Abstract
Lactic acid bacteria (LAB) and butyric acid bacteria (BAB) are commonly used as probiotics in swine production. However, their combined effect on post-weaning pigs has not been assessed. Therefore, here we investigated the individual and combined efficacy of dietary Enterococcus faecium and Clostridium butyricum on the growth and gut microbiota of post-weaning pigs at a commercial farm. Four independent trials were conducted, in each of which five pens containing 10 pigs were assigned to one of five treatments: C, basal diet; L, basal diet + live E. faecium; D, basal diet + heat-killed E. faecium; M, basal diet + C. butyricum; or L+M, basal diet + live E. faecium + C. butyricum. Each trial was conducted over a 90-day period that was divided into two phases (Phase 1, days 0–40 post-weaning; and Phase 2, days 40–90 post-weaning), with the probiotics being supplemented only during Phase 1. Ten pigs in each pen were used for body weight (BW) analysis and fecal samples were collected from five or six of these pigs. In addition, the fecal samples from one randomly selected trial were used for gut microbiota analysis. We found that pigs in the L, D, and L+M treatment groups had a significantly higher BW than those in C (p < 0.05) but pigs in the L+M treatment group had a similar BW to those in the L and M groups. Furthermore, there were no significant differences in alpha diversity among the treatments but the beta diversity (weighted UniFrac distances) showed distinct clustering patterns, with pigs in C having discrete microbiota from those in all of the probiotics treatment groups except D (C vs. L, q = 0.04; C vs. M, q = 0.06; C vs. L+M, q = 0.06). These findings indicate that dietary supplementation with live or heat-killed E. faecium enhances growth performance in pigs but there is no synergistic effect when E. faecium is used in combination with C. butyricum. Furthermore, the addition of live E. faecium and C. butyricum to the diet of pigs may change the structure of the gut microbiota.
Keywords: body weight, Clostridium butyricum, Enterococcus faecium, gut microbiota, probiotics, swine
Introduction
Antibiotics are often added to the diets of pigs to reduce the occurrence of diseases such as diarrhea and to improve growth performance. However, there is growing concern about the risk of the emergence of antibiotic-resistant bacteria and the residual effects of antibiotics in meat products. Subsequently, the subtherapeutic use of antibiotics as growth promoters has been banned in Europe since 2006 (Council Regulation EC 70/524/ EEC). Therefore, there has been increased interest in finding a suitable alternative to antibiotics, with probiotics receiving considerable attention.
Lactic acid bacteria (LAB), such as Lactobacillus, Bacillus, and Enterococcus, are among the most commonly used probiotics for pigs. Many studies have shown that the use of Enterococcus faecium has a positive effect on the health and performance of pigs. For example, Mallo et al. (1) reported that dietary supplementation with 106 CFU/g E. faecium improved the gut microbiota balance, growth, and feed conversion of piglets, and Zhao et al. (2) demonstrated that the use of E. faecium improved growth performance in weanling pigs. However, some studies have yielded inconsistent results–for example, Broom et al. (3) showed that supplementation of the post-weaning diet with E. faecium did not affect growth performance or gastrointestinal bacterial populations in piglets. Therefore, it is likely that the effect of dietary E. faecium in pigs depends on the diet composition, diversity of bacterial species and strains, as well as environmental factors.
Butyric acid bacteria (BAB), such as Clostridium, have also been considered as possible candidates for use as probiotics in swine production. Clostridium butyricum is a butyric acid-producing, Gram-positive anaerobe found in soil and in the intestines of healthy animals and humans (4) that has been shown to have beneficial effects on animal health and performance, and it is commonly used as a feed additive in Asia and Europe. Furthermore, Takahashi et al. (5) recently reported that the use of C. butyricum may improve the zootechnical performance of weaned piglets.
Enterococcus faecium and C. butyricum have been widely used for animal production in Japan. Enterococcus is facultative anaerobic bacteria, while Clostridium is obligate anaerobe bacteria. Due to the characteristics, the two bacteria grow within different sites of the gut. Enterococcus can form biofilm microcolonies throughout the gastrointestinal tract (6), but Clostridium mainly grow in the distal intestine, cecum and colon (7). Due to their differing characteristics, the two bacteria do not compete for nutrients as they have different ecological niches and it is expected that the combined use of these probiotics would have highly synergistic effects. However, there have been no reports on the combined effects of LAB and BAB on post-weaning pigs. Therefore, the aim of this study was to investigate the efficacy of dietary E. faecium and C. butyricum, both separately and in combination, on the growth and gut microbiota of post-weaning pigs at a commercial farm.
Materials and Methods
Probiotics
Four probiotic powder products (Products 1–4) containing live or heat-killed E. faecium strain NHRD IHARA (EFNH), or live C. butyricum strain MIYAIRI 588 (CBM588) were manufactured by a commercial company (Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan). The concentrations of probiotics in the products are shown in Table 1.
Table 1.
Concentrations of Enterococcus faecium and Clostridium butyricum in the probiotic powder products.
| Probiotic | Product 1 | Product 2 | Product 3 | Product 4 |
|---|---|---|---|---|
| Live E. faecium NHRD IHARA (cfu/g) | 1.2 × 109 | – | – | 1.2 × 109 |
| Heat-killed E. faecium NHRD IHARA (cells/g) | – | 1.1 × 109 | – | – |
| Live C. butyricum MIYAIRI 588 (cfu/g) | – | – | 9.6 × 107 | 2.0 × 108 |
Study Design
This study was conducted at a commercial farm in Japan. A total of 200 pigs (Landrace × Large White × Duroc) with initial age of 30.0 ± 1.0 days old were used in the experiment, which involved four independent trials. All trials were started at the first days after weaning of the pigs. In each trial, 50 pigs were housed in five pens at a density of 10 pigs per pen and each pen was randomly assigned to one of five treatment groups: C, basal diet without probiotics; L, basal diet supplemented with 1 g/kg of Product 1 containing live E. faecium; D, basal diet supplemented with 1 g/kg of Product 2 containing heat-killed E. faecium; M, basal diet supplemented with 1 g/kg of Product 3 containing live C. butyricum; and L+M, basal diet supplemented with 1 g/kg of Product 4 containing live E. faecium + C. butyricum. Each trial was conducted over a 90-day period that was divided into two phases (Phase 1, days 0–40 post-weaning; and Phase 2, days 40–90 post-weaning), with the probiotics being supplemented only during Phase 1. Phase 2 was designed to investigate whether effects of the probiotics were sustained or not. The pigs were fed diets A or B, which were for growing pigs and for adult pigs, respectively (Table 2), and as a basal diet during Phases 1 and 2, respectively. These diets and water were offered ad libitum. The experiments were exempted from ethic evaluations because all animals were commercially raised and limited to body weight and microbiological evaluations.
Table 2.
Chemical compositions (%) of diets A and B.
| Diet A | Diet B | |
|---|---|---|
| Crude protein | 18.0 | 14.0 |
| Ether extract | 3.0 | 2.5 |
| Crude fiber | 5.0 | 4.5 |
| Crude ash | 8.0 | 7.5 |
| Calcium | 0.55 | 0.55 |
| Phosphorus | 0.40 | 0.45 |
| Total digestible nutrients | 80.0 | 78.0 |
Sample Collection
In each trial, 10 pigs in each pen were used for body weight (BW) analysis. The BW of each animal was measured on the first day of Phase 1 (T1) and on the final days of Phases 1 and 2 (T2, and T3, respectively). Since the weighed pigs were not of the same age, the BW on T2 and T3 were estimated from the measured values by linear approximation, as previously described (8). In addition, fecal samples were collected from five or six of the weighed pigs in each pen on T1, T2, and T3, lyophilized, and stored at −20°C until further analysis.
DNA Extraction
The fecal samples from one randomly selected trial were used to analyze the gut microbiota in each treatment group. This included 30 samples on T1 (n = 6 for each treatment group) and 29 samples on T2 and T3 (n = 5 for C; n = 6 for all other groups). Bacterial DNA was extracted from the lyophilized feces following a previously described procedure (9, 10) with modification (8). Briefly, fecal samples (20 mg) were washed three times in Tris-EDTA (ethylenediaminetetraacetic acid) buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA; pH 8.0). Subsequently, the samples were resuspended in a solution containing 500 μL of Tris-EDTA buffer and 100 μL of 10% sodium dodecyl sulfate. Glass beads (300 mg; diameter, 0.1 mm) and 600 μL of buffer-saturated phenol were added. Then the mixture was vortexed for 10 s using Micro Smash MS-100R (TOMY SEICO CO., LTD, Tokyo, Japan) followed by incubation at 65°C for 10 min. This step was repeated twice. After the step, 550 μL of the supernatant was subjected to isopropanol precipitation. The extracted DNA was suspended in 200 μL of Tris-EDTA buffer. Subsequently the DNA was purified using a High Pure PCR Template Kit (Roche Inc., Basel, Switzerland) according to the manufacture's instruction. Finally the DNA was suspended in 50 μL elution buffer and stored at −20°C until further analysis.
16S rRNA Gene Amplification and High-Throughput Sequencing
The extracted DNA was amplified using the specific primer pair 341F/805R [341F: 5′-CCTACGGGNGGCWGCAG-3′; 805R: 5′-GACTACHVGGGTATCTAATCC-3′; (11)], which targets the V3–V4 region of the 16S rRNA bacterial gene. This primer set contained the Illumina MiSeq sequencing adapter (forward primer: AATGATACGGCGACCACCGAGATCTACAC; reverse primer: CAAGCAGAAGACGGCATACGAGAT) and a unique barcode sequence that allowed all of the samples to be pooled for Illumina MiSeq sequencing. Each 50 μL of polymerase chain reaction (PCR) mixture contained 1 μL of sample DNA, 21 μL of MilliQ® water, 25 μL of 2X MightyAmp® Buffer, 1 μL of forward primer (5 μM), 1 μL of reverse primer (5 μM), and 1 μL (1.25 U) of MightyAmp DNA Polymerase (Takara, Tokyo, Japan). The following thermal cycling conditions were used: initial denaturation at 98°C for 2 min, followed by 35 cycles at 95°C for 10 s, 60°C for 15 s, and 68°C for 40 s, and final elongation at 72°C for 5 min. The PCR products were checked on a 2% agarose gel for correct product size formation.
Amplicons of 16S rDNA were purified using SPRI select beads (Beckman-Coulter Inc., USA) with a 0.62 × ratio of beads to sample volume. These libraries were quantified using the Quantus™ Fluorometer (Promega, Tokyo, Japan) and pooled in equal amounts of DNA. The pooled library was then sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA, USA), which generated paired 300-bp reads.
After sequencing, six samples from T1 (C, n = 3; L, n = 2; L+M, n = 1) were excluded from the downstream analyses owing to their low numbers of reads.
Sequence data obtained from the present study have been deposited DDBJ under accession no DRA007951.
Preprocessing and Analysis
Raw sequence data were analyzed with QIIME 2 version 2018.2 (http://qiime2.org). The data were first demultiplexed and processed using DADA2 (12) for quality filtering and the construction of amplicon sequence variants (ASVs), which are analogous to traditional operational taxonomic units (OTUs). The ASVs were then summarized in feature tables, which were rarefied to a depth of 20,000 ASVs using the qiime feature-table rarefy command to avoid the bias that occurs with increasing depth. Representative sequences were aligned with MAFFT (13) using the qiime alignment command and were used to construct a phylogenetic tree with FastTree2 using the qiime phylogeny command (14). Alpha diversity, which was represented by the Chao1 estimator and the Shannon index, and beta diversity, which was represented by the weighted UniFrac distances, were estimated using the q2-diversity plugin in QIIME 2. Principal coordinates analysis (PCoA) based on weighted UniFrac distances was also performed using QIIME 2 and visualized with Emperor (15). The taxonomy of the sequence variants was assigned using the QIIME 2 q2-feature-classifier plugin against the Greengenes13.8 99% OTUs full-length sequences (16).
Statistical Analyses
The BW data were analyzed using analysis of variance and analysis of covariance with the generalized linear model procedure in R 3.5.0. Normal distribution was evaluated by Shapiro–Wilk test and the homogeneity of variance was tested by Levene's test. The model for the analysis of BW on T1 included treatment and trial fixed effects, while the models for T2 included treatment and trial fixed effects as well as the BW on T1 as a covariate. For analysis of the BW on T1 and T2, the TukeyHSD method was used for multiple comparisons. For analyzing the BW on T3, Games-Howell's test was used for multiple comparisons because of heteroscedasticity of the data (Levene's test; p < 0.05). Differences were considered statistically significant at p < 0.05.
Differences in the relative abundances of microbial taxa among the treatment groups were tested using the Kruskal–Wallis test followed by the post hoc Mann–Whitney U-test in R 3.5.0. The Kruskal–Wallis test for multiple pairwise comparisons was also performed to analyze statistical differences in alpha diversity (Chao1 and Shannon index), while permutational multivariate analysis of variance was used with 999 permutations to evaluate differences in weighted UniFrac distances in QIIME 2. In all tests, the p-values were adjusted for multiple testing using the Benjamin–Hochberg procedure (q-values) and differences among the means were considered statistically significant at q < 0.10.
Results
Growth Performance
The total of 9 pigs (C = 6, L = 3) were sick during Phase 2 or 3, and the pigs were excluded from analyzing body weight. There was no significant difference in the corrected BW of pigs among treatment groups on T1 or T3 (Table 3). On T2, pigs in the L, D, and L+M treatment groups had a significantly higher BW than those in C (p < 0.05), but pigs in the L+M treatment group had a similar BW to those in the L and M groups.
Table 3.
Corrected body weight (kg) of the post-weaning pigs in each treatment group.
| Time | C | L | D | M | L+M |
|---|---|---|---|---|---|
| T1 | 10.4 ± 1.61 | 10.0 ± 1.10 | 10.2 ± 0.74 | 10.2 ± 1.43 | 10.2 ± 1.25 |
| T2 | 31.6 ± 5.04a | 33.3 ± 3.49b | 32.9 ± 3.28b | 31.9 ± 4.42ab | 32.9 ± 3.89b |
| T3 | 72.7 ± 8.66 | 74.6 ± 6.43 | 74.2 ± 6.86 | 73.1 ± 8.40 | 74.5 ± 5.56 |
Values are means ± standard deviations.
Means with different superscript letters within a column are significantly different (p < 0.05).
T1, First day of Phase 1 (day 0 post-weaning); T2, final day of Phase 1 (day 40 post-weaning); T3, final day of Phase 2 (day 90 post-weaning).
C, Basal diet; L, basal diet + 1 g/kg of Product 1 containing live Enterococcus faecium; D, basal diet + 1 g/kg of Product 2 containing heat-killed E. faecium; M, basal diet + 1 g/kg of Product 3 containing live Clostridium butyricum; L+M, basal diet + 1 g/kg of Product 4 containing live E. faecium + C. butyricum.
Alpha Diversity
There were no significant differences in alpha diversity (Chao1 and Shannon index) among the treatment groups on T1, T2, or T3 (Table 4).
Table 4.
Alpha diversity of the gut microbiota of post-weaning pigs in each treatment group.
| C | L | D | M | L+M | |
|---|---|---|---|---|---|
| T1 | |||||
| Chao1 | 537.82 ± 34.50 | 399.30 ± 150.02 | 479.69 ± 78.74 | 429.35 ± 101.11 | 351.57 ± 62.52 |
| Shannon | 7.24 ± 0.18 | 6.33 ± 0.67 | 6.58 ± 0.92 | 6.70 ± 0.35 | 5.93 ± 1.30 |
| T2 | |||||
| Chao1 | 696.43 ± 83.54 | 637.49 ± 78.95 | 740.50 ± 159.08 | 668.73 ± 74.58 | 649.71 ± 187.31 |
| Shannon | 7.51 ± 0.25 | 7.04 ± 0.35 | 7.39 ± 0.58 | 7.25 ± 0.27 | 7.27 ± 0.70 |
| T3 | |||||
| Chao1 | 347.83 ± 45.04 | 423.13 ± 103.97 | 656.42 ± 214.88 | 436.53 ± 124.64 | 463.35 ± 136.83 |
| Shannon | 6.40 ± 0.21 | 6.70 ± 0.52 | 6.77 ± 0.69 | 6.32 ± 0.68 | 6.46 ± 0.48 |
Values are means ± standard deviations. T1, First day of Phase 1 (day 0 post-weaning); T2, final day of Phase 1 (day 40 post-weaning); T3, final day of Phase 2 (day 90 post-weaning).
C, Basal diet; L, basal diet + 1 g/kg of Product 1 containing live Enterococcus faecium; D, basal diet + 1 g/kg of Product 2 containing heat-killed E. faecium; M, basal diet + 1 g/kg of Product 3 containing live Clostridium butyricum; L+M, basal diet + 1 g/kg of Product 4 containing live E. faecium + C. butyricum.
Beta Diversity
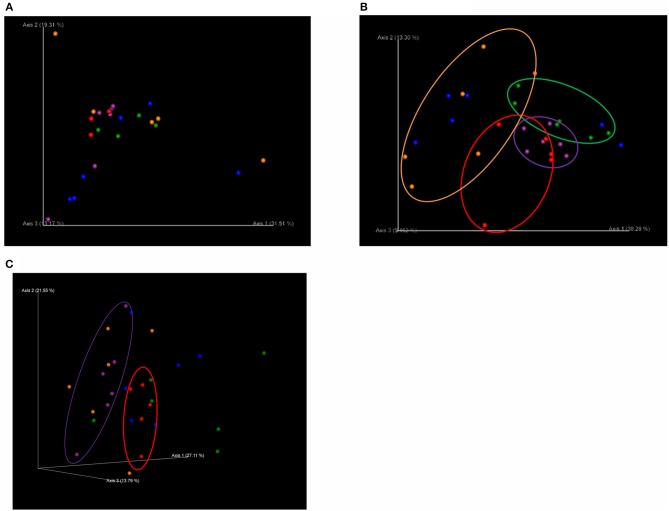
There was no significant difference in beta diversity (weighted UniFrac distances) among treatments on T1 (Figure 1A). However, on T2, the weighted UniFrac distances showed distinct clustering patterns that separated the microbiota of pigs in C from those in each of the dietary probiotics treatment groups except D (C vs. L, q = 0.04; C vs. M, q = 0.06; C vs. L+M, q = 0.06), as well as pigs in M from those in L+M (q = 0.02) and pigs in L from those in M and L+M (q = 0.03 for each) (Figure 1B). The microbiota of pigs in M was also different from that of pigs in C on T3 (q = 0.08) (Figure 1C).
Figure 1.
Principal coordinates analysis (PCoA) plots based on weighted UniFrac distances of the bacterial community structures in the gut microbiota of post-weaning pigs that were fed probiotics-supplemented diets or a control diet on (A) the first day of Phase 1 (T1; day 0 post-weaning), (B) the final day of Phase 1 (T2; day 40 post-weaning), and (C) the final day of Phase 2 (T3; day 90 post-weaning). 3D PCoA plots were visualized using the program Emperor. Red: basal diet (C); green: basal diet + 1 g/kg of Product 1 containing live Enterococcus faecium (L); blue: basal diet + 1 g/kg of Product 2 containing heat-killed E. faecium (D); purple: basal diet + 1 g/kg of Product 3 containing live Clostridium butyricum (M); orange: basal diet + 1 g/kg of Product 4 containing live E. faecium + C. butyricum (L+M).
Taxonomic Composition
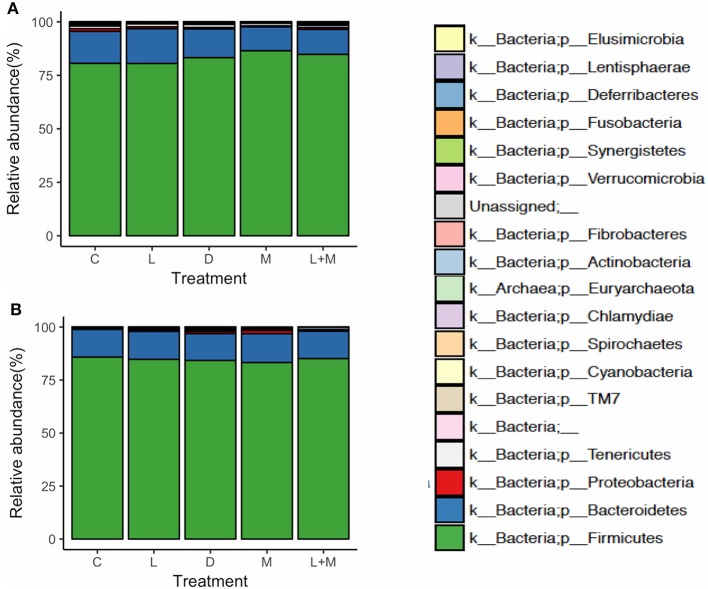
On T2 and T3, Firmicutes was the most dominant phylum in the gut microbiota of all treatment groups followed by Bacteroidetes (Figure 2), with these two phyla representing over 95% of the total microbiota. On T2, the proportions of five phyla (Table 5) and 11 genera (Table 6) significantly differed among treatments. At the phylum level, there was no significant difference in the relative abundance of Bacteroidetes among treatments but there was a significantly higher relative abundance of Firmicutes in M than in C, L, or D and of Cyanobacteria in C than in L, M, or L+M. At the genus level, there was a significantly higher abundance of Lactobacillus in C, M, and L+M than in L or D, of Chlamydia in C, M, and L+M than in L, and of Treponema in C and L+M than in L or M. On T3, the proportions of two phyla (Table 5) and eight genera (Table 6) significantly differed among treatments, with significantly higher abundances of the phylum Actinobacteria, unidentified genera in the Coriobacteriaceae family, and other Coriobacteriaceae in D, M, and L+M than in C, and of Megasphaera in M and L+M than in the other treatment groups.
Figure 2.
Relative abundances at the phylum level of the gut microbiota in post-weaning pigs that were fed probiotics-supplemented diets or a control diet on (A) the final day of Phase 1 (T2; day 40 post-weaning) and (B) the final day of Phase 2 (T3; day 90 post-weaning).
Table 5.
Relative abundances (%) of bacterial phyla that exhibited significant differences among treatment groups (q < 0.10) in the gut microbiota of post-weaning pigs.
| Treatment | q-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Time | Phylum | C | L | D | M | L+M | <0.05 | <0.10 |
| T2 | Chlamydiae | 0.20 ± 0.24 | 0.00 ± 0.00 | 0.02 ± 0.04 | 0.02 ± 0.02 | 0.13 ± 0.04 | L vs. C L+M vs. L, D, M |
L vs. M |
| Cyanobacteria | 0.24 ± 0.16 | 0.06 ± 0.06 | 0.09 ± 0.07 | 0.04 ± 0.01 | 0.04 ± 0.02 | C vs. L, M, L+M | ||
| Firmicutes | 80.6 ± 2.9 | 80.5 ± 5.7 | 83.3 ± 2.6 | 86.5 ± 1.2 | 84.8 ± 3.7 | M vs. C, L, D | ||
| Proteobacteria | 1.27 ± 0.73 | 0.94 ± 0.29 | 0.76 ± 0.33 | 0.48 ± 0.06 | 0.88 ± 0.55 | L vs. M | ||
| Spirochaetes | 0.22 ± 0.23 | 0.03 ± 0.03 | 0.14 ± 0.13 | 0.03 ± 0.05 | 0.15 ± 0.11 | C, L+M vs. L, M | ||
| T3 | Actinobacteria | 0.15 ± 0.22 | 0.21 ± 0.34 | 0.75 ± 0.56 | 0.79 ± 0.82 | 1.03 ± 0.79 | C, L vs. D, M, L+M | |
| Tenericutes | 0.16 ± 0.16 | 0.50 ± 0.47 | 0.26 ± 0.30 | 0.011 ± 0.012 | 0.018 ± 0.022 | L vs. M, L+M | ||
Values are means ± standard deviations. The significance of differences was evaluated using the Kruskal–Wallis test followed by the post hoc Mann–Whitney U-test with the Benjamin–Hochberg false discovery rate correction (q < 0.10). T2, Final day of Phase 1 (day 40 post-weaning); T3, final day of Phase 2 (day 90 post-weaning).
C, Basal diet; L, basal diet + 1 g/kg of Product 1 containing live Enterococcus faecium; D, basal diet + 1 g/kg of Product 2 containing heat-killed E. faecium; M, basal diet + 1 g/kg of Product 3 containing live Clostridium butyricum; L+M, basal diet + 1 g/kg of Product 4 containing live E. faecium + C. butyricum.
Table 6.
Relative abundances (%) of bacterial genera that exhibited significant differences among treatment groups (q < 0.10) in the gut microbiota of post-weaning pigs.
| Treatment | q-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Phylum | Genus | C | L | D | M | L+M | < 0.05 | < 0.10 |
| T2 | Bacteroidetes | Parabacteroides | 0.27 ± 0.16 | 0.12 ± 0.10 | 0.25 ± 0.11 | 0.05 ± 0.02 | 0.18 ± 0.14 | M vs. C, D, L+M | |
| Chlamydiae | Chlamydia | 0.20 ± 0.24 | 0.00 ± 0.00 | 0.02 ± 0.04 | 0.02 ± 0.02 | 0.13 ± 0.04 | L vs. C, L+M vs. L, D, M |
L vs. M | |
| Cyanobacteria | o_YS2;f_;g_ | 0.24 ± 0, 16 | 0.06 ± 0.06 | 0.09 ± 0.07 | 0.04 ± 0.01 | 0.04 ± 0.02 | C vs. L, M, L+M | ||
| Firmicutes | Lactobacillus | 5.44 ± 2.51 | 1.98 ± 1.18 | 1.98 ± 0.99 | 9.18 ± 5.24 | 6.87 ± 4.66 | L, D vs. M, L+M | C vs. L, D | |
| Firmicutes | Dorea | 1.13 ± 0.25 | 0.61 ± 0.18 | 0.83 ± 0.59 | 1.37 ± 0.48 | 1.45 ± 0.73 | C, M, L+M vs. L | ||
| Firmicutes | f_Peptostreptococcaceae;g_ | 0.41 ± 0.09 | 0.78 ± 0.21 | 0.54 ± 0.21 | 0.77 ± 0.29 | 0.42 ± 0.22 | C, L+M vs. L, M | ||
| Firmicutes | Faecalibacterium | 4.01 ± 1.95 | 2.36 ± 0.46 | 2.58 ± 2.02 | 3.48 ± 0.95 | 1.66 ± 0.51 | M vs. L+M | L vs. M | |
| Firmicutes | Mitsuokella | 0.14 ± 0.12 | 0.37 ± 0.54 | 0.62 ± 0.81 | 0.59 ± 0.17 | 0.02 ± 0.03 | C vs. M L+M vs. L, M |
||
| Firmicutes | L7A_E11 | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.06 | 0.02 ± 0.01 | 0.06 ± 0.03 | L, L+M vs. M | ||
| Proteobacteria | o_RF32;f_;g_ | 0.05 ± 0.04 | 0.006 ± 0.007 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.04 ± 0.05 | C vs. D | C vs. L, M M vs. L+M |
|
| Spirochaetes | Treponema | 0.22 ± 0.23 | 0.03 ± 0.03 | 0.14 ± 0.13 | 0.03 ± 0.05 | 0.15 ± 0.11 | C, L+M vs. L, M | ||
| T3 | Actinobacteria | f_Coriobacteriaceae;_ | 0.001 ± 0.002 | 0.013 ± 0.031 | 0.06 ± 0.40 | 0.038 ± 0.022 | 0.083 ± 0.045 | C vs. D, M, L+M L vs. D, L+M |
|
| Actinobacteria | f_Coriobacteriaceae;g_ | 0.036 ± 0.025 | 0.048 ± 0.080 | 0.17 ± 0.10 | 0.22 ± 0.18 | 0.36 ± 0.29 | C vs. D, M, L+M L vs. L+M |
L vs. D | |
| Actinobacteria | Collinsella | 0.013 ± 0.014 | 0.013 ± 0.033 | 0.087 ± 0.068 | 0.053 ± 0.027 | 0.16 ± 0.15 | C, L vs. D, M, L+M M vs. L+M |
||
| Bacteroidetes | f_S24-7;g_ | 0.25 ± 0.16 | 0.50 ± 0.31 | 0.90 ± 0.76 | 0.86 ± 0.90 | 1.20 ± 0.52 | C vs. L+M | ||
| Firmicutes | Megasphaera | 5.95 ± 1.29 | 4.04 ± 4.28 | 7.21 ± 2.02 | 13.46 ± 2.84 | 12.55 ± 3.75 | C, L, D vs. M, L+M | ||
| Firmicutes | Mogibacterium | 0.052 ± 0.040 | 0.069 ± 0.051 | 0.011 ± 0.010 | 0.003 ± 0.008 | 0.012 ± 0.020 | L vs. M | C vs. M L vs. D, L+M |
|
| Proteobacteria | Escherichia | 0.028 ± 0.063 | 0.003 ± 0.006 | 0.068 ± 0.080 | 0.89 ± 2.17 | 0.076 ± 0.17 | L vs. D | ||
| Tenericutes | o_RF39;f_;g_ | 0.16 ± 0.15 | 0.48 ± 0.47 | 0.24 ± 0.28 | 0.011 ± 0.012 | 0.018 ± 0.021 | L vs. M, L+M | ||
Values are means ± standard deviations. “o,” “f,” and “g” indicate the order, family, and genus, respectively. Some operational taxonomic units (OTUs) could not be assigned to a genus because the sequence was absent from the database. The significance of differences was evaluated using the Kruskal–Wallis test followed by the post hoc Mann–Whitney U-test with the Benjamin–Hochberg false discovery rate correction (q < 0.10). T2, Final day of Phase 1 (day 40 post-weaning); T3, final day of Phase 2 (day 90 post-weaning).
Discussion
In this study, we examined the effects of dietary supplementation with E. faecium and C. butyricum, both individually and in combination, on the growth and gut microbiota composition of post-weaning pigs. We found that the addition of live or heat-killed E. faecium to the pigs' diet during the post-weaning period improved their growth performance, with no significant difference between the two treatments. Similarly, Sukegawa et al. (8) reported that dietary supplementation with heat-killed E. faecium enhanced the growth of post-weaning pigs. Heat-killed bacterium cannot produce any metabolites. The bacterial components might have positive effect on the gut, resulting in increasing BW. On the other hand, supplementation with C. butyricum did not have any effect on growth performance in the present study. This contrasts with the findings of Takahashi et al. (5), who reported that the use of C. butyricum may improve the zootechnical performance of weaned piglets. The effect of dietary probiotics varies depending on factors such as location and the microbiota of the host. Moreover, in the present study, the amount of feeding C. butyricum was 9.6 × 104 cfu/g feed, which was less than that of the previous study. For these reasons, the BW of pigs fed C. butyricum might have not increased contrast to that of control treatment. Following completion of the probiotics administration period, there was no significant difference in the BW of pigs among treatments, indicating that there was no residual effect of the probiotics on growth performance. Beneficial effects of these probiotics might be diminished by ceasing probiotics, resulting in the growth performance of pigs in probiotic treatments returning back to normal. The result implies that the bacteria were not able to persist in the intestine.
We expected that the combined use of E. faecium and C. butyricum would have a highly synergistic effect on the pigs due to the different characteristics of LAB and BAB. However, we found that pigs in the L+M treatment group had a similar BW to those in the L and M groups, indicating that the use of single probiotics had a similar effect on growth performance to the combined use of E. faecium and C. butyricum. Similarly, Han et al. (17) reported that the combined supplementation of broilers with C. butyricum and L. plantarum had no significant effect on growth performance. Many studies have demonstrated that dietary LAB and BAB have positive effects on factors other than growth parameters in animals, such as intestinal morphometric parameters. For example, the length of the intestinal villi is improved by feeding pigs LAB (18–20) and broilers BAB (21). Moreover, Long et al. (22) reported that the combined use of LAB and BAB could further improve the length of the intestinal villi in mice. Therefore, since we were unable to investigate factors associated with intestinal health in the present study, we cannot rule out the possibility that the combined use of LAB and BAB has synergistic effects on factors other than growth parameters and the microbiota of post-weaning pigs.
We also explored the effects of dietary supplemented probiotics on the diversity of the gut microbiota in post-weaning pigs, using Chao1 to indicate the bacterial richness and the Shannon index to reflect the bacterial diversity. We found no significant differences in alpha diversity among treatments. Previous studies have obtained varying results on the effects of probiotics on alpha diversity–for example, Chae et al. (23) showed that the administration of E. faecium to weaned piglets increased the bacterial richness, while Li et al. (24) showed that dietary supplementation with E. faecalis decreased the bacterial richness. Thus, the effects of probiotics on the alpha diversity of the gut microbiota appears to vary depending on the microorganism strain, diet, and environmental factors.
To confirm this lack of alteration of the intestinal microbiota by these dietary probiotics, we performed PCoA based on weighted UniFrac distances. Interestingly, this showed that there were significant changes in microbial community structure following the administration of E. faecium and C. butyricum. Furthermore, significant differences in beta diversity were still observed between M and C following completion of the probiotics treatment period, indicating that the effect of dietary C. butyricum on the structure of the microbiota was sustained.
Many researchers have reported that Firmicutes and Bacteroidetes are the most dominant phyla in pig fecal samples (23, 25–27) and our findings supported this. Bacteria in the phylum Firmicutes are fiber digesters and produce short-chain fatty acids (SCFA) from dietary compounds (28), resulting in improved growth performance. Although there was no significant difference in the BW of pigs between M and the other treatment groups, supplementation with only C. butyricum increased the proportion of Firmicutes on T2. Bacteria in the genus Lactobacillus (phylum Firmicutes) are beneficial in the intestine, producing bacteriocins, organic acids, and hydrogen peroxide (29), and many studies have shown that dietary probiotics increase the relative abundance of Lactobacillus in pigs (23, 30, 31). Furthermore, we previously found that dietary supplementation with heat-killed E. faecium increased the proportion of Lactobacillus (8). However, in the present study, the relative abundance of Lactobacillus was significantly lower in pigs that had been supplemented with live or heat-killed E. faecium than in the control, and in a separate study conducted at another commercial farm, we found that pigs had similar proportions of Lactobacillus in their gut microbiota regardless of whether their diets were supplemented with live, heat-killed, or no E. faecium (data not shown). Therefore, it appears that the effect of live and heat-killed E. faecium on Lactobacillus may vary depending on the microbiota of the host and the dietary probiotic used, and it is not necessarily related to a reduction in Lactobacillus.
Bacteria in the phylum Chlamydiae are associated with a broad range of diseases in swine and Pollmann et al. (32) reported that dietary E. faecium reduced the incidence of Chlamydia infection. Similarly, in the present study, we found that dietary supplementation with live E. faecium decreased the proportion of Chlamydia. Furthermore, the administration of live E. faecium and C. butyricum decreased the proportion of Treponema (phylum Spirochaetes), suggesting that these probiotics inhibited the growth of these bacteria, which are associated with colitis (33).
Dietary supplementation with C. butyricum has also been shown to increase the relative abundance of Megasphaera (phylum Firmicutes) (34), which includes Megasphaera elsdenii, an intestinal lactate- and sugar-fermenting species (35) that produces SCFAs, which are important for the energy balance of animals (27). Similarly, we found that the proportion of Megasphaera in the gut microbiota was significantly higher in the M and L+M treatment groups than in C on T3. We did not, however, observe any significant difference among treatments on T2, the reason for which is unclear but may be related to dietary C. butyricum affecting the growth of Megasphaera. After completion of the probiotics treatment period, there was no significant difference in the relative abundance of any taxonomic group except Cyanobacteria between L+M and C, suggesting that combined administration of E. faecium and C. butyricum did not have a synergistic effect on the composition of the gut microbiota.
In conclusion, we demonstrated that dietary supplementation with live or heat-killed E. faecium during the post-weaning period enhanced the growth performance of pigs and that the use of E. faecium or C. butyricum altered the structure of the microbiota. However, we did not observe any synergistic effect of their combined use on growth performance and taxonomic composition. Further studies are required to evaluate the combined effects of these probiotics on other factors, such as the intestinal health of pigs.
Author Contributions
YS performed the experiments, analyzed the data, and wrote the manuscript. YK and SR analyzed the data. KO, MT, and TF designed the study. SS designed the study and analyzed the data. All authors discussed the results and approved the final manuscript.
Conflict of Interest Statement
YS, SR, SS, and TF were employed by NH Foods Ltd. YK, KO, and MT were employed by Miyarisan Pharmaceutical Co., Ltd.
References
- 1.Mallo JJ, Rioperez J, Honrubia P. The addition of Enterococcus faecium to diet improves piglet's intestinal microbiota and performance. Livest Sci. (2010) 133:176–8. 10.1016/j.livsci.2010.06.057 [DOI] [Google Scholar]
- 2.Zhao P, Zhang Z, Lan R, Li T, Kim I. Comparison of efficacy of lactic acid bacteria complex and Enterococcus faecium DSM 7134 in weanling pigs. J Appl Anim Res. (2018) 46:888–92. 10.1080/09712119.2017.1420655 [DOI] [Google Scholar]
- 3.Broom LJ, Miller HM, Kerr KG, Knapp JS. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res Vet Sci. (2006) 80:45–54. 10.1016/j.rvsc.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol. (2004) 41:219–26. 10.1016/j.femsim.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, McCartney E, Knox A, Francesch M, Oka K, Wada K, et al. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim Sci J. (2018) 89:895–905. 10.1111/asj.13006 [DOI] [PubMed] [Google Scholar]
- 6.Barnes AMT, Dale JL, Chen Y, Manias DA, Greenwood Quaintance KE, Karau MK, et al. Enterococcus faecalis readily colonizes the entire gastrointestinal tract and forms biofilms in a germ-free mouse model. Virulence. (2017) 8:282–96. 10.1080/21505594.2016.1208890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato R, Tanaka M. Intestinal distribution and intraluminal localization of orally administered Clostridium butyricum in rats. Microbiol Immunol. (1997) 41:665–71. 10.1111/j.1348-0421.1997.tb01909.x [DOI] [PubMed] [Google Scholar]
- 8.Sukegawa S, Ihara Y, Yuge K, Rao S, Oka K, Arakawa F, et al. Effects of oral administration of heat-killed Enterococcus faecium strain NHRD IHARA in post-weaning piglets. Anim Sci J. (2014) 85:454–60. 10.1111/asj.12163 [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto M, Hayashi H, Benno Y. Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol Immunol. (2003) 47:133–42. 10.1111/j.1348-0421.2003.tb02796.x [DOI] [PubMed] [Google Scholar]
- 10.Matsuki T, Watanabe K, Fujimoto J, Takada T. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. (2004) 70:7220–8. 10.1128/AEM.70.12.7220-7228.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herlemann DPR, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. (2011) 5:1571–9. 10.1038/ismej.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. (2016) 13:581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. (2002) 30:3059–66. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price MN, Dehal PS, Arkin AP. FastTree 2 - approximately maximum-likelihood trees for large alignments. PLoS ONE. (2010) 5:e9490. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience. (2013) 2:2–5. 10.1186/2047-217X-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald D, Price MN, Goodrich J, Nawrocki EP, Desantis TZ, Probst A, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. (2012) 6:610–8. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J, Wang Y, Song D, Lu Z, Dong Z, Miao H, et al. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric Immunol. (2018) 29:797–807. 10.1080/09540105.2018.1457013 [DOI] [Google Scholar]
- 18.Suo C, Yin Y, Wang X, Lou X, Song D, Wang X, et al. Effects of lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet Res. (2012) 8:89. 10.1186/1746-6148-8-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galeano JAC, Herrera AL, Suescún JP. The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Rev Fac Nac Agron Medell. (2016) 69:7803–11. 10.15446/rfna.v69n1.54748 [DOI] [Google Scholar]
- 20.Dowarah R, Verma AK, Agarwal N, Patel BHM, Singh P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest Sci. (2017) 195:74–9. 10.1016/j.livsci.2016.11.006 [DOI] [Google Scholar]
- 21.Zhang L, Zhang L, Zhan X, Zeng X, Zhou L, Cao G, et al. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. (2016) 7:1–9. 10.1186/s40104-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long M, Yang S, Li P, Song X, Pan J, He J, et al. Combined use of C. butyricum Sx-01 and L. salivarius C-1-3 improves intestinal health and reduces the amount of lipids in serum via modulation of gut microbiota in mice. Nutrients. (2018) 10:810. 10.3390/nu10070810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae JP, Pajarillo EAB, Oh JK, Kim H, Kang DK. Revealing the combined effects of lactulose and probiotic enterococci on the swine faecal microbiota using 454 pyrosequencing. Microb Biotechnol. (2016) 9:486–95. 10.1111/1751-7915.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Niu Q, Wei Q, Zhang Y, Ma X, Kim SW, et al. Microbial shifts in the porcine distal gut in response to diets supplemented with Enterococcus Faecalis as alternatives to antibiotics. Sci Rep. (2017) 7:1–10. 10.1038/srep41395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, et al. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci USA. (2012) 109:15485–90. 10.1073/pnas.1205147109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H, et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep. (2015) 5:1–7. 10.1038/srep09938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran H, Anderson CL, Bundy JW, Fernando SC, Miller PS, Burkey TE. Effects of spray-dried porcine plasma on fecal microbiota in nursery pigs. J Anim Sci. (2018) 96:1017–31. 10.1093/jas/skx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molist F, Manzanilla EG, Pérez JF, Nyachoti CM. Coarse, but not finely ground, dietary fibre increases intestinal Firmicutes: Bacteroidetes ratio and reduces diarrhoea induced by experimental infection in piglets. Br J Nutr. (2012) 108:9–15. 10.1017/S0007114511005216 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Han Y, Zhao J, Zhou Z, Fan H. Consuming fermented distillers' dried grains with solubles (DDGS) feed reveals a shift in the faecal microbiota of growing and fattening pigs using 454 pyrosequencing. J Integr Agric. (2017) 16:900–10. 10.1016/S2095-3119(16)61523-X [DOI] [Google Scholar]
- 30.Pajarillo EAB, Chae JP, Balolong MP, Kim HB, Park C-S, Kang D-K. Effects of probiotic Enterococcus faecium NCIMB 11181 administration on swine fecal microbiota diversity and composition using barcoded pyrosequencing. Anim Feed Sci Technol. (2015) 201:80–8. 10.1016/j.anifeedsci.2015.01.011 [DOI] [Google Scholar]
- 31.Balasubramanian B, Lee SI, Kim IH. Inclusion of dietary multi-species probiotic on growth performance, nutrient digestibility, meat quality traits, faecal microbiota and diarrhoea score in growing–finishing pigs. Ital J Anim Sci. (2018) 17:100–6. 10.1080/1828051X.2017.1340097 [DOI] [Google Scholar]
- 32.Pollmann M, Nordhoff M, Pospischil A, Tedin K, Wieler LH. Effects of a probiotic strain of Enterococcus faecium on the rate of natural Chlamydia infection in swine. Society. (2005) 73:4346–53. 10.1128/IAI.73.7.4346-4353.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mølbak L, Klitgaard K, Jensen TK, Fossi M, Boye M. Identification of a novel, invasive, not-yet-cultivated Treponema sp. in the large intestine of pigs by PCR amplification of the 16S rRNA gene. J Clin Microbiol. (2006) 44:4537–40. 10.1128/JCM.01537-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Chen X, Liu P, Zhao J, Sun J, Guan W, et al. Dietary Clostridium butyricum induces a phased shift in fecal microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model. J Agric Food Chem. (2018) 66:5157–66. 10.1021/acs.jafc.8b01253 [DOI] [PubMed] [Google Scholar]
- 35.Marounek M, Fliegrova K, Bartos S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. (1989) 55:1570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]