Abstract
Background
The duration of heightened stroke risk after acute myocardial infarction (MI) remains uncertain.
Methods and Results
We performed a retrospective cohort study using claims between 2008 and 2015 from a nationally representative 5% sample of Medicare beneficiaries aged ≥66 years. Both acute MI and ischemic stroke were ascertained using previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), diagnosis codes. To exclude periprocedural strokes from percutaneous coronary intervention, we did not count strokes occurring during an acute MI hospitalization. Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or September 30, 2015. We fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjustment for demographics, stroke risk factors, and Charlson comorbidities. We used the corresponding survival probabilities to compute the hazard ratio in each 4‐week interval after discharge. Confidence intervals were computed using the nonparametric bootstrap method. Among 1 746 476 eligible beneficiaries, 46 182 were hospitalized for acute MI and 80 466 for ischemic stroke. After adjustment for demographics, stroke risk factors, and Charlson comorbidities, the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (hazard ratio: 2.7; 95% confidence interval, 2.3–3.2), remained elevated during weeks 5 to 8 (hazard ratio: 2.0; 95% confidence interval, 1.6–2.4) and weeks 9 to 12 (hazard ratio: 1.6; 95% confidence interval, 1.3–2.0), and was no longer significantly elevated afterward.
Conclusions
Acute MI is associated with an elevated risk of ischemic stroke that appears to extend beyond the 1‐month window that is currently considered the at‐risk period.
Keywords: myocardial infarction; stroke; stroke prevention; stroke, ischemic
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Myocardial Infarction
Clinical Perspective
What Is New?
Acute myocardial infarction is associated with an elevated risk of ischemic stroke that extends beyond the 1‐month time window that is currently considered the at‐risk period.
What Are the Clinical Implications?
The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiological classification systems and clinical trial selection criteria.
Introduction
Stroke is a serious complication of acute myocardial infarction (MI).1, 2 Patients with ischemic stroke after acute MI have greater morbidity and higher rates of both short‐ and long‐term mortality compared with patients without stroke.1, 3, 4, 5, 6, 7 Acute MI is considered an etiological cause of ischemic stroke if it occurs within 1 month of the stroke,8, 9 and ongoing randomized clinical trials evaluating embolic strokes of undetermined source exclude patients with acute MI only during this time frame.10, 11 However, prior studies on the relationship between acute MI and ischemic stroke have not elucidated the precise duration of heightened stroke risk.6, 7, 12, 13, 14 We therefore aimed to assess the magnitude and duration of ischemic stroke risk following acute MI in a large, heterogeneous sample of patients. We hypothesized that acute MI would be independently associated with a heightened risk of ischemic stroke beyond the 1‐month window that is currently considered the at‐risk period.8, 9
Methods
Design
We performed a retrospective cohort study using inpatient and outpatient claims data from a 5% sample of Medicare beneficiaries. The US Centers for Medicare and Medicaid Services (CMS) provide health insurance to a large majority of American residents once they reach 65 years of age. CMS data sets are available for research and include data on claims submitted by hospitals and providers in the course of Medicare beneficiaries’ clinical care.15 Each claim includes the date of service and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes. A unique and anonymous identifier code can link multiple claims for a given patient, allowing for a comprehensive and longitudinal analysis of each beneficiary's care over time. The data reported in this study are available upon reasonable request from the corresponding author. The Weill Cornell Medicine institutional review board approved this study and waived the requirement for informed consent.
Patient Population
In keeping with standard practice in analyzing Medicare data,15 we limited our cohort to beneficiaries with continuous coverage in traditional fee‐for‐service Medicare (both Parts A and B) for at least 1 year (or until death, if applicable). Because Medicare eligibility generally begins at age 65 years, we included only patients aged ≥66 years to allow time for beneficiaries to enter medical care and for their providers to document any preexisting medical comorbidities.
Measurements
Our exposure variable was acute MI, defined using ICD‐9‐CM code 410.x1 in the first or second hospital discharge diagnosis position. Hospitalizations for MI lasting >180 days or <3 days were excluded. This algorithm has been validated as having a positive predictive value of 94% for acute MI compared with detailed medical chart review.16 Patients with multiple visits for acute MI were entered into the analysis at the time of their initial visit for acute MI. To focus on the post‐MI time period and to exclude any strokes that may have been due to percutaneous coronary intervention for the acute MI, we excluded patients who had a claim for an ischemic stroke before or during the index hospitalization for acute MI.
We ascertained demographic characteristics including age, sex, and race from the CMS denominator file. The following vascular risk factors and comorbidities were ascertained from ICD‐9‐CM codes: hypertension, diabetes mellitus, atrial fibrillation, congestive heart failure, valvular heart disease, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, alcohol abuse, and tobacco use. We also used ICD‐9‐CM codes to ascertain Charlson comorbidities.17
Outcome
All patients were followed for the primary outcome of ischemic stroke, defined as a hospitalization with ICD‐9‐CM codes 433.x1, 434.x1, or 436 in any diagnosis code position in the absence of a primary discharge code for rehabilitation (V57) or any codes for subarachnoid hemorrhage (430), intracerebral hemorrhage (431), or trauma (800–804 and 850–854). This combination of diagnosis codes has been found to have sensitivity of 86% and specificity of 95% for ischemic stroke.18
Statistical Analysis
Patients’ baseline characteristics were compared using the χ2 test and the t test, as appropriate. Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or September 30, 2015 (introduction of ICD‐10‐CM). We fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke while adjusting for demographics, stroke risk factors, and Charlson comorbidities. Because the goal of the study was to isolate the association between MI and stroke, rather than to build a parsimonious prediction model, all covariates were included in the model regardless of statistical significance. We used the corresponding survival probabilities to compute the hazard ratio in each 4‐week interval after discharge. Confidence intervals (CIs) were computed using the nonparametric bootstrap function. We performed 4 sensitivity analyses. First, to exclude strokes potentially caused by coronary reperfusion procedures that occurred after discharge for acute MI, we performed a sensitivity analysis in which we censored patients at the time of cardiac catheterization or coronary bypass grafting after hospitalization for acute MI. We used the diagnostic code algorithms of previous studies and billing guidelines to identify cardiac catheterization or coronary artery bypass grafting procedures.19, 20, 21 Second, to exclude strokes potentially caused by atrial fibrillation, we performed a sensitivity analysis in which we censored patients with atrial fibrillation before or during the hospitalization for MI. Third, to exclude strokes potentially caused by atrial fibrillation that developed newly after MI, we performed a sensitivity analysis in which we censored patients at the time of atrial fibrillation that occurred after hospitalization for acute MI. Fourth, to evaluate the effect of misclassification among ICD‐9‐CM codes, we performed an additional sensitivity analysis in which we assumed a 15% rate of misclassification among both acute MI and acute ischemic stroke ICD‐9‐CM diagnosis codes. We also performed a subgroup analysis evaluating the risk of ischemic stroke by MI type: ST‐segment–elevation MI (STEMI) and non‐STEMI (NSTEMI). As per prior studies, STEMI was defined using ICD‐9‐CM codes 410.01, 410.11, 410.21, 410.31, 410.41, 410.51, 410.51, 410.61, 410.81, and 410.91, and NSTEMI was defined using ICD‐9‐CM code 410.71.22 Finally, using stroke as a time‐varying covariate, we evaluated the 1‐year mortality rate among patients with and without stroke after MI. Statistical analyses were performed using R version 3.3.1 (R Foundation for Statistical Computing). The threshold of statistical significance was set at α=0.05.
Cohort‐Crossover Analysis
To assess whether our findings might have been caused by a lack of adjustment for variables not available in Medicare claims data, we performed a cohort‐crossover analysis in which each patient served as his or her own control. Using the McNemar test for matched data, we compared the risk of ischemic stroke in successive 4‐week periods during the first year after MI versus the corresponding 4‐week periods 1 year later. To avoid immortal time bias, we limited our cohort to patients who either remained alive and insured for at least 24 months after the index hospitalization or who had a fatal stroke before 24 months. Similar to the primary analysis, we included only ischemic strokes occurring after discharge from the MI hospitalization.
Results
Among the 1 746 476 eligible beneficiaries in our sample, we identified 46 182 (2.6%) patients with acute MI. The mean age of patients with acute MI was 79.0±8.1 years. Compared with patients without acute MI, patients with acute MI were older, were more often male, and had higher rates of stroke risk factors (Table 1). Over a mean follow‐up of 4.6±2.2 years, 80 466 patients were hospitalized with an acute ischemic stroke. Patients with stroke were older, were more often female, and had a higher burden of stroke risk factors (Table 2).
Table 1.
Characteristics of a 5% Sample of Medicare Beneficiaries, Stratified by Presence of Acute MI
| Characteristic | Acute MI (n=46 182) | No Acute MI (n=1 700 294) |
|---|---|---|
| Age, y, mean±SD, y | 79.0±8.1 | 73.3±7.7 |
| Female | 23 466 (50.8) | 974 639 (57.3) |
| Race | ||
| White | 40 437 (87.6) | 1 463 628 (86.1) |
| Black | 3557 (7.7) | 133 570 (7.9) |
| Other | 2188 (4.7) | 103 096 (6.0) |
| Hypertension | 41 592 (90.1) | 858 565 (50.5) |
| Diabetes mellitus | 22 152 (48.0) | 344 821 (20.3) |
| Congestive heart failure | 24 226 (52.5) | 103 381 (6.1) |
| Peripheral vascular disease | 11 477 (24.9) | 101 564 (6.0) |
| Chronic obstructive pulmonary disease | 14 702 (31.8) | 173 310 (10.2) |
| Chronic kidney disease | 14 816 (32.1) | 77 857 (4.6) |
| Atrial fibrillation | 15 829 (34.3) | 125 056 (7.4) |
| Valvular disease | 13 334 (28.9) | 105 434 (6.2) |
| Tobacco use | 7808 (16.9) | 20 688 (1.2) |
| Alcohol use | 5586 (12.1) | 41 875 (2.5) |
Data are presented as n (%) unless otherwise specified. MI indicates myocardial infarction.
Table 2.
Characteristics of a 5% Sample of Medicare Beneficiaries, Stratified by Presence of Ischemic Stroke
| Characteristic | Ischemic Stroke (n=80 466) | No Ischemic Stroke (n=1 666 010) |
|---|---|---|
| Age, y, mean±SD | 77.5±7.8 | 73.3±7.7 |
| Female | 47 098 (58.5) | 951 007 (57.1) |
| Race | ||
| White | 67 110 (83.4) | 1 436 955 (86.3) |
| Black | 9145 (11.4) | 127 982 (7.7) |
| Other | 4211 (5.2) | 101 073 (6.0) |
| Hypertension | 54 550 (67.8) | 845 607 (50.8) |
| Diabetes mellitus | 24 745 (30.8) | 342 228 (20.5) |
| Congestive heart failure | 11 091 (13.8) | 116 516 (7.0) |
| Peripheral vascular disease | 9669 (12.0) | 103 372 (6.2) |
| Chronic obstructive pulmonary disease | 12 009 (14.9) | 176 003 (10.6) |
| Chronic kidney disease | 7532 (9.4) | 85 141 (5.1) |
| Atrial fibrillation | 13 272 (16.5) | 127 613 (7.7) |
| Valvular disease | 9117 (11.3) | 109 651 (6.6) |
| Tobacco use | 1686 (2.1) | 26 810 (1.6) |
| Alcohol use | 2950 (3.7) | 44 511 (2.7) |
Data are presented as n (%) unless otherwise specified.
After adjustment for demographics, stroke risk factors, and Charlson comorbidities, the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (hazard ratio: 2.7; 95% CI, 2.3–3.2), remained substantially elevated during weeks 5 to 8 (hazard ratio: 2.0; 95% CI, 1.6–2.4) and weeks 9 to 12 (hazard ratio: 1.6; 95% CI, 1.3–2.0), and was no longer significantly elevated afterward (Table 3, Figure).
Table 3.
Models Evaluating the Relationship Between Acute MI and Ischemic Stroke
| Weeks 0–4 | Weeks 5–8 | Weeks 9–12 | Weeks 13–16 | |
|---|---|---|---|---|
| Primary analysisa | 2.7 (2.3–3.2) | 2.0 (1.6–2.4) | 1.6 (1.3–2.0) | 1.2 (0.9–1.6) |
| Sensitivity analysis 1b | 5.0 (3.9–6.3) | 2.5 (1.7–3.3) | 1.9 (1.3–2.7) | 0.9 (0.5–1.5) |
| Sensitivity analysis 2c | 2.9 (2.4–3.6) | 1.9 (1.4–2.4) | 1.6 (1.2–2.0) | 1.4 (1.0–1.9) |
| Sensitivity analysis 3d | 3.1 (2.7–3.5) | 1.4 (1.1–1.7) | 1.1 (0.8–1.3) | 1.3 (1.0–1.7) |
| Sensitivity analysis 4e | 2.7 (2.3–3.2) | 2.0 (1.6–2.4) | 1.6 (1.3–2.0) | 1.3 (0.9–1.6) |
Data are reported as hazard ratio (95% confidence interval). MI indicates myocardial infarction.
Adjusted for demographics, stroke risk factors, and Charlson comorbidities.
In which we censored patients at the time of cardiac catheterization or coronary artery bypass grafting after discharge for acute MI.
In which we excluded patients with atrial fibrillation before or during the index hospitalization for acute MI.
In which we censored patients at the time of atrial fibrillation after discharge for acute MI.
In which we assumed a 15% rate of misclassification among diagnoses of acute MI and ischemic stroke International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis codes.
Figure 1.
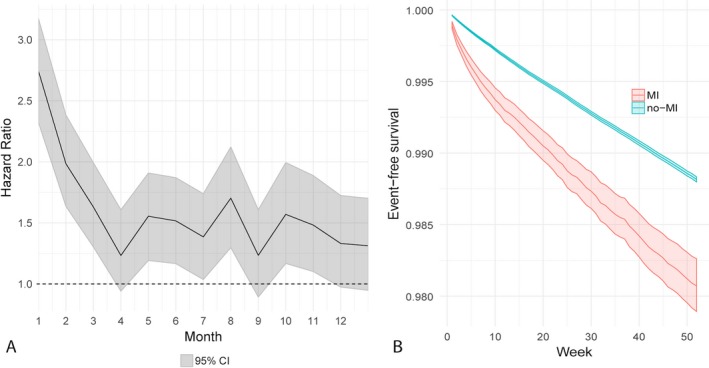
Temporal evolution of ischemic stroke risk after acute myocardial infarction (MI). A, Hazard ratios and 95% confidence intervals (CIs) from a Cox regression model of ischemic stroke after acute MI. The dotted line represents a hazard ratio of 1. B, Survival probabilities and associated 95% CIs for ischemic stroke in patients with and without acute MI.
Our results were unchanged in the first sensitivity analysis in which we censored patients at the time of cardiac catheterization or coronary bypass grafting after discharge for acute MI (Table 3). Our results were similar in the second sensitivity analysis in which we excluded patients with a prior or concurrent diagnosis of atrial fibrillation during the hospitalization for acute MI. In addition, our results were similar, although more attenuated, in the third sensitivity analysis in which we censored patients at the time of atrial fibrillation after discharge for acute MI (Table 3). Finally, our results were unchanged in the fourth sensitivity analysis in which we assumed a 15% rate of misclassification among both acute MI and ischemic stroke ICD‐9‐CM diagnosis codes (Table 3).
Of the 46 182 patients with MI, 18 637 (40%) had STEMI and 27 545 (60%) had NSTEMI. The risk of ischemic stroke was elevated for 12 weeks after both STEMI and NSTEMI and was no longer significantly elevated afterward (Table 4).
Table 4.
Subgroup Analysis Evaluating the Relationship Between MI Type and Ischemic Stroke
| Weeks 0–4 | Weeks 5–8 | Weeks 9–12 | Weeks 13–16 | |
|---|---|---|---|---|
| STEMI | 3.0 (2.4–3.9) | 2.0 (1.5–2.6) | 1.6 (1.2–2.1) | 1.1 (0.7–1.6) |
| NSTEMI | 2.6 (2.1–3.1) | 2.0 (1.6–2.5) | 1.7 (1.2–2.0) | 1.3 (1.0–1.7) |
Data are reported as hazard ratio (95% confidence interval) and adjusted for demographics, stroke risk factors, and Charlson comorbidities. MI indicates myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
We found that the 1‐year mortality rate in patients with acute MI but without stroke was 37.1% (95% CI, 36.7–37.5%). In contrast, among patients with MI and stroke, the 1‐year mortality rate was 51.5% (95% CI, 49.5–53.5%).
Finally, in our cohort‐crossover analysis, we found that the risk of stroke was highest in the first 4 weeks after acute MI compared with the corresponding 4‐week time period 1 year later. The risk of stroke remained substantially elevated through weeks 25 to 28 and was no longer significantly elevated afterward.
Discussion
In a large, heterogeneous group of Medicare beneficiaries, we found that acute MI is associated with an elevated risk of ischemic stroke that extends beyond the 1‐month time window that is currently considered the at‐risk period. The prolonged period of ischemic stroke risk was evident in patients with STEMI and NSTEMI and was independent of periprocedural strokes that may have occurred in the setting of coronary reperfusion therapies.
Prior guidelines and ongoing randomized trials consider acute MI to be an etiological cause of ischemic stroke if it occurs within 1 month of stroke.8, 9, 10, 11 Although prior studies have examined the association between acute MI and ischemic stroke, those studies did not delineate the duration of heightened stroke risk and the time at which stroke risk returns to baseline after acute MI. Furthermore, prior studies did not account for potential confounders such as stroke risk from coronary reperfusion therapies6, 7, 13, 14 and, in some cases, included the composite of ischemic stroke and intracerebral hemorrhage as the outcome.6, 7, 12 Our results build on prior studies by delineating the duration of ischemic stroke risk after acute MI, and our findings suggest that the currently accepted at‐risk period for stroke caused by acute MI should be redefined. Our study has clinical and scientific implications because it may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiological classification systems and clinical trial selection criteria.
The results of our study must be interpreted in light of its limitations. First, we lacked information regarding MI severity or location and findings of echocardiography or coronary angiography. Similarly, we lacked information regarding the severity, distribution, neuroimaging characteristics, or mechanism of the ischemic stroke. Second, we lacked data regarding antithrombotics and other stroke preventive medications and their adherence. Prior work suggests that patients are more compliant with antithrombotic therapy in the acute period after MI23; therefore, the association between acute MI and ischemic stroke may be more prolonged than what we found. Third, our use of administrative data may have led to misclassification of stroke or acute MI events. We attempted to mitigate this by using previously validated diagnosis code algorithms with high sensitivities and specificities.16, 18 Finally, our study was limited to patients aged >65 years; consequently, our findings may not be generalizable to younger patients, who may have different mechanisms or risk factors for cardiac injury. However, most acute MIs and ischemic strokes occur in patients aged >65 years.
Conclusion
Acute MI is associated with an elevated risk of ischemic stroke that appears to extend beyond the 1‐month window that is currently considered the at‐risk period.
Sources of Funding
Dr Merkler is supported by National Institutes of Health (NIH) grant KL2TR0002385 and the Leon Levy Foundation in Neuroscience. Dr Murthy is supported by the American Brain Foundation/American Academy of Neurology and the Leon Levy Foundation. Dr Navi is supported by NIH grant K23NS091395 and the Florence Gould Endowment for Discovery in Stroke. Dr Iadecola is supported by NIH grants R37NS089323, R01NS034179, R01NS037853, and R01 NS073666. Dr Kamel is supported by NIH grants K23NS082367, R01NS097443, and U01NS095869, as well as the Michael Goldberg Research Fund.
Disclosures
None.
Acknowledgments
The authors are grateful to Monica Chen for her editing and clerical assistance.
(J Am Heart Assoc. 2018;7:e010782 DOI: 10.1161/JAHA.118.010782)
An abstract of this work was presented at the International Stroke Conference, January 26, 2018, in Los Angeles, CA.
References
- 1. Brammas A, Jakobsson S, Ulvenstam A, Mooe T. Mortality after ischemic stroke in patients with acute myocardial infarction: predictors and trends over time in Sweden. Stroke. 2013;44:3050–3055. [DOI] [PubMed] [Google Scholar]
- 2. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta‐analysis. Am J Med. 2006;119:354.e1–9. [DOI] [PubMed] [Google Scholar]
- 3. Saczynski JS, Spencer FA, Gore JM, Gurwitz JH, Yarzebski J, Lessard D, Goldberg RJ. Twenty‐year trends in the incidence of stroke complicating acute myocardial infarction: Worcester Heart Attack Study. Arch Intern Med. 2008;168:2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dutta M, Hanna E, Das P, Steinhubl SR. Incidence and prevention of ischemic stroke following myocardial infarction: review of current literature. Cerebrovasc Dis. 2006;22:331–339. [DOI] [PubMed] [Google Scholar]
- 5. Cronin L, Mehta SR, Zhao F, Pogue J, Budaj A, Hunt D, Yusuf S. Stroke in relation to cardiac procedures in patients with non‐ST‐elevation acute coronary syndrome: a study involving >18 000 patients. Circulation. 2001;104:269–274. [DOI] [PubMed] [Google Scholar]
- 6. Kassem‐Moussa H, Mahaffey KW, Graffagnino C, Tasissa G, Sila CA, Simes RJ, White HD, Califf RM, Bhapkar MV, Newby LK; Symphony, nd SI . Incidence and characteristics of stroke during 90‐day follow‐up in patients stabilized after an acute coronary syndrome. Am Heart J. 2004;148:439–446. [DOI] [PubMed] [Google Scholar]
- 7. Budaj A, Flasinska K, Gore JM, Anderson FA Jr, Dabbous OH, Spencer FA, Goldberg RJ, Fox KA; Grace Investigators . Magnitude of and risk factors for in‐hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation. 2005;111:3242–3247. [DOI] [PubMed] [Google Scholar]
- 8. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 9. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 10. Diener HC, Easton JD, Granger CB, Cronin L, Duffy C, Cotton D, Brueckmann M, Sacco RL; Re‐Spect ESUS Investigators . Design of Randomized, double‐blind, Evaluation in secondary Stroke Prevention comparing the EfficaCy and safety of the oral Thrombin inhibitor dabigatran etexilate vs. acetylsalicylic acid in patients with Embolic Stroke of Undetermined Source (RE‐SPECT ESUS). Int J Stroke. 2015;10:1309–1312. [DOI] [PubMed] [Google Scholar]
- 11. National Institutes of Health . AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA). Available at: https://clinicaltrials.gov/ct2/show/NCT03192215. Accessed December 22, 2017.
- 12. Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community‐based study of stroke incidence after myocardial infarction. Ann Intern Med. 2005;143:785–792. [DOI] [PubMed] [Google Scholar]
- 13. Yaghi S, Pilot M, Song C, Blum CA, Yakhkind A, Silver B, Furie KL, Elkind MS, Sherzai D, Sherzai AZ. Ischemic stroke risk after acute coronary syndrome. J Am Heart Assoc. 2016;5:e002590 DOI: 10.1161/JAHA.115.002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sundboll J, Horvath‐Puho E, Schmidt M, Pedersen L, Henderson VW, Botker HE, Sorensen HT. Long‐term risk of stroke in myocardial infarction survivors: thirty‐year population‐based cohort study. Stroke. 2016;47:1727–1733. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Medicare and Medicaid Services . Medicare limited dataset files. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/. Accessed August 11, 2017.
- 16. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 17. Jimenez Caballero PE, Lopez Espuela F, Portilla Cuenca JC, Ramirez Moreno JM, Pedrera Zamorano JD, Casado Naranjo I. Charlson comorbidity index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. J Stroke Cerebrovasc Dis. 2013;22:e214–e218. [DOI] [PubMed] [Google Scholar]
- 18. Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 19. Blankenship JC, Bateman TM, Haines DE, Pearlman AS, Schoenfeld MH, Sigel CJ, Wolk MJ, Wood DL. ACC expert consensus document on ethical coding and billing practices for cardiovascular medicine specialists. American College of Cardiology. J Am Coll Cardiol. 1999;33:1076–1086. [DOI] [PubMed] [Google Scholar]
- 20. Clark MA, Bakhai A, Lacey MJ, Pelletier EM, Cohen DJ. Clinical and economic outcomes of percutaneous coronary interventions in the elderly: an analysis of Medicare claims data. Circulation. 2004;110:259–264. [DOI] [PubMed] [Google Scholar]
- 21. Gillum RF, Gillum BS, Francis CK. Coronary revascularization and cardiac catheterization in the United States: trends in racial differences. J Am Coll Cardiol. 1997;29:1557–1562. [DOI] [PubMed] [Google Scholar]
- 22. Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. 2017;48:2931–2938. [DOI] [PubMed] [Google Scholar]
- 23. Czarny MJ, Nathan AS, Yeh RW, Mauri L. Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol. 2014;37:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]


