Abstract
Background
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) is a main concern in burn care centers worldwide. The some reports of MRSA in Iran suggested that MRSA with type SCCmec III is common among burn patients. The aim of this study was to determine the prevalence, virulence genes, and antimicrobial susceptibility of the direct repeat units (dru) types of MRSA with SCCmec IIIA isolated from burn wounds in a burn care center in Tehran, Iran.
Methods
In total, 165 S. aureus isolates were collected from clinical samples. In order to detect MRSA isolates, the mecA gene was amplified through the polymerase chain reaction (PCR) method. Antimicrobial susceptibility was tested using the disc agar diffusion test. Moreover, the PCR method was applied to determine SCCmec types, virulence genes, and antimicrobial resistance genes. The dru region was sequenced and thereby, dru types and dru repeats were identified. A similarity matrix was used to create minimum spanning tree (MST).
Results
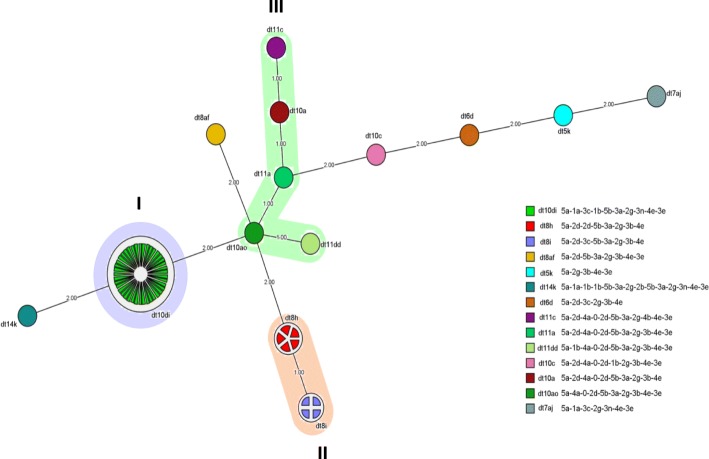
The prevalence of MRSA was 69% (114 out of 165 isolates). Most of MRSA isolates (61 out of 114, 53.5%) were SCCmec type IIIA. All MRSA isolates were vancomycin-susceptible and more than 68% of MRSA isolates with SCCmec type IIIA were mupirocin resistant. The successful dru typing of isolates with SCCmec type IIIA revealed fourteen different dru types. There were two new dru types, namely dt10di and dt7aj. MST analysis indicated the presence of the three clusters of dt10di (cluster I), dt8i-dt8 h (cluster II), and dt11c-dt10ao-dt11dd-dt11a-dt10a (cluster III). There were significant differences between clusters I and II respecting antimicrobial resistance pattern and virulence genes.
Conclusion
Three main dru clusters are prevalent in the study setting. The main dru types in the setting are dt10di, dt8i, and dt8 h. Dru typing can be used to differentiate MRSA strains with SCCmec IIIA.
Keywords: Methicillin-resistant Staphylococcus aureus, SCCmec typing, Dru type, Virulence factor
Background
Staphylococcus aureus (S. aureus) is one of the most common causes of infection among patients with severe burn wounds [1, 2]. Colonization of burn wounds with S. aureus can cause septicemia and substantially increase mortality rate [3]. The pathogenicity of S. aureus strains depends on different virulence factors such as Panton-Valentine leukocidin (PVL), staphylococcal enterotoxins, and hemolysins alpha, beta, gamma, and delta [4].
One of the major concerns in the treatment of infections is antimicrobial resistance, particularly to methicillin [5, 6]. Methicillin resistance is encoded by the mecA gene. In 2011, a second gene, mecC has been discovered that also causes methicillin/beta-lactam resistance. Both genes are situated on large, potentially mobile genetic elements, so-called SCCmec elements (staphylococcal cassette chromosome mec) [7, 8]. So far, thirteen main types of SCCmec have been identified [9]. These types differ from each other in size and genetic composition. Some studies reported that MRSA with SCCmec III is the most prevalent type of S. aureus in burn care centers in some countries of the world [2, 10–12]. The few reports of MRSA in Iran also suggested that MRSA with type SCCmec III is common among burn patients [13, 14]. mecC MRSA represent a recently recognised form of MRSA, encoding a divergent mec gene, which can colonise and cause disease in humans and a wide range of other host species [8].
A variable number of tandem repeats region including 40-bp of direct repeat units (dru) has been detected downstream to the mecA gene close to IS431 in the SCCmec element. The sequencing of this region can be used for detecting and subtyping methicillin-resistant S. aureus (MRSA) [15, 16]. A study reported that the dru typing of ST239 MRSA isolates provided the clearest distinction between SCCmec IIIA and III isolates [17]. Dru types are stable enough and hence, can be used in epidemiological analyses [16, 18].
Despite the high prevalence of MRSA with SCCmec III in burn care centers, there is limited information about its dru types. Therefore, the present study was conducted in a burn care center in Tehran, Iran, to determine the prevalence, virulence genes, and antimicrobial susceptibility of the dru types of MRSA with SCCmec IIIA isolated from burn wounds.
Materials and methods
Bacterial isolates
In total, 165 non-duplicate S. aureus isolates were collected using sterile swab from burn wound infections in a burn care center in Tehran, Iran. Sampling was done in four consecutive trimesters from June 2013 to June 2014. Isolates were primarily identified as S. aureus based on colony morphology, Gram staining, and catalase, coagulase, mannitol fermentation, and deoxyribonuclease tests [19]. Then, the identity of S. aureus isolates was confirmed through the amplification of the femA gene based on the polymerase chain reaction (PCR) method and using primers explained in an earlier work [20]. After that, in order to identify MRSA isolates, the mecA gene was detected using specific primers [21, 22]. Finally, MRSA isolates were subjected to further testing.
Antimicrobial susceptibility tests
The antimicrobial susceptibility of MRSA isolates was tested via the disc diffusion method on Mueller-Hinton agar based on the guideline recommended by the Clinical and Laboratory Standards Institute (CLSI) [23]. The discs used in this study were cotrimoxazole 25 μg, erythromycin 15 μg, clindamycin 2 μg, mupirocin 5 μg, rifampin 5 μg, linezolid 30 μg, and quinupristin-dalfopristin 15 μg (MAST, Merseyside, England). The microbroth dilution method was also used to determine the antimicrobial minimum inhibitory concentration (MIC) of oxacillin and vancomycin (Sigma, Steinheim, Germany). The control strain was S. aureus ATCC 29213. Moreover, the PCR method was employed to amplify the ermA, ermC, blaZ, and mupA genes using specific primers [24].
SCCmec typing and detection of virulence genes
SCCmec types were determined through the Multiplex-PCR as described elsewhere [21, 25]. The PCR method was used to detect the genes encoding haemolysins (hla, hlb), toxic shock syndrome toxin (tst), exfoliative toxin A (eta), staphylococcal enterotoxins (sea, seb and sec), and Panton–Valentine leukocidin (pvl) among isolates with SCCmec type III [22, 26, 27].
Dru typing
Dru region was detected using the primers HVR1:59 ACTATTCCCTCAGGCGTCC 39 and HVR2:59 GGAGTTAATCTACGTCTCATC 39 [28]. The sequencing of all PCR products was performed on both strands through the same primers used in the primary PCR. The ChromasPro software (Technelysium Pty, Australia) was employed to analyze and align sequences. New repeats were confirmed through re-sequencing. Then, the nomenclature published by Goering et al. [16] (available at www.dru-typing.org) was used to detect and name dru repeats (dr, 40 bps) and dru types (dt, the combination of dru repeats). A minimum spanning tree (MST) was also created via the BioNumerics software v. 7.6.1 (Applied Maths, Austin, USA) and distance intervals were created using a bin distance of 1.0%. Clustering was done based on the distances among dru types. Accordingly, dru types, separated by a single MST distance, were considered to be closely related to each other and hence, were assigned to an identical cluster.
Data analysis
Data were presented using the measures of descriptive statistics. Moreover, the Fisher’s exact test was conducted for categorical comparisons. The level of significance was set at less than 0.05.
Results
Among 165 S. aureus isolates, 114 (69%) were MRSA. Most of MRSA isolates (61/114; 53.5%) were SCCmec type IIIA. Also, twenty (17.5%) were identified as SCCmec type V, two (1.7%) as SCCmec type I, and 31 (27.2%) as non-typable.
All MRSA isolates showed susceptibility to vancomycin (MIC50 ≤ 1 μg/ml, MIC90 ≤ 2 μg/ml), while most MRSA isolates (68%) were resistant to mupirocin.
All MRSA isolates were resistant to cefoxitin and more than 73% of them were resistant to erythromycin and clindamycin. Moreover, around 53% of MRSA isolates were resistant to mupirocin and trimethoprim-sulfamethoxazole. However, only a few of MRSA isolates showed resistance to rifampin (22%), quinupristin/dalfopristin (2%), and linezolid (2%). The MIC of oxacillin in 100% of MRSA isolates was higher than 64 μg/mL and all of isolates were susceptible to vancomycin. The most prevalent antimicrobial resistance gene was blaZ which was found in 85% of isolates followed by ermA, mup and ermC which were found in 65, 64 and 57% of isolates, respectively. The most prevalent genes encoding virulence factors in MRSA isolates were hla (61%), hlb (44%), sea (23%) and seb (2%), respectively. The sec, eta, tst, and pvl genes were not detected in any of MRSA isolates in this study.
As Table 1 shows, all dru types of SCCmec type III were successfully identified, which included fourteen different dru types with fifteen dru repeats. Among the identified dru types, two were new (dt10di and dt7aj). The most prevalent dru types among SCCmec type IIIA isolates were dt10di, dt8 h, and dt8i. Each minor dru type was observed only in one isolate.
Table 1.
Antimicrobial resistance pattern, antibiotic resistance genes, virulence genes, and dru types in MRSA isolates with type III SCCmec
| Sampling Time | Sample no. | Dru type | Cluster | Antibiotic resistance | Antibiotic resistance genes | Virulence genes |
|---|---|---|---|---|---|---|
| First trimester | 1 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | sea |
| 2 | 10di | I | E, CD, TS, MUP | ermC, blaZ | hla, sea | |
| 3 | 11dd | III | E, CD, TS, SYN, MUP | ermA, mup | hla, hlb | |
| 4 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 5 | 7aj | – | E, CD, TS, MUP | ermA, ermC, blaZ | hla, sea | |
| 6 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 7 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 8 | 14 k | – | E, CD, TS, MUP | ermC, blaZ | sea | |
| 9 | 11c | III | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 10 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | hla, hlb, seb | |
| 11 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | hla, seb | |
| 12 | 11a | III | E, TS, MUP | blaZ, mup | – | |
| 13 | 5 k | – | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 14 | 10di | I | E, CD, TS, MUP | ermA, blaZ, mup | sea | |
| 15 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 16 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | – | |
| 17 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| Second trimester | 18 | 10di | I | E, CD, TS, MUP | ermC | – |
| 19 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 20 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 21 | 10di | I | E, CD, TS, MUP | blaZ | sea | |
| 22 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 23 | 10di | I | E, CD, TS, MUP | ermA, ermC, mup | hla, sea | |
| 24 | 10di | I | E, CD, TS, MUP | ermC, blaZ | hla, sea | |
| 25 | 10di | I | E, CD, TS, MUP | ermA, blaZ | Sea, seb | |
| 26 | 10ao | III | E, CD, MUP | ermA, ermC, mup | – | |
| 27 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, hlb, sea | |
| 28 | 10di | I | SYN, MUP | ermC, blaZ, mup | sea | |
| 29 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 30 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla | |
| 31 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | sea | |
| 32 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 33 | 8af | – | E, CD, RP, MUP | ermC, blaZ, mup | – | |
| 34 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| Third trimester | 35 | 10di | I | E, CD, TS, MUP | ermC, blaZ | – |
| 36 | 10di | I | E, CD, TS, MUP | ermA, ermC, mup | – | |
| 37 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 38 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, hlb, sea | |
| 39 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | hla, hlb, sea | |
| 40 | 10a | III | MUP | ermA, ermC, blaZ, mup | hla | |
| 41 | 10di | I | E, CD, TS, MUP | ermC, blaZ, mup | seb | |
| 42 | 10di | I | E, CD, TS, SYN, LZD, RP, MUP | ermA, ermC, blaZ, mup | – | |
| 43 | 10di | I | E, CD, TS, MUP | ermA, ermC | sea | |
| 44 | 10di | I | E, CD, TS, MUP | ermA, ermC | – | |
| 45 | 10di | I | E, CD, TS | ermA, ermC, blaZ, mup | hla, hlb | |
| 46 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | hla, sea | |
| 47 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ | hla, sea | |
| 48 | 10di | I | E, CD, TS, MUP | mup | – | |
| 49 | 10c | – | E | blaZ | sea | |
| Forth trimester | 50 | 8i | II | E, TS, MUP | ermA, ermC, blaZ, mup | hla, hlb |
| 51 | 8 h | II | – | blaZ, mup | hla | |
| 52 | 6d | – | – | ermA, blaZ, mup | hla, hlb | |
| 53 | 8i | II | CD | ermA, ermC, mup | hla, hlb | |
| 54 | 8i | II | E, CD, TS, MUP | ermA, ermC, blaZ | hla, hlb, sea | |
| 55 | 8 h | II | E, TS, RP, MUP | blaZ | hlb, sea | |
| 56 | 8 h | II | E, CD, TS, RP, MUP | ermA, ermC, blaZ, mup | sea | |
| 57 | 8 h | II | E, CD, TS, RP, MUP | ermC | – | |
| 58 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 59 | 8i | II | E, CD, TS, RP, MUP | ermA, ermC, blaZ | hla, hlb | |
| 60 | 10di | I | E, CD, TS, MUP | ermA, ermC, blaZ, mup | – | |
| 61 | 8 h | II | E, CD, TS, RP, MUP | ermC | – |
FOX: Cefoxitin, E: Erythromycin, CD: Clindamycin, TS: trimethoprim/ Sulfamethoxazole, RP: Rifampicin,
SYN: quinupristin/dalfopristin, LZD: Linezolid, MUP: Mupirocin
MST analysis revealed three clusters of SCCmec type IIIA, namely dt10di (cluster I), dt8i-dt8 h (cluster II), and dt11c-dt10ao-dt11dd-dt11a-dt10a (cluster III) (Fig. 1). Analysis of dru types indicated that cluster I was the most prevalent dru cluster in the first nine months of the study (i.e. from June 2013 to February 2014), while cluster II was the most prevalent cluster in the last trimester of the study (i.e. from March to May 2014) (Table 1).
Fig. 1.
Minimum Spanning Tree generated using the BioNumerics software representing the fourteen dru types and the fifteen dru repeats observed in the studied isolates. Numerical values on the branches indicate the similarities (MST distance) between different dru types. BioNumerics software created similarity values (termed bins) and converted these values into distance units. The bin unit distance was set at 1% (i.e., dru types at a distance of 1 on the MST had a similarity of more than 99%, while dru types at a distance of 2 had a similarity of 98–99%, and so on). Dru types were assigned to the same cluster (depicted in color) if they were separated by an MST distance of 1 (i.e. if they showed a similarity of at least 99%)
The results of antimicrobial susceptibility tests on clusters I and II indicated that resistance to rifampin in cluster II was significantly higher than cluster I (P = 0.003). Moreover, the results of virulence gene analysis illustrated the significantly higher prevalence of the virulence gene hlb in cluster II compared with cluster I (P = 0.01).
Analysis of antimicrobial susceptibility pattern indicated that 92% of isolates in cluster I were resistant to erythromycin, clindamycin, cotrimoxazole, and mupirocin. On the other hand, 44% of isolates in cluster II showed resistance to erythromycin, clindamycin, cotrimoxazole, rifampin, and mupirocin.
Analysis of the patterns of antimicrobial resistance gene also showed that the most prevalent patterns in clusters I and II were ermA + ermC + blaZ + mup and ermA + ermC + blaZ, respectively. Moreover, the greatest frequency of virulence gene patterns in these two clusters was respectively related to hla + sea and hla + hlb. However, the sec, eta, tst, and pvl genes were detected in none of the MRSA isolates.
Discussion
This study aimed to determine the prevalence, virulence genes, and antimicrobial susceptibility of the dru types of MRSA with SCCmec III isolated from burn wounds in a burn care center in Tehran, Iran. Study results illustrated that the prevalence of MRSA among patients with burn wounds was 69%. This prevalence rate is higher than the rates reported in earlier studies in Iran [20, 29, 30], except for a study in burn centers in Ahvaz which reported a prevalence rate of 80% [31]. Moreover, the prevalence of MRSA in the present study was higher than the rates reported in burn centers in the United States (32%), European countries (26%), and Australia (23%) [32–34]. This difference can be attributed to the differences in infection control policies used in different areas.
Our study also showed that a high proportion of MRSA isolates harbored SCCmec type IIIA. Similarly, studies in Iran and other Asian countries reported the high prevalence of SCCmec type IIIA [13, 35–37]. SCCmec types III and IIIA were also detected in Hungarian and Brazilian clones [36]. MRSA isolates with SCCmec types III can act as large reservoirs of enterotoxins and antimicrobial resistance and prevail in communities. Our previous survey shown that the sea, hla, fib and icaA were most frequent genes encoding virulence factors among MRSA with SCCmec type IIIA [4]. Therefore, accurate diagnosis and effective control strategies are essential to minimize their prevalence. Otherwise, they may become prevalent and cause serious consequences in a near future.
None of the MRSA isolates in the present study were resistant to vancomycin. Although MRSA non-resistance to vancomycin in our study supports the effectiveness of this antibiotic in managing MRSA, this antibiotic should be prescribed with great caution in order to prevent the emergence of vancomycin-resistant S. aureus.
The prevalence of MRSA resistance to mupirocin in the present study was 68%. However, earlier studies reported lower rates of MRSA resistance to mupirocin. For instance, studies on burn patients in England, India, and Iran reported that this rate was 5.1, 22.7, and 34%, respectively [31, 38, 39]. The use of mupirocin for treating burn wound infections caused by S. aureus might have caused resistance to mupirocin among MRSA isolates. Previous studies in the setting of the present study showed Pseudomonas aeruginosa as one the main causes of burn wound infection in recent years [40, 41]. Of course, there are no detailed data about the use of mupirocin in the study setting. However, a study reported a substantially high prevalence of mupirocin-resistant MRSA among patients previously treated with mupirocin. Similarly, a high likelihood of mupirocin resistance was observed among patients with Pseudomonas infection treated with cefepime [42]. Mupirocin is produced by the Gram-negative bacterium Pseudomonas fluorescens and hence, Pseudomonas is inherently resistant to mupirocin [43, 44]. Furthermore, the mupA gene, which mediates mupirocin resistance in Pseudomonas, can move between bacterial isolates and thereby, cause mupirocin resistance in other isolates such as MRSA [45–47].
Our results also indicated dt10di, dt8 h, and dt8i as the most prevalent dru types in MRSA with SCCmec type IIIA (Fig. 1). The dru type dt10di (i.e. cluster I) was mostly prevalent in the first nine months of the study, while the dru types dt8 h and dt8i (i.e. cluster II) were mostly prevalent in the last trimester. These findings may be due to the fact that the dru types dt8 h and dt8i might have been entered to the study setting in the last trimester or might have been emerged as a result of the polymorphism of dt10ao dru type. Unlike our results, a study in Malaysia reported nine dru types in SCCmec type III, the most prevalent of which was the dt13d dru type (41%) [17]. Moreover, a study in Scotland detected 25 dru types and 33 dru repeats, with the dt10a and dt7c as the most prevalent dru types, respectively [16]. The greater number of dru types and dru repeats in that study compared to our study can be due to the fact that samples in the present study were selected from a single hospital, while samples in that study were selected from different hospitals.
The results of the present study also indicated the higher prevalence of cluster II in the last three months of the study. This may denote the increasing prevalence of this cluster. Moreover, compared with cluster I, cluster II had higher resistance to rifampin. Besides, the presence of the hlb gene was more prevalent in cluster II. The most prevalent antimicrobial resistance pattern in cluster I was erythromycin+clindamycin+cotrimoxazole+mupirocin, while the most prevalent antimicrobial resistance pattern in cluster II was erythromycin+clindamycin+cotrimoxazole+rifampin+mupirocin. The latter finding confirms the higher resistance to rifampin in cluster II. Of course, the number of samples in cluster II was small and hence, further studies with larger samples are recommended.
Virulence gene patterns in clusters and dru types in the present study showed that the most prevalent virulence gene patterns in cluster I were hla + sea (39%) and sea (12%), while the most prevalent virulence gene pattern in cluster II was hla + hlb (33.3%) (Data were not presented). Besides, hla + hlb virulence genes in the dt8i dru type were more prevalent than the other dru types. Considering the significant roles of these genes in exacerbating skin infections, the prevalence of these strains can complicate the conditions of patients with burn wound infections. Of course, because of the small sample size in cluster II, drawing definitive conclusions in this area is impossible.
Conclusion
This study shows the high prevalence of SCCmec IIIA among MRSA strains isolated from burn wounds in a teaching hospital in Tehran, Iran. These strains are highly resistant to multiple antibiotics. The three most common dru types among these strains are dt10i, dt8 h, and dt8i. Clusters with these dru types significantly differ from each other respecting their antimicrobial resistance patterns.
Acknowledgements
We would like to thank Ms. Mahboobeh Sattarzadeh-Tabrizi, who assisted us with specimen collection. This research has been supported by Tehran University of Medical Sciences & health Services grant 97-01-30/38035.
Funding
This research has been supported by Tehran University of Medical Sciences & health Services (grant code: 97–01-30/38035).
Availability of data and materials
Please contact author for data requests.
Abbreviations
- ATCC
American Type Culture Collection
- bp
base pair
- CD
Clindamycin
- CLSI
Clinical and Laboratory Standards Institute
- DAD
Disk Agar Diffusion
- dru
direct repeat unit
- dt
dru type
- E
Erythromycin
- eta
exfoliative toxin A
- LZD
Linezolid
- MIC
Minimum Inhibitory Concentration
- MRSA
Methicillin-Resistant Staphylococcus aureus
- MST
Minimum Spanning Tree
- MUP
Mupirocin
- PCR
Polymerase Chain Reaction
- pvl
Panton–Valentine leukocidin
- RP
Rifampicin
- S. aureus
Staphylococcus aureus
- SCCmec
Staphylococcal Cassette Chromosome mec
- SYN
quinupristin/dalfopristin
- TS
trimethoprim/ Sulfamethoxazole
- tst
toxic shock syndrome toxin
- VRSA
Vancomycin-Resistant Staphylococcus aureus
Authors’ contributions
ME and FJ designed the study. MM and ME drafted the manuscript. RB performed data analysis. All authors provided intellectual input to the study and read and approved the final manuscript.
Ethics approval and consent to participate
This study has the formal approval of the Research Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (approval number: IR.TUMS.MEDICINE.REC.1397.235).
Consent for publication
Not Applicable.
Competing interest
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitra Motallebi, Email: motallebi.mitra@yahoo.com.
Fereshteh Jabalameli, Email: jabalamf@tums.ac.ir.
Reza Beigverdi, Email: r-beigverdi@tums.ac.ir.
Mohammad Emaneini, Email: emaneini@gmail.com.
References
- 1.Emaneini M, Beigverdi R, van Leeuwen WB, Rahdar H, Karami-Zarandi M, Hosseinkhani F, et al. Prevalence of methicillin-resistant Staphylococcus aureus isolated from burn patients in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist Netherlands. 2018;12:202–206. doi: 10.1016/j.jgar.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues MVP, Fortaleza CMCB, Riboli DFM, Rocha RS, Rocha C, da Cunha M de LR de S. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a burn unit from Brazil. Burns. Netherlands; 2013 Sep;39(6):1242–1249. [DOI] [PubMed]
- 3.Toscano Olivo TE, de Melo EC, Rocha C, Fortaleza CMCB. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus among patients from a burn unit in Brazil. Burns. Netherlands. 2009;35(8):1104–1111. doi: 10.1016/j.burns.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Motallebi M, Jabalameli F, Asadollahi K, Taherikalani M, Emaneini M. Spreading of genes encoding enterotoxins, haemolysins, adhesin and biofilm among methicillin resistant Staphylococcus aureus strains with staphylococcal cassette chromosome mec type IIIA isolated from burn patients. Microb Pathog. England. 2016;97:34–37. doi: 10.1016/j.micpath.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Shahsavan S, Emaneini M, Noorazar Khoshgnab B, Khoramian B, Asadollahi P, Aligholi M, et al. A high prevalence of mupirocin and macrolide resistance determinant among Staphylococcus aureus strains isolated from burnt patients. Burns. Netherlands. 2012;38(3):378–382. doi: 10.1016/j.burns.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini SS, Niakan M, Saderi H, Motallebi M, Taherikalani M, Asadollahi K, et al. Frequency of genes encoding erythromycin ribosomal methylases among Staphylococcus aureus clinical isolates with different D-phenotypes in Tehran. Iran Iran J Microbiol Iran. 2016;8(3):161–167. [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu K, Ito T, Tsubakishita S, Sasaki T, Takeuchi F, Morimoto Y, et al. Genomic Basis for Methicillin Resistance in Staphylococcus aureus. Infect Chemother. Korea (South); 2013 Jun;45(2):117–36. [DOI] [PMC free article] [PubMed]
- 8.Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22(1):42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig S, Johannesen T, Overballe-Petersen S, Larsen J, Larsen AR, Stegger M. Novel SCC mec type XIII (9A) identified in an ST152 methicillin-resistant Staphylococcus aureus. Infect Genet Evol. 2018;1:61. doi: 10.1016/j.meegid.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Asghar AH. Molecular characterization of methicillin-resistant Staphylococcus aureus isolated from tertiary care hospitals. Pakistan J Med Sci Pakistan. 2014;30(4):698–702. doi: 10.12669/pjms.304.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, et al. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. United States. 2006;50(3):1001–1012. doi: 10.1128/AAC.50.3.1001-1012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs PC, Kopp J, Hafner H, Kleiner U, Pallua N. MRSA-retrospective analysis of an outbreak in the burn Centre Aachen. Burns. Netherlands. 2002;28(6):575–578. doi: 10.1016/S0305-4179(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 13.Namvar AE, Afshar M, Asghari B, Rastegar LA. Characterisation of SCCmec elements in methicillin-resistant Staphylococcus aureus isolated from burn patients. Burns. Netherlands. 2014;40(4):708–712. doi: 10.1016/j.burns.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Parhizgari N, Khoramrooz SS, Malek Hosseini SAA, Marashifard M, Yazdanpanah M, Emaneini M, et al. High frequency of multidrug-resistant Staphylococcus aureus with SCCmec type III and Spa types t037 and t631 isolated from burn patients in southwest of Iran. APMIS Denmark. 2016;124(3):221–228. doi: 10.1111/apm.12493. [DOI] [PubMed] [Google Scholar]
- 15.Ryffel C, Bucher R, Kayser FH, Berger-Bachi B. The Staphylococcus aureus mec determinant comprises an unusual cluster of direct repeats and codes for a gene product similar to the Escherichia coli sn-glycerophosphoryl diester phosphodiesterase. J Bacteriol United States. 1991;173(23):7416–7422. doi: 10.1128/jb.173.23.7416-7422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goering RV, Morrison D, Al-Doori Z, Edwards GFS, Gemmell CG. Usefulness of mec-associated direct repeat unit (dru) typing in the epidemiological analysis of highly clonal methicillin-resistant Staphylococcus aureus in Scotland. Clin Microbiol Infect England. 2008;14(10):964–969. doi: 10.1111/j.1469-0691.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghaznavi-Rad E, Goering RV, Nor Shamsudin M, Weng PL, Sekawi Z, Tavakol M, et al. Mec-associated dru typing in the epidemiological analysis of ST239 MRSA in Malaysia. Eur J Clin Microbiol infect dis. Germany. 2011;30(11):1365–1369. doi: 10.1007/s10096-011-1230-1. [DOI] [PubMed] [Google Scholar]
- 18.Bartels MD, Boye K, Oliveira DC, Worning P, Goering R, Westh H. Associations between dru types and SCCmec cassettes. PLoS One United States. 2013;8(4):e61860. doi: 10.1371/journal.pone.0061860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connie R. Mahon, Donald C. Lehman GMJ. Textbook of Diagnostic Microbiology. 5th Edition. Saunders; 2014.
- 20.Fatholahzadeh B, Emaneini M, Gilbert G, Udo E, Aligholi M, Modarressi MH, et al. Staphylococcal cassette chromosome mec (SCCmec) analysis and antimicrobial susceptibility patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolates in Tehran. Iran Microb Drug Resist United States. 2008;14(3):217–220. doi: 10.1089/mdr.2008.0822. [DOI] [PubMed] [Google Scholar]
- 21.Soroush S, Jabalameli F, Taherikalani M, Amirmozafari N, Fooladi AAI, Asadollahi K, et al. Investigation of biofilm formation ability, antimicrobial resistance and the staphylococcal cassette chromosome mec patterns of methicillin resistant Staphylococcus epidermidis with different sequence types isolated from children. Microb Pathog. England. 2016;93:126–130. doi: 10.1016/j.micpath.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Emaneini M, Jabalameli L, Iman-Eini H, Aligholi M, Ghasemi A, Nakhjavani FA, et al. Multiple-locus variable number of tandem repeats fingerprinting (MLVF) and virulence factor analysis of methicillin resistant Staphylococcus aureus SCCmec type III. Polish J Microbiol Poland. 2011;60(4):303–307. [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute (CLSI).Available online: https://clsi.org/standards/products/microbiology/ (accessed on 10 September 2017). 2017.
- 24.Emaneini M, Bigverdi R, Kalantar D, Soroush S, Jabalameli F, Noorazar Khoshgnab B, et al. Distribution of genes encoding tetracycline resistance and aminoglycoside modifying enzymes in Staphylococcus aureus strains isolated from a burn center. Ann Burns Fire Disasters Italy. 2013;26(2):76–80. [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Kuwahara-Arai K, Katayama Y, Uehara Y, Han X, Kondo Y, et al. Staphylococcal cassette chromosome mec (SCCmec) analysis of MRSA. Methods Mol Biol United States. 2014;1085:131–148. doi: 10.1007/978-1-62703-664-1_8. [DOI] [PubMed] [Google Scholar]
- 26.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun United States. 2002;70(2):631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrotra M, Wang G, Johnson WM. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol. United States. 2000;38(3):1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi J, Miyanohara H, Nakajima T, Kitajima I, Yoshinaga M, Maruyama I, et al. Molecular typing of the methicillin resistance determinant (mec) of clinical strains of Staphylococcus based on mec hypervariable region length polymorphisms. J Lab Clin Med United States. 1995;126(1):29–35. [PubMed] [Google Scholar]
- 29.Hoseini Alfatemi SM, Motamedifar M, Hadi N, Sedigh Ebrahim Saraie H. Analysis of Virulence Genes Among Methicillin Resistant Staphylococcus aureus (MRSA) Strains. Jundishapur J Microbiol. Iran; 2014 Jun;7(6):e10741. [DOI] [PMC free article] [PubMed]
- 30.Askari E, Soleymani F, Arianpoor A, Tabatabai SM, Amini A, Naderinasab M. Epidemiology of mecA-methicillin resistant Staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. Iran J Basic Med Sci. 2012;15(5):1010–1019. [PMC free article] [PubMed] [Google Scholar]
- 31.Abbasi-Montazeri E, Khosravi AD, Feizabadi MM, Goodarzi H, Khoramrooz SS, Mirzaii M, et al. The prevalence of methicillin resistant Staphylococcus aureus (MRSA) isolates with high-level mupirocin resistance from patients and personnel in a burn center. Burns Netherlands. 2013;39(4):650–654. doi: 10.1016/j.burns.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Guggenheim M, Zbinden R, Handschin AE, Gohritz A, Altintas MA, Giovanoli P. Changes in bacterial isolates from burn wounds and their antibiograms: a 20-year study (1986-2005) Burns. Netherlands. 2009;35(4):553–560. doi: 10.1016/j.burns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Hodle AE, Richter KP, Thompson RM. Infection control practices in U.S. burn units. J burn care res. England. 2006;27(2):142–151. doi: 10.1097/01.BCR.0000203493.31642.79. [DOI] [PubMed] [Google Scholar]
- 34.Nimmo GR, Pearson JC, Collignon PJ, Christiansen KJ, Coombs GW, Bell JM, et al. Antimicrobial susceptibility of Staphylococcus aureus isolated from hospital inpatients, 2009: report from the Australian group on antimicrobial resistance. Commun Dis Intell Q Rep Australia. 2011;35(3):237–243. doi: 10.33321/cdi.2011.35.22. [DOI] [PubMed] [Google Scholar]
- 35.Aires de Sousa M, Crisostomo MI, Sanches IS, Wu JS, Fuzhong J, Tomasz A, et al. Frequent recovery of a single clonal type of multidrug-resistant Staphylococcus aureus from patients in two hospitals in Taiwan and China. J Clin Microbiol. United States; 2003 Jan;41(1):159–63. [DOI] [PMC free article] [PubMed]
- 36.Arakere G, Nadig S, Swedberg G, Macaden R, Amarnath SK, Raghunath D. Genotyping of methicillin-resistant Staphylococcus aureus strains from two hospitals in Bangalore, South India. J Clin Microbiol United States. 2005;43(7):3198–3202. doi: 10.1128/JCM.43.7.3198-3202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cirlan M, Saad M, Coman G, Bilal NE, Elbashier AM, Kreft D, et al. International spread of major clones of methicillin resistant Staphylococcus aureus: nosocomial endemicity of multi locus sequence type 239 in Saudi Arabia and Romania. Infect Genet Evol Netherlands. 2005;5(4):335–339. doi: 10.1016/j.meegid.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan PU, Miles K, Shetty N. Detection of methicillin and mupirocin resistance in Staphylococcus aureus isolates using conventional and molecular methods: a descriptive study from a burns unit with high prevalence of MRSA. J Clin Pathol England. 2002;55(10):745–748. doi: 10.1136/jcp.55.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudresh MS, Ravi GS, Motagi A, Alex AM, Sandhya P, Navaneeth BV. Prevalence of mupirocin resistance among staphylococci, its clinical significance and relationship to clinical use. J Lab Physicians India. 2015;7(2):103–107. doi: 10.4103/0974-2727.163127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidari H, Emaneini M, Dabiri H, Jabalameli F. Virulence factors, antimicrobial resistance pattern and molecular analysis of Enterococcal strains isolated from burn patients. Microb Pathog England. 2016;90:93–97. doi: 10.1016/j.micpath.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Salimi F, Eftekhar F. Prevalence of blaIMP, and blaVIM gene carriage in metallo-beta-lactamase-producing burn isolates of Pseudomonas aeruginosa in Tehran. Turkish J Med Sci Turkey. 2014;44(3):511–514. doi: 10.3906/sag-1302-67. [DOI] [PubMed] [Google Scholar]
- 42.Caffrey AR, Quilliam BJ, LaPlante KL. Risk factors associated with mupirocin resistance in meticillin-resistant Staphylococcus aureus. J Hosp Infect England. 2010;76(3):206–210. doi: 10.1016/j.jhin.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Yanagisawa T, Kawakami M. How does Pseudomonas fluorescens avoid suicide from its antibiotic pseudomonic acid?: evidence for two evolutionarily distinct isoleucyl-tRNA synthetases conferring self-defense. J Biol Chem United States. 2003;278(28):25887–25894. doi: 10.1074/jbc.M302633200. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. United States. 1985;27(4):495–498. doi: 10.1128/AAC.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis United States. 2009;49(6):935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 46.Ramsey MA, Bradley SF, Kauffman CA, Morton TM. Identification of chromosomal location of mupA gene, encoding low-level mupirocin resistance in staphylococcal isolates. Antimicrob Agents Chemother. United States. 1996;40(12):2820–2823. doi: 10.1128/AAC.40.12.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driscoll DG, Young CL, Ochsner UA. Transient loss of high-level mupirocin resistance in Staphylococcus aureus due to MupA polymorphism. Antimicrob Agents Chemother United States. 2007;51(6):2247–2248. doi: 10.1128/AAC.00241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.