Abstract
Background
Recent evidence from cohort studies and meta‐analyses suggests that the obesity paradox phenomenon may exist in patients with diabetes mellitus. The goal of this study was to assess the association between adverse events and obesity by using 2 different measures of obesity, body mass index (BMI; kg/m2) and waist circumference, in patients with a mean 10‐year history of type 2 diabetes mellitus.
Methods and Results
We used data from the ACCORD (the Action to Control Cardiovascular Risk in Diabetes) study to evaluate the relationship between obesity and adverse events in patients with a mean 10‐year history of type 2 diabetes mellitus. The primary outcome of this study was all‐cause mortality. Secondary outcomes were cardiac death, nonfatal myocardial infarction, and stroke. Patients who were class III obese with BMI ≥40 had the highest risk of all‐cause mortality, followed by patients with class II obesity, whereas overweight patients had the lowest risk. We found significant correlations between BMI and waist circumference (r=0.802). We observed that the relationships between waist circumference and primary and second end points were much like the relationships between BMI and primary and second end points (J‐shaped relationship for all‐cause mortality, V‐shaped relationship for cardiac death, U‐shaped relationship for nonfatal myocardial infarction, and reverse linear relationship for noncardiac death).
Conclusions
No evidence of the obesity paradox was observed in patients with a 10‐year history of diabetes mellitus. Class III obese patients showed the highest risk of adverse events (all‐cause mortality, cardiac death, nonfatal myocardial infarction, and noncardiac death). BMI and waist circumference showed similar relationships with adverse events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000620.
Keywords: all‐cause death, body mass index, diabetes mellitus, obesity paradox, waist circumference
Subject Categories: Diabetes, Type 2
Clinical Perspective
What Is New?
We studied the relationship between 2 measures of obesity (body mass index and waist circumference) and adverse events in patients with a mean 10‐year history of type 2 diabetes mellitus.
What Are the Clinical Implications?
No evidence of the obesity paradox was observed in patients with a 10‐year history of diabetes mellitus.
Class III obese patients with body mass index ≥40 had the highest risk of adverse events (all‐cause mortality, cardiac death, nonfatal myocardial infarction, and noncardiac death), followed by patients with class II obesity. Overweight patients had the lowest risk.
Body mass index and waist circumference showed similar relationships with adverse events.
The prevalence of obesity has almost doubled over the past 3 decades and has been become a health problem worldwide.1, 2 Although obesity is associated with an increased risk of coronary atherosclerotic heart disease and related cardiovascular risk factors, such as hypertension and diabetes mellitus (DM), multiple clinical trials have demonstrated that obesity offers a striking protective effect over “normal” body mass index (BMI; kg/m2), a phenomenon known as the obesity paradox.3, 4, 5 This protective role of obesity has been demonstrated in different populations, including patients with coronary artery disease, heart failure, and atrial fibrillation.6, 7, 8 Recent evidence from cohort research and meta‐analyses suggests that the obesity paradox may also exist in patients with DM.9, 10 In those studies, patients who were a normal weight at the time of diagnosis of DM had higher all‐cause mortality than those patients who were overweight or obese.9 However, whether the protective role of obesity in type 2 DM (T2DM) decreases with the progress of DM is still in dispute. So et al found a V‐shaped relationship of BMI with mortality in Chinese populations with a mean 5‐year duration of DM.11 Kokkinos observed an inverse linear relationship of BMI with mortality in black and white populations with unspecified durations of DM.10 Cardiovascular disease (CVD) is the leading cause of death and the single biggest driver of healthcare costs in patients with T2DM. However, it usually takes ≥10 years after diagnosis for patients with DM to develop CVD. Prior studies have focused mainly on newly diagnosed T2DM patients and those with a short history of T2DM. The relationships between obesity and mortality in these populations have been described previously.9, 12 Little is known about the relationship between obesity and adverse events in T2DM patients with a long history of DM. Furthermore, previous studies have included relatively small sample sizes and small proportions of patients with extreme obesity (BMI ≥40), which makes their conclusions less convincing.3, 5, 9, 12 Recent studies have focused on abdominal obesity, as evaluated by waist circumference.13, 14 However, no relevant study has investigated the relationship among obesity, waist circumference, and adverse clinical outcomes. In the current study, we sought to evaluate the relationship between obesity (defined by both BMI and waist circumference) and adverse events in this population with a mean 10‐year history of T2DM.
Method
The national database data used for this study, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of the agreement with the Biological Specimen and Data Repository Information Coordinating Center (BioLINCC). Ethics approval and consent to participate were not applicable.
Data Collection and Study Population
The rationale and design of the ACCORD (the Action to Control Cardiovascular Risk in Diabetes) trial have been described previously and the results published.15, 16, 17 Briefly, ACCORD was a multicenter randomized controlled trial performed to determine whether intensive control of blood glucose, blood pressure, and lipids could reduce the incidence of CVD in T2DM patients at high risk of CVD. The trial recruited 10 251 volunteers with T2DM whose mean age was 62 years. At the time of recruitment, participants had T2DM for a median of 10 years. They had a mean glycated hemoglobin (HbA1c) level of 8.3%. Some also had a history of previous CVD or CVD risk factors (dyslipidemia, hypertension, current status as a smoker, or obesity). The participants were recruited from 77 clinical centers across the United States and Canada. Surprisingly, intensive control of blood glucose, blood pressure, and lipids was found to have a neutral effect on death and nonfatal cardiovascular events but increased cardiac death.18
Exposure Variable
This study is based on the ACCORD trial derived from BioLINCC. We excluded 6 patients for whom no BMI data were available. We further excluded 3 patients in the underweight category because of the small proportion in this category (0.03%). BMI is defined as their weight in kilograms divided by the square of their height in meters. Patients were classified according to BMI standards: normal, 18.5 to <25; overweight, 25 to <30; class I obese, 30 to <35; class II obese, 35 to <40; and class III obese, BMI ≥40. Waist circumference was determined according to the NHANES (the National Health and Nutrition Examination Survey) III protocol during normal minimal respiration by placing a measuring tape around the waist just above the uppermost lateral border of the iliac crest.13, 19 The primary outcome of the present study was all‐cause mortality. Secondary outcomes were cardiac death, nonfatal myocardial infarction (MI), and stroke, which have been defined previously.15, 17
Statistical Analysis
Baseline characteristics of patients across the categories were summarized as frequencies and percentages for categorical variables and as means and standard deviations or interquartile range for continuous variables, depending on whether data distribution was normal (assessed by normal Q‐Q plots). Categorical variables were compared using χ2 analysis, and continuous variables were compared by ANOVA test or Mann–Whitney U test, according to distribution type. Cox proportional hazards regression models that included statistically significant covariates and clinically meaningful confounders were performed to investigate the relationship between BMI and clinical end points. The adjusted event hazard associated with each BMI stratum was compared with the class III obese group (BMI ≥40). Survival curves from Cox regression models, inclusive of the 5 BMI groups defined earlier, with statistically significant covariates and clinically meaningful confounders as co‐covariates, were performed using class III obese patients as references. In the multivariable model, we adjusted sex, age, race, hypertension, smoking, hyperlipidemia, previous cardiovascular events, previous heart failure, proteinuria, depression, heart rate, systolic blood pressure, diastolic blood pressure, total cholesterol, low‐density lipoprotein, high‐density lipoprotein, glycated hemoglobin, and glomerular filtration rate, according to previous studies.11, 20, 21, 22 A cubic spline smoothing technique was used to study the shape of the relationship of BMI and waist circumference with the logarithm of the relative risk of adverse events adjusted for the above‐mentioned confounding factors. The correlation between BMI and waist circumference was determined using the Pearson correlation coefficient (r). All statistical tests were 2‐sided, and P<0.05 was considered statistically significant. All analyses were performed using IBM‐SPSS (v22; IBM Corp), Empower (X&Y Solutions), and R (http://www.R-project.org).
Results
Baseline Characteristics
The proportions of included participants with T2DM by BMI category were as follows: 8.39% normal weight, 28.9% overweight, 32.9% class I obese, 20.2% class II obese, and 9.61% class III obese. The mean age was 64.0±7.53 years, and the majority (61.4%) of the patients were male. Class III obese participants with DM were >3 years younger than their normal‐weight counterparts. Baseline demographic, clinical, laboratory examination, and medication information is shown in Table 1 according to BMI groups. Obese patients were younger and more likely to have cardiovascular risk factors, specifically hypertension, hyperlipidemia, and proteinuria, than were normal‐weight patients. Class III obese participants with DM were more likely to be women and to develop depression. Smoking and previous cardiovascular events decreased, whereas other traditional cardiac risk factors, duration of DM, and rate of depression increased across increasing categories of obesity. Obese patients were more likely to accept evidence‐based therapies, including aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors, and thiazolidinedione than were normal‐weight patients.
Table 1.
Baseline Demographic, Clinical, and Laboratory Examination Characteristics
| BMI Category (kg/m2) | P Value | |||||
|---|---|---|---|---|---|---|
| Normal Weight, <25 | Overweight, 25 to <30 | Class I Obese, 30 to <35 | Class II Obese, 35 to <40 | Class III Obese, ≥40 | ||
| n | 860 | 2962 | 3368 | 2069 | 985 | |
| BMI, kg/m2 | 23.4±1.36 | 27.8±1.37 | 32.4±1.43 | 37.2±1.43 | 42.3±1.61 | 0.000 |
| Age, y | 64.0±7.53 | 63.9±6.95 | 62.9±6.51 | 61.6±6.00 | 60.9±5.78 | 0.000 |
| Sex, % | 0.000 | |||||
| Male | 555 (64.5) | 2024 (68.3) | 2156 (64.0) | 1139 (55.1) | 421 (42.7) | |
| Female | 306 (35.5) | 938 (31.7) | 1212 (36.0) | 930 (44.9) | 564 (57.3) | |
| Race | 0.000 | |||||
| White | 355 (41.2) | 1698 (57.3) | 2216 (65.8) | 1428 (69.0) | 692 (70.3) | |
| Nonwhite | 506 (58.8) | 1264 (32.8) | 1152 (34.2) | 641 (31.0) | 293 (37.6) | |
| Median duration of DM, y | 11.8±7.8 | 11.6±8.0 | 10.2±7.30 | 10.4±7.17 | 10.4±7.59 | 0.000 |
| Hypertension | 592 (68.8) | 2178 (73.5) | 2543 (75.5) | 1618 (78.2) | 790 (80.2) | 0.000 |
| Hyperlipidemia | 556 (64.7) | 2085 (70.4) | 2423 (71.9) | 1409 (68.1) | 687 (69.7) | 0.000 |
| Previous cardiovascular events | 282 (32.8) | 1099 (37.1) | 1219 (36.2) | 694 (33.5) | 311 (31.6) | 0.000 |
| Current smoker | 182 (21.2) | 468 (15.8) | 430 (12.8) | 244 (11.8) | 102 (10.4) | 0.000 |
| Previous heart failure | 35 (4.10) | 117 (4.00) | 133 (3.90) | 136 (6.60) | 71 (7.20) | 0.000 |
| Proteinuria | 146 (17.0) | 524 (17.7) | 651 (19.3) | 469 (22.7) | 243 (24.7) | 0.000 |
| Depression | 113 (13.1) | 563 (19.0) | 783 (23.2) | 614 (29.7) | 348 (35.3) | 0.000 |
| Waist circumference, cm | 86.8±7.23 | 97.8±7.89 | 107.9±8.51 | 117.1±9.48 | 125.5±10.0 | 0.000 |
| HR, beats/min | 72.0±11.8 | 71.5±11.6 | 73.3±11.8 | 73.6±11.6 | 75.1±11.8 | 0.000 |
| SBP, mm Hg | 136±18.2 | 137±16.8 | 136±17.0 | 136.4±17.1 | 136±17.3 | 0.523 |
| DBP, mm Hg | 72.7±10.7 | 73.6±10.5 | 75.8±10.6 | 75.9±10.5 | 77.3±10.8 | 0.000 |
| Glycated hemoglobin, % | 8.29±1.06 | 8.29±1.03 | 8.29±1.03 | 8.31±1.03 | 8.33±1.07 | 0.290 |
| GFR, mL/min | 93.3±37.7 | 90.7±27.9 | 90.6±24.2 | 91.7±26.4 | 90.1±27.1 | 0.043 |
| FPG, mg/dL | 174.7±66.3 | 172.8±54.9 | 175.8±55.3 | 177.4±54.7 | 177±.54.7 | 0.061 |
| Cholesterol, mg/dL | ||||||
| Total | 184.3±42.6 | 180.7±41.8 | 183.6±42.4 | 185.0±40.4 | 186.0±41.9 | 0.001 |
| LDL | 108.1±35.1 | 104.3±33.3 | 104.8±34.1 | 104.8±33.3 | 106.3±33.9 | 0.021 |
| HDL | 46.6±14.6 | 41.9±11.4 | 41.0±11.2 | 41.2±11.0 | 41.8±11.1 | 0.000 |
| Urinary albumin, mg/dL | 10.8±36.0 | 8.70±27.1 | 10.0±36.1 | 12.2±45.3 | 11.3±36.6 | 0.015 |
| Medications | ||||||
| Insulin | 57 (6.6) | 295 (10.0) | 367 (10.9 | 267 (12.9) | 154 (15.6) | 0.000 |
| Metformin | 534 (62) | 1862 (62.9) | 2211 (65.7) | 1324 (64.0) | 622 (63.1) | 0.112 |
| Sulfonylurea | 493 (57.3) | 1648 (55.6) | 1806 (53.6) | 1068 (51.6) | 458 (46.5) | 0.000 |
| Thiazolidinedione | 137 (6.1) | 566 (25.1) | 742 (22.0) | 534 (25.8) | 277 (28.1) | 0.001 |
| β‐Blocker | 200 (23.4) | 839 (28.3) | 1030 (33.5) | 696 (33.7) | 312 (31.7) | 0.000 |
| ACEI | 399 (46.7) | 1642 (55.5) | 1834 (54.7) | 1159 (56.1) | 530 (53.9) | 0.000 |
| Statin | 508 (59.6) | 1920 (65.0) | 2172 (64.8) | 1302 (63.1) | 595 (60.5) | 0.005 |
| Aspirin | 405 (47.5) | 1594 (54.0) | 1907 (57.0) | 1135 (55.0) | 534 (54.4) | 0.000 |
Data are shown as mean±SD or n (%) except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Crude Events Rates
During a mean follow‐up of 9.43±2.37 years, 1950 patients died (669 patients from cardiac death), 936 patients (9.1%) survived nonfatal MI, and 516 patients developed stroke (5.0%). Crude clinical outcomes among BMI categories are shown in Table 2. The rates of all adverse events across all categories were similar.
Table 2.
Crude Primary and Secondary End Points Among BMI Categories
| BMI Category (kg/m2) | P Value | |||||
|---|---|---|---|---|---|---|
| Normal Weight, <25 | Overweight, 25 to <30 | Class I Obese, 30 to <35 | Class II Obese, 35 to <40 | Class III Obese, ≥40 | ||
| All‐cause mortality | 169 (19.6) | 567 (19.1) | 612 (18.2) | 409 (19.8) | 197 (19.1) | 0.533 |
| Cardiac death | 59 (6.9) | 171 (5.8) | 215 (6.4) | 152 (7.3) | 71 (7.2) | 0.195 |
| Nonfatal MI | 83 (9.6) | 282 (9.5) | 290 (8.6) | 191 (9.2) | 88 (9.1) | 0.739 |
| Noncardiac death | 110 (12.8) | 396 (13.4) | 397 (11.8) | 257 (12.4) | 126 (12.8) | 0.445 |
Data are shown as n (%). BMI indicates body mass index; MI, myocardial infarction.
Multivariate Adjusted Analyses
After adjusting statistically significant covariates and clinically meaningful confounders, patients who were class III obese with BMI ≥40 had the highest risk of all‐cause mortality, followed by patients with class II obesity, whereas overweight patients had the lowest risk (Figure 1A). The adjusted hazard ratio ranged from 0.665 (95% confidence interval, 0.561–0.788) to 0.863 (95% confidence interval, 0.727–1.025) compared with the reference group. The same pattern was also observed for cardiac death. The highest cardiac death rate was also in the class III obese group, followed by that in the patients with class II obesity, whereas the lowest cardiac death rate was in the overweight patients (Figure 1B). No significant differences were noted between groups with respect to the incidence of nonfatal MI (Figure 1C). The highest noncardiac death rate was also in the class III obese group, followed by the patients with class II obesity, whereas the lowest noncardiac death rate was among normal‐weight patients (Figure 1D). Figure 2 shows statistically significant covariates and clinically meaningful confounders adjusted survival curves for all‐cause mortality, cardiac death, nonfatal cardiac events, and noncardiac death based on the 5 predefined categories of BMI.
Figure 1.

Primary and secondary end points by body mass index category. A, All‐cause mortality. B, Cardiac death. C, Nonfatal MI. D, Noncardiac death. All models were adjusted for sex, age, race, hypertension, smoking, hyperlipidemia, previous cardiovascular events, previous heart failure, proteinuria, depression, heart rate, systolic blood pressure, diastolic blood pressure, total cholesterol, low‐density lipoprotein, high‐density lipoprotein, glycated hemoglobin, and glomerular filtration rate. CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction.
Figure 2.

Survival curves for primary and secondary end points for 5 predefined body mass index categories after adjusting multivariate rates. A, All‐cause mortality. B, Cardiac death. C, Nonfatal MI. D, Noncardiac death. All models were adjusted for cofounders in Figure 1. MI indicates myocardial infarction.
BMI as a Continuous Variable
Overweight patients with lower risk of multivariate‐adjusted rates across the entire group followed a J‐shaped pattern in terms of all‐cause mortality, with the highest rates observed in class III obese patients and the lowest in overweight patients (Figure 3A). A V‐shaped relationship was observed between BMI and cardiac death, where event rate decreased sharply with increasing BMI between 20 and 27, and then began to increase sharply at BMI >27 (Figure 3B). BMI was related to nonfatal MI in a U‐shaped manner after adjusting for confounding factors, where the nadir of the risk curve was at BMI 33 (Figure 3C). The risk of noncardiac death was found to increase with BMI (Figure 3D).
Figure 3.
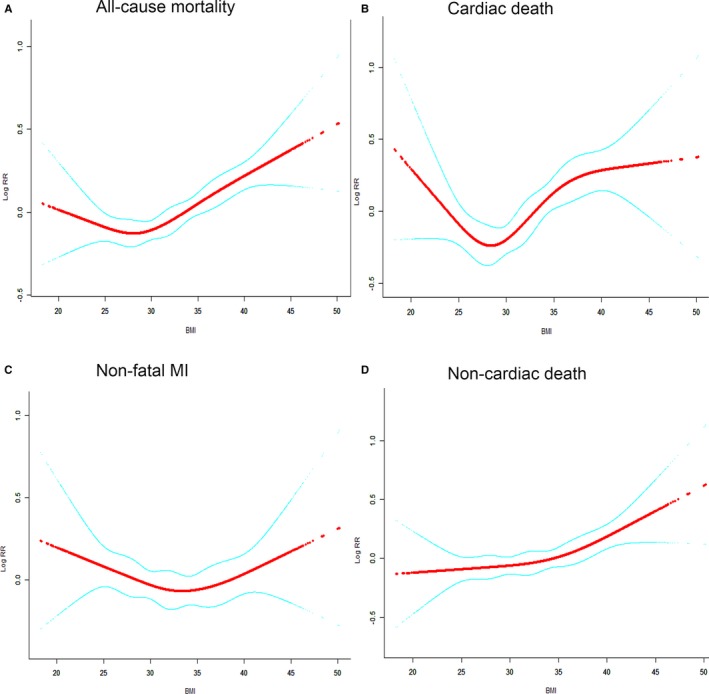
Smooth spline curves of BMI for the estimation of risk of primary and second end points after adjusting multivariate rates. A, All‐cause mortality. B, Cardiac death. C, Nonfatal MI. D, Noncardiac death. Red line denotes fitted curves, and blue line represents 95% confidence intervals for the association between BMI and adverse events. All models were adjusted for cofounders in Figure 1. BMI indicates body mass index; MI, myocardial infarction.
Waist Circumference as a Continuous Variable
We found significant correlations between BMI and waist circumference (r=0.802; Figure 4). When we used waist circumference as a continuous variable to study the continuous relationship between waist circumference and primary and secondary end outcomes after adjusting for multivariate rates, we observed relationships similar to those between BMI and primary and secondary end points (J‐shaped relationship for all‐cause mortality, V‐shaped relationship for cardiac death, U‐shaped relationship for nonfatal MI, and reverse linear relationship for noncardiac death; Figure 5).
Figure 4.

Correlation and agreement between body mass index (BMI) and waist circumference.
Figure 5.

Smooth spline curves of waist circumference for the estimation of risk of primary and second end points after adjusting the confounding factors. A, All‐cause mortality. B, Cardiac death. C, Nonfatal MI. D, Noncardiac death. Red line denotes fitted curves, and blue line represents 95% confidence intervals for the association between waist circumference and adverse events. All models were adjusted for cofounders in Figure 1. MI indicates myocardial infarction.
Discussion
This study was performed using the data from a large randomized controlled trial with a median follow‐up of 9.4 years. We studied the recent controversial issue of the obesity paradox in the context of all‐cause mortality, cardiac death, nonfatal MI, and noncardiac death in T2DM patients. Our results, benefiting as they do from our use of a large randomized controlled trial with a long duration of follow‐up and a relatively large proportion of obese patients, reasonably dispel any notion of an obesity paradox for T2DM patients.
Although the reasons for an obesity paradox are not clear, the characteristics of included patients could be involved. Zamorta et al23 compared 2527 heart failure patients with and without DM. The obesity paradox was present in heart failure patients without DM but not in those with DM, as if DM had removed this phenomenon from patients with heart failure. Other baseline characteristics may have had significant interactions with BMI and so affected Zamorta and colleagues’ conclusions. Overweight and obese patients tend to be younger, and younger patients tend to have better prognoses. Overweight and obese patients usually also have more comorbidities, which lead to more aggressive treatment of cardiovascular risk factors, increasing the likelihood of improving prognosis.
Abi Khalil et al24 categorized 5005 T2DM patients with acute heart failure in groups with BMI <20 (2%), 20 to 24.9 (20.8%), 25 to 29.9 (36.6%), 30 to 34.9 (23.3%), and ≥35 (17.6%), with 12 months of follow‐up. The results showed that BMI was inversely correlated with mortality. Furthermore, in this study we included relatively few participants with class II or III obesity (BMI ≥35). Thus, this result is not very informative regarding any relationship between extreme obesity and adverse events. Furthermore, Das et al25 assessed the impact of extreme obesity (class III obesity) on the outcomes of patients with ST‐segment–elevation MI. Patients with extreme obesity suffered higher mortality than their counterparts, a finding that is consistent with our results. Some cohort studies6, 20 found a U‐shaped relation between BMI and all‐cause mortality. The nadirs of these curves were around a BMI of 30 to 35. If these studies include more class I obese patients (the nadir of the curve) and relatively few class II or III obesity patients in future studies, a reverse relationship or the obesity paradox might appear.
Both underweight status and extreme obesity might increase the incidence of mortality.6, 26 Patients who are underweight may have severe undiagnosed diseases.23, 27 Conversely, extremely obese patients usually have an increased risk of coronary atherosclerotic heart disease and related cardiovascular risk factors, such as hypertension and DM.
Obesity was not determined solely using BMI but also by waist circumference, which provides information on body fat distribution. Most of these overweight or obese patients were abdominally obese. A recent study found that the similarly significant associations between BMI and cardiovascular risk factors, such as hypertension, hyperlipidemia, and DM, are also true of waist circumference.13 A study performed by Flegal and Graubard found that waist circumference and BMI predicted virtually the same number of all‐cause deaths.28 Our research found similar results compared with previous studies.
Limitations
Our analyses should be viewed in the context of some limitations. BMI and waist circumference were calculated at admission without reevaluation during the follow‐up period. However, the change in weight was small in overweight or obese patients.29 We did not have enough patients who were extremely lean (BMI ≤18.5) to draw conclusions between BMI and adverse events in these participants.
Conclusion
Data from the ACCORD study revealed no evidence of the obesity paradox in patients with a 10‐year history of DM. Class III patients have the highest risk of adverse events (all‐cause mortality, cardiac death, nonfatal MI, and noncardiac death). BMI and waist circumference had a similar relationship to adverse events.
Author Contributions
Hu and Xing designed the study and provided methodological expertise. Huang and Pei drafted the article. Chen and Peng drafted the tables and figures.
Disclosures
None.
Acknowledgments
All authors read, provided critical feedback, and approved the final article and agree to publish.
(J Am Heart Assoc. 2018;7:e010512 DOI: 10.1161/JAHA.118.010512.)
References
- 1. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. [DOI] [PubMed] [Google Scholar]
- 2. Williams R, Alexander G, Armstrong I, Baker A, Bhala N, Camps‐Walsh G, Cramp ME, de Lusignan S, Day N, Dhawan A, Dillon J, Drummond C, Dyson J, Foster G, Gilmore I, Hudson M, Kelly D, Langford A, McDougall N, Meier P, Moriarty K, Newsome P, O'Grady J, Pryke R, Rolfe L, Rice P, Rutter H, Sheron N, Taylor A, Thompson J, Thorburn D, Verne J, Wass J, Yeoman A. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391:1097–1107. [DOI] [PubMed] [Google Scholar]
- 3. Moscarella E, Spitaleri G, Brugaletta S, Sentí FS, Pernigotti A, Ortega‐Paz L, Cequier A, Iñiguez A, Serra A, Jiménez‐Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Valgimigli M, Serruys PW, Sabaté M. Impact of body mass index on 5‐year clinical outcomes in patients with ST‐segment elevation myocardial infarction after everolimus‐eluting or bare‐metal stent implantation. Am J Cardiol. 2017;120:1460–1466. [DOI] [PubMed] [Google Scholar]
- 4. Holroyd EW, Sirker A, Kwok CS, Kontopantelis E, Ludman PF, De Belder MA, Butler R, Cotton J, Zaman A, Mamas MA. The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British Cardiovascular Intervention Society Registry. JACC Cardiovasc Interv. 2017;10:1283–1292. [DOI] [PubMed] [Google Scholar]
- 5. Joyce E, Hoogslag GE, Kamperidis V, Debonnaire P, Katsanos S, Mertens B, Marsan NA, Bax JJ, Delgado V. Relationship between myocardial function, body mass index, and outcome after ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Imaging. 2017;10:e005670. [DOI] [PubMed] [Google Scholar]
- 6. Angerås O, Albertsson P, Karason K, Råmunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. [DOI] [PubMed] [Google Scholar]
- 7. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, Jacob S. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123:646–651. [DOI] [PubMed] [Google Scholar]
- 8. Gupta PP, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Can J Cardiol. 2015;31:195–202. [DOI] [PubMed] [Google Scholar]
- 9. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell‐Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. BMI‐mortality paradox and fitness in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2012;35:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. So WY, Yang X, Ma RC, Kong AP, Lam CW, Ho CS, Cockram CS, Ko GT, Chow CC, Wong V, Tong PC, Chan JC. Risk factors in V‐shaped risk associations with all‐cause mortality in type 2 diabetes—The Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2008;24:238–246. [DOI] [PubMed] [Google Scholar]
- 12. Thomas G, Khunti K, Curcin V, Molokhia M, Millett C, Majeed A, Paul S. Obesity paradox in people newly diagnosed with type 2 diabetes with and without prior cardiovascular disease. Diabetes Obes Metab. 2014;16:317–325. [DOI] [PubMed] [Google Scholar]
- 13. Abbasi F, Blasey C, Reaven GM. Cardiometabolic risk factors and obesity: does it matter whether BMI or waist circumference is the index of obesity. Am J Clin Nutr. 2013;98:637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail‐Beigi F, Feeney P. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i–43i. [DOI] [PubMed] [Google Scholar]
- 16. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Grimm RH, Margolis KL, Probstfield JL, Simons‐Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 17. Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail‐Beigi F, Grimm RH, Probstfield JL, Simons‐Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nine‐year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 20. Benderly M, Boyko V, Goldbourt U. Relation of body mass index to mortality among men with coronary heart disease. Am J Cardiol. 2010;106:297–304. [DOI] [PubMed] [Google Scholar]
- 21. Medina‐Inojosa JR, Somers VK, Thomas RJ, Jean N, Jenkins SM, Gomez‐Ibarra MA, Supervia M, Lopez‐Jimenez F. Association between adiposity and lean mass with long‐term cardiovascular events in patients with coronary artery disease: no paradox. J Am Heart Assoc. 2018;7:e007505 DOI: 10.1161/JAHA.117.007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan MD, O'Connor P, Feeney P, Hire D, Simmons DL, Raisch DW, Fine LJ, Narayan KM, Ali MK, Katon WJ. Depression predicts all‐cause mortality: epidemiological evaluation from the ACCORD HRQL substudy. Diabetes Care. 2012;35:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zamora E, Lupón J, Enjuanes C, Pascual‐Figal D, de Antonio M, Domingo M, Comín‐Colet J, Vila J, Peñafiel J, Farré N, Alonso N, Santesmases J, Troya M, Bayés‐Genís A. No benefit from the obesity paradox for diabetic patients with heart failure. Eur J Heart Fail. 2016;18:851–858. [DOI] [PubMed] [Google Scholar]
- 24. Abi Khalil C, Sulaiman K, Singh R, Jayyousi A, Asaad N, AlHabib KF, Alsheikh‐Ali A, Al‐Jarallah M, Bulbanat B, AlMahmeed W, Dargham S, Ridha M, Bazargani N, Amin H, Al‐Motarreb A, AlFaleh H, Elasfar A, Panduranga P, Al Suwaidi SJ. BMI is inversely correlated to the risk of mortality in patients with type 2 diabetes hospitalized for acute heart failure: findings from the Gulf aCute heArt failuRE (Gulf‐CARE) registry. Int J Cardiol. 2017;241:262–269. [DOI] [PubMed] [Google Scholar]
- 25. Das SR, Alexander KP, Chen AY, Powell‐Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in‐hospital outcomes of 50,149 patients with ST‐Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body‐mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body‐mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 28. Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr. 2009;89:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fadl YY, Krumholz HM, Kosiborod M, Masoudi FA, Peterson PN, Reid KJ, Weintraub WS, Buchanan DM, Spertus JA. Predictors of weight change in overweight patients with myocardial infarction. Am Heart J. 2007;154:711–717. [DOI] [PubMed] [Google Scholar]


