Abstract
Background
Proteomic studies of follicular fluid (FF) exist for several species, including the horse; however, the seasonal influence on FF proteome has not been explored in livestock. The application of high-throughput proteomics of FF in horse has the potential to identify seasonal variations of proteins involved in follicle and oocyte growth.
Methods
This study (i) profiles the proteomes of equine FF collected from dominant growing follicles during the spring anovulatory season (SAN), and spring (SOV), summer (SUM), and fall (FOV) ovulatory seasons; and (ii) identifies season-dependent regulatory networks and associated key proteins.
Results
Regardless of season, a total of 90 proteins were identified in FF, corresponding to 63, 72, 69, and 78 proteins detected in the SAN, SOV, SUM, and FOV seasons, respectively. Fifty-two proteins were common to all seasons, a total of 13 were unique to either season, and 25 were shared between two seasons or more. Protein-to-protein interaction (PPI) analysis indicated the likely critical roles of plasminogen in the SAN season, the prothrombin/plasminogen combination in SUM, and plasminogen/complement C3 in both SOV and FOV seasons. The apolipoprotein A1 appeared crucial in all seasons. The present findings show that FF proteome of SUM differs from other seasons, with FF having high fluidity (low viscosity).
Conclusions
The balance between the FF contents in prothrombin, plasminogen, and coagulation factor XII proteins favoring FF fluidity may be crucial at the peak of the ovulatory season (SUM) and may explain the reported lower incidence of hemorrhagic anovulatory follicles during the SUM season.
Electronic supplementary material
The online version of this article (10.1186/s12958-019-0473-z) contains supplementary material, which is available to authorized users.
Keywords: Ovary; Follicle; Seasonality; Proteomics; Mares, horses
Background
Within the existing efforts to improve fertility in livestock and companion animals, a different knowledge is sought after to achieve a greater reproduction rate, especially for females. A lack of high-quality oocytes and reliable reproductive biomarkers [1, 2] represents an obstacle toward the success. Meanwhile, the high dynamic in the composition of the follicular fluid (FF) during follicular growth [3, 4] creates an opportunity for the identification of key molecules that are critical for oocyte developmental competence acquisition. Furthermore, the situation is even more challenging in horses, due to the obvious reproductive seasonality [4].
The ovarian activity of non-pregnant mares is continuously changing throughout the year, presenting periods of intense activity during summer, low activity during winter (deep anestrus phase), and irregular activity during spring and fall transitional seasons [5–7]. Results of previous studies have shown differences in preovulatory follicle diameter and blood flow [8–10], and hormonal concentrations among seasons [9]. Studying the effect of season on ovarian activity is important not only for better understanding of the follicular dynamics in mares but also for improving our knowledge regarding the follicular environment and biological processes associated with oocyte maturation and ovulation during different seasons of the year. Numerous reports have suggested the constant changes of the FF composition related to the physiological status of the growing follicle [11, 12], the physiological and health conditions of the animal [13–15], and the reproductive seasonality variation [16, 17]. These changes can influence the quality of growing oocytes and their readiness for successful fertilization and subsequent embryo development [18–20]. Moreover, investigating the seasonal variation of equine FF composition may help to better comprehend the mechanisms governing oocyte and follicle maturation, facilitating, therefore, assisted reproductive techniques.
High-throughput technologies (e.g., genomics, metabolomics, and proteomics) allow for in-depth investigations of complex samples such as FF, with potential for new biomarker discoveries, or strategies for intrafollicular treatment. Large-scale proteomics approaches (gel-based and gel-free) were applied to either profile or compare global proteomes of FF in cows [21, 22], humans [23, 24], pigs [25, 26], dogs [27], and horses [28, 29]. Currently, the knowledge regarding equine FF proteome is deficient, and its relationship with fertility in mares is still unknown. Furthermore, the relationship of the equine FF dynamics composition with the reproductive seasonality in horses remains to be determined [4].
The aims of the present study were to (i) use the shotgun (gel-free) approach to evaluate the proteome profiles of equine FF collected from ovarian follicles (30–34 mm in diameter) at different seasons of the year (spring anovulatory or SAN, spring ovulatory or SOV, summer or SUM, and fall ovulatory or FOV); and (ii) apply comparative bioinformatics analyses to identify potential regulatory network differences.
Methods
Animals
Seventeen Quarter horse mares, 8 to 14 years old and weighing 400 to 600 kg, were housed on pasture in the northern hemisphere (37° 42′ 37.53“ N, 89° 13’ 9.50” W),
under natural light conditions, with free access to fresh water and trace-mineralized salt. Animals were handled in accordance with the US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (https://grants.nih.Gov /grants/olaw /references/phspol.htm #US GovPrinciples). This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Southern Illinois University.
Ultrasonographic examination and seasonal groups
Follicular fluids were collected from dominant growing follicles during various seasons of the same year: – March, as Spring Anestrus (SAN) representing the transition season when dominant anovulatory follicles are found after the deep anestrus season and before the spring ovulatory season; − Between April and May, as Spring Ovulatory (SOV) representing the beginning of the ovulatory season, with regular cycling of mares; − Between June and July, as Summer (SUM) representing the middle of the ovulatory season, with maximum of ovarian cyclic activity expected; − and September, as Fall Ovulatory (FOV) representing the final period of the ovulatory season befor the transition to the fall anovulatory and deep anestrus seasons. In all four seasons, follicles ≥6 mm in diameter were ablated, as previously described [30], to induce a new follicular wave, allowing, therefore, proper tracking of growing/healthy follicles. During the SAN season, after follicle ablation, follicles of the new induced wave were daily tracked using an ultrasound machine (Aloka SSD-900; Aloka Co, Ltd., Wallingford, CT, USA) equipped with a multi-frequency 5–10 MHz linear array transducer (Aloka UST-5821–7.5). Samples of FF (n = 6 follicles) were collected when the follicles reached 30–34 mm in diameter. During SOV, SUM, and FOV, mares were monitored daily with ultrasonography until ovulation; thereafter, follicle ablation was performed on day 10–12 after ovulation (day 0 = ovulation) and follicle tracking of the new induced wave was performed daily to collect FF when a dominant follicle reached 30–34 mm in diameter. Samples of FF were aspirated during SOV, SUM, and FOV seasons (n = 6, 6, and 12 follicles, respectively). In all seasons, the presence of uterine edema (estrus-like) and the absence of a corpus luteum detected through ultrasonography at the moment of FF collection did qualify the animal for the procedure.
Follicular fluid collection
Samples of FF were collected using transvaginal ultrasound-guided follicle aspiration as recently reported [10]. Samples were immediately centrifuged at 1600 x g (10 min at 4 °C), followed by a second centrifugation at 3200 x g (15 min at 4 °C) of resulting supernatants. Only clear FF samples, without any visible trace of blood contamination (presence of red blood cells) were stored at − 80 °C until analyses.
Electrophoresis of follicular-fluid proteins
Optimal isolation of frozen-thawed equine FF proteins was tested through various equine FF:Acetone:Trichloroacetic Acid (TCA) mixture ratios (5:4:1, 1:4:0, and 1.7:3.3:0). Mixtures were incubated (overnight, − 20 °C), centrifuged (9500 g, 10 min, 4 °C), and supernatants were discarded. Cold acetone (1 ml, kept at − 20 °C) was added to each pellet and sample mixtures were vortexed (20 min), centrifuged (9500 g, 10 min, 4 °C), and resulting supernatants (acetone) were discarded. After three repetitions, pellets were dried under the fume hood and resuspended in the nanopure water. All protein samples were subjected to albumin depletion according to the manufacturer’s instruction (ProteoExtract Albumin removal kit; Calbiochem EMD Biosciences, Darmstadt, Germany). Depleted protein samples were mixed with acetone in a 1:4 ratio (v/v - FF:Acetone), precipitated overnight at 4 °C, and washed twice with acetone by successive centrifugations (9500 g, 10 min, 4 °C). Final protein samples were quantified (NanoDrop spectrophotometer; Thermo Scientific, Grand Island, NY, USA) and aliquots of each sample were mixed with sample buffer and loaded into wells of a 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Gels were run as previously described [31], followed by staining with Coomassie blue R-250 reagent to visualize the protein bands.
Liquid chromatography-mass spectrometry (LC-MS) analysis of follicular-fluid proteins
Extracted FF proteins of each mare were determined (NanoDrop spectrophotometer, ThermoFisher Scientific) and equal amounts of proteins of two to four mares were pooled for each season (SAN, SOV, SUM, and FOV). For proteomic analyses, three independent pools (100 μg protein each) were constituted for each season. Pooled samples were precipitated overnight with 100% acetone (1:5 ratio), washed two times with 100% acetone, air-dried, and stored at − 20 °C. Prior to in-solution digestion, protein precipitates were dissolved in 100 μl of 100 mM ammonium/5% acetonitrile, reduced with 1/10 volume of 100 mM dithiothreitol (DTT) for 15 min at 65 °C, and alkylated with 1/10 volume of 10 mM iodoacetamide (IAA) for 30 min at room temperature in dark. Digestion was carried out with Trypsin/Lys-C Mix (Promega, Madison, WI) at 37 °C overnight. Samples were freeze-dried and protein tryptic digest was resuspended in 0.1% (v/v) formic acid, 2.0% (v/v) acetonitrile. Aliquots of peptides representing two micrograms of protein were subjected to LC-MS analysis as described previously [32]. Briefly, peptides were separated using Ultimate 3000 HPLC system and reversed phase C18, 75 μm × 150 mm column (both Thermo Fisher Scientific), via 170 min long, nonlinear, constant flow (0.3 μl/ml) gradient of acetonitrile (in 0.1% formic acid) as follows: 2–55% for 125 min, 95% for 15 min, 2% for 30 min. Raw mass spectral data were collected by LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) working in the result dependent acquisition (RDA) mode of 18 scan events: one MS scan (m/z range: 300–1700) followed by 17 MSMS scans for the 17 most intense ions detected in MS scan, with dynamics exclusion allowed.
Protein identification and bioinformatics analyses
The raw files were searched using the SEQUEST algorithm of the Proteome Discoverer 1.1.0 software (Thermo Fisher Scientific) as described previously [33]. Variable modifications were considered as follows: cysteine carbamidomethylation (+ 57.021), methionine oxidation (+ 15.995), methionine dioxidation (+ 31.990). The spectral data were matched against target and decoy databases to allow for calculation of false discovery rates (FDR). The NCBI (www.ncbi.nlm.nih.gov) Equus caballus taxonomy referenced protein database (36,108 entries as of August 2017) served as the target database, while its reversed copy (created automatically by the software) served as a decoy database. The search results were filtered by FDR < 1% for high-confidence protein identification. Proteins were functionally annotated (Gene ontology or GO, Enrichment, KEGG pathway, and protein-protein interactions) using the online tools of Agbase (http://agbase.arizona.edu/), DAVID (Database for Annotation, Visualization and Integrated Discovery; DAVID Bioinformatics Resources 6.8; https://david.ncifcrf.gov/home.jsp), and STRING (https://string-db.org/cgi/input.pl?sessionId=LyvanBxDO3QN&input_page_show_search=on) using the default settings.
Results
Sample preparation prior to proteomic analysis
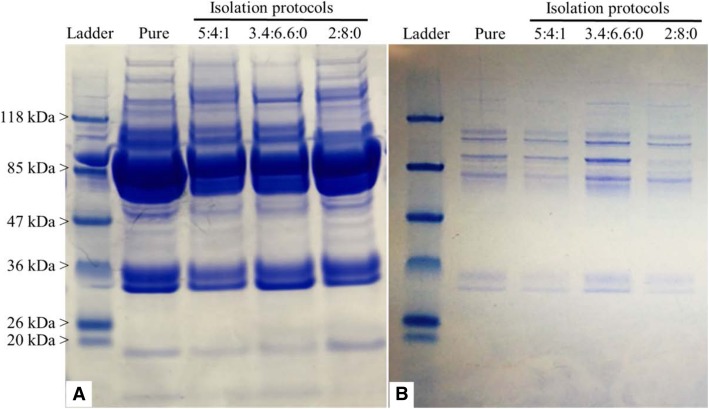
The protein concentrations of pure FF derived from all seasons (SAN, SOV, SUM, and FOV) averaged 39.2 ± 0.4, 38 ± 0.3, 38.3 ± 0.4, and 39 ± 0.4 μg/μl, respectively. The use of pure FF samples (33.2 ± 0.4 μg/μl) for protein precipitation tests (in 5:4:1, 1.7:3.3:0, and 1:4:0 solvent ratios) resulted in decreased protein concentrations (5.8 ± 0.1, 7.9 ± 0.2, and 22.8 ± 0.4 μg/μl, respectively), while the additional albumin depletion procedure led to lesser protein concentrations in all tested FF groups (0.1 ± 0.01, 0.14 ± 0.01, and 0.41 ± 0.02 μg/μl for 5:4:1, 1.7:3.3:0, and 1:4:0 solvent ratios, respectively). Representative electrophoresis gels of both precipitated (Fig. 1a) and precipitated/depleted proteins (Fig. 1b) indicate comparable protein profiles across samples. Although the depletion of pure FF samples (33.2 ± 0.4 μg/μl) produced lower protein concentrations (0.59 ± 0.2 μg/μl), the recovery rate and gel electrophoresis protein profiles were satisfactory for further proteomic analysis.
Fig. 1.
Follicular fluid (FF) protein isolation through combined precipitation and depletion approach. Representative gel electrophoresis of equine FF submitted to four different Acetone-TCA-based protein precipitation protocols (a), followed by albumin depletion (b) are shown. Gels were stained with Coomassie blue to visualize the protein bands, showing decreased protein amounts following both precipitation and depletion. Utilization of pure FF revealed higher protein recovery following depletion. Extraction protocols (5:4:1, 3.4:6.6:0, and 2:8:0) corresponded to FF:Acetone:TCA, respectively
Total proteins identified
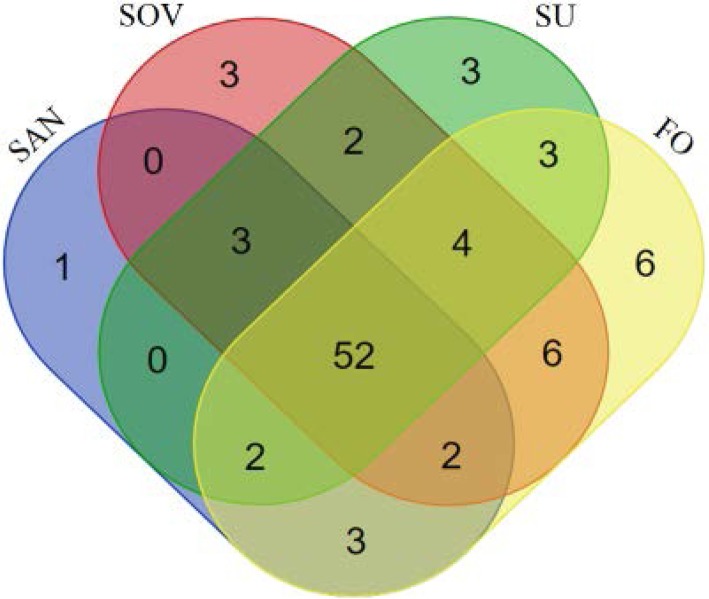
All identified proteins are summarized (Table 1). The totals of 63, 72, 69, and 78 proteins were identified with high confidence (FDR < 1%) in SAN, SOV, SUM, and FOV samples, respectively. Approximately 87% of proteins were annotated with the NCBI-non redundant database, and 13% with ENSEMBL. The Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) indicates 52 proteins shared across all seasons, 25 proteins detected in two or three different seasons, and 13 unique proteins identified in a specific season (one for SAN, three for SOV, three for SUM, and six for FOV; Fig. 2). Overall, a total of 90 proteins were detected in the FF samples across all seasons. Proteins found in each intersection of the Venn diagram are listed in a textual output (Table 2), and all seasonal proteome datasets with full protein annotations are provided as supplementary data (Additional file 1: Table S1).
Table 1.
Seasonal variation of equine follicular fluid proteome
| Reproductive seasons | Number of detected proteins | |||
|---|---|---|---|---|
| N | NCBI annotated (%) | ENSEMBL annotated (%) | ||
| Partially | Fully | |||
| Spring anovulatory (SAN) | 63 | 42 (66.7) | 12 (19.0) | 9 (14.3) |
| Spring ovulatory (SOV) | 72 | 48 (66.7) | 14 (19.4) | 10 (13.9) |
| Summer (SUM) | 69 | 47 (68.1) | 13 (18.8) | 9 (13.0) |
| Fall ovulatory (FOV) | 78 | 49 (62.8) | 18 (23.1) | 11 (14.1) |
Fig. 2.
Venn diagram representation of proteins identified in equine follicular fluid across seasons. Spring anovulatory (SAN), spring ovulatory (SOV), summer (SUM or SU) and fall ovulatory (FOV or FO) seasons
Table 2.
Equine follicular fluid proteins distribution across four reproductive seasons
There were no shared proteins among the following groups: SAN/SOV and SAN/SU. SAN spring anovulatory, SOV spring ovulatory, SUM summer, FOV fall ovulatory
Functional classification, protein enrichment, and pathways analyses
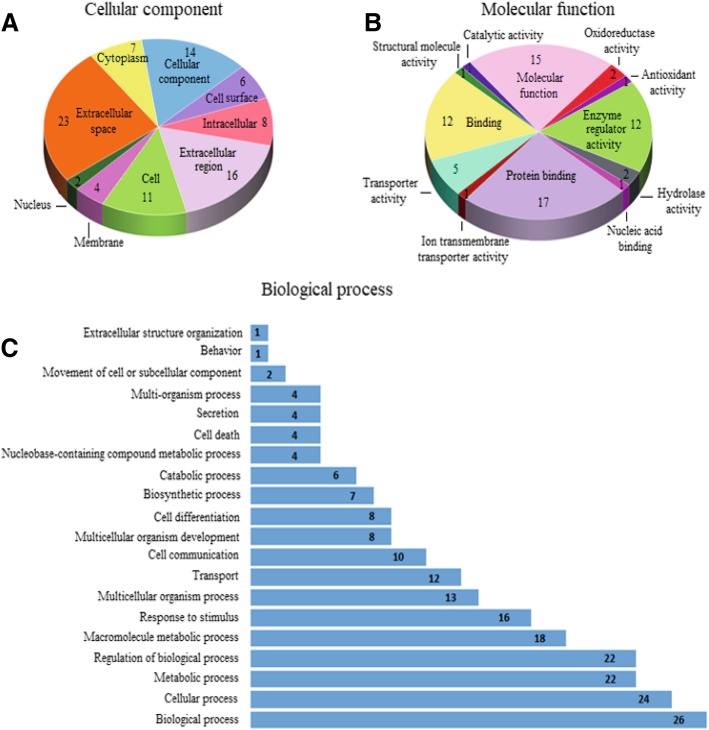
For functional classification, GO annotation was available for 88.5 to 91.7% of identified proteins across the season datasets. Proteins were classified into three GO categories as cellular components (CC), molecular functions (MF), and biological processes (BP). Regardless of season, proteins were distributed within 9–10, 12, and 20 GO terms associated with CC, MF, and BP, respectively. The functional categorization of shared proteins and the observed quantitative variations in GO terms constituting each functional category are shown (Fig. 3).
Fig. 3.
Functional categorization of proteins shared across all seasons. Distribution of total protein per gene ontology (GO) terms in the Cellular component (a), Molecular function (b), and Biological process (c) categories
Irrespective of the season, GO terms associated with extracellular components (space, region, cell surface, membrane, and proteinaceous) represented approximately 54% of the total annotations within the CC category (Table 3). These specific GO terms were 6x to 9x enriched (P < 10− 5, FDR < 0.01). Other GO names such as “blood microparticle” and “fibrinogen complex” were significantly enriched (>104x; P < 10− 4, FDR < 0.01) in our datasets. Similarly, the GO terms associated with binding (binding, protein, and nucleic acid) were highly represented (~ 49%) within the MF category (Table 4), and only the “serine-type endopeptidase inhibitor activity” GO term revealed significant enrichment across all seasons (46-63x; P < 10− 16, FDR < 0.01). Nonetheless, several GO terms associated with binding (e.g., heparin, cholesterol, phosphatidylcholine, and copper ion) and enzymatic activity (e.g., cholesterol transporter, phosphatidylcholine-sterol O-acyltransferase activator, structural molecule, cysteine-type endopeptidase inhibitor) were significantly enriched (>6x; P < 0.05), but with higher FDR (> 0.01). As for the BP category across seasons, approximately 70, 8, 5, and 4% of total annotations were respectively associated with “processes”, “response to stimulus”, “transport”, and “cell communication” (Table 5). Meanwhile, the “acute-phase response”, “fibrinolysis”, and “positive regulation of cholesterol esterification” GO terms were significantly enriched in all seasonal samples (>62x; P < 10− 3, FDR < 0.01), and the “blood coagulation” GO term was substantially enriched in the SUM samples only (36x; P < 10− 3; FDR < 0.01). Finally, the “complement and coagulation cascades” KEGG pathway was significantly enriched (50-54x; P < 10− 3; FDR < 0.01), regardless of the reproductive season.
Table 3.
Cellular component of equine follicular fluid proteins across four different reproductive seasons
| GO terms / GO names | Reproductive seasons | Shared | |||
|---|---|---|---|---|---|
| SAN n (%) |
SOV n (%) |
SUM n (%) |
FOV n (%) |
proteins n (%) |
|
| GO:0005615 / Extracellular space | 25 (24.0) | 29 (23.8) | 29 (26.9) | 32 (25.4) | 23 (25.3) |
| GO:0005575 / Cellular component | 17 (16.3) | 21 (17.2) | 18 (16.7) | 22 (17.5) | 14 (15.4) |
| GO:0005576 / Extracellular region | 17 (16.3) | 19 (15.6) | 19 (17.6) | 21 (16.7) | 16 (17.6) |
| GO:0005623 / Cell | 14 (13.5) | 17 (13.9) | 13 (12.0) | 16 (12.7) | 11 (12.1) |
| GO:0005622 / Intracellular | 9 (8.7) | 12 (9.8) | 10 (9.3) | 11 (8.7) | 8 (8.8) |
| GO:0005737 / Cytoplasm | 7 (6.7) | 8 (6.6) | 7 (6.5) | 8 (6.3) | 7 (7.7) |
| GO:0009986 / Cell surface | 6 (5.8) | 6 (4.9) | 6 (5.6) | 6 (4.8) | 6 (6.6) |
| GO:0016020 / Membrane | 6 (5.8) | 6 (4.9) | 4 (3.7) | 6 (4.8) | 4 (4.4) |
| GO:0005634 / Nucleus | 3 (2.9) | 3 (2.5) | 2 (1.9) | 3 (2.4) | 2 (2.2) |
| GO:0005578 / Proteinaceous extracellular matrix | – | 1 (0.8) | – | 1 (0.8) | – |
| Total annotations | 104 (100) | 122 (100) | 108 (100) | 126 (100) | 91 (100) |
SAN spring anovulatory, SOV spring ovulatory, SUM summer, FOV fall ovulatory
Table 4.
Molecular function of equine follicular fluid proteins across four different reproductive seasons
| GO terms / GO names | Reproductive seasons | Shared | |||
|---|---|---|---|---|---|
| SAN n (%) |
SOV n (%) |
SUM n (%) |
FOV n (%) |
proteins n (%) |
|
| GO:0005488 / Binding | 22 (26.2) | 25 (27.2) | 25 (27.2) | 29 (27.9) | 21 (27.6) |
| GO:0005515 / Protein binding | 18 (19.6) | 18 (19.6) | 19 (20.7) | 20 (19.2) | 17 (19.0) |
| GO:0003674 / Molecular function | 16 (19.6) | 16 (17.4) | 17 (18.5) | 20 (19.2) | 15 (18.1) |
| GO:0030234 / Enzyme regulator activity | 12 (14.3) | 12 (13.0) | 13 (14.1) | 13 (12.5) | 12 (11.2) |
| GO:0005215 / Transporter activity | 5 (6.0) | 6 (6.5) | 5 (5.4) | 6 (5.8) | 5 (5.2) |
| GO:0016787 / Hydrolase activity | 2 (2.4) | 4 (4.3) | 2 (2.2) | 4 (3.8) | 2 (3.4) |
| GO:0005198 / Structural molecule activity | 2 (2.4) | 4 (4.3) | 3 (3.3) | 3 (2.9) | 1 (4.3) |
| GO:0016491 / Oxidoreductase activity | 2 (2.4) | 2 (2.2) | 3 (3.3) | 3 (2.9) | 2 (1.7) |
| GO:0003676 / Nucleic acid binding | 2 (2.4) | 1 (1.1) | 2 (2.2) | 2 (1.9) | 1 (3.4) |
| GO:0003824 / Catalytic activity | 1 (1.2) | 2 (2.2) | 1 (1.1) | 2 (1.9) | 1 (2.6) |
| GO:0015075 / Ion transmembrane transporter activity | 1 (1.2) | 1 (1.1) | 1 (1.1) | 1 (1.0) | 1 (0.9) |
| GO:0016209 / Antioxidant activity | 1 (1.2) | 1 (1.1) | 1 (1.1) | 1 (1.0) | 1 (0.9) |
| Total annotations | 84 (100) | 92 (100) | 92 (100) | 104 (100) | 79 (100) |
SAN spring anovulatory, SOV spring ovulatory, SUM summer, FOV fall ovulatory
Table 5.
Biological process of equine follicular fluid proteins across four different reproductive seasons
| GO terms / GO names | Reproductive seasons | Shared | |||
|---|---|---|---|---|---|
| SAN n (%) |
SOV n (%) |
SUM n (%) |
FOV n (%) |
proteins n (%) |
|
| GO:0008150 / Biological process | 31 (13.2) | 35 (13.8) | 32 (12.7) | 40 (13.7) | 26 (12.3) |
| GO:0009987 / Cellular process | 26 (11.1) | 29 (11.5) | 28 (11.2) | 32 (11.0) | 24 (11.3) |
| GO:0050789 / Regulation of biological process | 25 (10.7) | 28 (11.1) | 27 (10.8) | 30 (10.3) | 22 (10.4) |
| GO:0008152 / Metabolic process | 24 (10.3) | 25 (9.9) | 26 (10.4) | 30 (10.3) | 22 (10.4) |
| GO:0043170 / Macromolecule metabolic process | 20 (8.5) | 20 (7.9) | 20 (8.0) | 24 (8.2) | 18 (8.5) |
| GO:0050896 / Response to stimulus | 19 (8.1) | 20 (7.9) | 19 (7.6) | 25 (8.6) | 16 (7.5) |
| GO:0032501 / Multicellular organism process | 14 (6.0) | 17 (6.7) | 16 (6.4) | 18 (6.2) | 13 (6.1) |
| GO:0006810 / Transport | 12 (5.1) | 14 (5.5) | 13 (5.2) | 16 (5.5) | 12 (5.7) |
| GO:0007154 / Cell communication | 10 (4.3) | 10 (4.0) | 11 (4.4) | 11 (3.8) | 10 (4.7) |
| GO:0007275 / Multicellular organism development | 9 (3.8) | 10 (4.0) | 10 (4.0) | 11 (3.8) | 8 (3.8) |
| GO:0030154 / Cell differentiation | 9 (3.8) | 9 (3.6) | 10 (4.0) | 11 (3.8) | 8 (2.8) |
| GO:0009058 / Biosynthetic process | 8 (3.4) | 7 (2.8) | 8 (3.2) | 9 (3.1) | 7 (3.3) |
| GO:0009056 / Catabolic process | 6 (2.6) | 7 (2.8) | 6 (2.4) | 8 (2.7) | 6 (2.8) |
| GO:0006139 / Nucleobase-containing compound metabolic process | 5 (2.1) | 4 (1.6) | 5 (2.0) | 6 (2.1) | 4 (1.9) |
| GO:0008219 / Cell Death | 4 (1.7) | 4 (1.6) | 5 (2.0) | 5 (1.7) | 4 (1.9) |
| GO:0046903 / Secretion | 4 (1.7) | 4 (1.6) | 5 (2.0) | 5 (1.7) | 4 (1.9) |
| GO:0051704 / Multi-organism process | 4 (1.7) | 4 (1.6) | 6 (2.4) | 5 (1.7) | 4 (1.9) |
| GO:0006928 / Movement of cell or subcellular component | 2 (0.9) | 3 (1.2) | 2 (0.8) | 2 (0.7) | 2 (0.9) |
| GO:0043062 / Extracellular structure organization | 1 (0.4) | 1 (0.4) | 1 (0.4) | 2 (0.7) | 1 (0.5) |
| GO:0007610 / Behavior | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.3) | 1 (0.5) |
| Total annotations | 234 (100) | 253 (100) | 251 (100) | 291 (100) | 212 (100) |
SAN spring anovulatory, SOV spring ovulatory, SUM summer, FOV fall ovulatory
Comparison between reproductive seasons
1Qualitative and quantitative differences in GO terms constituting each functional category were found across seasons (Tables 3-5). GO terms associated with “extracellular space” (in CC category) and “protein binding” (in MF category) increased in SUM compared to other seasonal groups. In contrast, GO terms associated with “membrane” and “nucleus” (in CC category), “transporter activity” (in MF category), and “response to stimulus” (in BP category) were decreased in SUM. Moreover, the proportions of annotations associated with “transporter activity” in MF and “response to stimulus” in BP were higher in SOV and FOV, respectively, compared to other seasons. Finally, GO terms associated with “intracellular” (in CC category), “binding”, “hydrolase activity”, and “structural molecule activity” (in MF category), and “biological process”, and “multicellular organism process” (in BP category) were lower in SAN compared to SOV.
Protein-protein interaction (PPI) network analyses
The PPI analysis was performed for each season, including the shared protein dataset. For each dataset, three major PPI K-means clustering were obtained with high confidence interaction score (> 0.7) and significant PPI enrichment (P < 10− 16). A representative PPI network generated from shared dataset is shown (Fig. 4). The three main clusters (circles) and related key proteins having higher numbers of interactions are indicated. Cluster 1 (green in Fig. 4) revealed F2 protein (or prothrombin) with the greatest interactions in SAN (n = 12), SOV (n = 14), FOV (n = 13), and shared (n = 12) protein datasets, while the combination of F2 (n = 13) with PLG (plasminogen, n = 11) appeared as the main players in the SUM dataset. In cluster 2 (blue in Fig. 4), plasminogen had the highest number of interactions in SAN and shared datasets (n = 10), while the combination of both plasminogen (n = 10) and ENSECAG00000000339 (complement C3) with four and five interactions may have important roles during SOV and FOV seasons, respectively. Contrarily in the SUM dataset, the F12 (coagulation factor XII) protein appeared as the main player with 12 interactions. Finally, the cluster 3 (red in Fig. 4) revealed APOA1 (apolipoprotein 1) protein as the key player in all seasons.
Fig. 4.
Protein-to-Protein Interaction network of proteins shared across all seasons
Discussion
The current study uses a gel-free technique to provide unique proteomic datasets of equine FF of dominant growing follicles (30–34 mm in diameter) during the SAN, SOV, SUM, and FOV seasons. The existence of seasonal proteins in a similar follicle class, reported herein for the first time, suggests potential critical roles of FF proteins during the folliculogenesis and maybe oogenesis in the equine species. Likewise, various biological functions, protein enrichment, and protein interaction networks are reported to have been influenced by the seasonal variations.
Follicular fluid proteins isolation
Combination of procedures, such as protein precipitation and depletion of high-abundant proteins, are routinely used to enhance the quality of starting samples for proteomic analyses [28, 34, 35]. In this study, all tested precipitation protocols (FF:Acetone:TCA ratio of 5:4:1, 1:4:0, and 1.7:3.3:0) resulted in expected lower protein concentrations (30 to 82% losses) that were exacerbated by a further depletion of high-abundance serum protein (about 99% losses). Interestingly, the electrophoretic profiles of protein samples were generally comparable, regardless of the procedure. Crude equine FF samples maintained the highest protein concentrations following depletion, with only 30% loss, from 33.2 ± 0.4 μg/μl to 22.8 ± 0.4 μg/μl. Thereafter, the 1:4:0 precipitation ratio appeared the most suitable with lesser protein loss, which was consistent with a previous report Santa et al. [36].
Proteome description
The gel-free LC-MS proteomics has been successfully used in previous studies of FF of stock animals [21, 23, 26]. The present study applied strict filters (FDR < 1%, and the minimum of two unique peptides per protein) to obtain proteins with high confidence identification, which may explain the slightly lower number of detected proteins (90 vs. 113) when compared to available FF mare proteome [28]. In addition, the proteome dataset of the current study contains fewer proteins in comparison to other monovular species such as humans (158 to 1079; [24, 37–40]), and dairy cattle (113 to 219; [15, 21]). Nonetheless, the aforementioned proteomic studies were generally performed with FF samples obtained from follicles of different sizes and unknown physiological statuses (i.e., growing and regressing follicles), under different technical approaches (e.g., gel-based or gel-free) and protein call stringencies (e.g., false discovery rate and peptides). The full annotation (87% with NCBI and 13% with ENSEMBL) of all detected proteins offers opportunities for in-depth investigations, such as the dynamic composition of the equine FF proteome and the relationship with oocyte quality. To the best of our knowledge, this is the first study providing essential clues of the FF proteome variations to enable a further understanding of the impact of different seasons on fertility of the mare, and maybe of other livestock.
Proteins specifics to seasons
Among the 90 proteins, a core set of 52 was detected across all seasons. It is expected that these proteins may have essential roles during folliculogenesis [39] and oogenesis [2] processes, as previously reported in humans. In contrast, the examined seasons were characterized by subsets of proteins that may serve as potential biomarkers of seasonal fertility in mares. For instance, the BPI (Bactericidal/Permeability-Increasing) fold-contain Family A member 2 (BPIFA2) precursor was found only in SOV, SUM, and FOV (ovulatory seasons). The BPI is an endogenous antibiotic protein that belongs to the family of mammalian lipopolysaccharide (LPS)-binding and lipid transport protein. The BPIFA2 is known to have a role in the innate immune responses and was reported to inhibit the formation of biofilm by pathogenic gram-negative bacteria in the respiratory tract [41]. In this regard, although the function for BPI in FF is still unknown, the presence of BPIFA2 during the ovulatory seasons may be important to protect the female genital tract (e.g., oviduct). Also, few reports have found that BPI is expressed in the testis and epididymis of mice and appears to take part in the process of gamete interactions [42, 43].
In contrast, keratin-10 was detected in SAN samples only and may, therefore, be associated with the non-ovulatory seasons. Although keratin is considered a common contaminant in proteomic studies, the keratin-10 family member has been reported as a negative modulator of cell cycle progression throughout the Phospho-Inositol 3 kinase (PI3 kinase) signal transduction pathway [44]. Numerous studies have reported the participation of PI3 kinase in the follicle-stimulating hormone or progesterone-induced meiotic oocyte maturation in Xenopus [45, 46] and mouse [47, 48]. In the present study, eight keratin-like family members were present in different intersections of the Venn diagram, and their specific roles in the acquisition of the oocyte developmental competence remain to be unfolded.
Functional analyses
In this study, the interpretation of bioinformatics analyses focused only on proteins exhibiting thresholds of significance that were lower than 1% in both Benjamini-Hochberg and FDR analyses. About half of the protein annotations belonged to the Extracellular GO term, and only 10% of the total annotations were attributed to Intracellular localization, regardless of the proteome dataset. This distribution is expected, given the composition of the FF, known to contain secretions of follicle cells and blood plasma exudates. Thus, proteins attributed to Intracellular regions may be residues of the various catabolic processes and/or cell breakdown (apoptosis) of follicle cells (granulosa cells) that occur throughout the follicle growth [39, 49, 50]. Protein distributions within the present FF datasets are in agreement with previous reports in other species [24, 51, 52], but differ from the uniquely available report in horses [28], indicating 83% of protein annotations within the Extracellular region and 17% Intracellular. This difference may be due to either the mare breeds (Welsh pony vs. Quarter horse in the current study) or their proteome dataset generated from the combination of distinct follicle physiological stages.
Regarding protein functions, approximately 49% of the total annotations belonged to binding (protein binding and nucleic acid binding GO terms), and 32% corresponded to other cellular and molecular activities. This specific distribution is in agreement with previous studies in humans [24, 51], and reflects the participation of FF proteins in a variety of physiological functions associated with follicle and oocyte growth. In this study, several proteins belonging to the serine-type endopeptidase inhibitor activity, binding (heparin, cholesterol, and copper ion), and enzyme transporter (cholesterol) were significantly enriched across all seasons; those proteins have also been detected in other mono-ovulatory species [28, 52].
Proteins associated with inflammatory responses (immune system, coagulation, acute phase response signaling), a FF signature across studies and species such as humans [53], goats [52], cattle [21], and horses [28], were significantly enriched in our datasets. These proteins may participate in cascades of immune and coagulation formation (fibrin) /inhibition (anti-thrombin) responses having vital roles in follicle growth and oocyte transfer to the oviduct following ovulation. The anticoagulation function of the FF has been revealed to be essential during follicle growth and rupture [54]; moreover, in all of our datasets (SAN, SOV, SUM, and FOV), a significant enrichment in proteins associated with the coagulation cascade was noticed.
The functional categorization indicated a higher proportion of proteins associated with the “Extracellular space” GO name during the SUM season. The increase in FF protein content found in our study during the SUM season may have been due to an increase in ovarian vascularization/blood flow [9, 55, 56], likely favoring the entry of additional plasma proteins into the follicle. Interestingly, the SAN dataset exhibited lower numbers of proteins associated with “Intracellular” (in CC); “Hydrolase activity”, “Structural molecule activity”, and “Binding” (in MF); and “Biological process” and “Multicellular organism process” (in BP) than that of the SOV dataset. These differences may lead to further understanding of the differences in FF environment of dominant anovulatory versus ovulatory follicles during the SAN and SOV seasons, respectively.
Protein and pathway enrichments
Proteins associated with the “complement and coagulation cascades” pathway were significantly enriched in all seasons: 25.4% in SAN, 23.6% SOV, 23.2% SUM, and 25.6% FOV. Indeed, the complement system and inflammatory processes regulate follicle development and ovulation [57–59]. Numerous proteins are known to play essential roles during major events of the ovarian follicle [60]. These events involve a variety of proteolytic and metabolic processes that are mediated by several enzymes found in our datasets. Furthermore, the synthesis of some proteins may have been favored by the high number of protease inhibitors found in our study, such as fetuin-B, plasma protease C1 inhibitor, protein Z-dependent protease inhibitor, alpha-1-antiproteinase 2, alpha-1-antichymotrypsin, GDN peptidase inhibitor 7, inter-alpha-trypsin inhibitor heavy chain H1, H2 and H4, and SERPIN for serine-protease inhibitors. Among them, the SERPIN, a superfamily of protease inhibitors [61], are involved in follicle development and may regulate the follicular extracellular matrix remodeling [22]. On the other hand, many other proteins were associated with coagulation cascades. The presence of proteins such as antithrombin-III, alpha macroglobulin, plasminogen, alpha-2-antiplasmin, and fibrinogen indicates their participation in the controlling, modeling, and regulation of the coagulation pathway leading to healthy follicle growth.
Protein-protein interaction (PPI) networks
The PPI network information is one of the major fields in systems biology allowing for complex network analyses [62]. The PPI permitted the consolidation of the “coagulation cascade” (Fig. 5) as a main signature of the equine FF, as seen in all datasets (SAN, SOV, SUM, FOV, and shared proteins) and previous reports in various species [28, 52, 53]. Clustering analyses allowed the prediction of the combination of F12 (coagulation factor XII), F2 (prothrombin), and PLG (plasminogen) as the signature of equine FF proteins during SUM, while the F2-PLG-ENSECAG00000000339 (complement C3) combination had higher interactions in both the SOV and FOV seasons. In contrast, both F2 and PLG were mainly found during the SAN season. These observations are significant given the functions of the implicated proteins.
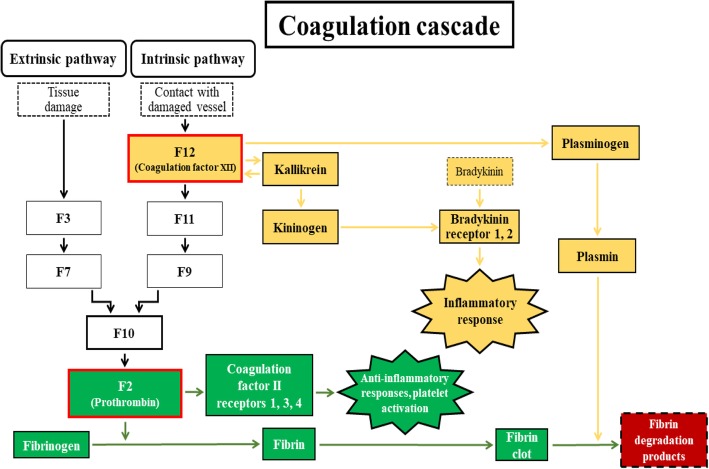
Fig. 5.
Schematized “coagulation cascade” pathway derived from proteins shared across all seasons. Adapted from KEGG Pathway Database of “Coagulation and Complement Cascade” (map04610; http://www.genome.jp/kegg/pathway.html). This diagram shows the roles of the F12 protein (Coagulation factor XII; yellow color), in the intrinsic pathway and the F2 protein (Prothrombin; green color), in the extrinsic pathway of the cascade, leading to the “fibrin degradation products”
Firstly, prothrombin or F2 is a glycoprotein and an essential component of the blood-clotting mechanism exerting effects through its mature form, thrombin, by interacting with specific receptors (or protease-activated receptors or ThRs) on the granulosa cell membrane [63, 64]. However, its contribution as an anti-inflammatory compound prone to induce hemorrhagic anovulatory follicles during the transitional reproductive seasons remains to be explored. Secondly, the coagulation factor XII or F12, however, is a pro-inflammatory protein interacting with prekallikrein to initiate a cascade of events leading to the release of bradykinin [65], which in turn increases the action of LH, contributes to follicular wall contraction [66], and favors ovulation [66–68]. These observations are supportive of the increased protein-protein interactions of the coagulation factor XII during SUM, having possible roles in the ovulation outcome in mares. Thirdly, the proteolytic factor plasminogen is capable of dissolving fibrin of blood clots and performs essential functions during reproductive processes such as extracellular matrix remodeling, modulating follicular development, corpus luteum formation, and weakening the follicle wall to promote ovulation [39, 53, 69–71]. Lastly, the high level of apolipoprotein-1 (APOA1) participates in the cholesterol and triglyceride transportation, having positive mitogenic and angiogenic effects [72], which is beneficial to follicle development.
In summary, this study describes, for the first time, the proteome profile of the equine FF collected during anovulatory (SAN) and ovulatory (SOV, SUM, and FOV) seasons. Functional analyses revealed differences that may be essential to better characterization of reproductive seasonality in mares. The findings show that SUM follicular fluid proteome of dominant follicles (30–34 mm) differs from other seasons and appears to be characterized by a higher fluidity. This former characteristic may allow a more efficient natural flux of biological factors to the oocyte, influencing its maturation, ovulation, and safe transport to the oviduct. While the “coagulation and complement” cascades were confirmed as the prime signatures of the FF proteome, the balance between prothrombin, plasminogen, and coagulation factor XII proteins seemed crucial for the fluidity of the FF at the peak moment of the ovulatory season.
Additional file
Table S1. All seasonal proteome datasets, with full protein annotations. (ODS 1926 kb)
Acknowledgements
The authors thank Dr. S B Park and Mrs. C S Steadman for their technical assistance during protein preparations and gel electrophoresis.
Funding
Funded by Southern Illinois University, Carbondale, IL; Institute of Genomics, Biocomputing and Bioinformatics at Mississippi State University; NIH MS-IDeA Network of Biomedical Research Excellence award 4P20GM103476–15;
and USDA-ARS Biophotonics Initiative (8–6402–3-018). The mass spectrometry proteomics analysis was performed at the Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University, with partial support from Mississippi Agriculture and Forestry Experimental Station. GAD is the recipient of a PhD sandwich from the Coordination for the Improvement of Higher Education Personnel (CAPES; grant #PPGMV-UFRRJ#88881.133485/2016–01), Brazil. GM I is the recipient of a PhD scholarship from the Ministry of Higher Education & Scientific Research, Baghdad, Iraq.
Availability of data and materials
All data generated and analyzed in this study are included in this published manuscript.
Authors’ contributions
GAD and GMI conducted the experiment, analyzed data, and wrote the first draft of the paper; OP, TP and DGP performed proteomics analyses; JMF and GAD conducted functional bioinformatic analyses; JCFJ, STW, and PLR assisted in experimental design and data interpretation; ELG, GMI, and JMF contributed to the design and execution of all experiments, and revised the manuscript. All authors read and approved the final draft.
Ethics approval
All animal care and experimental protocols used in this study were approved by the institutional animal care and the ethic committee of Southern Illinois University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Assidi M, Montag M, Van Der Ven K, Sirard M-A. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet. 2011;28(2):173–188. doi: 10.1007/s10815-010-9491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7(1):40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahiminiya S, Gérard N. Le liquide folliculaire chez les mammifères. Gynécologie Obstétrique & Fertilité. 2010;38(6):402–404. doi: 10.1016/j.gyobfe.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Ginther O. Pitfalls in animal reproduction research: how the animal guards nature's secrets. Theriogenology. 2013;80(3):169–175. doi: 10.1016/j.theriogenology.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Nagy P, Guillaume D, Daels P. Seasonality in mares. Anim Reprod Sci. 2000;60:245–262. doi: 10.1016/s0378-4320(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 6.Ginther O, Gastal E, Gastal M, Beg M. Seasonal influence on equine follicle dynamics. Anim Reprod. 2004;1(1):31–44. [Google Scholar]
- 7.Donadeu F, Watson E. Seasonal changes in ovarian activity: lessons learnt from the horse. Anim Reprod Sci. 2007;100(3–4):225–242. doi: 10.1016/j.anireprosci.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Ginther O. Folliculogenesis during the transitional period and early ovulatory season in mares. J Reprod Fertil. 1990;90(1):311–320. doi: 10.1530/jrf.0.0900311. [DOI] [PubMed] [Google Scholar]
- 9.Gastal E, Gastal M, Donadeu F, Acosta T, Beg M, Ginther O. Temporal relationships among LH, estradiol, and follicle vascularization preceding the first compared with later ovulations during the year in mares. Anim Reprod Sci. 2007;102(3–4):314–321. doi: 10.1016/j.anireprosci.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Ishak GM, Bashir ST, Dutra GA, Gastal GDA, Gastal MO, Cavinder CA, et al. In vivo antral follicle wall biopsy: a new research technique to study ovarian function at the cellular and molecular levels. Reprod Biol Endocrinol. 2018;16(1):71. doi: 10.1186/s12958-018-0380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arashiro EK, Palhao MP, Wohlres-Viana S, Siqueira LG, Camargo LS, Henry M, et al. In vivo collection of follicular fluid and granulosa cells from individual follicles of different diameters in cattle by an adapted ovum pick-up system. Reprod Biol Endocrinol. 2013;11(1):73. doi: 10.1186/1477-7827-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tungal SS, Swamy MN, Honnappa T, Bhaskaran R. Ovarian antral follicular dynamics and follicular fluid composition in non-descript goats of Karnataka. Theriogenology Insight. 2014;4(2):49. [Google Scholar]
- 13.Regiani T, Cordeiro FB. da Costa LdVT, Salgueiro J, Cardozo K, Carvalho VM et al. follicular fluid alterations in endometriosis: label-free proteomics by MSE as a functional tool for endometriosis. Syst Biol Reprod Med. 2015;61(5):263–276. doi: 10.3109/19396368.2015.1037025. [DOI] [PubMed] [Google Scholar]
- 14.Turco L, Guimarães E, Cordeiro FB, de Carvalho Lopes PH, Gozzo FC, Pilau EJ, et al. Proteomic analysis of follicular fluid from women with and without endometriosis: new therapeutic targets and biomarkers. Mol Reprod Dev. 2013;80(6):441–450. doi: 10.1002/mrd.22180. [DOI] [PubMed] [Google Scholar]
- 15.Zachut M, Sood P, Levin Y, Moallem U. Proteomic analysis of preovulatory follicular fluid reveals differentially abundant proteins in less fertile dairy cows. J Proteome. 2016;139:122–129. doi: 10.1016/j.jprot.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, Derar R, Hussein H. Seasonal variation of the ovarian follicular dynamics and luteal functions of sheep in the subtropics. Theriogenology. 2006;66(2):463–469. doi: 10.1016/j.theriogenology.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Yie S-M, Brown G, Liu G-Y, Collins J, Daya S, Hughes E, et al. Melatonin and steroids in human pre-ovulatory follicular fluid: seasonal variations and granulosa cell steroid production. Hum Reprod. 1995;10(1):50–55. doi: 10.1093/humrep/10.1.50. [DOI] [PubMed] [Google Scholar]
- 18.Valckx S, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, et al. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. 2014;12(1):13. doi: 10.1186/1477-7827-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüssow KP, Rátky J, Torner H, et al. Et al. follicular and oocyte development in gilts of different age. Acta Vet Hung. 2002;50(1):101–110. doi: 10.1556/AVet.50.2002.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Algriany O, Bevers M, Schoevers E, Colenbrander B, Dieleman S. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology. 2004;62(8):1483–1497. doi: 10.1016/j.theriogenology.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Ferrazza RA, Garcia HDM, EMdS S, Mihm Carmichael M, FFd S, Burchmore R, et al. Quantitative proteomic profiling of bovine follicular fluid during follicle development. Biol Reprod. 2017;97(6):835–849. doi: 10.1093/biolre/iox148. [DOI] [PubMed] [Google Scholar]
- 22.Fu Q, Huang Y, Wang Z, Chen F, Huang D, Lu Y, et al. Proteome profile and quantitative proteomic analysis of buffalo (Bubalusbubalis) follicular fluid during follicle development. Int J Mol Sci. 2016;17(5):618. doi: 10.3390/ijms17050618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Spiessens C, D’Hooghe T, Peeraer K, Carpentier S. Follicular fluid biomarkers for human in vitro fertilization outcome: proof of principle. Proteome Sci. 2016;14(1):17. doi: 10.1186/s12953-016-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh JW, Kim SK, Cho KC, Kim MS, Suh CS, Lee JR, et al. Proteomic analysis of human follicular fluid in poor ovarian responders during in vitro fertilization. Proteomics. 2017;17(6). [DOI] [PubMed]
- 25.Bijttebier J, Tilleman K, Dhaenens M, Deforce D, Van Soom A, Maes D. Comparative proteome analysis of porcine follicular fluid and serum reveals that excessive α2-macroglobulin in serum hampers successful expansion of cumulus-oocyte complexes. Proteomics. 2009;9(19):4554–4565. doi: 10.1002/pmic.200900270. [DOI] [PubMed] [Google Scholar]
- 26.Ducolomb Y, González-Márquez H, Fierro R, Jiménez I, Casas E, Flores D, et al. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology. 2013;79(6):896–904. doi: 10.1016/j.theriogenology.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Fahiminiya S, Reynaud K, Labas V, Batard S, Chastant-Maillard S, Gérard N. Steroid hormones content and proteomic analysis of canine follicular fluid during the preovulatory period. Reprod Biol Endocrinol. 2010;8(1):132. doi: 10.1186/1477-7827-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahiminiya S, Labas V, Roche S, Dacheux J-L, Gérard N. Proteomic analysis of mare follicular fluid during late follicle development. Proteome Sci. 2011;9(1):54. doi: 10.1186/1477-5956-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrucci B, Wolf C, Arlas T, Santos G, Estanislau J, Fiala S, et al. Proteomics of mare follicular fluid during follicle development. Journal of Equine Veterinary Science. 2014;34(1):115–116. [Google Scholar]
- 30.Gastal E, Gastal M, Bergfelt D, Ginther O. Role of diameter differences among follicles in selection of a future dominant follicle in mares. Biol Reprod. 1997;57(6):1320–1327. doi: 10.1095/biolreprod57.6.1320. [DOI] [PubMed] [Google Scholar]
- 31.Feugang JM, Rodriguez-Munoz JC, Willard ST, Bathgate RA, Ryan PL. Examination of relaxin and its receptors expression in pig gametes and embryos. Reprod Biol Endocrinol. 2011;9:10. doi: 10.1186/1477-7827-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takáč T, Vadovič P, Pechan T, Luptovčiak I, Šamajová O, Šamaj J. Comparative proteomic study of Arabidopsis mutants mpk4 and mpk6. Sci Rep. 2016;6:28306. doi: 10.1038/srep28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takáč T, Šamajová O, Pechan T, Luptovčiak I, Šamaj J. Feedback microtubule control and microtubule-actin cross-talk in Arabidopsis revealed by integrative proteomic and cell biology analysis of KATANIN 1 mutants. Mol Cell Proteomics. 2017;16(9):1591–1609. doi: 10.1074/mcp.M117.068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feist P, Hummon AB. Proteomic challenges: sample preparation techniques for microgram-quantity protein analysis from biological samples. Int J Mol Sci. 2015;16(2):3537–3563. doi: 10.3390/ijms16023537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L, He L, Fountoulakis M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A. 2004;1023(2):317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Santa C, Anjo SI, Manadas B. Protein precipitation of diluted samples in SDS-containing buffer with acetone leads to higher protein recovery and reproducibility in comparison with TCA/acetone approach. Proteomics. 2016;16(13):1847–1851. doi: 10.1002/pmic.201600024. [DOI] [PubMed] [Google Scholar]
- 37.Lewandowska AE, Macur K, Czaplewska P, Liss J, Łukaszuk K, Ołdziej S. Qualitative and quantitative analysis of proteome and Peptidome of human follicular fluid using multiple samples from single donor with LC–MS and SWATH methodology. J Proteome Res. 2017;16(8):3053–3067. doi: 10.1021/acs.jproteome.7b00366. [DOI] [PubMed] [Google Scholar]
- 38.Shen X, Liu X, Zhu P, Zhang Y, Wang J, Wang Y, et al. Proteomic analysis of human follicular fluid associated with successful in vitro fertilization. Reprod Biol Endocrinol. 2017;15(1):58. doi: 10.1186/s12958-017-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, et al. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteome. 2013;87:68–77. doi: 10.1016/j.jprot.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi L, Gagliardi A, Landi C, Focarelli R, De Leo V, Luddi A, et al. Protein pathways working in human follicular fluid: the future for tailored IVF? Expert Rev Mol Med. 2016;18:e9. doi: 10.1017/erm.2016.4. [DOI] [PubMed] [Google Scholar]
- 41.Gakhar L, Bartlett JA, Penterman J, Mizrachi D, Singh PK, Mallampalli RK, et al. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS One. 2010;5(2):e9098. doi: 10.1371/journal.pone.0009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K, Liu Y, Xia X, Wang L, Lu M, Hu Y, et al. Bactericidal/permeability-increasing protein in the reproductive system of male mice may be involved in the sperm–oocyte fusion. Reproduction. 2013;146(2):135–144. doi: 10.1530/REP-13-0127. [DOI] [PubMed] [Google Scholar]
- 43.Mou L, Xie N. Male infertility-related molecules involved in sperm-oocyte fusion. J Reprod Dev. 2017;63(1):1–7. doi: 10.1262/jrd.2016-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKCζ mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21(21):7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muslin AJ, Klippel A, Williams LT. Phosphatidylinositol 3-kinase activity is important for progesterone-induced Xenopus oocyte maturation. Mol Cell Biol. 1993;13(11):6661. doi: 10.1128/mcb.13.11.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagowski CP, Myers JW, Ferrell JE. The classical progesterone receptor associates with p42 MAPK and is involved in phosphatidylinositol 3-kinase signaling inXenopus oocytes. J Biol Chem. 2001;276(40):37708–37714. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]
- 47.Hoshino T, Shimizu K, Honda T, Kawakatsu T, Fukuyama T, Nakamura T, et al. A novel role of Nectins in inhibition of the E-cadherin–induced activation of Rac and formation of cell-cell Adherens junctions. Mol Biol Cell. 2004;15(3):1077–1088. doi: 10.1091/mbc.E03-05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makker A, Goel MM, Nigam D, Mahdi AA, Das V, Agarwal A, et al. Aberrant Akt activation during implantation window in infertile women with intramural uterine fibroids. Reprod Sci. 2017;25(8):1243–1253. doi: 10.1177/1933719117737844. [DOI] [PubMed] [Google Scholar]
- 49.Markstrom E, Svensson E, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis--dependence on follicle differentiation. Reproduction. 2002;123(1):23–30. doi: 10.1530/rep.0.1230023. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126(4):415–424. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 51.Zamah AM, Hassis ME, Albertolle ME, Williams KE. Proteomic analysis of human follicular fluid from fertile women. Clin Proteomics. 2015;12(1):5. doi: 10.1186/s12014-015-9077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paula ARJ, Lobo MDP, Monteiro-Moreira ACO, Moreira RA, Melo CHS, Souza-Fabjan JMG, et al. Proteomic analysis of follicular fluid from tropically-adapted goats. Anim Reprod Sci. 2018;188:35–44. doi: 10.1016/j.anireprosci.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Jarkovska K, Martinkova J, Liskova L, Halada P, Moos J, Rezabek K, et al. Proteome mining of human follicular fluid reveals a crucial role of complement cascade and key biological pathways in women undergoing in vitro fertilization. J Proteome Res. 2010;9(3):1289–1301. doi: 10.1021/pr900802u. [DOI] [PubMed] [Google Scholar]
- 54.de Agostini AI, Dong J-C, de Vantéry AC, Ramus M-A, Dentand-Quadri I, Thalmann S, et al. Human follicular fluid heparan sulfate contains abundant 3-O-sulfated chains with anticoagulant activity. J Biol Chem. 2008;283(42):28115–28124. doi: 10.1074/jbc.M805338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acosta T, Beg M, Ginther O. Aberrant blood flow area and plasma gonadotropin concentrations during the development of dominant-sized transitional anovulatory follicles in mares. Biol Reprod. 2004;71(2):637–642. doi: 10.1095/biolreprod.104.028498. [DOI] [PubMed] [Google Scholar]
- 56.Watson E, Al-Zi'abi M. Characterization of morphology and angiogenesis in follicles of mares during spring transition and the breeding season. Reproduction. 2002;124(2):227–234. [PubMed] [Google Scholar]
- 57.Anahory T, Dechaud H, Bennes R, Marin P, Lamb NJ, Laoudj D. Identification of new proteins in follicular fluid of mature human follicles. Electrophoresis. 2002;23(7–8):1197–1202. doi: 10.1002/1522-2683(200204)23:7/8<1197::AID-ELPS1197>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 58.Ebisch IM, Thomas CM, Wetzels AM, Willemsen WN, Sweep FC, Steegers-Theunissen RP. Review of the role of the plasminogen activator system and vascular endothelial growth factor in subfertility. Fertil Steril. 2008;90(6):2340–2350. doi: 10.1016/j.fertnstert.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Hanrieder J, Zuberovic A, Bergquist J. Surface modified capillary electrophoresis combined with in solution isoelectric focusing and MALDI-TOF/TOF MS: a gel-free multidimensional electrophoresis approach for proteomic profiling—exemplified on human follicular fluid. J Chromatogr A. 2009;1216(17):3621–3628. doi: 10.1016/j.chroma.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 60.Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 61.Silverman GA, Bird PI, Carrell RW, Coughlin PB, Gettins PG, Irving JI, et al. The serpins are an expanding superfamily of structurally similar but funtionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276(36):33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 62.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(D1):D447–DD52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldsack NR, Chambers RC, Dabbagh K, Laurent GJ. Molecules in focus thrombin. Int J Biochem Cell Biol. 1998;30(6):641–646. doi: 10.1016/s1357-2725(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 64.Roach LE, Petrik JJ, Plante L, LaMarre J, Gentry PA. Thrombin generation and presence of thrombin receptor in ovarian follicles. Biol Reprod. 2002;66(5):1350–1358. doi: 10.1095/biolreprod66.5.1350. [DOI] [PubMed] [Google Scholar]
- 65.Hofman Z, de Maat S, Hack CE, Maas C. Bradykinin: inflammatory product of the coagulation system. Clin Rev Allergy Immunol. 2016;51(2):152–161. doi: 10.1007/s12016-016-8540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellberg P, Larson L, Olofsson J, Hedin L, Brännström M. Stimulatory effects of bradykinin on the ovulatory process in the in vitro-perfused rat ovary. Biol Reprod. 1991;44(2):269–274. doi: 10.1095/biolreprod44.2.269. [DOI] [PubMed] [Google Scholar]
- 67.Brännström M, Hellberg P. Bradykinin potentiates LH-induced fofficular rupture in the rat ovary perfused in vitro. Hum Reprod. 1989;4(5):475–481. doi: 10.1093/oxfordjournals.humrep.a136930. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimura Y, Espey L, Hosoi Y, Adachi T, Atlas S, Ghodgaonkar R, et al. The effects of bradykinin on ovulation and prostaglandin production by the perfused rabbit ovary. Endocrinology. 1988;122(6):2540–2546. doi: 10.1210/endo-122-6-2540. [DOI] [PubMed] [Google Scholar]
- 69.Cao M, Nicola E, Portela VM, Price CA. Regulation of serine protease inhibitor-E2 and plasminogen activator expression and secretion by follicle stimulating hormone and growth factors in non-luteinizing bovine granulosa cells in vitro. Matrix Biol. 2006;25(6):342–354. doi: 10.1016/j.matbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Beers WH. Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell. 1975;6(3):379–386. doi: 10.1016/0092-8674(75)90187-7. [DOI] [PubMed] [Google Scholar]
- 71.Bianchi L, Gagliardi A, Campanella G, Landi C, Capaldo A, Carleo A, et al. A methodological and functional proteomic approach of human follicular fluid en route for oocyte quality evaluation. J Proteome. 2013;90:61–76. doi: 10.1016/j.jprot.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 72.von Otte S, Paletta JR, Becker S, König S, Fobker M, Greb RR, et al. Follicular fluid high density lipoprotein-associated sphingosine 1-phosphate is a novel mediator of ovarian angiogenesis. J Biol Chem. 2006;281(9):5398–5405. doi: 10.1074/jbc.M508759200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All seasonal proteome datasets, with full protein annotations. (ODS 1926 kb)
Data Availability Statement
All data generated and analyzed in this study are included in this published manuscript.