Some qualitative similarities exist between the results of the present study and those reported by Wistrand-Yuen et al. (2018). Important among these, both studies report altered mutational spectra based on selection strength. However, in contrast to published results, the results of our study demonstrate that the enrichment of high-level resistance need not be the result of additive or synergistic interactions between low-resistance conferring mutations. Instead, this bias may also be a direct fall-out of altered fitness effects of canonical resistance-conferring mutations by intermediate adaptive changes.
The preferential enrichment of high-level rifampicin resistant mutants by intermittent exposure to low drug levels has important clinical implications. First, it has been argued that selection of resistance at sublethal antibiotic concentrations is likely to be a sequential process involving the accumulation of several low-benefit low-cost mutations, eventually resulting in high-level resistance (Andersson and Hughes 2014; Sandegren 2014). In the case of rifampicin resistance, this does not appear to be the case. Indeed, the His526Tyr, His526Gln, Asp516Gly, and Ser531Phe mutations that were isolated from our alternate-low selection lines are all clinically relevant (O’Neill et al. 2006; Koch et al. 2014). Second, resistant strains that evolved under low intermittent antibiotic exposure had no cost when compared to wild type, which would facilitate their spread. Third, the preferential enrichment of high-level resistant mutants over low-level resistant mutants can potentially reduce the diversity of bacteria within patients. There is some evidence to suggest that pathogenic bacteria colonize the host by clonal proliferation (McVicker et al. 2014). In the absence of any other competing clones, high-level resistant mutants would have a higher chance of successfully establishing infection. Finally, it has been demonstrated that high-level rifampicin resistance (in particular, resistance conferred by mutations at His526 and Ser531 positions of rpoB), but not low-level resistance in M. tuberculosis, is correlated with cross resistance to rifabutin and KRM-1648 (Yang et al. 1998; Cavusoglu et al. 2004), complicating therapeutic intervention greatly.
We find that the results of this study have application for a variety of systems when viewed from the generalist/specialist adaptation point of view. Low-rifampicin induced phenotypes, namely fimbriation and SCV formation, had higher fitness than the ancestor in the presence and absence of antibiotic, and, hence, may be considered as “generalists.” On the other hand, mutationally acquired drug-resistance that confers a selective advantage only in the presence of rifampicin may be considered as “specialists.” The selection of generalists rather than specialists early in the alternate-low lineages is in agreement with a large body of evidence suggesting that fluctuating environments favor generalists (Cooper and Lenski 2010; Condon et al. 2014; de Vos et al. 2015; Karve et al. 2015; Melbinger and Vergassola 2015). Interestingly, though alternate-low and alternate-high selections were both fluctuating environments, the former favored generalists while the latter favored specialists. We think that this disparity is explained by the fact that the generalists that evolved in our experiments do not have a detectable advantage over the ancestor at high drug pressure, and, hence, even though the selection environment faced by the alternate-high lineages was temporally fluctuating, the evolutionary trajectories they followed were biased toward the evolution of specialists that can tolerate high rifampicin levels. Based on these considerations, we propose that, while strong directional selection prefers “specialist” adaptations, despite the costs associated with specialization, mild selection strength environments may prefer “generalist” adaptations. Specialization can occur in generalists too, though the fitness landscape of mutations that mediate specialization in generalist and naïve backgrounds may vary significantly. As a result, the strength of selection is an important parameter to consider when predicting how tussles between competing genotypes would play out during evolution.
Adaptation to novel environments is a hallmark of all living organisms. Though our study used the evolution of rifampicin resistance in E. coli under low antibiotic pressure as a model, the finding that relatively generic adaptations can modify the fitness effects of mutations, is universally relevant. This study highlights the role of environment is determining which adaptive trajectory is followed by an evolving population. In summary, we believe that the results presented here significantly enhance our understanding of the selection for resistance at sublethal antibiotic concentrations and also reveal a new facet of the role of selection for fitness under drug-free conditions in shaping the emergence of antibiotic resistance.
Acknowledgments
The authors acknowledge Sanket Shelke and Aishwarya Venkataravi for technical assistance. Deepak Barua is acknowledged for critically reading this manuscript. This project was funded by the Department of Science and Technology (DST), Government of India and the Indian Institute of Science Education and Research (IISER), Pune. N.M. is a recipient of a DST-INSPIRE faculty fellowship. S.B. is a recipient of a DST-INSPIRE scholarship. S.H. is a recipient of a scholarship from the IISER, Pune, India. The authors declare no conflicts of interests.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7553237.
Communicating editor: L. Wahl
Literature Cited
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I., 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 82: 5724–5727. 10.1073/pnas.82.17.5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aka S. T., Haji S. H., 2015. Sub-MIC of antibiotics induced biofilm formation of Pseudomonas aeruginosa in the presence of chlorhexidine. Braz. J. Microbiol. 46: 149–154. 10.1590/S1517-838246120140218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose P. G., Bhavnani S. M., Ellis-Grosse E. J., Drusano G. L., 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin. Infect. Dis. 51: S103–S110. 10.1086/653057 [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Hughes D., 2012. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updat. 15: 162–172. 10.1016/j.drup.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Hughes D., 2014. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 12: 465–478. 10.1038/nrmicro3270 [DOI] [PubMed] [Google Scholar]
- Avalos Vizcarra I., Hosseini V., Kollmannsberger P., Meier S., Weber S. S., et al. , 2016. How type 1 fimbriae help Escherichia coli to evade extracellular antibiotics. Sci. Rep. 6: 18109 10.1038/srep18109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J. M., Wrande M., Hughes D., 2014. Acetate availability and utilization supports the growth of mutant sub-populations on aging bacterial colonies. PLoS One 9: e109255 10.1371/journal.pone.0109255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman J., Andersson D. I., 2000. The cost of antibiotic resistance from a bacterial perspective. Drug Resist. Updat. 3: 237–245. 10.1054/drup.2000.0147 [DOI] [PubMed] [Google Scholar]
- Campbell E. A., Korzheva N., Mustaev A., Murakami K., Nair S., et al. , 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104: 901–912. 10.1016/S0092-8674(01)00286-0 [DOI] [PubMed] [Google Scholar]
- Cavusoglu C., Karaca-Derici Y., Bilgic A., 2004. In-vitro activity of rifabutin against rifampicin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Clin. Microbiol. Infect. 10: 662–665. 10.1111/j.1469-0691.2004.00917.x [DOI] [PubMed] [Google Scholar]
- Condon C., Cooper B. S., Yeaman S., Angilletta M. J., Jr., 2014. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 68: 720–728. 10.1111/evo.12296 [DOI] [PubMed] [Google Scholar]
- Cooper T. F., Lenski R. E., 2010. Experimental evolution with E. coli in diverse resource environments. I. Fluctuating environments promote divergence of replicate populations. BMC Evol. Biol. 10: 11 10.1186/1471-2148-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L., 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos M. G., Dawid A., Sunderlikova V., Tans S. J., 2015. Breaking evolutionary constraint with a tradeoff ratchet. Proc. Natl. Acad. Sci. USA 112: 14906–14911. 10.1073/pnas.1510282112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel E., Kranzler M., Stark T. D., Hofmann T., Ehling-Schulz M., 2015. The endospore-forming pathogen Bacillus cereus exploits a small colony variant-based diversification strategy in response to aminoglycoside exposure. MBio 6: e01172–e01215. 10.1128/mBio.01172-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman O., Goldberg A., Ronin I., Shoresh N., Balaban N. Q., 2014. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513: 418–421. 10.1038/nature13469 [DOI] [PubMed] [Google Scholar]
- Gally D. L., Bogan J. A., Eisenstein B. I., Blomfield I. C., 1993. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J. Bacteriol. 175: 6186–6193. 10.1128/jb.175.19.6186-6193.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibyan L., Huang T., Kim M., Wolff E., Nguyen A., et al. , 2003. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amst.) 2: 593–608. 10.1016/S1568-7864(03)00024-7 [DOI] [PubMed] [Google Scholar]
- Gullberg E., Cao S., Berg O. G., Ilback C., Sandegren L., et al. , 2011. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 7: e1002158 10.1371/journal.ppat.1002158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Laureti L., Crussard S., Abida H., Rodriguez-Rojas A., et al. , 2013. Beta-Lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat. Commun. 4: 1610 10.1038/ncomms2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjifrangiskou M., Gu A. P., Pinkner J. S., Kostakioti M., Zhang E. W., et al. , 2012. Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J. Bacteriol. 194: 6195–6205. 10.1128/JB.01012-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. R., Iles J. C., MacLean R. C., 2011. The fitness cost of rifampicin resistance in Pseudomonas aeruginosa depends on demand for RNA polymerase. Genetics 187: 817–822. 10.1534/genetics.110.124628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. W., Mah T. F., 2017. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 41: 276–301. 10.1093/femsre/fux010 [DOI] [PubMed] [Google Scholar]
- Hasman H., Schembri M. A., Klemm P., 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182: 1089–1095. 10.1128/JB.182.4.1089-1095.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh A., Hill C., Mokhtar J., Novotna G., Tran N., et al. , 2011. Genome-wide dynamics of a bacterial response to antibiotics that target the cell envelope. BMC Genomics 12: 226 10.1186/1471-2164-12-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Andersson D. I., 2015. Evolutionary consequences of drug resistance: shared principles across diverse targets and organisms. Nat. Rev. Genet. 16: 459–471. 10.1038/nrg3922 [DOI] [PubMed] [Google Scholar]
- Jefferson K. K., 2004. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236: 163–173. 10.1111/j.1574-6968.2004.tb09643.x [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A., 1989. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J. Bacteriol. 171: 5229–5231. 10.1128/jb.171.9.5229-5231.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve S. M., Daniel S., Chavhan Y. D., Anand A., Kharola S. S., et al. , 2015. Escherichia coli populations in unpredictably fluctuating environments evolve to face novel stresses through enhanced efflux activity. J. Evol. Biol. 28: 1131–1143. 10.1111/jeb.12640 [DOI] [PubMed] [Google Scholar]
- Klemm P., 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5: 1389–1393. 10.1002/j.1460-2075.1986.tb04372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Mizrahi V., Warner D. F., 2014. The impact of drug resistance on Mycobacterium tuberculosis physiology: what can we learn from rifampicin? Emerg. Microbes Infect. 3: e17 10.1038/emi.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Reisman I., Ronin I., Gefen O., Braniss I., Shoresh N., et al. , 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355: 826–830. 10.1126/science.aaj2191 [DOI] [PubMed] [Google Scholar]
- Lim J. K., Gunther N. W. t., Zhao H., Johnson D. E., Keay S. K., et al. , 1998. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 66: 3303–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone J. G., 2015. Role of small colony variants in persistence of Pseudomonas aeruginosa infections in cystic fibrosis lungs. Infect. Drug Resist. 8: 237–247. 10.2147/IDR.S68214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicker G., Prajsnar T. K., Williams A., Wagner N. L., Boots M., et al. , 2014. Clonal expansion during Staphylococcus aureus infection dynamics reveals the effect of antibiotic intervention. PLoS Pathog. 10: e1003959 10.1371/journal.ppat.1003959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbinger A., Vergassola M., 2015. The impact of environmental fluctuations on evolutionary fitness functions. Sci. Rep. 5: 15211 10.1038/srep15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk A. H., Wong A., Kassen R., 2015. The fitness costs of antibiotic resistance mutations. Evol. Appl. 8: 273–283. 10.1111/eva.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Quiroz R. C., Silva C. A., Molina C. F., Leiva L. E., Reyes-Cerpa S., et al. , 2015. Exposure to sub-inhibitory concentrations of cefotaxime enhances the systemic colonization of Salmonella Typhimurium in BALB/c mice. Open Biol. 5:pii: 150070. 10.1098/rsob.150070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. R., Isabella V. M., Lewis K., 2014. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 68: 1–12. 10.1007/s00248-013-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita J. M., Arias C. A., 2016. Mechanisms of antibiotic resistance. Microbiol. Spectr. 4 10.1128/microbiolspec.VMBF-0016-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T., Matsumoto T., Horiuchi K., Kasuga E., Natori T., et al. , 2018. Characterization of clinically isolated thymidine-dependent small-colony variants of Escherichia coli producing extended-spectrum beta-lactamase. J. Med. Microbiol. 67: 33–39. 10.1099/jmm.0.000634 [DOI] [PubMed] [Google Scholar]
- Nguyen U. T., Harvey H., Hogan A. J., Afonso A. C., Wright G. D., et al. , 2014. Role of PBPD1 in stimulation of Listeria monocytogenes biofilm formation by subminimal inhibitory beta-lactam concentrations. Antimicrob. Agents Chemother. 58: 6508–6517. 10.1128/AAC.03671-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira N. M., Martinez-Garcia E., Xavier J., Durham W. M., Kolter R., et al. , 2015. Biofilm formation as a response to ecological competition. PLoS Biol. 13: e1002191 (erratum: PLoS Biol. 13: e1002232). 10.1371/journal.pbio.1002191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S. K., Cars O., 2007. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin. Infect. Dis. 45: S129–S136. 10.1086/519256 [DOI] [PubMed] [Google Scholar]
- O’Neill A. J., Huovinen T., Fishwick C. W., Chopra I., 2006. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob. Agents Chemother. 50: 298–309. 10.1128/AAC.50.1.298-309.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S., 1984. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J. Bacteriol. 160: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S. L., Suleman L., Vuotto C., Donelli G., 2015. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 64: 323–334. 10.1099/jmm.0.000032 [DOI] [PubMed] [Google Scholar]
- Pigliucci M., 2010. Genotype-phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 557–566. 10.1098/rstb.2009.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour N. K., Dusane D. H., Dhakephalkar P. K., Zamin F. R., Zinjarde S. S., et al. , 2011. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 62: 328–338. 10.1111/j.1574-695X.2011.00818.x [DOI] [PubMed] [Google Scholar]
- Pränting M., Andersson D. I., 2011. Escape from growth restriction in small colony variants of Salmonella typhimurium by gene amplification and mutation. Mol. Microbiol. 79: 305–315. 10.1111/j.1365-2958.2010.07458.x [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Kriegeskorte A., Kahl B. C., Becker K., Löffler B., et al. , 2014. Staphylococcus aureus small colony variants (SCVs): a road map for the metabolic pathways involved in persistent infections. Front. Cell. Infect. Microbiol. 4: 99 10.3389/fcimb.2014.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q., Toll-Riera M., Heilbron K., Preston G. M., MacLean R. C., 2016. The genomic basis of adaptation to the fitness cost of rifampicin resistance in Pseudomonas aeruginosa. Proc. Biol. Sci. 283: 20152452. 10.1098/rspb.2015.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro R. S., Costa H., Gordo I., 2016. Macrophage adaptation leads to parallel evolution of genetically diverse Escherichia coli small-colony variants with increased fitness in vivo and antibiotic collateral sensitivity. Evol. Appl. 9: 994–1004. 10.1111/eva.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M. G., 2000. Compensatory evolution in rifampin-resistant Escherichia coli. Genetics 156: 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Verdugo A., Gaut B. S., Tenaillon O., 2013. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol. Biol. 13: 50 10.1186/1471-2148-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E., Cimdins A., Lüthje P., Brauner A., Sjöling A., et al. , 2018. “It’s a gut feeling” - Escherichia coli biofilm formation in the gastrointestinal tract environment. Crit. Rev. Microbiol. 44: 1–30 (erratum: Crit. Rev. Microbiol. 44: i). 10.1080/1040841X.2017.1303660 [DOI] [PubMed] [Google Scholar]
- Sandegren L., 2014. Selection of antibiotic resistance at very low antibiotic concentrations. Ups. J. Med. Sci. 119: 103–107. 10.3109/03009734.2014.904457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos V., Hirshfield I., 2016. The physiological and molecular characterization of a small colony variant of Escherichia coli and its phenotypic rescue. PLoS One 11: e0157578 10.1371/journal.pone.0157578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stentebjerg-Olesen B., Chakraborty T., Klemm P., 2000. FimE-catalyzed off-to-on inversion of the type 1 fimbrial phase switch and insertion sequence recruitment in an Escherichia coli K-12 fimB strain. FEMS Microbiol. Lett. 182: 319–325. 10.1111/j.1574-6968.2000.tb08915.x [DOI] [PubMed] [Google Scholar]
- Travisano M., Lenski R. E., 1996. Long-term experimental evolution in Escherichia coli. IV. Targets of selection and the specificity of adaptation. Genetics 143: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr L., Kreis C. A., Hoerr V., Flint L., Hachmeister M., et al. , 2016. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J. Antimicrob. Chemother. 71: 438–448. 10.1093/jac/dkv371 [DOI] [PubMed] [Google Scholar]
- Van Acker H., Van Dijck P., Coenye T., 2014. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 22: 326–333. 10.1016/j.tim.2014.02.001 [DOI] [PubMed] [Google Scholar]
- von Eiff C., 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents 31: 507–510. 10.1016/j.ijantimicag.2007.10.026 [DOI] [PubMed] [Google Scholar]
- Wagner G. P., Zhang J., 2011. The pleiotropic structure of the genotype-phenotype map: the evolvability of complex organisms. Nat. Rev. Genet. 12: 204–213. 10.1038/nrg2949 [DOI] [PubMed] [Google Scholar]
- Wei Q., Tarighi S., Dotsch A., Haussler S., Musken M., et al. , 2011. Phenotypic and genome-wide analysis of an antibiotic-resistant small colony variant (SCV) of Pseudomonas aeruginosa. PLoS One 6: e29276 10.1371/journal.pone.0029276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistrand-Yuen E., Knopp M., Hjort K., Koskiniemi S., Berg O. G., et al. , 2018. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 9: 1599 10.1038/s41467-018-04059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Li X., Gunawardana M., Maguire K., Guerrero-Given D., et al. , 2014. Beta- lactam antibiotics stimulate biofilm formation in non-typeable haemophilus influenzae by up-regulating carbohydrate metabolism. PLoS One 9: e99204 (erratum: PLoS One 9: e115784). 10.1371/journal.pone.0099204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Koga H., Ohno H., Ogawa K., Fukuda M., et al. , 1998. Relationship between antimycobacterial activities of rifampicin, rifabutin and KRM-1648 and rpoB mutations of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 42: 621–628. 10.1093/jac/42.5.621 [DOI] [PubMed] [Google Scholar]
- Zhou J., Dong Y., Zhao X., Lee S., Amin A., et al. , 2000. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J. Infect. Dis. 182: 517–525. 10.1086/315708 [DOI] [PubMed] [Google Scholar]
- Zhou Y. N., Jin D. J., 1998. The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like “stringent” RNA polymerases in Escherichia coli. Proc. Natl. Acad. Sci. USA 95: 2908–2913. 10.1073/pnas.95.6.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Whole genome sequencing data has been deposited to GenBank under the project ID: PRJNA494985 (BioSample accessions: SAMN10187669, SAMN10187670, SAMN10187671). Supplemental figures have been submitted to Figshare. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7553237.