Abstract
Background
Prior research has focused on early outcomes after congenital heart surgery, but less is known about later risks. We aimed to determine the late causes of death among children (<21 years of age) surviving their initial congenital heart surgery.
Methods and Results
This is a retrospective cohort study from the Pediatric Cardiac Care Consortium, a US‐based registry of interventions for congenital heart defects (CHD). Excluding patients with chromosomal anomalies or inadequate identifiers, we matched those surviving their first congenital heart surgery (1982–2003) against the National Death Index through 2014. Causes of death were obtained from the National Death Index to calculate cause‐specific standardized mortality ratios (SMRs). Among 31 132 patients, 2527 deaths (8.1%) occurred over a median follow‐up period of 18 years. Causes of death varied by time after surgery and severity of CHD but, overall, 69.9% of deaths were attributed to the CHD or another cardiovascular disorder, with a SMR for CHD/cardiovascular disorder of 67.7 (95% confidence interval: 64.5–70.8). Adjusted odds ratios revealed increased risk of death from CHD/cardiovascular disorder in females [odds ratio=1.28; 95% confidence interval (1.04–1.58); P=0.018] with leading cardiovascular disorder contributing to death being cardiac arrest (16.8%), heart failure (14.8%), and arrhythmias (9.1%). Other major causes of death included coexisting congenital malformations (4.7%, SMR: 7.0), respiratory diseases (3.6%, SMR: 8.2), infections (3.4%, SMR: 8.2), and neoplasms (2.1%, SMR: 1.9).
Conclusions
Survivors of congenital heart surgery face long‐term risks of premature mortality mostly related to residual CHD pathology, heart failure, and arrhythmias, but also to other noncardiac conditions. Ongoing monitoring is warranted to identify target factors to address residual morbidities and improve long‐term outcomes.
Keywords: congenital heart disease, mortality, outcomes research, surgery
Subject Categories: Congenital Heart Disease, Mortality/Survival
Clinical Perspective
What Is New?
Persistently elevated cardiovascular disorder–related risk across all severity forms of congenital heart defects (CHD) suggests that postoperative cardiovascular sequelae continue to impose a significant burden despite improvements in the surgical management of CHD.
Excess mortality from non‐CHD/noncardiovascular disorder causes remains unchanged over time, suggesting limited progress in the management or prevention of these additional non‐CHD morbidities.
What Are the Clinical Implications?
Myocardial protection during congenital heart surgery, emergence of new medications, provision of implantable defibrillators to those at risk for sudden arrhythmic death, and interdisciplinary clinical investigation may all attenuate the late risks from postoperative morbidities in the population with repaired CHD.
Introduction
Children undergoing congenital heart surgery (CHS) are at risk for both early and late mortality, but most prior research has focused on survival to hospital discharge. Studies of long‐term outcomes have shown elevated risk for premature mortality across all forms of congenital heart defects (CHD), and defined high‐risk groups by CHD characteristics.1, 2, 3, 4 We recently examined this excess risk for survivors of CHS in the Pediatric Cardiac Care Consortium (PCCC), a large US‐based registry for interventions for CHD5 and found the standardized mortality ratio (SMR) to range from 4.3 times (95% confidence interval [CI] 3.7–5.0) for mild diseases, to 5.8 for moderate (95% CI: 4.2–7.9), 12.4 for severe two‐ventricle (95% CI: 11.5–13.4) and 35 times (95% CI: 33–38) above the general population.4 Less is known, though, about the eventual causes of death (COD) among patients surviving to hospital discharge after the initial CHS or after their final corrective or palliative surgery.1, 6, 7, 8, 9
Patients operated for CHD may be at risk for premature mortality related to abnormal cardiac function and arrhythmias, systemic and pulmonary vascular abnormalities, and impaired lung function.7, 8, 9, 10, 11, 12 In addition, they frequently have coexisting extracardiac abnormalities and predispositions for neurologic and cerebrovascular disorders, gastrointestinal/hepatic, renal, endocrine, and neoplastic disorders.13, 14, 15, 16, 17, 18, 19, 20 Patients with operated CHDs are also exposed to other risks such as additional interventions, diagnostic radiation,21, 22 chronic use of medications,23 and psycho‐emotional sequences24, 25 leading to suicidal behavior or alcoholism.26 Furthermore, CHD patients are concurrently exposed to the same cardiovascular and other risk factors as the general population and may react differently to conditions such as aging and pregnancy.10, 18, 27 These exposures may create an additive organ injury leading to increased morbidity above the general population.28 Consequently, CHD patients are expected to experience different COD than the general population.
We recently reported the 25‐year survival outcomes of 35 998 children undergoing CHS in the United States between 1982 and 2003 by linking the PCCC with the US National Death Index (NDI).5 Long‐term survival was decreased across all forms of CHD including even the mildest lesions.4 We now examine the COD in this cohort to evaluate differential risks that may inform targeted surveillance for specific patient groups.
Methods
We conducted a retrospective cohort study using data from the PCCC registry enriched with prospectively collected data through linkage with the NDI. The study was approved by the Institutional Review Boards of the University of Minnesota and Emory University, by the NDI, and by the state birth registries of Minnesota, Arkansas, Ohio, South Carolina, and Missouri with waiver for informed consent for patients enrolled in the PCCC up to April 15, 2003, the date stricter Health Insurance Portability and Accountability Act rules took effect. The data, analytic methods, and study materials will be made available upon request from the corresponding author to qualified individuals completing necessary training requirements as set by the Institutional Review Board of Emory University. Shared data will be free of identifiers to protect the rights and privacy of the individuals who participate in this project as required by the Health Insurance Portability and Accountability Act Privacy Rule, and any local, state, and federal laws and regulations.
PCCC Registry
Details of the creation, activities, and function of the PCCC have been described before.5, 29 We queried the PCCC registry for patients who (1) were US residents; (2) underwent first CHS in a US PCCC center between January 1, 1982 and April 15, 2003; (3) were <21 years of age at the time of surgery; (4) survived to discharge after the CHS; and (5) had adequate identifiers for matching with the NDI.30, 31 We excluded low‐birth‐weight infants (<2.5 kg at the time of surgery) with isolated patent ductus arteriosus ligation and patients with known chromosomal abnormalities because of COD associated with the underlying condition.
We abstracted demographic and clinical variables including sex, age at first surgery, year of first surgery, type of surgery, and CHD diagnosis. Information about race was obtained from the PCCC, linkage to state birth records, or death records obtained from the NDI. The subset with race information available was classified as “black,” “white,” or “other race.”
Assignment of Cardiac Diagnosis and Classification of Defects
Each patient is assigned one primary diagnosis using a severity‐based list of CHD and the operative strategy for the first reported CHS (Data S1).31 We grouped conditions as mild, moderate, and severe lesions. The severe category was subdivided into single‐ (1V) and two‐ventricle lesions (2V). Further subclassification of two‐ventricular conditions into 1 of 8 categories was based on anatomo‐pathophysiologic characteristics. If more than one CHD is present, patients are classified by the hierarchically most severe diagnosis. Lesions with coexistence of different pathophysiologies are classified as complex lesions to distinguish them from the plain forms of the primary CHD lesion.31
Death Ascertainment and Causes of Death Classification
Death was ascertained from the PCCC and by matching to NDI records through December 31, 2014.30, 31 COD were provided by the NDI Plus, both as underlying and multiple or contributing COD (Data S1).32 All COD are given as International Classification of Disease (ICD) codes.33 Between 1982 to 1988, the NDI used the ICD‐9 revision, but beginning in 1999, the ICD‐10 revision is used. Because the PCCC includes data from both ICD revisions, all ICD‐10 codes were recoded to ICD‐9 for tabulation purposes.
Underlying COD were grouped in the following major ICD categories: (1) congenital heart disease (CHD); (2) diseases of the circulatory system (other than CHD) (termed herein cardiovascular disorders or CVD); (3) congenital malformations; (4) diseases of the respiratory system; (5) external causes of injury and poisoning; (6) infectious diseases; (7) neoplasms; and (8) other, where all other medical causes were lumped together. COD were compared across sexes and different age‐strata (selected to match the Centers for Disease Control and Prevention reports of annual mortality), and to the general US population (Table S1).
Deaths by multiple COD were classified: (1) as CHD‐associated death, when there is at least 1 ICD code related to CHD; (2) as CVD‐associated death, when there is no ICD code related to CHD but at least 1 code related to CVD; or (3) as non‐CHD/non‐CVD death when there are no codes related to CHD or CVD.
US Mortality Data
Mortality data for the US population for the years 1982 to 2014 were downloaded from the CDC “Wonder” website and comprise age‐, sex‐, and year‐specific death rates per 100 000 people.34
Statistical Analysis
Underlying COD classification was compared across sexes within age‐group strata using χ2 tests. Time of death from first surgery was categorized (<90 days, 90–365 days, 1–4 years, 5–9 years, 10–14 years, and >15 years) and treated as an ordinal variable to understand trends in cause‐specific mortality. Associations between underlying COD and time from surgery were examined using a Cochran‐Armitage test for trend.
SMRs were used to quantify the cause‐specific rate of mortality in this CHD population compared with an age‐, sex‐, and calendar‐year‐matched US population (for additional information see Data S1).31 Multivariable logistic regression was used to assess the association between race or sex and a specific COD. Models contained the overall effect of race or sex and other potential confounders: year of death, age at death, severity of CHD, sex, and race, as applicable.
All analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, NC) and an interactive figure was created using plotly.35 Statistical significance was assessed at the 0.05 level unless otherwise noted.
Results
Characteristics of Study Population
Among the 35 998 patients who met inclusion criteria, we excluded 4866 (13.5%) patients with a known chromosomal abnormality. Patients excluded because of chromosomal anomalies tended to be younger at their first surgery, were more likely to be female, and have two‐ventricle lesions with L‐R physiology. The final cohort consisted of 31 132 patients, from 47 centers, discharged alive following their initial CHS.
A total of 2527 deaths (8.1%) occurred following discharge after the first CHS over a median follow‐up period of 18.1 years (interquartile range: 14.5–22.2). Among the deaths, 1030 (40.8%) were female and 1497 were male (59.2%). Infants (<1 year of age) accounted for the largest share of deaths (n=994, 39.3%) followed by children aged 1 to 4 years (n=633, 25.0%). Median age at death was 1.8 years (interquartile range: 0.5–12.8). The median age of the cohort at the end of the study period was 20.9 years (interquartile range: 16.3–26.3) (Table 1).
Table 1.
Summary of Patient Characteristics in the PCCC Cohort
| Overall (N=31 132) | Died (N=2527) | |
|---|---|---|
| Median age at surgery (y) (IQR) | 0.96 (0.17–4.22) | 0.16 (0.02–1.03) |
| Sex | ||
| Females | 14 695 (47.2%) | 1030 (40.8%) |
| Males | 16 437 (52.8%) | 1497 (59.2%) |
| Race | ||
| White | 9421 (80.6%) | 1885 (76.5%) |
| Black | 1948 (16.7%) | 500 (20.3%) |
| Other | 327 (2.8%) | 79 (3.2%) |
| Missing | 19 439 | 63 |
| Physiology | ||
| Two‐ventricle lesions | ||
| L‐R Shunt | 12 361 (39.7%) | 419 (16.6%) |
| ASD | 5565 (17.9%) | 134 (5.3%) |
| PDA | 2784 (9.0%) | 79 (3.1%) |
| VSD (simple) | 3496 (11.2%) | 126 (5.0%) |
| CCAVC (simple) | 515 (1.7%) | 80 (3.2%) |
| LHOL | 5286 (17.0%) | 331 (13.1%) |
| Cor‐Tri | 74 (0.2%) | 3 (0.1%) |
| MS | 69 (1.3%) | 14 (0.6%) |
| AS/Sub‐AS | 1263 (4.1%) | 88 (3.5%) |
| CoA | 3668 (11.8%) | 191 (7.6%) |
| IAA | 212 (0.7%) | 35 (1.4%) |
| APVR | 1436 (4.6%) | 65 (2.6%) |
| TAPVR | 672 (2.2%) | 50 (2.0%) |
| PAPVR | 764 (2.5%) | 15 (0.6%) |
| RVOTO | 3596 (11.6%) | 242 (9.6%) |
| PS/Sub‐PS | 697 (2.2%) | 33 (1.3%) |
| PA/IVS | 198 (0.6%) | 22 (0.9%) |
| TOF | 2701 (8.7%) | 187 (7.4%) |
| TGA physiology (d‐TGA simple) | 1545 (5.0%) | 113 (4.5%) |
| Complete mixing (TAC) | 204 (0.7%) | 48 (1.9%) |
| Complex lesions | 2510 (8.1%) | 345 (13.7%) |
| Complex CAVC | 44 (0.1%) | 23 (0.9%) |
| Complex d‐TGA | 228 (0.7%) | 72 (2.9%) |
| Complex VSD | 1620 (5.2%) | 77 (3.1%) |
| Complex TOF | 618 (2.0%) | 173 (6.9%) |
| Miscellaneous | 1846 (5.9%) | 178 (7.0%) |
| l‐TGA (2V) | 200 (0.6%) | 48 (1.9%) |
| MR/AI | 388 (1.3%) | 40 (1.6%) |
| TVA | 158 (0.5%) | 28 (1.1%) |
| Other | 1100 (3.5%) | 62 (2.5%) |
| SV | 2348 (7.5%) | 786 (31.1%) |
| Left heart | 988 (3.2%) | 250 (9.9%) |
| Right heart | 806 (2.6%) | 352 (13.9%) |
| Other | 554 (1.8%) | 184 (7.3%) |
| Severity (two‐ventricle lesions) | ||
| Mild | 10 974 (35.3%) | 307 (12.2%) |
| Moderate | 10 833 (34.8%) | 584 (23.1%) |
| Severe 2V | 4252 (13.7%) | 593 (23.5%) |
| N/A | 2725 (8.8%) | 257 (10.2%) |
| Era | ||
| Early (1982–1992) | 9057 (29.1%) | 1063 (42.1%) |
| Mid (1993–1997) | 10 356 (33.3%) | 788 (31.2%) |
| Late (1998–2003) | 11 719 (37.6%) | 676 (26.8%) |
Numbers in parentheses express % unless otherwise specified. 2V indicates 2 ventricles; APVR, abnormal pulmonary venous return; AS/Sub‐AS, aortic stenosis/subaortic stenosis; ASD, atrial‐septal defect; CCAVC, complete common atrioventricular canal; CoA, coarctation of the aorta; Cor‐Tri, cor‐triatriatum; IAA, interrupted aortic arch; IQR, interquartile range; LHOL, left heart obstructive lesions; L‐R Shunt, left‐to‐right shunt lesions; MR/AI, mitral regurgitation/aortic insufficiency; MS, mitral stenosis; N/A, not classifiable; PA/IVS, pulmonary atresia with intact ventricular septum; PAPVR, partial APVR; PCCC, Pediatric Cardiac Care Consortium; PDA, patent ductus arteriosus; PS/Sub‐PS, pulmonary stenosis/subpulmonary stenosis; RVOTO, right ventricular outflow tract obstruction; SV, single ventricle; TAC, truncus arteriosus communis; TAPVR, total APVR; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; TVA, tricuspid valve anomaly; VSD, ventricular septal defect.
Underlying COD
The underlying COD was CHD in 58.8% of deaths, with most of these (79.5%) occurring before 5 years of age. Other frequent COD included cardiovascular disorders (CVD) (11.1%), and external causes of injury (8.2%) (Figure 1) followed by coexisting noncardiac congenital anomalies (4.7%), respiratory diseases (3.6%), infections (3.4%), and neoplasms (2.1%). All other conditions were less frequent and accounted for the remaining 8.2% of deaths combined (Table S2).
Figure 1.
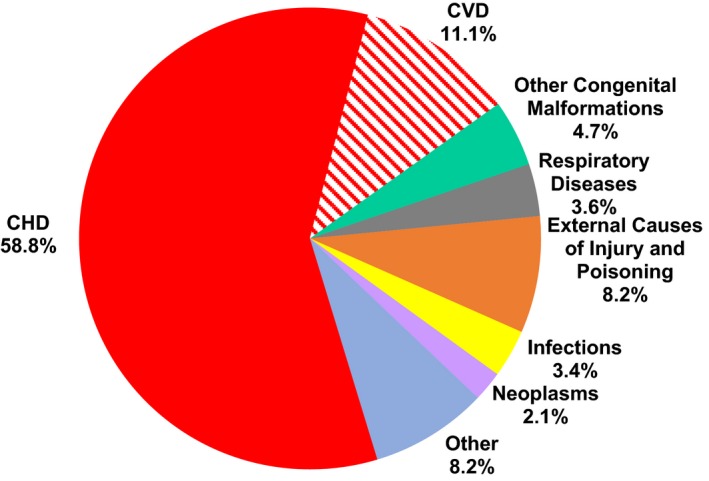
Underlying cause of death in patients undergoing congenital heart surgery. CHD indicates congenital heart defects; CVD, cardiovascular disorders.
CHD/CVD was the leading underlying COD in all patients up to 34 years of age; however, the relative percentage of deaths caused by CHD declined for both sexes as patients aged, while all other causes became more common after 20 years of age (Figure 2 and Table S3). Landmark analysis based on time after the first CHS revealed a similar trend with significant drop of the percentage of deaths caused by CHD at the 90‐day, 1‐year, and 5‐year mark followed by a slower rate of decline thereafter (Table S4). An almost reverse trend with increased percentage of deaths caused by CVD, neoplasms, and external causes was noted with longer follow‐up time post initial CHS. There is also a milder trend towards higher incidence of fatal respiratory conditions over time, while the percentage of deaths from associated malformations and infections remained relatively constant over the follow‐up period.
Figure 2.

Underlying cause of death by age and sex (Female: A, Male: B). CHD indicates congenital heart defects; CVD, cardiovascular disorders.
Contributing COD
Overall, when examining contributing COD, over two thirds of deaths (n=1722, 68.1%) had at least 1 ICD code defining CHD as the underlying or multiple COD (Figure 3A, 3B, and Table S5). CVD was listed as multiple COD in over half of all CHD‐related deaths (54.1%) but only in 29.5% of the non‐CHD/non‐CVD deaths. The most frequent CVD listings were cardiac arrest (16.8%), heart failure (14.8%), and arrhythmias/conduction abnormalities (9.1%). Comparing frequency of multiple COD in patients over age 5 years (relative to those younger) revealed a relative decrease in cardiac arrest of 22.0% and heart failure by 19.5%, but an increase in arrhythmias by 38.8% and pulmonary heart disease by 13.8% (Table S6). Deaths coded as unrelated to CHD or CVD (Figure 3A and Table S7) were identified in 424 patients (6.8%). Most of these deaths were attributed to external causes but a significant number of them were attributed to coexisting malformations, respiratory diseases, and neoplasms (9%–10% each).
Figure 3.
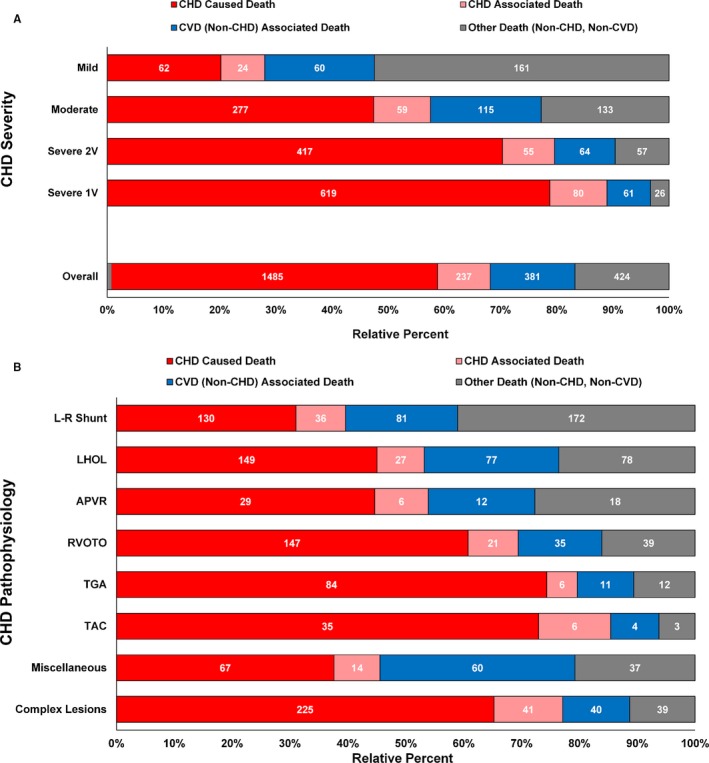
A, Underlying or multiple causes of death by CHD severity. 1V, 1 ventricle; 2V, 2 ventricles. B, Underlying or multiple causes of death by CHD pathophysiology. APVR indicates abnormal pulmonary venous return; CHD, congenital heart defects; CVD, cardiovascular disorders; L‐R Shunt, left‐to‐right shunt lesions; LHOL, left heart obstructive lesions; RVOTO, right ventricular outflow tract obstruction; TAC, truncus arteriosus communis; TGA, transposition of the great arteries. Length of bars represents the relative percentage of death from a specific cause while the counts reflect the actual number of deaths in each category.
Comparison to the General US Population
Underlying COD was compared with the general US population, adjusted for age, sex, and year of death (Table 2). In patients with operated CHD, the risk of death caused by CHD or CVD was 67.7 times higher (95% CI: 64.5–70.8) than the general population. The separate SMRs from CHD or CVD are displayed in Table S8. The overall SMR for CHD/CVD ranged from 17.6 for patients with mild CHD to 157.1 for severe two‐ventricle and 501.9 for single ventricle (SV). The increased SMR for CHD/CVD death peaked among death events between 1 and 4 years of age, but declined steadily thereafter, reaching 7.58 (95% CI: 5.63–9.53) among deaths between 25 and 34 years of age. SMRs for underlying COD by major physiology groups are presented in Table S9.
Table 2.
Cause‐Specific SMR for Major Category Groupsa
| Underlying Cause of Death | Group | N | SMR (95%) | 95% CI | P Value |
|---|---|---|---|---|---|
| CHD or CVD | Overall | 1765 | 67.7 | (64.5–70.8) | <0.001 |
| <1 y | 819 | 107.9 | (100.5–115.3) | <0.001 | |
| 1–4 y | 469 | 201.4 | (183.2–219.6) | <0.001 | |
| 5–9 y | 117 | 118.5 | (97.1–140.0) | <0.001 | |
| 10–14 y | 104 | 84.7 | (68.4–101.0) | <0.001 | |
| 15–19 y | 118 | 50.9 | (41.7–60.1) | <0.001 | |
| 20–24 y | 70 | 32.4 | (24.8–40.0) | <0.001 | |
| 25–34 y | 58 | 7.58 | (5.63–9.53) | <0.001 | |
| Mild CHD | 97 | 17.6 | (14.1–21.1) | <0.001 | |
| Moderate CHD | 360 | 56.3 | (50.5–62.1) | <0.001 | |
| Severe 2V CHD | 465 | 157.1 | (142.8–171.4) | <0.001 | |
| Severe 1V CHD | 673 | 501.9 | (463.9–539.8) | <0.001 | |
| Females | 748 | 85.3 | (79.2–91.3) | <0.001 | |
| Males | 1017 | 58.7 | (55.1–62.4) | <0.001 | |
| Early Erab | 632 | 167.6 | (154.5–180.7) | <0.001 | |
| Mid Erab | 506 | 101.6 | (92.8–110.5) | <0.001 | |
| Late Erab | 475 | 81.3 | (74.0–88.6) | <0.001 | |
| Other congenital malformations | Overall | 118 | 7.00 | (5.74–8.27) | <0.001 |
| <1 y | 46 | 3.83 | (2.73–4.94) | <0.001 | |
| 1–4 y | 36 | 20.5 | (13.8–27.2) | <0.001 | |
| 5–9 y | 8 | 9.42 | (2.89–15.9) | 0.012 | |
| 10–14 y | 5 | 7.28 | (0.90–13.7) | 0.054 | |
| 15–19 y | 8 | 14.1 | (4.32–23.8) | 0.009 | |
| 20–24 y | 7 | 17.9 | (4.63–31.1) | 0.013 | |
| 25–34 y | 8 | 15.2 | (4.66–25.7) | 0.008 | |
| Mild CHD | 27 | 7.38 | (4.60–10.2) | <0.001 | |
| Moderate CHD | 38 | 6.76 | (4.61–8.91) | <0.001 | |
| Severe 2V CHD | 20 | 4.68 | (2.63–6.73) | <0.001 | |
| Severe 1V CHD | 21 | 9.96 | (5.70–14.2) | <0.001 | |
| Females | 48 | 6.78 | (4.86–8.69) | <0.001 | |
| Males | 70 | 7.17 | (5.49–8.85) | <0.001 | |
| Early Erab | 31 | 6.60 | (4.28–8.24) | <0.001 | |
| Mid Erab | 43 | 8.45 | (5.94–11.00) | <0.001 | |
| Late Erab | 29 | 4.81 | (3.06–6.56) | <0.001 | |
| Respiratory diseases | Overall | 92 | 8.24 | (6.55–9.92) | <0.001 |
| <1 y | 27 | 10.9 | (6.78–15.0) | <0.001 | |
| 1–4 y | 25 | 14.8 | (9.02–20.6) | <0.001 | |
| 5–9 y | 8 | 8.50 | (2.61–14.39) | 0.013 | |
| 10–y | 6 | 5.51 | (1.10–9.92) | 0.045 | |
| 15–19 y | 7 | 6.71 | (1.74–11.68) | 0.024 | |
| 20–24 y | 10 | 9.70 | (3.69–15.72) | 0.005 | |
| 25–34 y | 8 | 3.50 | (1.07–5.92) | 0.043 | |
| Mild CHD | 22 | 5.96 | (3.47–8.45) | <0.001 | |
| Moderate CHD | 23 | 5.96 | (3.52–8.39) | <0.001 | |
| Severe 2V CHD | 24 | 14.3 | (8.55–19.9) | <0.001 | |
| Severe 1V CHD | 14 | 18.7 | (8.90–28.5) | <0.001 | |
| Females | 40 | 8.77 | (6.05–11.49) | <0.001 | |
| Males | 52 | 7.87 | (5.73–10.01) | <0.001 | |
| Early Erab | 26 | 10.5 | (6.48–14.6) | <0.001 | |
| Mid Erab | 19 | 7.49 | (4.12–10.86) | <0.001 | |
| Late Erab | 29 | 11.0 | (7.00–15.0) | <0.001 | |
| Infections | Overall | 85 | 8.16 | (6.42–9.89) | <0.001 |
| <1 y | 27 | 14.1 | (8.76–19.4) | <0.001 | |
| 1–4 y | 28 | 18.8 | (11.9–25.8) | <0.001 | |
| 5–9 y | 1 | 1.36 | (0–4.01) | 0.793 | |
| 10–14 y | 8 | 13.7 | (4.21–23.2) | 0.009 | |
| 15–19 y | 11 | 16.5 | (6.75–26.3) | 0.002 | |
| 20–24 y | 5 | 5.40 | (0.67–10.1) | 0.068 | |
| 25–34 y | 2 | 0.61 | (0–1.46) | 0.373 | |
| Mild CHD | 12 | 3.38 | (1.47–5.29) | 0.015 | |
| Moderate CHD | 20 | 5.51 | (3.15–8.07) | <0.001 | |
| Severe 2V CHD | 22 | 16.5 | (9.57–23.3) | <0.001 | |
| Severe 1V CHD | 24 | 41.2 | (24.7–57.7) | <0.001 | |
| Females | 41 | 9.86 | (6.84–12.87) | <0.001 | |
| Males | 44 | 7.03 | (4.95–9.10) | <0.001 | |
| Early Erab | 18 | 7.43 | (4.00–10.9) | <0.001 | |
| Mid Erab | 29 | 13.1 | (8.30–17.8) | <0.001 | |
| Late Erab | 23 | 12.3 | (7.24–17.3) | <0.001 | |
| Neoplasms | Overall | 53 | 1.91 | (1.39–2.43) | <0.001 |
| <1 y | 1 | 2.51 | (0–7.44) | 0.547 | |
| 1–4 y | 9 | 3.54 | (1.23–5.87) | 0.031 | |
| 5–9 y | 7 | 1.99 | (0.52–3.47) | 0.188 | |
| 10–14 y | 8 | 2.43 | (0.75–4.11) | 0.096 | |
| 15–19 y | 10 | 2.85 | (1.08–4.61) | 0.040 | |
| 20–24 y | 7 | 2.27 | (0.59–3.94) | 0.140 | |
| 25–34 y | 8 | 0.98 | (0.30–1.66) | 0.949 | |
| Mild CHD | 18 | 1.66 | (0.89–2.43) | 0.092 | |
| Moderate CHD | 16 | 1.67 | (0.85–2.49) | 0.109 | |
| Severe 2V CHDc | 10 | 3.78 | (1.44–6.12) | 0.020 | |
| Severe 1V CHDc | 3 | 2.82 | (0–6.01) | 0.264 | |
| Females | 23 | 1.82 | (1.08–2.57) | 0.030 | |
| Males | 30 | 1.99 | (1.23–2.70) | <0.001 | |
| Early Erab | 12 | 2.57 | (1.11–4.00) | 0.035 | |
| Mid Erab | 12 | 2.39 | (1.04–3.75) | 0.044 | |
| Late Erab | 13 | 2.56 | (1.18–3.98) | 0.027 | |
| External causes | Overall | 207 | 1.08 | (0.93–1.23) | 0.293 |
| <1 y | 17 | 4.75 | (2.49–7.01) | 0.001 | |
| 1– 4 y | 22 | 1.72 | (1.00–2.44) | 0.049 | |
| 5– 9 y | 30 | 3.28 | (2.10–4.44) | <0.001 | |
| 10–14 y | 16 | 1.38 | (0.70–2.05) | 0.272 | |
| 15–19 y | 44 | 0.96 | (0.68–1.25) | 0.792 | |
| 20–24 y | 42 | 0.95 | (0.66–1.23) | 0.708 | |
| 25–34 y | 32 | 0.55 | (0.36–0.74) | <0.001 | |
| Mild CHD | 70 | 1.00 | (0.76–1.23) | 0.978 | |
| Moderate CHD | 68 | 0.98 | (0.75–1.21) | 0.869 | |
| Severe 2V CHD | 25 | 1.34 | (0.82–1.87) | 0.201 | |
| Severe 1V CHD | 16 | 2.38 | (1.22–3.55) | 0.020 | |
| Females | 40 | 1.05 | (0.76–1.36) | 0.759 | |
| Males | 157 | 1.09 | (0.91–1.26) | 0.301 | |
| Early Erab | 55 | 1.78 | (1.31–2.25) | 0.001 | |
| Mid Erab | 39 | 1.22 | (0.84–1.61) | 0.253 | |
| Late Erab | 43 | 1.46 | (1.03–1.90) | 0.039 | |
| Other medical causes | Overall | 207 | 2.06 | (1.76–2.34) | <0.001 |
| <1 y | 57 | 0.98 | (0.73–1.24) | 0.907 | |
| 1–4 y | 44 | 7.62 | (5.37–9.87) | <0.001 | |
| 5–9 y | 18 | 5.21 | (2.80–7.61) | <0.001 | |
| 10–14 y | 19 | 4.79 | (2.64–6.95) | <0.001 | |
| 15–19 y | 25 | 4.48 | (2.72–6.24) | <0.001 | |
| 20–24 y | 24 | 3.85 | (2.31–5.38) | <0.001 | |
| 25–34 y | 20 | 1.41 | (0.78–2.03) | 0.192 | |
| Mild CHD | 61 | 2.38 | (1.78–2.96) | <0.001 | |
| Moderate CHD | 59 | 1.75 | (1.30–2.20) | 0.001 | |
| Severe 2V CHD | 27 | 1.25 | (0.78–1.72) | <0.001 | |
| Severe 1V CHD | 35 | 3.36 | (2.25–4.47) | <0.001 | |
| Females | 80 | 2.05 | (1.60–2.50) | <0.001 | |
| Males | 127 | 2.06 | (1.70–2.42) | <0.001 | |
| Early Erab | 53 | 2.20 | (1.60–2.79) | <0.001 | |
| Mid Erab | 60 | 2.41 | (1.80–3.02) | <0.001 | |
| Late Erab | 52 | 1.71 | (1.25–2.18) | 0.003 |
1V indicates 1 ventricle; 2V, 2 ventricles; CHD, congenital heart defects; CI, confidence interval; CVD, cardiovascular disorder; SMR, standardized mortality ratio.
Overall SMR and SMRs by era and CHD severity are adjusted for sex, year of death, and age at death. SMRs by sex are adjusted for age at death and year of death. SMRs by age strata are adjusted for year of death and sex.
Deaths occurring after 15 years of follow‐up were not included in the SMR calculations for era. This truncation was necessary for making follow‐up time comparable among eras. As a result, death counts across eras do not sum to total death count for a specific cause.
Combined SMR for severe CHD: 3.50 (95% CI: 1.6–5.51) (P=0.001).
Significantly increased SMRs were also noted for other COD such as associated congenital malformations (SMR 7.0; 95% CI: 5.74–8.27), respiratory diseases (SMR 8.24; 95% CI: 6.55–9.92), infections (SMR 8.16; 95% CI: 6.42–9.89), and neoplasms (SMR 1.91; 95% CI: 1.39–2.43) (Table 2). Additionally, there were age‐specific differences in risk of death caused by neoplasms and external causes. The higher risk from neoplasms affected only ages 1 to 4 years and 15 to 19 years, most notably in the group with severe CHD. Finally, risk of death from external causes did not differ overall from the general population, but it was increased for ages <10 years and decreased for ages 25 to 34 years. In a sensitivity analysis excluding patients with an ICD code referring to events associated with surgical care (some of which may refer to operations for CHD), the differential risk persisted for those aged 5 to 9 years and 25 to 34 years (Table S10). Of interest, the proportion of deaths from motor vehicle accidents was higher, while the proportion for homicide/assaults was lower, for CHD patients >20 years of age compared with the general population (Table S11).
Comparison of Causes of Death by Patient Characteristics
The risk of death caused by CHD/CVD was higher in women (SMR in females 85.3; 95% CI: 79.2–91.3 versus men 58.7; 95% CI: 55.1–62.4) (Table 2). Adjusted odds ratios between females and males revealed increased odds of death from a CHD or CVD in females [odds ratio=1.28; 95% CI (1.04–1.58); P=0.018]. Women had higher percentage of deaths caused by contribution from pulmonary heart disease, while men were more prone to arrhythmias, but after adjustment for severity none of these reached significance (Table S5). Stratified analysis by age did not reveal any significant difference between females and males during the reproductive age between 20 and 34 years of age. In addition, there were only 3 events coded as deaths associated with pregnancy, childbirth, and the puerperium.
Adjusted odds ratios for COD by race demonstrated that blacks had mild decrease in the odds of deaths from neoplasms relative to whites, but increased odds of death from “other” causes. There was no difference in the percentage of the various contributing causes of death between whites and blacks (Table S12).
Most deaths in patients with moderate and severe CHD were associated with CHD/CVD (57.5% for moderate, 79.6% for severe two‐ventricle, and 88.9% for SV) (Figure 3A and Table S13). For patients with mild CHD, 47.5% of death records included CHD or CVD among the multiple causes of death. Patent ductus arteriosus was the condition with the lowest frequency of CHD/CVD–associated deaths. Frequently reported CVDs as multiple COD included heart failure, cardiac arrest, and arrhythmias, but with considerable variation across the spectrum of CHD (Table S14). The in‐depth analysis of the multiple COD is outside of the scope of this report.
An era effect was observed on the CHD/CVD–related risk of death with progressive decline over time across all categories of CHD. This decline was driven mostly by decreases in CHD‐related mortality, while CVD‐related risk of death remained relatively constant over time (Table S15).
Discussion
Risk of Death From CHD and Cardiovascular Causes of Death
Our data show the majority of premature mortality in patients surviving to hospital discharge after CHS is CHD related and occurs before 5 years of age (Figure S1) with a gradual decline as the follow‐up time increases after the first operation. Much of this may reflect perioperative mortality following subsequent procedures, as patients undergoing staged surgical strategies are often reoperated within this age range.
Mortality from cardiovascular conditions not directly linked to the underlying CHD was the next most frequent cause of death, and was higher than the general population across all severity groups, even for mild forms of CHD. The excess risk for CHD/CVD mortality persisted throughout the follow‐up period, but gradually decreased over time.
Among the CVD conditions contributing to death, heart failure, arrhythmias, and conduction abnormalities were most frequent, highlighting the significant residual cardiovascular morbidity. Pulmonary heart disease, cerebrovascular conditions, and myocardial ischemia were less frequently reported. Over time after CHS, there was a shift from CHD and cardiac arrests towards more heart failure and arrhythmia‐related deaths. As expected, there was considerable variation in the underlying and contributing COD between individual lesions, reflecting the differences in their underlying physiology, severity, and residual abnormalities. The detailed analysis of this variation is outside the scope of this report.
The higher risk of death from CHD in females after discharge parallels our observations of in‐hospital deaths after CHS.36 A potential cause for that differential risk of death caused by CHD may be pregnancy‐related events; however, the available number of events could not confirm this hypothesis. There were no sex differences for CVD‐related deaths besides a trend towards more pulmonary heart disease–related deaths in females consistent with their reported higher incidence of pulmonary hypertension.37
Non‐CHD/Non‐CVD Causes of Death
In contrast to the CHD/CVD causes, risk of death from noncardiovascular causes generally increased with age. Among these causes, excess risk above the general population was noted for respiratory and infectious conditions. This is not surprising given the close relationship between cardiovascular and respiratory health, as well the increased vulnerability of these patients to infectious processes.38, 39, 40 Similarly, the increased risk of death from neoplastic processes in patients with operated CHD has been observed before.19, 38 However, the large number of patients in our cohort allowed us to demonstrate an age‐dependent risk of death from neoplasms and identify groups at highest risk. This excess risk may reflect increased incidence of certain forms of cancer, or increased vulnerability to complications of cancer treatment, or both.41, 42, 43
Risk of death from coexisting congenital anomalies was consistently increased across all age groups and likely reflects the high incidence of such extracardiac abnormalities in this group of patients.44
Interestingly, an age‐dependent differential risk was noted for external causes of death. Not previously described, the higher risk from external causes in the group of 5 to 9 years of age suggests increased vulnerability to injuries and accidents during young childhood, when little control can be successfully applied over such exposures. On the other hand, the risk from external causes is moderated in the 25 to 34 years of age group, possibly because of activity restrictions, self‐imposed limitations, decreased involvement in risky behaviors, or self‐selection of a less‐at‐risk subgroup. Suicidal risk was elevated in an adult cohort with tetralogy of Fallot in Taiwan,26 but was not a substantial source of death in our cohort.
Trends in Modality of Death
Comparing eras in this cohort demonstrates a promising trend towards decreasing CHD mortality. On the other hand, the persistently elevated CVD‐related risk in each era and across all severity forms of CHD suggests that postoperative cardiovascular sequelae continue to impose a significant burden despite improvements in treating CHD. Moreover, the excess mortality from other causes remained unchanged over time, suggesting limited progress in the management or prevention of these additional non‐CHD morbidities.
Comparison With Other Studies
Our findings are similar to those from the population‐based study in Finland,8 the only other large study of modes of death among patients with operated CHD. This study included about 11 000 patients and spanned over 60 years; however, most of these patients had lesions of mild or uncharacterized severity, and only a few of them have complex or single‐ventricle forms of CHD. In contrast to the Finnish study, we found the effect of era on CHD‐related mortality was seen across most CHD lesions and not just ventricular septal defect, perhaps related to the larger sample size or ability to distinguish CHD‐ versus CVD‐related deaths in our cohort.
Other studies on this subject are not directly comparable to the PCCC and Finnish studies because they include a mixed population of adult‐only patients with operated and unoperated CHD.7, 9, 12, 34, 45, 46, 47, 48 However, despite these methodological differences, the overall distribution of COD was very similar, at least for the lesions with sufficient numbers of patients.
Limitations
Limitations of this study are those inherent to its registry‐based, retrospective nature. As a result, the number of eligible patients who survive to discharge by era may reflect the number and size of centers contributing data to the registry during the different era as well as the increased number of patients operated and surviving operations over time. In addition, information regarding subsequent procedures, residual defects, socioeconomic data, and lifestyle exposures is limited.
An additional limitation is the quality of COD codes available in the NDI‐Plus; few deaths in our cohort fell in the unknown category, so this is unlikely to significantly affect the findings of our study, but there are other known limitations of this resource including risk for misclassification of chronic conditions, attribution errors, and use of mode instead of actual COD.49 Nevertheless, the NDI has been used extensively to understand COD across a wide range of conditions and has been generally found to provide meaningful results.50 Of importance for our study comparing COD between different pathophysiologies, there is no reason to believe that misattribution of COD would have been differential by lesion.
Despite these limitations, the concordance of our findings with similar studies supports the validity of the methodology used. As the PCCC contains a much larger number of patients than previously reported studies, the data suggest that our approach can be used with confidence to understand the specific risks associated with even the rarest CHD for which other cohorts contain very few events or no data at all.
Operative techniques and medical care continue to evolve; thus, long‐term outcomes and cause‐specific risks are expected to change over time. Experience has shown that apart from conditions with radical changes in their management, treatment for many conditions evolves gradually without major shifts in risk. Continuous monitoring of our cohort will allow us to identify major risks in survivors with operated CHD and focus on strategies that will reduce their hazards.
Conclusions
Survivors of CHS face long‐term risks for premature mortality, with deaths attributed most often to residual CHD pathology, heart failure, and arrhythmias, but also to other noncardiac conditions. As a result, ongoing monitoring of this population is warranted and additional research is needed to identify modifiable factors that can be targeted to address residual morbidities and improve long‐term outcomes.
Sources of Funding
This study was supported by National Heart, Lung, and Blood Institute R01 HL122392 and NIH CTSA Award UL1TR000114.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods.
Table S1. ICD‐9 and ICD‐10 Codes Associated with Cause of Death Groupings
Table S2. Breakdown of “Other” Underlying Causes of Death
Table S3. Underlying Cause of Death by Age‐Sex Stratification
Table S4. Underlying Cause of Death by Time Since Initial Congenital Heart Surgery
Table S5. Frequency of CHD and CVD Codes as Contributing Causes of Death
Table S6. Summary Statistics for Multiple Causes of Death*
Table S7. Underlying Cause of Death in Patients With Non‐CHD/non‐CVD‐Associated Death
Table S8. SMRs for Congenital Heart Defects (CHD) or Cardiovascular Disorders (CVD)
Table S9. SMR for Underlying Causes of Death by Major Physiology Group
Table S10. SMR for External Causes of Death Excluding Surgical Misadventures
Table S11. List of External Causes of Death by Age Group and Estimates from Age‐Matched US Population of Deaths by External Causes from 1981 to 2014
Table S12. Distribution of Underlying and Multiple Causes of Death by Race
Table S13. Modes of Death by CHD Group
Table S14. Underlying and Contributing Causes of Death by CHD Group
Table S15. Era Effect on the SMR for CVD/CHD‐Associated Death by Physiology Group
Figure S1. Distribution of cumulative causes of death after the first congenital heart surgery (CHS).
Acknowledgments
We thank the program directors and data collection coordinators from the participating Pediatric Cardiac Care Consortium (PCCC) centers; without their effort and dedication, this work could not have been completed. Susan Anderson and Brian Harvey were especially instrumental in the management of PCCC and initial linkage of this cohort with mortality information.
(J Am Heart Assoc. 2018;7:e010624 DOI: 10.1161/JAHA.118.010624)
References
- 1. Erikssen G, Liestol K, Seem E, Birkeland S, Saatvedt KJ, Hoel TN, Dohlen G, Skulstad H, Svennevig JL, Thaulow E, Lindberg HL. Achievements in congenital heart defect surgery: a prospective, 40‐year study of 7038 patients. Circulation. 2015;131:337–46; discussion 346. [DOI] [PubMed] [Google Scholar]
- 2. Larsen SH, Olsen M, Emmertsen K, Hjortdal VE. Interventional treatment of patients with congenital heart disease: nationwide Danish experience over 39 years. J Am Coll Cardiol. 2017;69:2725–2732. [DOI] [PubMed] [Google Scholar]
- 3. Raissadati A, Nieminen H, Jokinen E, Sairanen H. Progress in late results among pediatric cardiac surgery patients: a population‐based 6‐decade study with 98% follow‐up. Circulation. 2015;131:347–353. [DOI] [PubMed] [Google Scholar]
- 4. Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas LK. Trends in long‐term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vinocur JM, Moller JH, Kochilas LK. Putting the Pediatric Cardiac Care Consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes. 2012;5:577–579. [DOI] [PubMed] [Google Scholar]
- 6. Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population‐based study. J Am Coll Cardiol. 2007;50:1263–1271. [DOI] [PubMed] [Google Scholar]
- 7. Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–1116. [DOI] [PubMed] [Google Scholar]
- 8. Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E. Late causes of death after pediatric cardiac surgery: a 60‐year population‐based study. J Am Coll Cardiol. 2016;68:487–498. [DOI] [PubMed] [Google Scholar]
- 9. Zomer AC, Vaartjes I, Uiterwaal CS, van der Velde ET, van den Merkhof LF, Baur LH, Ansink TJ, Cozijnsen L, Pieper PG, Meijboom FJ, Grobbee DE, Mulder BJ. Circumstances of death in adult congenital heart disease. Int J Cardiol. 2012;154:168–172. [DOI] [PubMed] [Google Scholar]
- 10. Lui GK, Rogers IS, Ding VY, Hedlin HK, MacMillen K, Maron DJ, Sillman C, Romfh A, Dade TC, Haeffele C, Grady SR, McElhinney DB, Murphy DJ, Fernandes SM. Risk estimates for atherosclerotic cardiovascular disease in adults with congenital heart disease. Am J Cardiol. 2017;119:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot N, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11:e102–e165. [DOI] [PubMed] [Google Scholar]
- 12. Engelings CC, Helm PC, Abdul‐Khaliq H, Asfour B, Bauer UM, Baumgartner H, Kececioglu D, Korten MA, Diller GP, Tutarel O. Cause of death in adults with congenital heart disease—an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol. 2016;211:31–36. [DOI] [PubMed] [Google Scholar]
- 13. Madsen NL, Marino BS, Woo JG, Thomsen RW, Videboek J, Laursen HB, Olsen M. Congenital heart disease with and without cyanotic potential and the long‐term risk of diabetes mellitus: a population‐based follow‐up study. J Am Heart Assoc. 2016;5:e003076 DOI: 10.1161/JAHA.115.003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dimopoulos K, Diller GP, Koltsida E, Pijuan‐Domenech A, Papadopoulou SA, Babu‐Narayan SV, Salukhe TV, Piepoli MF, Poole‐Wilson PA, Best N, Francis DP, Gatzoulis MA. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–2328. [DOI] [PubMed] [Google Scholar]
- 15. Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, Sainani NI, Hill AJ, Odze RD, Johncilla ME, Ukomadu C, Gauvreau K, Valente AM, Landzberg MJ; Alliance for Adult Research in Congenital Cardiology I . Liver health in adults with Fontan circulation: a multicenter cross‐sectional study. J Thorac Cardiovasc Surg. 2017;153:656–664. [DOI] [PubMed] [Google Scholar]
- 16. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, Cetta F. 40‐year follow‐up after the Fontan operation: long‐term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. [DOI] [PubMed] [Google Scholar]
- 17. Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385–2394. [DOI] [PubMed] [Google Scholar]
- 18. Webb CL, Jenkins KJ, Karpawich PP, Bolger AF, Donner RM, Allen HD, Barst RJ; Congenital Cardiac Defects Committee of the American Heart Association Section on Cardiovascular Disease in the Young . Collaborative care for adults with congenital heart disease. Circulation. 2002;105:2318–2323. [DOI] [PubMed] [Google Scholar]
- 19. Gurvitz M, Ionescu‐Ittu R, Guo L, Eisenberg MJ, Abrahamowicz M, Pilote L, Marelli AJ. Prevalence of cancer in adults with congenital heart disease compared with the general population. Am J Cardiol. 2016;118:1742–1750. [DOI] [PubMed] [Google Scholar]
- 20. Opotowsky AR, Moko LE, Ginns J, Rosenbaum M, Greutmann M, Aboulhosn J, Hageman A, Kim Y, Deng LX, Grewal J, Zaidi AN, Almansoori G, Oechslin E, Earing M, Landzberg MJ, Singh MN, Wu F, Vaidya A. Pheochromocytoma and paraganglioma in cyanotic congenital heart disease. J Clin Endocrinol Metab. 2015;100:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen S, Liu A, Gurvitz M, Guo L, Therrien J, Laprise C, Kaufman JS, Abrahamowicz M, Marelli AJ. Exposure to low‐dose ionizing radiation from cardiac procedures and malignancy risk in adults with congenital heart disease. Circulation. 2018;137:1334–1345. [DOI] [PubMed] [Google Scholar]
- 22. Beausejour Ladouceur V, Lawler PR, Gurvitz M, Pilote L, Eisenberg MJ, Ionescu‐Ittu R, Guo L, Marelli AJ. Exposure to low‐dose ionizing radiation from cardiac procedures in patients with congenital heart disease: 15‐year data from a population‐based longitudinal cohort. Circulation. 2016;133:12–20. [DOI] [PubMed] [Google Scholar]
- 23. Koos R, Mahnken AH, Muhlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kuhl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–749. [DOI] [PubMed] [Google Scholar]
- 24. Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–164. [DOI] [PubMed] [Google Scholar]
- 25. Deng LX, Khan AM, Drajpuch D, Fuller S, Ludmir J, Mascio CE, Partington SL, Qadeer A, Tobin L, Kovacs AH, Kim YY. Prevalence and correlates of post‐traumatic stress disorder in adults with congenital heart disease. Am J Cardiol. 2016;117:853–857. [DOI] [PubMed] [Google Scholar]
- 26. Chiu SN, Wang JK, Chen HC, Lin MT, Wu ET, Chen CA, Huang SC, Chang CI, Chen YS, Chiu IS, Chen CL, Wu MH. Long‐term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–125. [DOI] [PubMed] [Google Scholar]
- 27. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high‐risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–38. [DOI] [PubMed] [Google Scholar]
- 28. Gurvitz M, Burns KM, Brindis R, Broberg CS, Daniels CJ, Fuller SM, Honein MA, Khairy P, Kuehl KS, Landzberg MJ, Mahle WT, Mann DL, Marelli A, Newburger JW, Pearson GD, Starling RC, Tringali GR, Valente AM, Wu JC, Califf RM. Emerging research directions in adult congenital heart disease: a report from an NHLBI/ACHA Working Group. J Am Coll Cardiol. 2016;67:1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vinocur JM, Menk JS, Connett J, Moller JH, Kochilas LK. Surgical volume and center effects on early mortality after pediatric cardiac surgery: 25‐year North American experience from a multi‐institutional registry. Pediatr Cardiol. 2013;34:1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spector LG, Menk JS, Vinocur JM, Oster ME, Harvey BA, St Louis JD, Moller J, Kochilas LK. In‐hospital vital status and heart transplants after intervention for congenital heart disease in the pediatric cardiac care consortium: completeness of ascertainment using the national death index and united network for organ sharing datasets. J Am Heart Assoc. 2016;5:e003783 DOI: 10.1161/JAHA.116.003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L. Trends in long‐term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bilgrad R, National Center for Health Statistics. National death index plus: coded causes of death: supplement to the national death index user's manual. Hyattsville, MD: Division of Vital Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention;1999.
- 33. Shi G, Zhu Z, Chen J, Ou Y, Hong H, Nie Z, Zhang H, Liu X, Zheng J, Sun Q, Liu J, Chen H, Zhuang J. Total anomalous pulmonary venous connection: the current management strategies in a pediatric cohort of 768 patients. Circulation. 2017;135:48–58. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention, National Center for Health Statistics . Compressed Mortality File 1999–2015 on CDC WONDER Online Database, released December 2016. Data are from the Compressed Mortality File 1999‐2015 Series 20 No. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed June 5, 2017.
- 35. Collaborative data science. Plotly Technologies Inc. Montréal, QC, 2015. https://plot.ly.
- 36. Kochilas LK, Vinocur JM, Menk JS. Age‐dependent sex effects on outcomes after pediatric cardiac surgery. J Am Heart Assoc. 2014;3:e000608 DOI: 10.1161/JAHA.113.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 38. Raissadati A, Nieminen H, Sairanen H, Jokinen E. Outcomes after the Mustard, Senning and arterial switch operation for treatment of transposition of the great arteries in Finland: a nationwide 4‐decade perspective. Eur J Cardiothorac Surg. 2017;52:573–580. [DOI] [PubMed] [Google Scholar]
- 39. Alonso‐Gonzalez R, Borgia F, Diller GP, Inuzuka R, Kempny A, Martinez‐Naharro A, Tutarel O, Marino P, Wustmann K, Charalambides M, Silva M, Swan L, Dimopoulos K, Gatzoulis MA. Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation. 2013;127:882–890. [DOI] [PubMed] [Google Scholar]
- 40. Mylotte D, Rushani D, Therrien J, Guo L, Liu A, Guo K, Martucci G, Mackie AS, Kaufman JS, Marelli A. Incidence, predictors, and mortality of infective endocarditis in adults with congenital heart disease without prosthetic valves. Am J Cardiol. 2017;120:2278–2283. [DOI] [PubMed] [Google Scholar]
- 41. Altmann AE, Halliday JL, Giles GG. Associations between congenital malformations and childhood cancer. A register‐based case‐control study. Br J Cancer. 1998;78:1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andreassi MG, Ait‐Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long‐term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–2708. [DOI] [PubMed] [Google Scholar]
- 43. Cohen S, Liu A, Gurvitz M, Guo L, Therrien J, Laprise C, Kaufman JS, Abrahamowicz M, Marelli AJ. Exposure to low‐dose ionizing radiation from cardiac procedures and malignancy risk in adults with congenital heart disease. Circulation. 2018;137:1334–1345. [DOI] [PubMed] [Google Scholar]
- 44. Stoll C, Dott B, Alembik Y, Roth MP. Associated noncardiac congenital anomalies among cases with congenital heart defects. Eur J Med Genet. 2015;58:75–85. [DOI] [PubMed] [Google Scholar]
- 45. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 46. Oliver JM, Gallego P, Gonzalez AE, Garcia‐Hamilton D, Avila P, Alonso A, Ruiz‐Cantador J, Peinado R, Yotti R, Fernandez‐Aviles F. Impact of age and sex on survival and causes of death in adults with congenital heart disease. Int J Cardiol. 2017;245:119–124. [DOI] [PubMed] [Google Scholar]
- 47. Silka MJ, Hardy BG, Menashe VD, Morris CD. A population‐based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245–251. [DOI] [PubMed] [Google Scholar]
- 48. Pillutla P, Shetty KD, Foster E. Mortality associated with adult congenital heart disease: trends in the US population from 1979 to 2005. Am Heart J. 2009;158:874–879. [DOI] [PubMed] [Google Scholar]
- 49. Aggarwal B, Ellis SG, Lincoff AM, Kapadia SR, Cacchione J, Raymond RE, Cho L, Bajzer C, Nair R, Franco I, Simpfendorfer C, Tuzcu EM, Whitlow PL, Shishehbor MH. Cause of death within 30 days of percutaneous coronary intervention in an era of mandatory outcome reporting. J Am Coll Cardiol. 2013;62:409–415. [DOI] [PubMed] [Google Scholar]
- 50. Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Ann Epidemiol. 2001;11:46–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. ICD‐9 and ICD‐10 Codes Associated with Cause of Death Groupings
Table S2. Breakdown of “Other” Underlying Causes of Death
Table S3. Underlying Cause of Death by Age‐Sex Stratification
Table S4. Underlying Cause of Death by Time Since Initial Congenital Heart Surgery
Table S5. Frequency of CHD and CVD Codes as Contributing Causes of Death
Table S6. Summary Statistics for Multiple Causes of Death*
Table S7. Underlying Cause of Death in Patients With Non‐CHD/non‐CVD‐Associated Death
Table S8. SMRs for Congenital Heart Defects (CHD) or Cardiovascular Disorders (CVD)
Table S9. SMR for Underlying Causes of Death by Major Physiology Group
Table S10. SMR for External Causes of Death Excluding Surgical Misadventures
Table S11. List of External Causes of Death by Age Group and Estimates from Age‐Matched US Population of Deaths by External Causes from 1981 to 2014
Table S12. Distribution of Underlying and Multiple Causes of Death by Race
Table S13. Modes of Death by CHD Group
Table S14. Underlying and Contributing Causes of Death by CHD Group
Table S15. Era Effect on the SMR for CVD/CHD‐Associated Death by Physiology Group
Figure S1. Distribution of cumulative causes of death after the first congenital heart surgery (CHS).


