Abstract
Background
Ticagrelor reduced cardiovascular death, myocardial infarction (MI), or stroke in patients with prior MI in PEGASUS‐TIMI 54 (Prevention of Cardiovascular Events [eg, Death From Heart or Vascular Disease, Heart Attack, or Stroke] in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin). MI can occur in diverse settings and with varying severity; therefore, understanding the types and sizes of MI events prevented is of clinical importance.
Methods and Results
MIs were adjudicated by a blinded clinical events committee and categorized by subtype and fold elevation of peak cardiac troponin over the upper limit of normal. A total of 1042 MIs occurred in 898 of the 21 162 randomized patients over a median follow‐up of 33 months. The majority of the MIs (76%) were spontaneous (Type 1), with demand MI (Type 2) and stent thrombosis (Type 4b) accounting for 13% and 9%, respectively; sudden death (Type 3), percutaneous coronary intervention–related (Type 4a) and coronary artery bypass graft–related (Type 5) each accounted for <1%. Half of MIs (520, 50%) had a peak troponin ≥10x upper limit of normal and 21% of MIs (220) had a peak troponin ≥100× upper limit of normal. A total of 21% (224) were ST‐segment–elevation MI STEMI. Overall ticagrelor reduced MI (4.47% versus 5.25%, hazard ratio 0.83, 95% confidence interval 0.72–0.95, P=0.0055). The benefit was consistent among the subtypes, including a 31% reduction in MIs with a peak troponin ≥100× upper limit of normal (hazard ratio 0.69, 95% confidence interval 0.53–0.92, P=0.0096) and a 40% reduction in ST‐segment elevation MI (hazard ratio 0.60, 95% confidence interval 0.46–0.78, P=0.0002).
Conclusions
In stable outpatients with prior MI, the majority of recurrent MIs are spontaneous and associated with a high biomarker elevation. Ticagrelor reduces the MI consistently among subtypes and sizes including large MIs and ST‐segment elevation MI.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01225562.
Keywords: antiplatelet therapy, myocardial infarction, ST‐segment elevation myocardial infarction, ticagrelor, troponin
Subject Categories: Coronary Artery Disease, Thrombosis, Acute Coronary Syndromes
Short abstract
See Article by https://doi.org/10.1161/JAHA.118.010996
Clinical Perspective
What Is New?
Debates regarding the duration of dual antiplatelet therapy often center around the concept of stent protection.
This article demonstrates that in high‐risk patients with prior myocardial infarction (MI), recurrent events are primarily large, spontaneous, de novo events rather than stent thrombosis and that long‐term ticagrelor consistently reduces recurrent MI regardless of size or cause.
What Are the Clinical Implications?
When evaluating patients for long‐term secondary prevention with ticagrelor, patient risk profile should be considered.
High‐risk patients with prior MI have long‐term risk of recurrent spontaneous events including large MIs and ST‐segment–elevation MI.
Long‐term ticagrelor significantly reduces MI regardless of cause including large MIs, ST‐segment–elevation MI, and the composite of fatal MI or sudden cardiac death.
These findings should be considered when weighing the risks and benefits of long‐term therapy with ticagrelor.
Introduction
Patients with prior myocardial infarction (MI) are at long‐term heightened risk of recurrent ischemic events.1 PEGASUS‐TIMI 54 (Prevention of Cardiovascular Events [eg, Death From Heart or Vascular Disease, Heart Attack, or Stroke] in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin) demonstrated that long‐term secondary prevention with ticagrelor, added to aspirin, reduced the primary composite end point of MI, stroke, or cardiovascular death by ≈15%.2 Overall, the magnitude of relative risk reduction was similar for all of the components of the composite end point, including cardiovascular death and ischemic stroke; however, the most frequent outcome was recurrent MI.
The definition and natural history of MI has evolved over the past decade.3 The measurement of circulating cardiac troponin, measured with increasingly sensitive assays that are able to detect very small levels of myocardial injury, has become central to the diagnosis of MI. Because of the increasing sensitivity of troponin assays, events categorized as MI reflect a broadening spectrum of events in terms of cause, size, and severity.4 In addition, events reported in clinical trials may differ from those commonly coded in public data sets.5 In acute populations undergoing intervention, for example, serial biomarkers may detect largely procedural MI, although differentiating procedural injury from index events may be challenging.6, 7, 8 Understanding and further characterizing MI events is therefore important in understanding their clinical significance, particularly in the context of therapies with safety concerns such as bleeding.9
We hypothesized that in the stable high‐risk PEGASUS‐TIMI 54 population, recurrent MI would be largely spontaneous and associated with significant elevation in circulating cardiac biomarkers. In addition, we hypothesized that the benefits of ticagrelor for reducing MI would be present across cause and size of MI but would be particularly notable for MI clearly mediated by coronary thrombosis such as ST‐segment–elevation MI (STEMI).
Methods
Study Population
PEGASUS‐TIMI 54 randomized patients with prior MI to ticagrelor 60 mg twice daily, ticagrelor 90 mg twice daily, or placebo, all on a background of low‐dose aspirin. The protocol was approved by the relevant ethics committee at each participating site. Written informed consent was obtained from all the patients. The design10 and primary results of the trial have been published.2 Patients aged at least 50 years were enrolled with a spontaneous MI occurring 1 to 3 years before enrollment and at least 1 of the following additional high‐risk features: age ≥65 years, diabetes mellitus requiring medication, a second prior spontaneous MI, multivessel coronary artery disease, or chronic renal dysfunction, defined as a creatinine clearance <60 mL/min as estimated by the Cockroft‐Gault equation. Patients were ineligible if there was planned use of a P2Y12 receptor antagonist or anticoagulant therapy during the study period; if they had a bleeding disorder, a history of intracranial bleeding, a central nervous system tumor, or an intracranial vascular abnormality; or if they had had gastrointestinal bleeding within the previous 6 months or major surgery within the previous month.
End Points
The primary efficacy end point was MI, and subtypes of MI in this article. All potential events were adjudicated by a clinical events committee, which was blinded to treatment allocation. All confirmed MI events were further categorized by the clinical events committee into clinical subtype as defined in the Universal Definition of MI as well as the associated peak troponin elevation (available in 88%). Stent thrombosis (Type 4b MI) was adjudicated through collection and formal review of coronary angiograms. Type 2 MI events had to meet criteria for a spontaneous MI as defined in the charter, not meet criteria for another MI subtype, and had to occur in the setting of a clinical picture consistent with ischemia caused by either increased oxygen demand or decreased supply, (eg, coronary artery spasm, coronary embolism, anemia, arrhythmias, hypertension, or hypotension). Although even low‐level elevations in cardiac troponin have been associated with adverse prognosis in patients with acute coronary syndrome,11 elevations of at least 10‐fold have generally been regarded as indicating larger events and enable detection of important events in the setting of procedures such as bypass surgery.3 In order to evaluate the consistency of benefit for ticagrelor across MI size, serial thresholds were examined also including 25×, 50×, 100×, and 200× the upper limit of normal. In addition, events were categorized into the core clinical categories of STEMI and non–ST segment–elevation MI with ECGs collected by the clinical events committee (in 1021 of 1042 or 98.6% of events). Additional efficacy end points included fatal MI, sudden cardiac death (presumed largely coronary in origin in a post‐MI population) and the combination of these 2.
Statistical Considerations
Cumulative event rates at 3 years following randomization were calculated by the complement of Kaplan–Meier survival estimates. The treatment comparisons were based on the Cox proportional‐hazards model including treatment assignment as an indicator variable. Since this was a large randomized trial, no additional adjustment was made in this model. We have also examined the risks of MI and subtypes of MI in the context of competing risks for consistency. The proportional hazards assumption was assessed by creating an interaction of the treatment assignment indicator and a function of survival time and included in the model. All models met this assumption. Efficacy analyses were performed on an intention‐to‐treat basis. Patients could have more than 1 MI during follow‐up, so analyses evaluating subtypes of MI included the first MI of that subtype. The data for this analysis will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The analytic methods are described and the study materials (trial protocol) have been published with primary results. Results are summarized by hazard ratios and 95% confidence intervals and all reported P values are 2‐sided. No adjustment was made for the significance level for multiple testing. Analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, NC) and R software version 3.2.2 (http://www.r-project.org).
Results
A total of 21 162 patients were randomized with 7067 to placebo, 7045 to ticagrelor 60 mg twice daily, and 7050 to ticagrelor 90 mg twice daily. A total of 1042 MI events occurred in a total of 898 patients at a median of 460 days (interquartile range 222–721) following randomization. Patients who had MI during the trial were older, had a greater prevalence of risk factors, had more severe vascular disease, and were more likely to have had an index non–ST segment–elevation MI relative to STEMI (Table S1). For patients randomized to placebo, the rate of recurrent MI at 3 years was 5.2% or 1.7% annualized.
Types and Sizes of MI
The majority of MI events that occurred during follow‐up were spontaneous atherothrombotic (76%, Type 1, Figure 1A). A minority were secondary to demand (13%, Type 2) or stent thrombosis (9%, Type 4b). Only 6 MI events were associated with troponin elevation in the setting of percutaneous intervention (Type 4a) and there was 1 MI event occurring in the setting of coronary artery bypass surgery (Type 5). When evaluating by subtype in the placebo arm, the highest rate was for spontaneous MI (4.1%, Figure S1). Rates of all other subtypes were <1% over 3 years (Figure S1).
Figure 1.
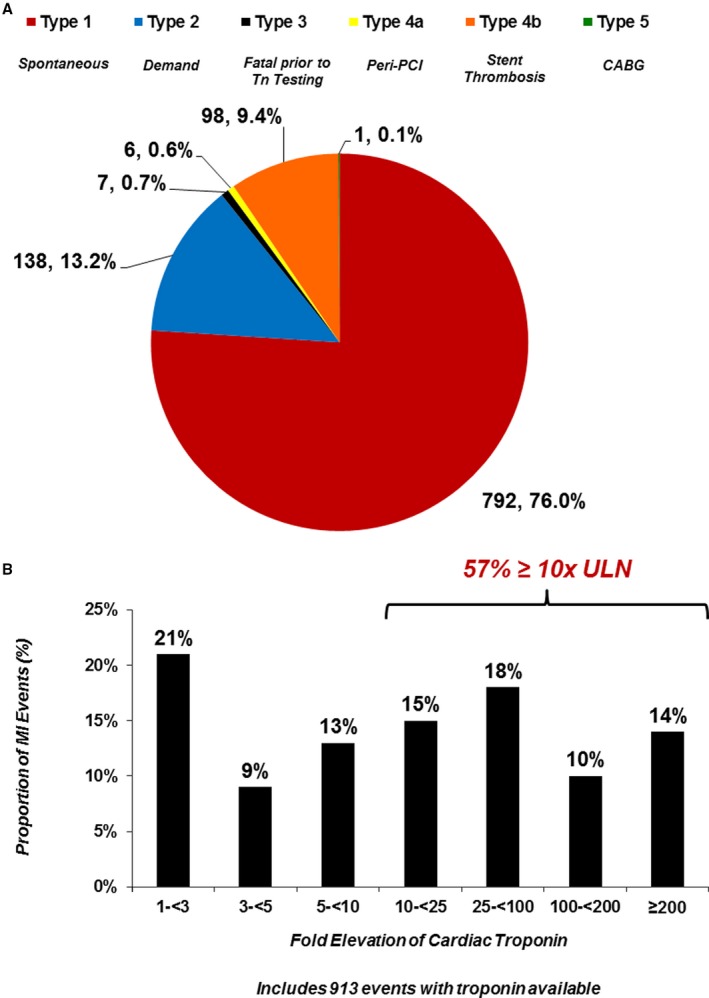
A, Distribution of myocardial infarction events occurring during follow‐up by Universal Definition of MI subtype. B, Distribution of myocardial infarction events occurring during follow‐up by fold elevation in peak biomarker (includes 913 events with troponin available). CABG indicates coronary artery bypass grafting surgery; MI, myocardial infarction; PCI, percutaneous coronary intervention; Tn, troponin; ULN, upper limit of normal.
There was a broad distribution of peak troponin elevation associated with MI events (Figure 1B). The majority of MI events were associated with a peak troponin elevation of 10 times the upper limit of normal or more.
Efficacy of Ticagrelor
Both doses of ticagrelor significantly reduced the risk of MI (ticagrelor 60 mg, hazard ratio (HR) 0.84, 95% confidence interval (CI) 0.72–0.98, P=0.031; ticagrelor 90 mg, HR 0.81, 95% CI 0.69–0.95, P=0.010). The effect of ticagrelor at reducing MI was evident early and continued through the 3 years of follow‐up (Figure 2). When stratifying by the Universal Definition of Myocardial Infarction subtypes (Figure 3), the benefit of ticagrelor appeared consistent for spontaneous (Type 1), demand (Type 2), and angiographically verified stent‐thrombosis‐related (Type 4b) with insufficient numbers of Type 3 (n=7), Type 4a (n=6), and Type 5 (n=1) MI to evaluate efficacy.
Figure 2.
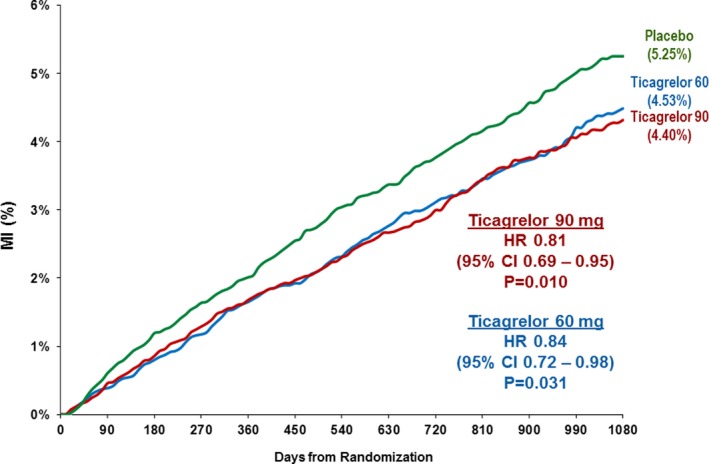
Myocardial infarction occurring over 3 years by randomized treatment. CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction.
Figure 3.
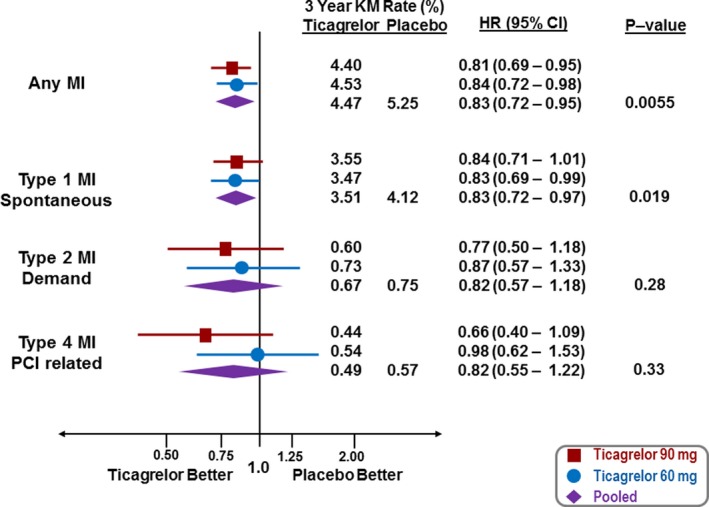
Reduction in MI by treatment (red—ticagrelor 90 mg twice daily, blue—ticagrelor 60 mg twice daily, purple—ticagrelor doses pooled) for MI overall and by MI subtype. CI indicates confidence interval; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction; PCI, percutaneous coronary intervention.
When evaluating by peak troponin as a measure of MI size, there was a consistent benefit with ticagrelor with increasing elevation of peak troponin (Figure 4). This included a 31% risk reduction in MIs with at least a 100‐fold elevation of peak troponin (ticagrelor doses pooled, HR 0.69, 95% CI 0.53–0.92; ticagrelor 60 mg, HR 0.63, 95% CI 0.45–0.89; ticagrelor 90 mg, HR 0.76, 95% CI 0.55–1.04, Figure 4). The proportion of first MI events with a troponin elevation ≥50 times the upper limit of normal was significantly lower with ticagrelor versus placebo (30% versus 37%, P=0.049), with the difference even greater for spontaneous (Type 1) MIs with a troponin elevation ≥50 times the upper limit of normal (27% versus 36%, P=0.019).
Figure 4.
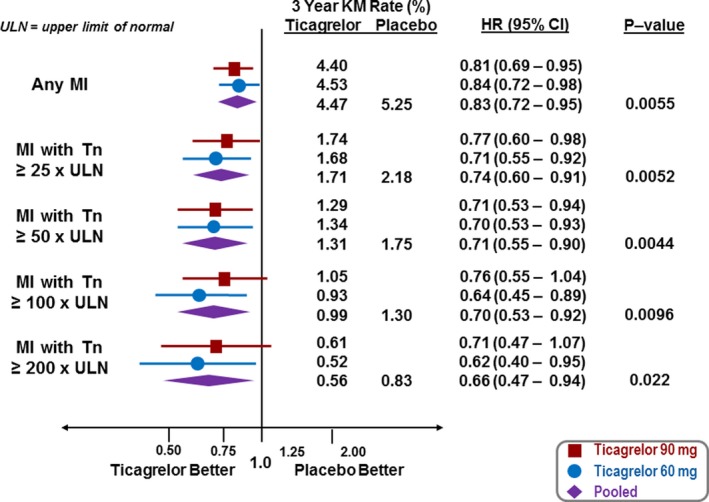
Reduction in MI by treatment (red—ticagrelor 90 mg twice daily, blue ticagrelor 60 mg twice daily, purple—ticagrelor doses pooled) for MI overall and by fold elevation of peak biomarker. CI indicates confidence interval; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction; Tn, troponin; ULN, upper limit of normal.
MI was also stratified by the core clinical subtypes of STEMI and non–ST‐segment–elevation MI. Patients randomized to placebo experienced STEMI at a roughly linear rate during follow‐up with no apparent plateau over time (Figure 5). Ticagrelor significantly reduced STEMI by 40% (ticagrelor doses pooled: HR 0.60, 95% CI 0.46–0.78, P=0.0002), with consistent efficacy with both the 60 mg and 90 mg ticagrelor doses (Figure 5). The incidence of non–ST segment–elevation MI also tended to be lower (ticagrelor doses pooled: HR 0.91, 95% CI 0.77–1.06).
Figure 5.
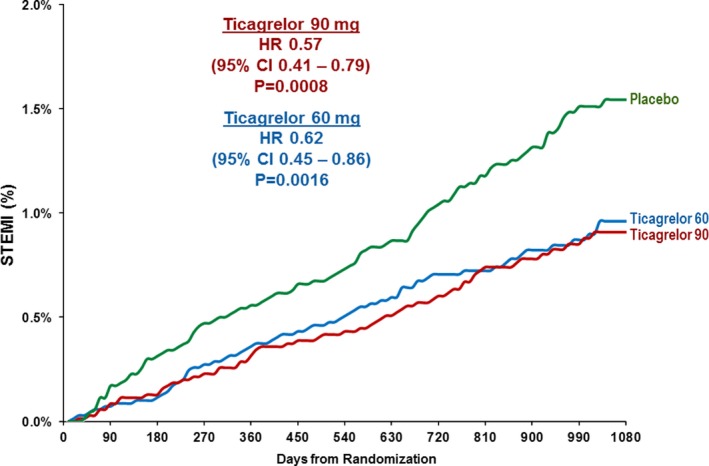
STEMI occurring over 3 years by randomized treatment. CI indicates confidence interval; HR, hazard ratio; STEMI, ST‐segment–elevation myocardial infarction.
Fatal MI or Sudden Cardiac Death
A total of 330 patients had a fatal MI (n=61, 5.85% of all MIs) or sudden cardiac death (n=269). Ticagrelor reduced the risk of these events by 23% (HR 0.77, 95% CI 0.62–0.96, P=0.020), with similar effects on fatal MI (HR 0.72, 95% CI 0.43–1.20) and on sudden cardiac death (HR 0.78, 95% CI 0.61–0.997, Figure S2).
Discussion
This analysis demonstrates 3 major findings: first, that the risk of recurrent MI over 3 years in well‐treated patients with history of MI >1 year prior exceeds 5% (≈1.7% per year); secondly, that even in the era of sensitive troponin assays, these recurrent events in stable secondary prevention populations are largely new spontaneous events and that demand‐related myocardial injury and stent complications accounted for only a minority of MIs; and finally, that long‐term treatment with ticagrelor on a background of aspirin significantly reduces the incidence of recurrent MI by 15% to 20%, reduces STEMI by 40%, and reduces the risk of fatal MI or sudden cardiac death by 23%.
In PEGASUS‐TIMI 54, patients were purposely selected in the stable phase at least 1 year from MI.2 Patients who were unstable, critically ill, or planned for procedures were excluded. In addition, utilization of indicated background therapy was high, with >99% using aspirin, >90% receiving statins, and >80% on β‐blockers. Nevertheless, recurrent MI continued to occur at a roughly linear rate even 3 years after randomization. This observation underscores the ongoing risk of spontaneous plaque rupture in the post‐MI population in spite of intensive medical therapy.
When evaluating the rate of recurrent MI, it is important to understand the subtype of event. As troponin has evolved to become the key component of the definition of MI and as troponin assays have continued to improve in sensitivity and specificity, there is debate about the clinical relevance of MI, particularly when occurring in the context of critical illness (demand or Type 2 MI) or elective percutaneous coronary intervention (procedural or Type 4 MI).4, 12 Although degree of biomarker elevation may represent several factors beyond the event itself (eg, time to diagnosis, door‐to‐balloon time), more than half of the MI events that occurred were associated with cardiac biomarker levels more than 10× the upper limit of normal. The current analysis demonstrates that the majority of MI events ascertained and adjudicated in a modern clinical trial are spontaneous and associated with significant elevation in circulating biomarkers.
Long‐term secondary prevention with ticagrelor 60 mg twice daily or 90 mg twice daily significantly reduced the risk of recurrent MI by 15% to 20%. This benefit was consistent when restricting to only spontaneous (Type 1) MIs as well as when restricting to large MIs with a 100‐fold or greater elevation of cardiac troponin. Although there is debate as to the meaning of biomarker cut points in determining the severity of MI, STEMI is broadly recognized as a severe, life‐threatening event. We observed that the benefit of ticagrelor appeared of even greater magnitude for STEMI with a 40% reduction, an observation that is intuitive given the high levels of platelet P2Y12 receptor inhibition achieved by both doses of ticagrelor and the important role of the platelet P2Y12 receptor in amplifying and sustaining arterial thrombus sufficiently to achieve complete arterial occlusion.13, 14, 15
The benefit of ticagrelor in reducing STEMI and large MI translated into a reduction in MI‐related mortality either as fatal MI or sudden death. These data support the position that potent antithrombotic strategies in high‐risk populations have the potential to reduce the mortality related to MI even beyond the benefits of currently utilized preventive strategies.
There are several limitations to this analysis. Systemic serial sampling of biomarkers was not performed after coronary interventions, which may have led to underascertainment of periprocedural (percutaneous coronary intervention or coronary artery bypass grafting surgery) MI. This, however, would lead to an underestimate of overall risk and might underestimate the absolute benefit of ticagrelor in this population. During the course of the trial, the Third Universal Definition of MI was published and modified the definition of Type 4a MI and added Type 4c MI (because of restenosis). Type 4a was made more stringent, increasing the threshold of troponin to 5× upper limit of normal and requiring additional evidence of ischemia (eg, syndrome, angiographic evidence, ECG changes); however, because there were only 6 Type 4a MIs, this change would not impact the findings of this analysis and therefore events were not re‐adjudicated with the updated definition. In addition, Type 4c MI was not captured. Similarly, there are limitations with the definition and the ability to discriminate Type 2 MI from other subtypes. As outlined in the Methods section, events classified as Type 2 MI had to meet criteria as an MI and had to occur in a setting consistent with supply–demand mismatch. Nonetheless, it is possible that some of these events were primarily thrombotic in cause or had a thrombotic component, an observation that would be supported by the trend towards benefit with ticagrelor in this analysis. Overall, only 13% of MI events were classified as Type 2 and inclusion of some Type 1 events would only reduce frequency in this population, underscoring the observation that Type 1 events are the predominant cause of MI in this population. An additional limitation is the use of different troponin assays of differing sensitivity across sites. Utilization of high thresholds (eg, 100‐fold elevations) in peak troponin was intended to select for large events regardless of assay, and differing assays would not impact the consistent findings shown with STEMI.
Conclusions
In stable outpatients with a history of MI, the majority of recurrent MI events are spontaneous and associated with a high biomarker elevation. Ticagrelor significantly reduces the incidence of MI consistently among different subtypes and biomarker sizes, with the greatest absolute reduction in spontaneous MIs, large MIs, and STEMI.
Sources of Funding
This study was supported by a grant to Brigham and Women's Hospital from AstraZeneca.
Disclosures
The TIMI Study Group has received significant research grant support from Accumetrics, Amgen, AstraZeneca, Beckman Coulter, Bristol‐Myers Squibb, CV Therapeutics, Daiichi Sankyo Co Ltd, Eli Lilly and Co, GlaxoSmithKline, Integrated Therapeutics, MedImmune, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho‐Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi‐Aventis, Sanofi‐Synthelabo, Siemens Medical Solutions, and Singulex. Bonaca reports consulting for AstraZeneca, Merck, Aralez, Bayer. Wiviott reports grants and consulting fees from AstraZeneca, Bristol Myers Squibb, Eisai, Arena, Merck, Eli Lilly, and Daiichi Sankyo, consulting fees from Aegerion, Angelmed, Janssen, Xoma, ICON Clinical, Boston Clinical, Boehringer Ingelheim, grants from Sanofi‐Aventis, and from Amgen. Dr Wiviott's wife is an employee of Merck. Morrow reports Research Grants: Abbott Laboratories, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GlaxoSmithKline, Merck, Novartis, Roche Diagnostics, Takeda. Consultant/Advisory Board; Abbott Laboratories, Aralez, Bayer, Merck, Peloton, Roche Diagnostics, and Verseon. Steg reports significant research grants from Merck, Sanofi, and Servier; other personal fees and nonfinancial support from AstraZeneca, Sanofi, Servier; personal fees from Amarin, Amgen, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Lilly, Merck, Janssen, Novartis, Pfizer, GSK, and Regeneron. Hamm reports consulting and speaker fees for AstraZeneca, Daiichi‐Sankyo, The Medicines Company, Boehringer Ingelheim, Bayer, Novartis, and SanofiAventis. Bhatt reports the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. Storey reports research grants, consultancy fees and honoraria from AstraZeneca; research grants and consultancy fees from PlaqueTec; consultancy fees from Actelion, Avacta, Bayer, Bristol Myers Squibb/Pfizer alliance, Novartis, The Medicines Company, and ThermoFisher Scientific. Cohen reports grants and personal fees from AstraZeneca, during the conduct of the study; personal fees from Merck, personal fees from Janssen, personal fees from Maquet, personal fees from malpractice attorneys, grants from Janssen, grants from Edwards, personal fees from Merck, personal fees from BMS/Pfizer, personal fees from Janssen, personal fees from BI, personal fees from Lilly, outside the submitted work. Budaj reports grants and personal fees from AstraZeneca, during the conduct of the study; grants and personal fees from GlaxoSmithKline, Bristol Myers Squibb/Pfizer; grants from Sanofi‐Aventis, Boehringer Ingelheim, Novartis, Eisai, outside the submitted work. Nicolau reports grants and personal fees from AstraZeneca, during the conduct of the study; grants from Jansen, grants, personal fees, and nonfinancial support from Sanofi, nonfinancial support from Bayer, grants and personal fees from Boeheringer Ingelheim, and grants from BMS, outside the submitted work. Parkhomenko reports grants and personal fees from AstraZeneca, during the conduct of the study; grants and personal fees from Bristol Myers Squibb/Pfizer, Sanofi‐Aventis, Bayer, Janssen; and research grants from Servier, Daiichi‐Sankyo, outside the submitted work. Dellborg reports personal fees from AstraZeneca, during the conduct of the study; and personal fees from Boehringer Ingelheim, Novartis, and Amgen, outside the submitted work. Werf reports personal fees from AstraZeneca for lectures and advisory board meetings. Jensen is an employee of AstraZeneca. Johanson is an employee of AstraZeneca. Braunwald reports grant support to institution from AstraZeneca. Sabatine reports research grant support through Brigham and Women's Hospital from: Abbott Laboratories; Amgen; AstraZeneca; Critical Diagnostics; Daiichi‐Sankyo; Eisai; Genzyme; Gilead; GlaxoSmithKline; Intarcia; Janssen Research Development; MedImmune; Merck; Novartis; Poxel; Pfizer; Roche Diagnostics; Takeda (All >$10 000 per year). Consulting for: Alnylam; Amgen; AstraZeneca; Cubist; CVS Caremark; Esperion; Intarcia; Ionis; Medicines Company; MedImmune; Merck; MyoKardia; Zeus Scientific (all ≤$10 000 per year except Amgen, Esperion, and Ionis). The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics by Recurrent Myocardial Infarction
Figure S1. Cumulative incidence of myocardial infarction subtypes occurring in patients randomized to placebo (on aspirin monotherapy) during follow‐up.
Figure S2. Ticagrelor and fatal myocardial infarction, sudden cardiac death, and the composite of both.
(J Am Heart Assoc. 2018;7:e009260 . DOI: 10.1161/JAHA.118.009260)
References
- 1. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 2. Bonaca MP, Bhatt DL, Steg PG, Storey RF, Cohen M, Im K, Oude Ophuis T, Budaj A, Goto S, Lopez‐Sendon J, Diaz R, Dalby A, Van de Werf F, Ardissino D, Montalescot G, Aylward P, Magnani G, Jensen EC, Held P, Braunwald E, Sabatine MS. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS‐TIMI 54. Eur Heart J. 2016;37:1133–1142. [DOI] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 4. Tricoci P. Consensus or controversy?: evolution of criteria for myocardial infarction after percutaneous coronary intervention. Clin Chem. 2017;63:82–90. [DOI] [PubMed] [Google Scholar]
- 5. Diaz‐Garzon J, Sandoval Y, Smith SW, Love S, Schulz K, Thordsen SE, Johnson BK, Driver B, Jacoby K, Carlson MD, Dodd KW, Moore J, Scott NL, Bruen CA, Hatch R, Apple FS. Discordance between ICD‐coded myocardial infarction and diagnosis according to the universal definition of myocardial infarction. Clin Chem. 2017;63:415–419. [DOI] [PubMed] [Google Scholar]
- 6. Morrow DA, Wiviott SD, White HD, Nicolau JC, Bramucci E, Murphy SA, Bonaca MP, Ruff CT, Scirica BM, McCabe CH, Antman EM, Braunwald E. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel‐Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009;119:2758–2764. [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON‐TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 8. Cavender MA, Bhatt DL, Stone GW, White HD, Steg PG, Gibson CM, Hamm CW, Price MJ, Leonardi S, Prats J, Deliargyris EN, Mahaffey KW, Harrington RA; CHAMPION PHOENIX Investigators . Consistent reduction in periprocedural myocardial infarction with cangrelor as assessed by multiple definitions: findings from CHAMPION PHOENIX (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition). Circulation. 2016;134:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidd SK, Bonaca MP, Braunwald E, De Ferrari GM, Lewis BS, Merlini PA, Murphy SA, Scirica BM, White HD, Morrow DA. Universal classification system type of incident myocardial infarction in patients with stable atherosclerosis: observations from thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events (TRA 2 degrees P)‐TIMI 50. J Am Heart Assoc. 2016;5:e003237 DOI: 10.1161/JAHA.116.003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, Held P, Jensen EC, Sabatine MS. Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin‐thrombolysis in myocardial infarction 54 (PEGASUS‐TIMI 54) trial. Am Heart J. 2014;167:437–444.e5. [DOI] [PubMed] [Google Scholar]
- 11. Bonaca M, Scirica B, Sabatine M, Dalby A, Spinar J, Murphy SA, Jarolim P, Braunwald E, Morrow DA. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55:2118–2124. [DOI] [PubMed] [Google Scholar]
- 12. Gaggin HK, Liu Y, Lyass A, van Kimmenade RR, Motiwala SR, Kelly NP, Mallick A, Gandhi PU, Ibrahim NE, Simon ML, Bhardwaj A, Belcher AM, Harisiades JE, Massaro JM, D'Agostino RBS, Januzzi JL Jr. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation. 2017;135:116–127. [DOI] [PubMed] [Google Scholar]
- 13. Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Storey RF, Angiolillo DJ, Bonaca MP, Thomas MR, Judge HM, Rollini F, Franchi F, Ahsan AJ, Bhatt DL, Kuder JF, Steg PG, Cohen M, Muthusamy R, Braunwald E, Sabatine MS. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS‐TIMI 54 Trial. J Am Coll Cardiol. 2016;67:1145–1154. [DOI] [PubMed] [Google Scholar]
- 15. Wang K, Zhou X, Zhou Z, Tarakji K, Carneiro M, Penn MS, Murray D, Klein A, Humphries RG, Turner J, Thomas JD, Topol EJ, Lincoff AM. Blockade of the platelet P2Y12 receptor by AR‐C69931MX sustains coronary artery recanalization and improves the myocardial tissue perfusion in a canine thrombosis model. Arterioscler Thromb Vasc Biol. 2003;23:357–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics by Recurrent Myocardial Infarction
Figure S1. Cumulative incidence of myocardial infarction subtypes occurring in patients randomized to placebo (on aspirin monotherapy) during follow‐up.
Figure S2. Ticagrelor and fatal myocardial infarction, sudden cardiac death, and the composite of both.


