Abstract
Background
Exaggerated blood pressure response during exercise predicts future hypertension and cardiovascular events in general population and different patients groups. However, its clinical and prognostic implications in patients with aortic stenosis have not been previously evaluated.
Methods and Results
We retrospectively studied 301 patients with moderate to severe asymptomatic aortic stenosis (aged 65±12 years) who underwent echocardiography and a modified Bruce exercise treadmill test. An exaggerated blood pressure response was defined as peak systolic blood pressure ≥190 mm Hg. An abnormal blood pressure response (either blunted or exaggerated) was found in 58% of patients and abnormal left ventricular geometry in 82%. There was no difference in the rates of abnormal blood pressure responses between patients with moderate and severe aortic stenosis ([exaggerated blood pressure response: 21% versus 22%, P=0.876] and [blunted blood pressure response: 35% versus 40%, P=0.647]). Patients with exaggerated blood pressure response (21%) were more likely to be older, have hypertension, higher pretest systolic blood pressure, left ventricular ejection fraction and mass, and increased arterial stiffness (all P<0.05). In a multivariate logistic regression analysis, an exaggerated blood pressure response was associated with higher pulse pressure/stroke volume index (odds ratio 2.45, 95% confidence interval 1.02–6.00, P=0.037) and left ventricular mass (odds ratio 2.04, 95% confidence interval 1.23–3.38, P=0.012) independent of age, hypertension, aortic annulus and left atrium diameter, and left ventricular ejection fraction.
Conclusions
In those with aortic stenosis, exaggerated blood pressure was strongly related to higher resting blood pressure values, left ventricular mass, and increased arterial stiffness independent of hypertension.
Keywords: aortic stenosis, exaggerated blood pressure response, exercise treadmill test, hypertension, outcome
Subject Categories: Exercise Testing, Echocardiography, Blood Pressure
Clinical Perspective
What Is New?
Abnormal blood pressure (BP) response to exercise testing was found in more than half of patients with moderate or severe aortic stenosis.
In particular exaggerated BP response was identified as a potential harmful response, and was strongly related to higher resting BP, left ventricular mass, and increased arterial stiffness independent of hypertension.
What Are the Clinical Implications?
An exaggerated BP response to exercise testing in aortic stenosis patients may identify patients with poorly controlled hypertension.
These patients may benefit from strict BP control to reduce vascular load and subsequent risk of cardiovascular complications.
Introduction
Exercise treadmill testing (ETT) provides clinically important diagnostic and prognostic information in different patient populations. In patients with asymptomatic moderate or severe aortic stenosis (AS), this includes both symptoms and heart rate and blood pressure (BP) responses to exercise.1, 2 Symptoms revealed by ETT are a class I indication for surgery while a fall in systolic BP is a class IIa indication.1, 2 However some asymptomatic patients may exhibit an exaggerated BP response (EBPR) to exercise, irrespective of the BP values at rest or of a history of hypertension. In the general population this phenomenon is associated with a greater risk of incident hypertension3, 4, 5 as well as of cardiovascular morbidity and mortality.4, 6, 7 Although arterial hypertension is the most common comorbidity in patients with AS,8, 9, 10 the significance of EBPR in AS is not known. The aim of the present study was to assess the clinical implications and prognostic value of EBPR in moderate or severe AS using data from the EXTAS (Exercise Testing in AS) cohort study.
Methods
Data Availability
The data, analytic methods, and study materials will not be made freely available to other researchers for purposes of reproducing the results or replicating the procedure since the complete study data set contains potentially identifying data.
Patient Population
The EXTAS study is a retrospective analysis of data gathered prospectively between January 2000 and May 2017. A total of 651 patients aged >18 years with moderate (effective orifice area 1.0–1.5 cm2) or severe (effective orifice area <1.0 cm2) AS1, 2 were assessed in a dedicated heart valve clinic at Guy's and St Thomas’ Hospital. During a median follow‐up of 25 months (mean 34.9±34.6 months) only 7 patients were lost to follow‐up. All patients underwent ETT at presentation and most were restudied when their AS crossed the threshold between moderate and severe, and thereafter annually. The present analysis focuses on the baseline data including ETT, echocardiography, and clinical characteristics. Patients were excluded from the study if they declared spontaneous symptoms justifying surgery (n=283), had co‐existent additional valve disease of more than moderate severity (12 patients with severe mitral regurgitation, 4 with severe mitral stenosis, and 5 with severe aortic regurgitation) or had an inability to exercise owing to comorbidities such as peripheral vascular disease (n=2), chronic obstructive pulmonary disease (n=24), anemia (n=2), or immobility or severe arthritis (n=3). The remaining 316 patients (49%) were apparently asymptomatic by history and eligible for ETT. Of these, peak systolic BP was not recorded in 15 patients, leaving 301 patients eligible for inclusion in the present analysis. Hypercholesterolemia was defined as treatment with lipid‐lowering drugs. Resting clinic BP before ETT was measured with a semiautomatic device with the patient resting for 5 to 10 minutes in the sitting position. Hypertension was defined from a history of elevated BP values, past or current treatment with antihypertensive agents or a BP at the baseline clinic visit >140/90 mm Hg.8 Before surgical or transcutaneous aortic valve replacement (AVR), all patients underwent conventional coronary angiography. Coronary artery disease was defined as previous myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention, or angiographic evidence of coronary artery disease (>70% stenosis of ≥1 main epicardial arteries). The study was approved by the local Institutional Review Board (Study Protocol no. 7461/2017), and the requirement about the informed consent was waived. The study was managed and conducted in accordance with the Declaration of Helsinki, and latest Good Clinical Practice guidelines.
Exercise Treadmill Test Protocol
The method for ETT have previously been described in detail.8 Briefly, ETTs were performed according to American College of Cardiology/American Heart Association practice guidelines using a Bruce protocol modified by 2 warm‐up stages.11, 12 The test was stopped prematurely for symptoms (significant breathlessness or any chest constriction or dizziness), progressive ventricular ectopy >3 beats, new atrial fibrillation, a sustained fall in systolic BP >20 mm Hg from the previous stage or >5 mm ST‐segment depression. The following measures were recorded: exercise time, exercise capacity in metabolic equivalents (METs), maximum rise in systolic BP and maximum fall from peak, ST‐segment depression in millimeters. METs were calculated from the speed and gradient of the treadmill by the machine's software using the formula (METS=[(speed×0.1)+(gradient/100×1.8×speed)+3.5]/3.5), where speed is measured in m/s and gradient as a percentage. One MET is usually defined as the energy expended at rest which is equal to a body oxygen consumption of nearly 3.5 mL per kilogram of body weight for an average adult.13 Blunted BP response was defined as a sustained fall in systolic BP ≥20 mm Hg below the previous stage or a failure to rise from the baseline level.14 An EBPR was defined as a peak systolic BP ≥190 mm Hg during ETT. Abnormal BP response was defined as either blunted BP response or EBPR.
Transthoracic Echocardiography
Echocardiographic data were obtained using commercially available ultrasound systems (Vingmed system 5, 7, 9 GE Medical, Milwaukee, Wisconsin, USA and a Philips “Epiq 7” cardiac ultrasound machine). The severity of AS, left ventricular (LV) wall thicknesses, chamber dimensions, stroke volume and ejection fraction were measured according to the prevalent European and United States guidelines of the time.15 The recommended methodology did not change over the period of the study. Although the thresholds for moderate and severe AS changed in 2009,15 these were standardized as part of our retrospective analysis. Mean transaortic resistance was calculated as 1.333×(mean transaortic pressure difference/mean transaortic flow) in dyne s/cm5.16 LV hypertrophy was diagnosed according to the prognostically validated cutoff values of LV mass >46.7 g/m2.7 in women and 49.2 g/m2.7 in men, respectively, and relative wall thickness as LV posterior wall thickness/LV internal radius at end‐diastole, and considered increased if ≥0.43.17 Systemic arterial distensibility was derived from the ratio of stroke volume index (SVi) divided by central pulse pressure (PP) (SV/PP) (mL/m2 per mm Hg), where central PP was calculated from brachial PP: central PP=(brachial PP×0.49)+(age×0.30)+7.11.9, 18 A low systemic arterial distensibility was defined as SVi/PP ≤0.6 mL/m2 per mm Hg. The ratio of PP to SVi (PP/SVi) was used as an indirect measure of systemic arterial stiffness.19
Study End Points
All‐cause mortality and AVR (either surgical or via a transcatheter approach) were recorded during follow‐up. Deaths were confirmed by reviewing the electronic patient record or a death certificate with September 19, 2017 as the censoring date. Follow‐up time was calculated from the baseline ETT until AVR, death or censoring.
Statistical Analyses
SPSS version 24.0 (IBM Corporation, Armonk, NY) was used for data management and statistical analyses. Continuous variables were tested for normality of distribution and presented as mean±SD. Comparison between the 3 groups was done by analysis of variance with Scheffe's post‐hoc test for continuous variables and a general linear model with Sidak's post‐hoc test for categorical variables. The predictors of EBPR were first identified in univariable binary logistic regression models, reported as odds ratio and 95% confidence interval (CI). Then based on univariate associations of a P<0.10, predictors of EBPR were selected and included in the multivariate logistic regression model. The bivariate association between peak systolic blood pressure and pretest systolic blood pressure was assessed with Pearson's correlation coefficient. Kaplan–Meier curves were used to examine cumulative event rates, and the difference between groups was tested using a log‐rank test. Cox proportional Hazard models were used to assess the association between EBPR and outcomes. Non‐linear associations between pre‐ETT systolic BP, peak systolic BP during ETT, difference between pre‐ETT systolic BP and peak systolic BP, and ratio of peak‐by pre‐ETT systolic BP and all‐cause mortality were explored by fitting the Cox proportional hazard model with penalized smoothing splines. A P<0.05 was considered to be statistically significant.
Results
Baseline Characteristics and Patterns of Blood Pressure Responses During Exercise
The mean age of the 301 patients was 65±12 years, and 67% were men. At baseline the AS was moderate in 200 (66%) and severe in 101 (34%). Two‐hundred eighty‐two (94%) were in sinus rhythm, 13 (4%) in atrial fibrillation and 6 (2%) were paced. An abnormal BP response during the baseline ETT was found in 174 (58%) patients. Of these, 64 (37%) exhibited an EBPR and 110 (63%) a blunted BP response. There was no difference in the rates of abnormal BP responses between those patients with moderate and severe AS ([EBPR: 21% versus 22%, P=0.876] and [blunted BP response: 35% versus 40%, P=0.647]). Patients with EBPR were more likely to be older or have documented hypertension, as well as higher clinic systolic BP, LV ejection fraction and LV mass (all P<0.05) (Table 1). On average, clinic systolic BP was 21 mm Hg higher in patients with EBPR compared with those with a normal BP response to ETT. PP/SVi, an indirect measure of systemic arterial stiffness, was also significantly higher in patients with EBPR compared with those with a normal BP response to ETT (Table 1). A low‐systemic arterial distensibility (SVi/PP ≤0.6 mL/m2 per mm Hg) was observed in 21% (n=63) of the entire study population, the prevalence being nearly 3‐fold as high in patients with EBPR compared with those with normal BP response to ETT (Table 1). A Pearson test for bivariate correlation showed that resting pretest systolic BP correlated closely with peak systolic BP during the ETT and was significantly higher in patients with EBPR compared with those with a normal BP response to ETT (Figure 1, Table 1). The average peak systolic BP was 202±10 in patients with EPBR versus 165±17 mm Hg in those with a normal BP response (Table 1). Furthermore, a statistically significant correlation was found between LV mass index and peak systolic BP during the ETT (r=0.22, P=0.018), but not with pretest systolic BP (r=0.13, P=0.064). In the entire study population, the prevalence of abnormal LV geometry was 82%, most commonly concentric LV hypertrophy (Figure 2). There was no difference in exercise duration, metabolic equivalents or severity of AS between patients with a normal BP response and EBPR (all P=NS), but patients with a blunted BP response had a significantly lower performance on ETT compared with patients with a normal BP response (P<0.01) (Table 1).
Table 1.
Characteristics of the Study Population According to BP Response During Exercise Treadmill Test
| Normal BP Response (n=127) | Blunted BP Response (n=110) | Exaggerated BP Response (n=64) | P Value | |
|---|---|---|---|---|
| Demographic and clinical data | ||||
| Age, y | 63±12 | 67±13* | 68±10* | 0.004 |
| Men/women | 63/37 | 68/32 | 70/30 | 0.533 |
| Body mass index, kg/m2 | 28.9±13.7 | 27.5±4.5 | 28.2±4.7 | 0.601 |
| Current and ex‐smokers, n (%) | 64 (50.5) | 45 (41) | 35 (54) | 0.239 |
| Clinic systolic BP, mm Hg | 135±16 | 142±18* | 156±19* , † | <0.001 |
| Clinic diastolic BP, mm Hg | 82±12 | 82±12 | 83±15 | 0.712 |
| Hypertension, n (%) | 77 (61) | 85 (77) | 54 (84) | 0.001 |
| Antihypertensive treatment, n (%) | 69 (55) | 77 (70) | 47 (73) | 0.039 |
| Coronary artery disease, n (%) | 58 (46) | 52 (47) | 38 (59) | 0.289 |
| Diabetes mellitus, n (%) | 15 (12) | 19 (17) | 8 (12) | 0.535 |
| Hypercholesterolemia, n (%) | 81 (64) | 70 (64) | 47 (73) | 0.468 |
| Echocardiographic data | ||||
| Left atrium dimension, cm | 3.7±0.6 | 3.8±0.8 | 3.9±0.6 | 0.298 |
| Aortic root diameter, cm | 3.3±0.5 | 3.3±0.5 | 3.3±0.5 | 0.859 |
| Aortic annulus diameter, cm | 2.1±0.2 | 2.1±0.3 | 2.0±0.2 | 0.124 |
| LV end‐diastolic diameter, cm | 4.4±0.6 | 4.6±0.7 | 4.6±0.7 | 0.197 |
| Interventricular septal thickness, cm | 1.3±0.3 | 1.3±0.3 | 1.4±0.3 | 0.157 |
| LV mass index, g/m2.7 | 48.2±14.9 | 51.3±18.1 | 58.7±19.1* | 0.007 |
| LV hypertrophy, n (%) | 61 (48) | 56 (51) | 44 (68) | 0.027 |
| LV ejection fraction, % | 61±6 | 59±8 | 62±6‡ | 0.013 |
| Mean transaortic resistance, dyne s/cm5 | 198±80 | 185±78 | 195±98 | 0.872 |
| Mean aortic gradient, mm Hg | 34±14 | 35±13 | 34±12 | 0.897 |
| Effective orifice area, cm2 | 0.95±0.22 | 0.91±0.22 | 0.95±0.22 | 0.281 |
| Doppler stroke volume index, mL/m2 | 43±14 | 42±14 | 45±10 | 0.533 |
| PP/SVi, mm Hg/mL per m2 | 1.27±0.48 | 1.55±0.52* | 1.71±0.68* | <0.001 |
| SVi/PP, mL/m2 per mm Hg | 0.85±0.28 | 0.75±0.27 | 0.73±0.22 | 0.023 |
| SVi/PP ≤0.6 mL/m2 per mm Hg, n (%) | 12 (10) | 34 (31) | 18 (29) | 0.002 |
| Exercise treadmill testing data | ||||
| Systolic BP before exercise, mm Hg | 133±15 | 144±19 | 153±18* , † | <0.001 |
| Diastolic BP beforeexercise, mm Hg | 84±10 | 85±11 | 87±12 | 0.275 |
| Target heart rate achieved, % | 87±14 | 84±17 | 89±11‡ | 0.026 |
| Peak systolic BP, mm Hg | 165±17 | 147±20* | 202±10* , † | <0.001 |
| Peak diastolic BP, mm Hg | 90±15 | 86±15 | 100±16* , † | <0.001 |
| Peak heart rate, beat/min | 137±26 | 128±26* | 137±19 | 0.010 |
| Exercise duration, min | 10.5±4.5 | 8.9±4.4 | 9.9±3.9 | 0.014 |
| Metabolic equivalents (METs) | 9.3±4.5 | 8.0±4.6 | 8.7±4.4 | 0.064 |
BP indicates blood pressure; LV, left ventricular; PP/SVi, pulse pressure/stroke volume index.
*P<0.01 vs normal BP response.
† P<0.01, ‡ P<0.05 vs blunted BP response.
Figure 1.
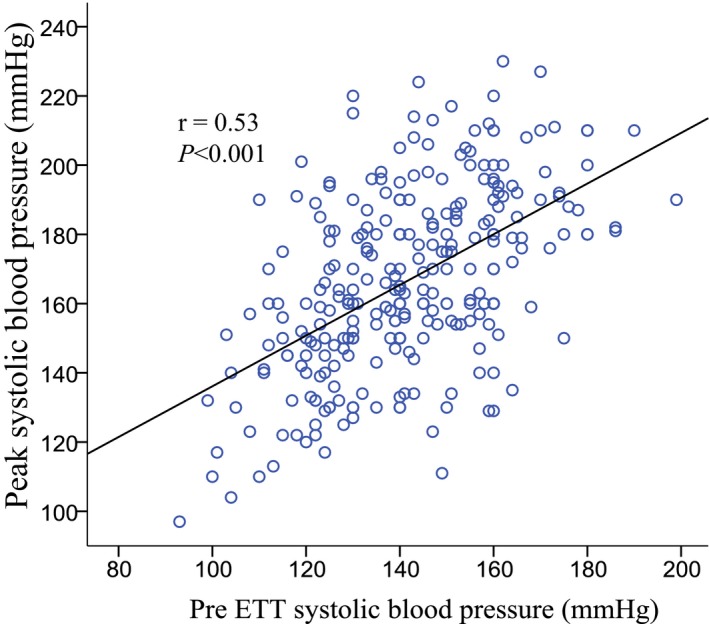
Scatterplot of peak systolic blood pressure during ETT and pre ETT systolic blood pressure. ETT indicates exercise treadmill test.
Figure 2.
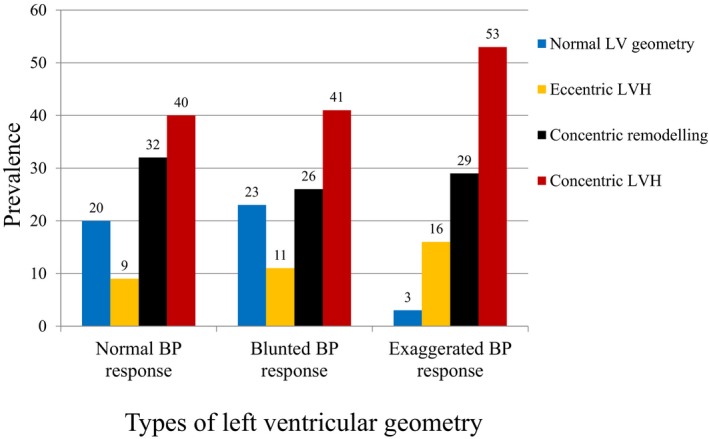
The prevalence of the types of left ventricular geometry according to BP response during exercise treadmill test. BP indicates blood pressure; LV, left ventricular; LVH, left ventricular hypertrophy.
Predictors of Exaggerated Blood Pressure Response
In a univariate logistic regression analysis, higher age, the presence of hypertension, abnormal LV geometry, higher systolic BP, LV mass, and higher ejection fraction and PP/SVi, but lower systemic arterial distensibility were all associated with the presence of EBPR (Table 2). In a multivariate logistic regression analysis, EBPR was associated with higher PP/SVi and LV mass independent of age, hypertension, aortic annulus and left atrium diameter, and LV ejection fraction (Table 2). In the same multivariate model, when hypertension was replaced by clinic systolic BP, EBPR retained a significant association with higher systolic BP (odds ratio per 1 SD systolic BP 2.08, 95% CI 1.08–4.02, P=0.030) and higher LV mass (odds ratio per 1 SD LV mass 1.81, 95% CI 1.11–2.97, P=0.019) independent of age, aortic annulus and left atrium diameter, and LV ejection fraction.
Table 2.
Predictors of Exaggerated Blood Pressure Response in Univariate and Multivariate Logistic Regression Analyses in Moderate or Severe Aortic Stenosis
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | 1.02 (0.99–1.05) | 0.065 | 1.05 (1.00–1.10) | 0.046 |
| Male sex | 1.25 (0.67–2.28) | 0.461 | ||
| Weight, kg | 1.01 (0.99–1.03) | 0.415 | ||
| Body mass index, kg/m2 | 0.99 (0.96–1.03) | 0.838 | ||
| Clinic SBP (per 1SD [7.5 mm Hg] increase) | 2.93 (2.03–4.21) | <0.001 | ||
| Clinic DBP, mm Hg | 1.00 (0.98–1.03) | 0.703 | ||
| Pre‐test SBP (per 1SD [7.4 mm Hg] increase) | 2.39 (1.71–3.34) | <0.001 | ||
| Pre‐test DBP (per 1SD [7.7 mm Hg] increase) | 1.20 (0.90–1.58) | 0.216 | ||
| Hypertension | 2.48 (1.20–5.14) | 0.014 | 1.57 (0.50–4.88) | 0.437 |
| Antihypertensive treatment | 1.64 (0.79–3.41) | 0.189 | ||
| Diabetes mellitus | 0.83 (0.34–2.01) | 0.65 | ||
| Hypercholesterolemia | 2.43 (0.86–6.87) | 0.093 | ||
| Aortic annulus diameter, mm | 0.22 (0.05–0.94) | 0.040 | 1.14 (0.10–13.40) | 0.917 |
| LV ejection fraction (%) | 1.05 (1.00–1.10) | 0.045 | 1.05 (0.97–1.14) | 0.206 |
| Left atrium diameter, cm | 1.04 (0.99–1.09) | 0.088 | 0.96 (0.89–1.04) | 0.303 |
| Interventricular septal thickness, cm | 2.80 (0.94–8.33) | 0.064 | ||
| Posterior wall thickness, cm | 5.80 (1.50–22.42) | 0.011 | ||
| LV mass (per 1 SD [2.8 g] increase) | 1.43 (1.07–1.91) | 0.015 | 2.04 (1.23–3.38) | 0.012 |
| High LV mass, g/m2.7 | 2.19 (1.04–4.64) | 0.040 | ||
| LV hypertrophy | 2.19 (1.04–4.64) | 0.039 | ||
| Abnormal LV geometry | 10.23 (1.35–77.27) | 0.024 | ||
| Effective orifice area, cm2 | 1.65 (0.46–5.87) | 0.441 | ||
| Doppler stroke volume, mL/m2 | 1.00 (0.99–1.02) | 0.586 | ||
| PP/SVi, mm Hg/mL per m2 | 2.58 (1.63–4.88) | 0.004 | 2.47 (1.02–6.00) | 0.037 |
| Low SAD (SVi/PP ≤0.6 mL/m2 per mm Hg) | 2.48 (1.15–5.33) | 0.020 | ||
CI indicates confidence interval; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LV, left ventricular; OR, odds ratio; PWV, pulse wave velocity; SAD; systemic arterial distensibility; SBP, systolic blood pressure.
Prediction of Outcomes by ETT Measures
During a mean follow up period of 34.9±34.6 months 250 (84%) patients had events, 222 AVR and 28 deaths. AVR occurred in 79% (n=100) with a normal BP response, 71% (n=78) with a blunted BP response, and 72% (n=46) with EBPR, (P=0.339). Death occurred in 7 (6%) with a normal BP response, 16 (15%) with a blunted BP response, and 5 (8%) with EBPR (P=0.051). In a univariate Cox regression analysis, the presence of EBPR was neither associated with all‐cause mortality (hazard ratio [HR] 0.93, 95% CI 0.35–2.45, P=0.884) nor AVR (HR 1.04, 95% CI 0.75–1.43, P=0.838). However a Kaplan–Meier analysis by BP response, showed a trend towards a difference in survival between the groups (Log‐rank P=0.061) (Figure 3). Blunted BP response was associated with all‐cause mortality (HR 2.30, 95% CI 1.09–4.88, P=0.029) in univariate Cox regression analysis, but when adjusting for age, male sex, mean pressure gradient and hypertension, the association between blunted BP response and all‐cause mortality did not remain significant (HR 1.80, 95% CI 0.75–4.28, P=0.186).
Figure 3.
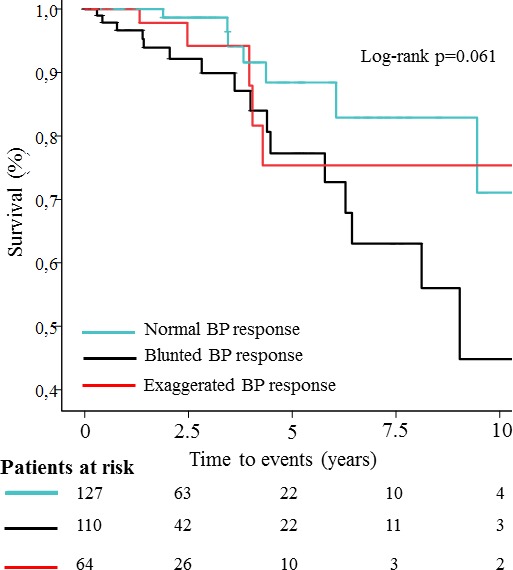
Kaplan–Meier plot illustrating the impact of exaggerated BP response on cumulative survival free from total mortality. BP indicates blood pressure.
In the unadjusted Cox proportional models, the difference between pretest systolic BP and peak systolic BP showed an inverse correlation with all‐cause mortality (Figure 4A). Mortality was highest if the BP rose by less than 20 mm Hg which applies patients with blunted BP response (Figure 4A). Peak systolic BP during ETT had no significant association with all‐cause mortality (Figure 4B) whilst pre‐ETT systolic BP showed a positive correlation, and the ratio of peak by pretest systolic BP an inverse correlation, with all‐cause mortality (Figure 4C and 4D). When patients with a blunted BP response were excluded from the survival analyses, comparing only EBPR versus normal BP response, the results did not change (all‐cause mortality: HR 1.70, 95% CI 0.54–5.36, P=0.368 and AVR: HR 1.01, 95% CI 0.70–1.44, P=0.973).
Figure 4.
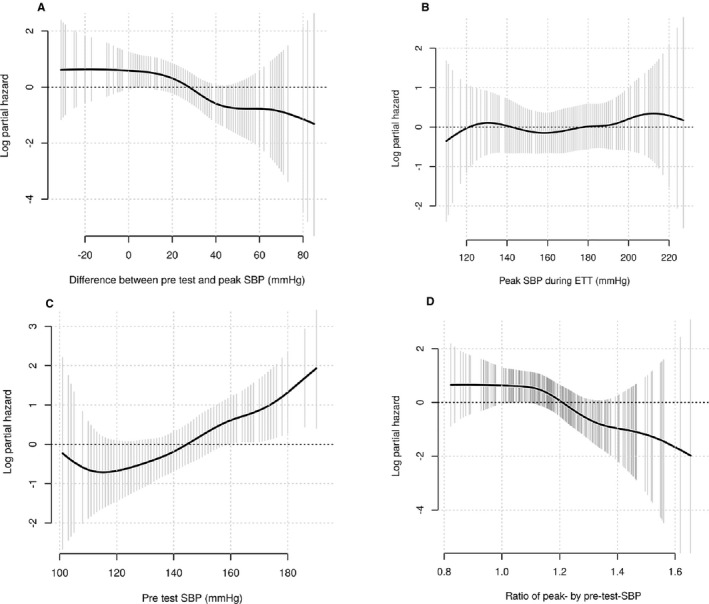
Smoothing spline estimates of potentially non‐linear relationships between total mortality and the difference between pretest and peak SBP (A), peak SBP during exercise (B), pretest SBP (C) and ratio of peak by pretest SBP (D). The solid line depicts the smoothed spline and the shaded area the 95% confidence interval. SBP indicates systolic blood pressure.
Discussion
In 301 patients with asymptomatic moderate or severe AS, an exaggerated BP response (EBPR) to ETT was seen in 64 (21%) patients. EBPR was more common in patients with higher resting BP values, increased LV mass, and increased systemic arterial stiffness. There was a trend towards a difference in survival across these 3 groups of normal, blunted and exaggerated BP response to exercise.
As expected the most frequent event was AVR, for which symptoms are a class I indication and a blunted BP response is a class IIa indication. However EPBR is not normally recorded and is not a recognized indication for AVR so it is not surprising that it was not prognostically important, although it was identified as potentially harmful response in our logistic regression models. By contrast EBPR has been repeatedly reported in studies on the general population3, 4, 5 as well as in hypertensive patients.20, 21, 22, 23, 24, 25 EBPR depends on age, the resting BP level and exercise intensity, and may also depend on increased sympathetic activity,20, 21 endothelial dysfunction and/or large artery stiffness.22, 23, 24, 25 There is no consensus on its definition. In a cohort of 1999 apparently healthy middle‐aged (40–59 years) Norwegian individuals,26 EBPR was defined as a systolic BP >200 mm Hg during a bicycle ergometer exercise test and predicted cardiovascular death and myocardial infarction.26 However in the Paris Prospective study III cohort of nearly 9000 patients aged 50 to 75 years, a peak systolic BP >150 mm Hg was used.27 This cutoff is probably too low since it is only in the resting range of 140 to 159 mm Hg defining the most common form of hypertension.28 The cutoff of 190 mm Hg we used to define EBPR was reasonable in view of the age of our population and the presence of AS.
Only a few small studies have investigated the hemodynamic responses to ETT in AS,29, 30, 31, 32 and all focused on revealed symptoms and a reduced BP response.14 We showed that EBPR was not rare in AS patients. It is likely that the BP response in AS is more complex than the general population and depends on LV contractile reserve and the resistance offered to LV outflow by the stenotic aortic valve. It is possible that the BP response can pseudonormalize because of a fall in stroke volume.33 Perlman et al showed that a rise in BP after transcutaneous aortic valve replacement (TAVI) was associated with better myocardial contractile reserve even in patients with reduced LV function at baseline.34 It is possible that a change in the BP response to exercise on serial ETT may reflect the natural history of the LV and valve in AS and provide further prognostic information.
In our patients we found a strong association between EBPR and higher resting BP, more frequent history of hypertension, greater LV mass and also increased systemic arterial stiffness. The LV is therefore exposed to at least 3 types of pressure overloads, 1 from AS per se, another from the concomitant hypertension, and a third from systemic arterial stiffness. In our study, a low systemic arterial distensibility (SVi/PP ≤0.6 mL/m2 per mm Hg) was 3‐fold more common in patients with EBPR compared with patients with a normal BP response to exercise. In a univariate logistic regression model, low systemic arterial distensibility was associated with a 2.5‐fold increased risk of EBPR, but in the adjusted model the significant association was not retained. Conversely, higher systemic arterial stiffness was associated with a 3‐fold increased risk of EBPR both in unadjusted and adjusted logistic regression models and was independent of hypertension. Our findings are in line with those demonstrated by the Framingham Heart Study showing that an increased systemic arterial stiffness may accelerate systolic BP elevation during exercise.35 Thus, the higher prevalence of systemic hypertension and arterial stiffness, as well as AS per se, may lead to abnormal LV geometry, impaired LV function, and worse clinical outcomes.9, 10, 36
We showed that LV mass was higher in patients with EBPR. Of note, only 3% of patients with EBPR had normal LV geometry compared with 20% in normal and 23% in blunted BP response groups. Similarly, more than a half of patients with EBPR had concentric LV hypertrophy. This suggests that EBPR might aid the decision to start antihypertensive therapy to avoid hypertension mediated target organ damage. Furthermore, the 5 deaths in patients with EBPR occurred relatively early in the first 4 years of follow‐up and all had elevated office and pretest systolic BP. By contrast, deaths occurred more gradually up to 10 years in patients with a normal BP response (Figure 3). This suggests that patients with high resting BP levels and EBPR were a high‐risk subgroup and might benefit from BP control. There is evidence that angiotensin‐converting enzyme inhibitors may preserve LV function reducing the rate of fibrosis and potentially prolonging the asymptomatic phase.37
Limitations
There were only 28 deaths in the entire study population, so the power for detecting a prognostic effect of EBPR was low. Further, owing to the retrospective design of the study with a heterogeneous study population it is difficult to exclude the impact of other unmeasured factors which may affect the relationship between EBPR and outcomes. Therefore, this analysis has to be regarded as hypothesis generating but it is strong enough to suggest that further prospective studies are warranted. Serial changes in BP response may also be instructive. Clinic BP before ETT was measured based on a real‐life clinical methodology which might be considered as a limitation for generalizing this in the research context.
Conclusion
In the EXTAS cohort study of moderate or severe AS, abnormal BP response was highly prevalent. In particular exaggerated BP response at baseline ETT was found in 21% of patients and was strongly related to higher resting BP, LV mass, and increased systemic arterial stiffness independent of hypertension, but could not predict adverse outcomes.
Disclosures
None.
Acknowledgments
We thank the participants of the EXTAS study for their important contributions.
(J Am Heart Assoc. 2018;7:e010735 DOI: 10.1161/JAHA.118.010735.)
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Manolio TA, Burke GL, Savage PJ, Sidney S, Gardin JM, Oberman A. Exercise blood pressure response and 5‐year risk of elevated blood pressure in a cohort of young adults: the CARDIA study. Am J Hypertens. 1994;7:234–241. [DOI] [PubMed] [Google Scholar]
- 4. Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol. 1998;51:29–35. [DOI] [PubMed] [Google Scholar]
- 5. Jae SY, Franklin BA, Choo J, Choi YH, Fernhall B. Exaggerated exercise blood pressure response during treadmill testing as a predictor of future hypertension in men: a longitudinal study. Am J Hypertens. 2015;28:1362–1367. [DOI] [PubMed] [Google Scholar]
- 6. Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol. 1995;26:1630–1636. [DOI] [PubMed] [Google Scholar]
- 7. Mottram PM, Haluska B, Yuda S, Leano R, Marwick TH. Patients with a hypertensive response to exercise have impaired systolic function without diastolic dysfunction or left ventricular hypertrophy. J Am Coll Cardiol. 2004;43:848–853. [DOI] [PubMed] [Google Scholar]
- 8. Saeed S, Rajani R, Seifert R, Parkin D, Chambers JB. Exercise testing in asymptomatic patients with moderate or severe aortic stenosis. Heart. 2018;104:1836–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saeed S, Senior R, Chahal NS, Lønnebakken MT, Chambers JB, Bahlmann E, Gerdts E. Lower trans‐aortic flow rate is associated with increased mortality in aortic valve stenosis. JACC Cardiovasc Imaging. 2017;10:912–920. [DOI] [PubMed] [Google Scholar]
- 10. Rieck ÅE, Cramariuc D, Boman K, Gohlke‐Bärwolf C, Staal EM, Lønnebakken MT, Rossebø AB, Gerdts E. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension. 2012;60:90–97. [DOI] [PubMed] [Google Scholar]
- 11. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 12. Bruce RA. Exercise testing methods and interpretation. Adv Cardiol. 1978;24:6–15. [DOI] [PubMed] [Google Scholar]
- 13. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. [DOI] [PubMed] [Google Scholar]
- 14. Magne J, Lancellotti P, Piérard LA. Exercise testing in asymptomatic severe aortic stenosis. JACC Cardiovasc Imaging. 2014;7:188–199. [DOI] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA Jr, Nakatani S, Quiñones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M. Echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves. J Am Soc Echocardiogr. 2009;22:975–1014. [DOI] [PubMed] [Google Scholar]
- 16. Das P, Rimington H, Smeeton N, Chambers J. Determinants of symptoms and exercise capacity in aortic stenosis: a comparison of resting haemodynamics and valve compliance during dobutamine stress. Eur Heart J. 2003;24:1254–1263. [DOI] [PubMed] [Google Scholar]
- 17. Gerdts E, Rossebø AB, Pedersen TR, Cioffi G, Lønnebakken MT, Cramariuc D, Rogge BP, Devereux RB. Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circ Cardiovasc Imaging. 2015;8:e003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chemla D, Hébert JL, Coirault C, Zamani C, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 19. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka S, Masuda T, Kamada Y, Hamazaki N, Kamiya K, Ogura MN, Maekawa E, Noda C, Yamaoka‐Tojo M, Ako J. Excessive SBP elevation during moderate exercise discriminates patients at high risk of developing left ventricular hypertrophy from hypertensive patients. J Hypertens. 2018;36:1291–1298. [DOI] [PubMed] [Google Scholar]
- 21. Kolasinska‐Kloch W, Furgala A, Banach T, Laskiewicz J, Thor PJ. Circadian heart rate variability in patients with primary arterial hypertension. Przegl Lek. 2002;59:752–755. [PubMed] [Google Scholar]
- 22. Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP, Ouyang P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. [DOI] [PubMed] [Google Scholar]
- 23. Olson KM, Augeri AL, Seip RL, Tsongalis GJ, Thompson PD, Pescatello LS. Correlates of endothelial function and the peak systolic blood pressure response to a graded maximal exercise test. Atherosclerosis. 2012;222:202–207. [DOI] [PubMed] [Google Scholar]
- 24. Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium‐derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004;102:87–96. [DOI] [PubMed] [Google Scholar]
- 25. Luscher TF. Imbalance of endothelium‐derived relaxing and contracting factors. A new concept in hypertension? Am J Hypertens. 1990;3:317–330. [DOI] [PubMed] [Google Scholar]
- 26. Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular death and myocardial infarction. Blood Press Monit. 1997;2:147–153. [PubMed] [Google Scholar]
- 27. Sharman JE, Boutouyrie P, Perier MC, Thomas F, Guibout C, Khettab H, Pannier B, Laurent S, Jouven X, Empana JP. Impaired baroreflex sensitivity, carotid stiffness, and exaggerated exercise blood pressure: a community‐based analysis from the Paris Prospective Study III. Eur Heart J. 2018;39:599–606. [DOI] [PubMed] [Google Scholar]
- 28. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 Task Force for the management of arterial hypertension of the European Society of Hypertension; Task Force for the management of arterial hypertension of the European Society of Cardiology. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2013;22:193–278. [DOI] [PubMed] [Google Scholar]
- 29. Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta‐analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol. 2009;104:972–977. [DOI] [PubMed] [Google Scholar]
- 30. Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart. 2001;86:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:377–382. [DOI] [PubMed] [Google Scholar]
- 32. Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis. 2002;11:204–209. [PubMed] [Google Scholar]
- 33. Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. [DOI] [PubMed] [Google Scholar]
- 34. Perlman GY, Loncar S, Pollak A, Gilon D, Alcalai R, Planer D, Lotan C, Danenberg HD. Post‐procedural hypertension following transcatheter aortic valve implantation: incidence and clinical significance. JACC Cardiovasc Interv. 2013;6:472–478. [DOI] [PubMed] [Google Scholar]
- 35. Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O'Donnell CJ, Mitchell GF, Vasan RS. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low‐gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013;128:1349–1353. [DOI] [PubMed] [Google Scholar]
- 37. Chambers J. The left ventricle in aortic stenosis. Evidence for the use of angiotensin‐converting enzyme inhibitors (education in heart). Heart. 2006;92:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data, analytic methods, and study materials will not be made freely available to other researchers for purposes of reproducing the results or replicating the procedure since the complete study data set contains potentially identifying data.


