Abstract
Background
Implantable cardioverter‐defibrillator (ICD) improves survival when used for primary or secondary prevention of sudden cardiac death. Whether the benefits of ICD in patients with atrial fibrillation (AF) are similar to those with normal sinus rhythm (NSR) is not well established. The aim of this study is to investigate whether ICD patients with AF are at higher risk of mortality and appropriate shock therapy compared with patients with NSR.
Methods and Results
Literature was searched and 25 observational studies with 63 283 patients were included in this meta‐analysis. We compared the outcomes of (1) all‐cause mortality and appropriate shock therapy among AF and NSR patients who received ICD for either primary or secondary prevention and (2) all‐cause mortality among AF patients with ICD versus guideline directed medical therapy. All‐cause mortality (odds ratio, 2.11; 95% confidence interval, 1.73–2.56; P<0.001) and incidence of appropriate shock therapy (odds ratio, 1.77; 95% confidence interval, 1.47–2.13; P<0.001) were significantly higher in ICD patients with AF as compared to NSR. There was no statistically significant mortality benefit from ICD compared with medical therapy in AF patients (odds ratio, 0.69; 95% confidence interval, 0.42–1.11; P=0.12) based on a separate meta‐analysis of 3 studies with 387 patients.
Conclusions
Overall mortality and appropriate shock therapy are higher in ICD patients with AF as compared with NSR. The impact of ICD on all‐cause mortality in AF patients when compared to goal‐directed medical therapy is unclear, and randomized controlled trials are needed comparing AF patients with ICD and those who have indications for ICD, but are only on medical therapy.
Keywords: atrial fibrillation, ejection fraction, heart failure, implantable cardioverter defibrillator
Subject Categories: Atrial Fibrillation, Sudden Cardiac Death, Catheter Ablation and Implantable Cardioverter-Defibrillator
Clinical Perspective
What Is New?
Implantable cardioverter‐defibrillator patients with atrial fibrillation appear to have higher appropriate shocks and overall mortality risk.
What Are the Clinical Implications?
Atrial fibrillation may be a marker of worse outcome in patients with implantable cardioverter‐defibrillator, and therefore implantable cardioverter‐defibrillator patients with atrial fibrillation may need tailored programming and close monitoring.
Introduction
Implantable cardioverter‐defibrillator (ICD) therapy has been shown to reduce sudden cardiac death and improve survival when used as primary prevention in selected heart failure (HF) patients with left ventricular dysfunction and secondary prevention in patients who survive previous cardiac arrest or have sustained ventricular tachycardia.1 ICD exerts this benefit by successfully detecting and terminating life‐threatening ventricular arrhythmias and thereby preventing sudden cardiac death. However, it is extremely important for the ICD device to precisely distinguish between atrial and ventricular arrhythmias before delivering a shock, given that inappropriate ICD shock therapy for atrial arrhythmias wrongly detected as ventricular arrhythmias is a common adverse event in patients with ICD.2
The rate of ICD implantation has increased in recent years; however, whether ICD is placed for primary or secondary prevention, atrial fibrillation (AF) is a frequently found supraventricular arrhythmia in these patients. Nonetheless, whether AF, which is an independent predictor of mortality in the general population,3 heralds worse outcomes in patients with ICD is not fully established.
Previously published studies are contradictory and offer little insight.4, 5, 6 Therefore, the aim of this meta‐analysis is: (1) to determine whether mortality benefit of ICD is similar in patients with AF and normal sinus rhythm (NSR) and (2) whether AF is an independent predictor of appropriate shock therapy.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Search Strategy
We followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines to conduct and report this meta‐analysis7 as illustrated in Figure 1. We searched PubMed, Biological Abstracts, CINAHL Plus with Full Text, Web of Science, the Cochrane Library, and Google Scholar (inception through November 30, 2017). “Atrial fibrillation,” “implantable cardioverter‐defibrillator,” and “shock” were the keywords used in this search. When available, filters/limiters were used to limit the search to clinical studies. Gray literature sources were not included. Titles and abstracts were independently reviewed by 2 authors (U.M., R.R.) and cross‐verified by a third reviewer (J.D.) for inclusion.
Figure 1.
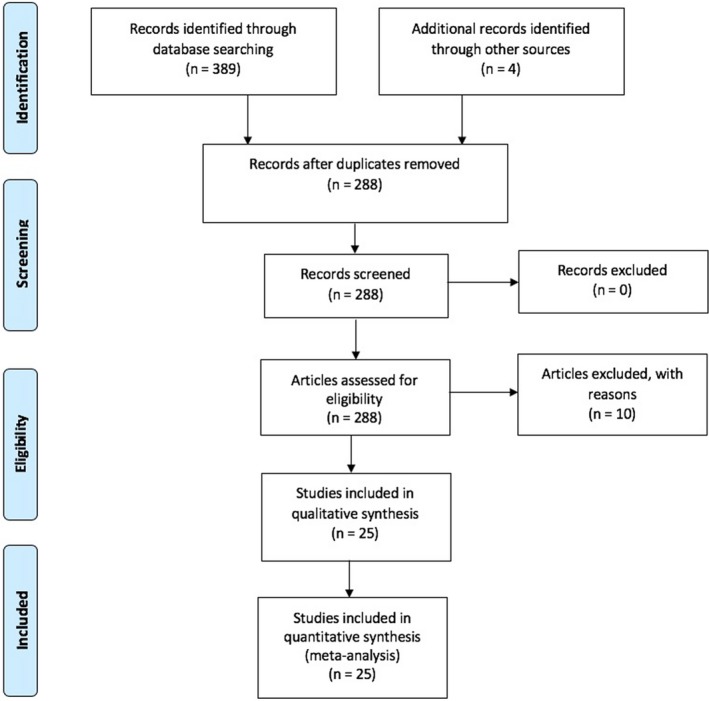
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram.
Inclusion Criteria
Observational studies (retrospective and prospective) reporting outcomes of all‐cause mortality and appropriate shock therapy in ICD patients with persistent or paroxysmal AF or atrial arrhythmias were included. Studies (subgroup analysis of original randomized controlled trials testing outcomes of ICDs) comparing outcomes in AF patients who meet criteria for ICD therapy, but are only on guideline‐directed medical therapy, and AF patients with ICD were also included in a separate meta‐analysis in this article.
Exclusion Criteria
Studies were excluded if they lacked a control group, included patients with cardiac resynchronization therapy, had inadequate data on baseline characteristics, were non‐English studies with no English translation, or only assessed inappropriate shock therapy.
Data Extraction and Quality Assessment of Studies
Data were independently extracted by 2 reviewers (U.M., R.R.) and cross‐verified by a third reviewer (J.D.). All disagreements between reviewers were resolved by consensus. We extracted data on study participants (sample size, age, sex, presence of hypertension, chronic kidney disease, diabetes mellitus, ischemic cardiomyopathy, dilated cardiomyopathy, and percent use of antiarrhythmics and beta‐blockers), study design, and follow‐up. Study characteristics are shown in Tables 1 and 2. The quality of each study and risk of bias was evaluated by the Newcastle–Ottawa quality assessment scale for nonrandomized studies. The following characteristics were assessed for sources of bias: (1) patient selection, including definitions of exposure and representation of the larger population; (2) comparability of study groups and controlling for confounding factors by design or analysis; and (3) assessment and documentation of outcome including duration and loss of follow‐up. Studies were graded as “poor” if they met 4 of the 9 criteria, “fair” if they met 5 to 6 criteria, and “good” if they met more than 6 criteria (Tables 1 and 2).
Table 1.
Study Characteristics of ICD Patients With and Without AF
| Study | Year | Follow Up (m) | Total Patients | Patient With AF | Patients With NSR | Age (y) | Male (%) | ICD Indication | LVEF (%) | AA in AF (%) | AA in NSR (%) | NYHA in AF | NYHA in NSR | Quality Assessmenta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Madhavan et al8 | 2016 | 40 | 253 | 115 | 138 | 68.3 | NS | PP | 32 | ··· | ··· | <3: 85b, ≥3: 15 | Good | |
| Grönefeld et al9 | 2000 | 20 | 229 | 38 | 191 | 64 | 82 | Unspecified | 37 | 21 | 13 | NS | NS | Good |
| Rienstra et al10 | 2007 | 31 | 290 | 83 | 207 | 63 | 81 | PP/SP | 29 | 45 | 36 | <3: 84, ≥3: 16 | <3: 90, ≥3: 10 | Good |
| Borleffs et al11 | 2010 | 28 | 913 | 250 | 663 | 67 | 79 | PP/SP | 32 | 24 | 10 | <3: 55, ≥3: 45 | <3: 66, ≥3: 34 | Good |
| Deneke et al12 | 2004 | 9.5 | 359 | 68 | 291 | 62.8 | 81 | PP/SP | 39 | 37.3 | 24.8 | <3: 81, ≥3: 29 | <3: 83, ≥3: 17 | Good |
| van Gelder et al4 | 2011 | 31 | 537 | 133 | 404 | 71 | 79 | PP | 28 | 20 | 11 | NS | NS | Good |
| Köbe et al13 | 2013 | 14 | 3261 | 607 | 2654 | 70.9 | 82 | PP/SP | 31 | 17.2 | 14.7 | <3: 78, ≥3: 22 | <3: 84, ≥3: 16 | Good |
| Smit et al14 | 2006 | 8 | 80 | 29 | 51 | 63 | 79 | PP | 24 | 24 | 18 | <3: 55, ≥3: 45 | <3: 51, ≥3: 39 | Good |
| Zareba et al15 | 2006 | 20 | 655 | 61 | 594 | 65 | NS | Unspecified | ··· | 14 | 5 | 2 to 4:73 | 2 to 4: 63 | Good |
| van Rees et al16 | 2011 | 60 | 1544 | 355 | 1189 | 61 | 79 | PP/SP | 35 | 20b | ··· | <3: 67b, ≥3: 33 | Good | |
| Bunch et al17 | 2009 | 12 | 1530 | 174 | 1356 | 68.7 | 81 | Unspecified | ··· | 27 | 7.2 | <3: 71, ≥3: 29 | <3: 76, ≥3: 24 | Good |
| Kraaier et al18 | 2013 | 41 | 647 | 183 | 464 | 64 | 81 | Unspecified | ··· | ··· | ··· | NS | NS | ··· |
| Ryan et al19 | 2001 | 24 | 321 | 92 | 229 | 65 | NS | Unspecified | ··· | ··· | ··· | NS | NS | ··· |
| Marijon et al20 | 2010 | 22 | 1030 | 277 | 753 | 63 | 89 | PP/SP | 36 | 54b | ··· | NS | NS | Good |
| Schernthaner et al21 | 2007 | 24.5 | 77 | 32 | 45 | 66 | NS | PP/SP | 34 | 13b | ··· | <3: 65b, ≥3: 35 | Good | |
| Desai et al6 | 2010 | 40 | 549 | 70 | 479 | 74 | NS | PP | 29 | 32b | ··· | 2 to 3: 69b, 4: 31 | Good | |
| Schefer et al22 | 2008 | 51 | 157 | 22 | 135 | 53 | 78 | PP/SP | 40 | 45b | ··· | <3: 74b, ≥3: 26 | Good | |
| Yang et al23 | 2012 | 29 | 148 | 20 | 128 | 53 | 86 | PP/SP | 51 | ··· | ··· | NS | NS | Good |
| Stein et al5 | 2009 | 11.4 | 1655 | 433 | 1222 | 64.4 | 83 | PP | ··· | 46b | ··· | <3: 69b, ≥3: 31 | Good | |
| Smith et al24 | 2011 | 31 | 427 | 112 | 315 | 58 | 79 | PP | 27 | 46b | ··· | <3: 82b, ≥3: 18 | Good | |
| Kraaier et al25 | 2013 | 12 | 861 | 207 | 654 | 62.7 | 79 | PP | 25 | ··· | ··· | NS | NS | Good |
| Hess et al26 | 2014 | 34.8 | 47 282 | 12 834 | 34 448 | 67 | NS | PP | 25 | ··· | ··· | <3: 63b, ≥3: 37 | Good | |
| Caputo et al27 | 2016 | 46 | 156 | 78 | 78 | 65 | NS | SP | 39 | 27b | ··· | NS | NS | Good |
AA indicates antiarrhythmic; AF, atrial fibrillation; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; Med, goal‐directed medical therapy; NS, not specified; NSR, normal sinus rhythm; PP, primary prevention; SP, secondary prevention.
Newcastle‐Ottawa Quality Assessment Scale: poor <4, fair 5 to 6, good >7.
All patients.
Table 2.
Study Characteristics of AF Patients With ICD and Goal‐Directed Medical Therapy
| Study | Year | Follow Up (m) | Total AF Patients | AF Patient With ICD | AF Patients on Med Therapy | Age (y) | Male (%) | ICD Indication | LVEF (%) | Use of AA (%) | NYHA Class (%) | Quality Assessmenta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zareba et al15 | 2006 | 20 | 102 | 61 | 41 | 65 | NA | Unspecified | ··· | 14 | 2 to 4:73 | Good |
| Singh et al28 | 2006 | 29 | 173 | 65 | 108 | 64 | 91 | Unspecified | 25 | 37 | <3: 60, ≥3: 40 | Good |
| Kadish et al29 | 2004 | 45.5 | 112 | 52 | 60 | 58 | NA | SP | 21 | 5.2 | <3: 79, ≥3: 21 | ··· |
AA indicates antiarrhythmic; AF, atrial fibrillation; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; Med therapy, goal‐directed medical therapy; SP, secondary prevention.
Newcastle–Ottawa Quality Assessment Scale: poor <4, fair 5 to 6, good >7.
Statistical Analysis
Risk estimates were used to examine the outcomes of mortality and appropriate shocks and their association with AF. These were derived from reported relative risks, odds ratios (ORs), hazard ratios, incident rate ratios, or standardized incidence ratios, together with corresponding 95% confidence intervals (CIs) from the original studies. Where necessary and possible, all metrics were converted to ORs. If both uni‐ and multivariate analyses were available, data from multivariate analyses were taken. Pooled ORs and 95% CIs were calculated using the DerSimonian–Laird random‐effects method.30 All tests were 2‐sided, and P<0.05 was deemed significant. Heterogeneity was assessed by the I2 statistic, which is the percentage of variation of study estimates beyond that which might be expected by chance alone. I2>50% was considered significant heterogeneity.31, 32 Potential publication bias was assessed by visual inspection of funnel plots, in which standard errors were plotted against log ORs, as well as Eggers’ regression intercept. All statistical analyses were performed using Comprehensive Meta‐Analysis (V3; Biostat Inc., Englewood, NJ). To remove publication and reporting bias, studies investigating AF specifically as a risk factor for appropriate shocks and mortality in patients with ICD were separated and analyzed for a prespecified sensitivity analysis, leaving out studies analyzing risk factors for mortality and appropriate shock with no a priori hypothesis.
Results
Search Results
The literature search yielded 389 articles. Hand‐searching identified 4 additional publications. After removing duplicate titles (n=105) and excluding irrelevant papers (n=11), 288 articles were assessed for eligibility. Of these, 25 observational studies4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 with 63 283 patients (n=16 390 ICD with AF; n=46 684 ICD with NSR; n=209 AF on medical therapy only) met the inclusion criteria for this meta‐analysis. Twenty‐four studies were available as full text; however, 118 was available only as an abstract. Follow‐up averaged 28.6 months (range, 8–60).
Both AF and NSR patients were age‐matched in studies (mean age, 64 years; range, 53–71); 93.4% of patients had ICD for primary prevention, and 6.6% patients had ICD as secondary prevention. Mean left ventricular (LV) ejection fraction was comparable between AF (31.2%) and NSR (31.8%). The relationship of AF and end points (all‐cause mortality and appropriate shock therapy) was analyzed based on a priori hypothesis by 13 studies,4, 9, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 28 whereas the rest of the 12 studies investigated AF as 1 of the several risk factors for these outcomes in ICD patients.
All‐Cause Mortality
Twenty‐two studies4, 5, 6, 8, 9, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 28 with 61 154 patients reported all‐cause mortality and were included for this meta‐analysis. Risk of all‐cause mortality was significantly higher in ICD patients with AF than ICD patients with NSR (OR, 2.11; 95% CI, 1.73–2.56; P<0.001; I2=75.46; Figure 2). Pooled analysis with a fixed‐effects model did not change the results (OR, 1.42; 95% CI, 1.35–1.49; P<0.001; I2=75.45; Figure S1). Funnel plot of these 22 studies showed significant publication bias (Figure S2). Therefore, we performed a preplanned sensitivity analysis by excluding studies that did not primarily look for all‐cause mortality based on presence or absence of AF, but found AF as a risk factor for mortality among other predictors. This sensitivity meta‐analysis of 13 studies showed a similar finding of significantly higher all‐cause mortality in AF patients with ICD than in NSR patients with ICD (OR, 2.00; 95% CI, 1.62–2.47; P<0.001; I2=46.91; Figure S3). A fixed‐effects model did not change the overall direction of the results (OR, 1.89; 95% CI, 1.65–2.15; P<0.001; I2=46.91; Figure S4). Funnel plot of these studies did not show any significant publication bias. We also performed another sensitivity analysis by excluding the only abstract,18 and found that results were unchanged with the risk of mortality being higher in ICD patients with AF than ICD patients with NSR (OR, 2.15; 95% CI, 1.75–2.63; P<0.001).
Figure 2.
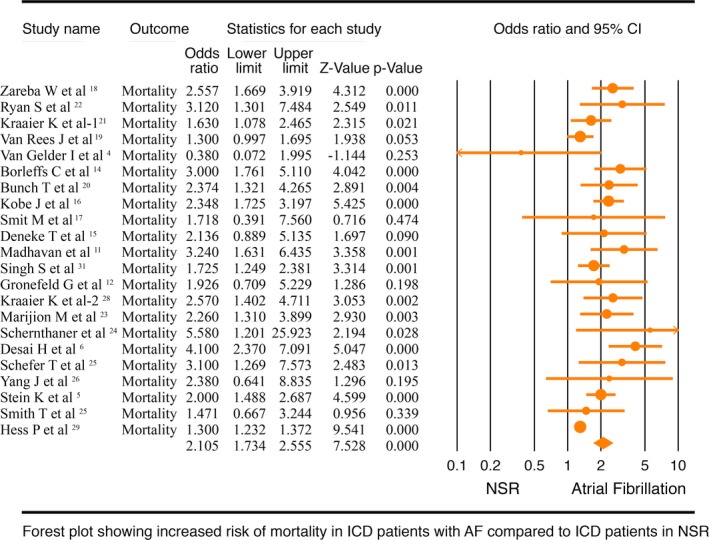
Forest plot comparing mortality in implantable cardioverter defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). CI indicates confidence interval.
We then performed a meta‐analysis of 3 studies15, 28, 29 with 387 patients that compared all‐cause mortality among AF patients with ICD to AF patients who met the criteria for ICD implantation, but were only on guideline‐directed medical therapy. The all‐cause mortality was comparable between AF patients with ICD and goal‐directed medical therapy (OR, 0.69; 95% CI, 0.42–1.11; P=0.12; I2=18.96; Figure 3). A fixed‐effects model did not change the outcome (OR, 0.70; 95% CI, 0.46–1.07; P=0.10; I2=18.96; Figure S5).
Figure 3.
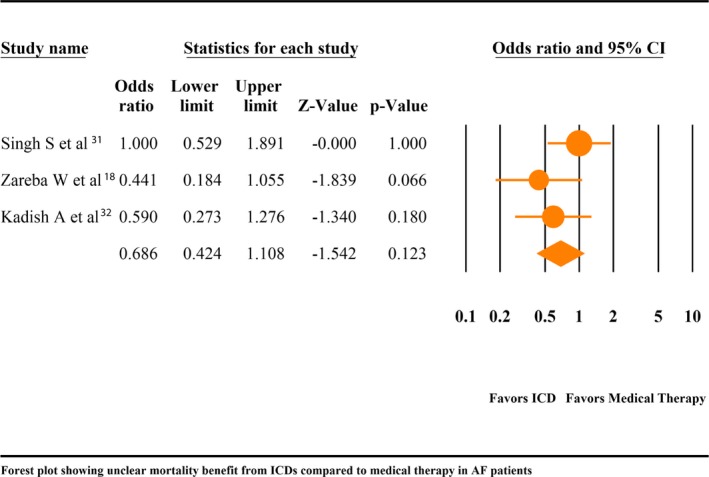
Forest plot comparing mortality in atrial fibrillation (AF) patients with implantable cardioverter defibrillator (ICD) vs goal‐directed medial therapy (GDMT). CI indicates confidence interval.
Appropriate Shock Therapy
Nine studies4, 9, 10, 11, 14, 15, 18, 27, 28 with 3680 patients assessed the association of AF and appropriate shock therapy among patients with ICD. Studies used an inconsistent protocol for ICD programming, which have been summarized in Table 3. Our meta‐analysis suggests that compared with NSR patients, AF patients with ICD are at higher risk of appropriate shock therapy (OR, 1.77; 95% CI, 1.47–2.13; P<0.001; I2=0.00; Figure 4). A separate fixed‐effects model did not change the outcome (OR, 1.77; 95% CI, 1.47–2.13; P<0.001; I2=0.00; Figure S6). No significant publication bias was noted on a funnel plot of these studies (Figure S7). A separate sensitivity analysis, where we excluded the only abstract,18 showed the same results (OR, 1.79; 95% CI, 1.46–2.19; P<0.001).
Table 3.
ICD Programing Protocol
| Study | ICD Programing |
|---|---|
| Borleffs et al11 | Unspecified |
| Caputo et al27 | Monitor Zone: >150 bpm. VT zone: 180 to 200 bpm. VF zone: >200 bpm |
| Grönefeld et al9 | Varied based on patients’ needs |
| Kraaier et al18 | Unspecified |
| Rienstra et al10 | VT zone: >150 bpm. VF zone: >200 bpm |
| Singh et al28 | Unspecified |
| Smit et al14 | VT zone: >150 bpm. VF zone: >200 bpm |
| van Gelder et al4 | Unspecified |
| Zareba et al15 | Unspecified |
bpm indicates beats per minute; ICD, implantable cardioverter‐defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 4.
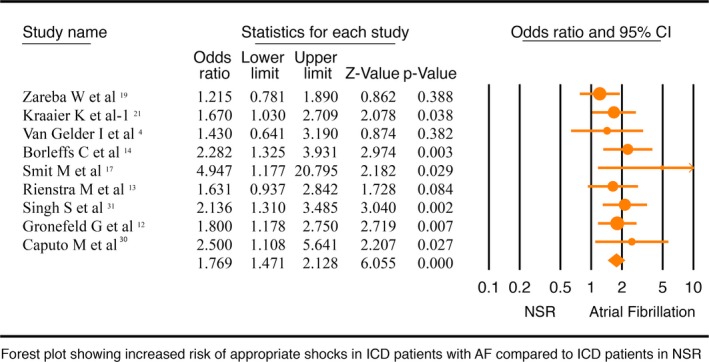
Forest plot comparing appropriate shock therapy in implantable cardioverter defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). CI indicates confidence interval.
Discussion
To our knowledge, this is the first meta‐analysis comparing survival benefit of ICD in AF and NSR patients. The principal finding of this meta‐analysis is that mortality (P<0.001) and appropriate shock therapy (P<0.001) are relatively higher in AF patients with ICD than NSR patients with ICD. In addition, the mortality benefit from ICD in AF patients, as compared with medical therapy alone, is currently unclear (P=0.12).
AF in the general population has been found to have an adverse prognosis and is independently associated with 1.5‐ to 1.9‐fold increased risk of mortality, as reported in the Framingham study.3 Similarly, the AVID (Antiarrhythmics Versus Implantable Defibrillators) registry analysis and the SOLVD (Studies of Left Ventricular Dysfunction) trial showed that AF is associated with an increased mortality risk in patients presenting with ventricular arrhythmias (relative risk, 1.2; CI=1.03–1.04; P=0.02)33 and in patients with LV dysfunction (relative risk, 1.34; CI=1.12–1.62; P=0.002),34 respectively. The reasons for adverse outcome in patients with AF is likely multifactorial and may include development and progression of HF, thromboembolic events, and use of antiarrhythmic drugs. Whether the presence of AF in patients with ICD with LV dysfunction and other comorbidities compounds the problem would be interesting to know. AF and HF are known to coexist, and their combination renders a poor prognosis.35 Thus, patients who are candidates for ICD placement with a combination of AF and HF are at a higher risk of mortality compared to those with HF alone. When indicated, ICD improves survival; however, our meta‐analysis on AF patients with ICD and goal‐directed medical therapy shows no difference in mortality. Based on available data from 3 studies and 387 patients, our pooled analysis suggests that there is no difference in mortality when comparing AF patients with ICD to those AF patients who otherwise meet criteria for ICD, but are only on goal‐directed medical therapy. It is important to note that the interpretation of the results of this particular analysis (with 3 studies and 387 patients) is limited because the lack of statistical significance may stem from a low statistical power. On the other hand, the comparison of similar‐sized trials that tested the benefit of ICD in the general population shows only 1 of 3 trials that showed a significant mortality benefit. The MADIT (Multicenter Automatic Defibrillator Implantation Trial)36 included 196 high‐risk patients (with unsustained ventricular tachycardias and other ventricular tachycardias not suppressed with Procainamide) than the typical primary prevention population and showed that overall mortality improved by 54% in the ICD arm, whereas the CASH (Cancer and Steroid Hormone) Study,37 a secondary prevention trial with 288 patients, showed a 23% (nonsignificant) reduction in mortality rates with ICD as compared with medical therapy alone. On the other hand, the CAT (Cardiomyopathy Trial),38 another primary prevention trial with 104 patients, showed no mortality benefit form ICD. In the CAT trial, there was only a 6% difference between groups (86% in ICD arm, 80% in control at 4 years), and the investigators attribute this lack of survival benefit to low event rate in the study. Perhaps a well‐powered, randomized trial comparing patients with high AF burden with ICD with those who need ICD, but are being managed medically, will shed more light.
It is well established that ICD prolongs survival by delivering shock to terminate life‐threatening arrhythmias; however, these shock therapies are not completely benign. Existing evidence shows that patients who receive defibrillator shock therapy have a higher mortality than those who do not.39, 40, 41, 42, 43 Direct myocardial damage from defibrillator shock therapy impairs cardiac function, leading to hemodynamic compromise and poor prognosis.44, 45, 46 Several studies have shown that AF independently increases the risk of inappropriate, as well as appropriate, shocks in patients with ICD.4, 16, 23, 47 AF was found to be the most common cause of inappropriate shock therapy in the MADIT‐II trial.48 Our study focuses on appropriate shocks and validates these findings, partially explaining the finding of increased mortality in ICD patients with AF compared to those without AF. It is, however, important to note that the MADIT RIT (Multicenter Automatic Defibrillator Implantation Trial‐Reduce Inappropriate Therapy) trial,49 that excluded permanent AF patients, showed reduction in inappropriate shocks and all‐cause mortality by programming ICD shocks only for tachyarrhythmias of 200 beats per minute or higher. However, studies included in our meta‐analysis were either published before the MADIT RIT, or ICD programing was based on specific needs of patients and did not specifically follow the MADIT RIT protocol. Although MADIT RIT programming decreased inappropriate shocks, mortality, and appropriate antitachycardia pacing therapy, it did not affect the incidence of appropriate shocks, which is shown to be increased in patients with AF in our analysis. Furthermore, in a substudy of the the MADIT RIT,50 although confirming a decrease in inappropriate ICD therapy with high‐rate programing even in patients with atrial arrhythmias, the inappropriate therapies were still significantly higher in patients with atrial arrhythmias compared to patients with NSR. It is notable, from MADIT RIT findings, that not all the appropriate shocks are required to terminate ventricular tachycardia/ventricular fibrillation; therefore, one can speculate that appropriate shocks in pre–MADIT RIT studies are likely overestimating the necessary appropriate shock therapy. Nonetheless, it is currently unclear whether MADIT RIT settings would have changed the results of this meta‐analysis. Therefore, this should be addressed in future randomized trials comparing high‐rate therapy to conventional therapy, specifically in patients with AF.
Furthermore, Klein et al, in the PROFIT (Prospective Analysis of Risk Factor for Appropriate ICD Therapy) study, investigated the predictors of ventricular arrhythmias in 250 ICD patients and reported a 1.8‐fold increased risk of ventricular arrhythmias at 2 years’ follow‐up in patients with AF.51 Similarly, an association of ventricular arrhythmias with AF was also reported in 2 different studies in patients with ICD.9, 52 One explanation for this could be the higher incidence of ventricular arrhythmias in AF patients attributed to shared risk factors, like ischemia, increased sympathetic tone, or increased LV filling pressures, and hemodynamic changes, like decreased cardiac output, which may lead to altered electrophysiological property of the ventricle causing appropriate shock delivery.14 Second, concomitant use of antiarrhythmic drugs for atrial arrhythmia can provoke ventricular arrhythmias because of their proarrhythmic potential and thereby further increasing the risk of mortality53, 54 and appropriate shocks with an ICD in place. A third possibility could be the development and progression of HF in AF patients given that lower ejection fraction has been associated with higher ICD‐unresponsive sudden cardiac death.20 It is important to note that several recent trials have shown that catheter ablation decreases AF burden and improves overall mortality, LV systolic function, and quality of life in AF patients with HF.55, 56, 57 The effect of catheter ablation and atrioventricular nodal ablation plus right ventricular pacing on outcomes of AF patients with ICD could not be ascertained from the included studies and will provide the basis for future randomized controlled trials.
Although ICD is an effective strategy to reduce sudden cardiac death, poor outcomes in AF patients raises a question regarding the benefit of ICD in AF patients and further extends the discussion of carefully reviewing the need for ICD placement on a case‐by‐case basis. This also warrants multicenter, randomized controlled trials of ICD versus medical management in, specifically, HF patients with AF and NSR for head‐to‐head comparison.
Limitations
Studies exploring predictors of mortality and appropriate shocks in patients with ICDs without an a priori hypothesis regarding AF offer a significant challenge attributed to the possibility of nonreporting of negative studies. To combat that, we performed a sensitivity analysis excluding these studies from the meta‐analysis and found similar results.
The included studies were observational or post‐hoc analyses of prospective, randomized trials, and studies not reporting adjusted outcome measurements were included in the study with unadjusted event rates introducing unknown confounders.
For appropriate shocks, device settings were variable across studies, and therefore it is unclear whether MADIT RIT high‐rate setting would have any impact on overall results.
Studies did not uniformly report AF type and whether AF was present at baseline or was new onset and detected by a device during the study, thereby limiting the interpretation of the results attributable to type of AF.
Conclusions
In conclusion, our meta‐analysis suggests that appropriate shock therapy and mortality are higher in AF patients with ICD as compared to NSR. With available data, the impact of ICD on all‐cause mortality in AF patients when compared with goal‐directed medical therapy is unclear. Randomized controlled trials comparing AF patients with ICD and those who have indications for ICD, but are only on medical therapy, are needed in this regard.
Disclosures
None.
Supporting information
Figure S1. Forest plot of studies comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S2. Funnel plot for all‐cause mortality.
Figure S3. Forest plot of studies with a priori hypothesis, comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR).
Figure S4. Forest plot of studies with a priori hypothesis, comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S5. Forest plot comparing mortality in AF patients with implantable cardioverter defibrillator (ICD) vs goal‐directed medical therapy (GDMT). A fixed‐effects model.
Figure S6. Forest plot comparing appropriate shock therapy in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S7. Funnel plot for appropriate shock therapy.
(J Am Heart Assoc. 2018;7:e010156 DOI: 10.1161/JAHA.118.010156.)
References
- 1. Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, Ferguson TB, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2013;61:e6–e75. [DOI] [PubMed] [Google Scholar]
- 2. Theuns DA, Klootwijk AP, Goedhart DM, Jordaens LJ. Prevention of inappropriate therapy in implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2004;44:2362–2367. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 4. van Gelder IC, Phan HM, Wilkoff BL, Brown ML, Rogers T, Peterson BJ, Birgersdotter‐Green UM. Prognostic significance of atrial arrhythmias in a primary prevention ICD population. Pacing Clin Electrophysiol. 2011;34:1070–1079. [DOI] [PubMed] [Google Scholar]
- 5. Stein KM, Mittal S, Gilliam FR, Gilligan DM, Zhong Q, Kraus SM, Meyer TE. Predictors of early mortality in implantable cardioverter‐defibrillator recipients. Europace. 2009;11:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desai H, Aronow WS, Ahn C, Gandhi K, Hussain S, Lai HM, Sharma M, Frishman WH, Cohen M, Sorbera C. Risk factors for appropriate cardioverter‐defibrillator shocks, inappropriate cardioverter‐defibrillator shocks, and time to mortality in 549 patients with heart failure. Am J Cardiol. 2010;105:1336–1338. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madhavan M, Waks JW, Friedman PA, Kramer DB, Buxton AE, Noseworthy PA, Mehta RA, Hodge DO, Higgins AY, Webster TL, Witt CM, Cha YM, Gersh BJ. Outcomes after implantable cardioverter‐defibrillator generator replacement for primary prevention of sudden cardiac death. Circ Arrhythm Electrophysiol. 2016;9:e003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grönefeld GC, Mauss O, Li YG, Klingenheben T, Hohnloser SH. Association between atrial fibrillation and appropriate implantable cardioverter defibrillator therapy: results from a prospective study. J Cardiovasc Electrophysiol. 2000;11:1208–1214. [DOI] [PubMed] [Google Scholar]
- 10. Rienstra M, Smit MD, Nieuwland W, Tan ES, Wiesfeld AC, Anthonio RL, Van den Berg MP, Van Veldhuisen DJ, van Gelder IC. Persistent atrial fibrillation is associated with appropriate shocks and heart failure in patients with left ventricular dysfunction treated with an implantable cardioverter defibrillator. Am Heart J. 2007;153:120–126. [DOI] [PubMed] [Google Scholar]
- 11. Borleffs CJ, van Rees JB, van Welsenes GH, van der Velde ET, van Erven L, Bax JJ, Schalij MJ. Prognostic importance of atrial fibrillation in implantable cardioverter‐defibrillator patients. J Am Coll Cardiol. 2010;55:879–885. [DOI] [PubMed] [Google Scholar]
- 12. Deneke T, Lawo T, Gerritse B, Lemke B. Mortality of patients with implanted cardioverter/defibrillators in relation to episodes of atrial fibrillation. Europace. 2004;6:151–158. [DOI] [PubMed] [Google Scholar]
- 13. Köbe J, Wasmer K, Andresen D, Kleemann T, Andresen D, Spitzer SG, Kleemann T, Jehle J, Spitzer SG, Jehle J, Brachmann J, Stellbrink C, Brachmann J, Stellbrink C, Hochadel M, Jochen S, Helmut K, Lars E. Impact of atrial fibrillation on early complications and one year‐survival after cardioverter defibrillator implantation: results from the German DEVICE registry. Int J Cardiol. 2013;168:4184–4190. [DOI] [PubMed] [Google Scholar]
- 14. Smit MD, Van Dessel PF, Rienstra M, Nieuwland W, Wiesfeld AC, Tan ES, Anthonio RL, Van Veldhuisen DJ, van Gelder IC. Atrial fibrillation predicts appropriate shocks in primary prevention implantable cardioverter‐defibrillator patients. Europace. 2006;8:566–572. [DOI] [PubMed] [Google Scholar]
- 15. Zareba W, Steinberg JS, McNitt S, Daubert JP, Piotrowicz K, Moss AJ. Implantable cardioverter‐defibrillator therapy and risk of congestive heart failure or death in MADIT II patients with atrial fibrillation. Heart Rhythm. 2006;3:631–637. [DOI] [PubMed] [Google Scholar]
- 16. van Rees JB, Borleffs CJW, de Bie MK, Stijnen T, van Erven L, Bax JJ, Schalij MJ. Inappropriate implantable cardioverter‐defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. [DOI] [PubMed] [Google Scholar]
- 17. Bunch TJ, Day JD, Olshansky B, Stolen KQ, Mullin CM. Newly detected atrial fibrillation in patients with an implantable cardioverter‐defibrillator is a strong risk marker of increased mortality. Heart Rhythm. 2009;6:2–8. [DOI] [PubMed] [Google Scholar]
- 18. Kraaier K, Van Rennes B, Oude Velthuis B, Dorman HG, Stevenhagen YJ, Van Opstal J, Scholten MF. Impact of atrial fibrillation in an implantable cardioverter defibrillator cohort. Eur Heart J. 2013;34:1408–1408. [Google Scholar]
- 19. Ryan S, Siemon G, Drogemuller A, Rameken M. 2 year follow‐up of 321 patients with an implantable cardioverter/defibrillator: comparison of patients with and without atrial fibrillation. Z Kardiol. 2001;90:906–915. [DOI] [PubMed] [Google Scholar]
- 20. Marijon E, Trinquart L, Otmani A, Leclercq C, Fauchier L, Chevalier P, Klug D, Defaye P, Lellouche N, Mansourati J, Deharo JC, Sadoul N, Anselme F, Maury P, Davy JM, Extramiana F, Hidden‐Lucet F, Probst V, Bordachar P, Mansour H, Chauvin M, Jouven X, Lavergne T, Chatellier G, Le Heuzey J. Predictors for short‐term progressive heart failure death in New York Heart Association II patients implanted with a cardioverter defibrillator—the EVADEF study. Am Heart J. 2010;159:659–664.e1. [DOI] [PubMed] [Google Scholar]
- 21. Schernthaner C, Pichler M, Strohmer B. Lower body mass index and atrial fibrillation as independent predictors for mortality in patients with implantable cardioverter defibrillator. Croat Med J. 2007;48:59–67. [PMC free article] [PubMed] [Google Scholar]
- 22. Schefer T, Wolber T, Binggeli C, Holzmeister J, Brunckhorst C, Duru F. Long‐term predictors of mortality in ICD patients with non‐ischaemic cardiac disease: impact of renal function. Europace. 2008;10:1052–1059. [DOI] [PubMed] [Google Scholar]
- 23. Yang JH, Byeon K, Yim HR, Park JW, Park SJ, Huh J, Kim JS, On YK. Predictors and clinical impact of inappropriate implantable cardioverter‐defibrillator shocks in Korean patients. J Korean Med Sci. 2012;27:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith T, Theuns DA, Caliskan K, Jordaens L. Long‐term follow‐up of prophylactic implantable cardioverter‐defibrillator‐only therapy: comparison of ischemic and nonischemic heart disease. Clin Cardiol. 2011;34:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraaier K, Scholten MF, Tijssen JG, Theuns DA, Jordaens LJ, Wilde AA, van Dessel PF. Early mortality in prophylactic implantable cardioverter‐defibrillator recipients: development and validation of a clinical risk score. Europace. 2013;16:40–46. [DOI] [PubMed] [Google Scholar]
- 26. Hess PL, Hellkamp AS, Peterson ED, Sanders GD, Al‐Khalidi HR, Curtis LH, Hammill BG, Pun PH, Curtis JP, Anstrom KJ, Hammill SC, Al‐Khatib SM. Survival after primary prevention implantable cardioverter‐defibrillator placement among patients with chronic kidney disease. Circ Arrhythm Electrophysiol. 2014;7:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caputo ML, Regoli F, Conte G, Adjibodou B, Svab S, Del Bufalo A, Moccetti T, Curti M, Klersy C, Auricchio A. Temporal trends and long term follow‐up of implantable cardioverter defibrillator therapy for secondary prevention: a 15‐year single‐centre experience. Int J Cardiol. 2017;228:31–36. [DOI] [PubMed] [Google Scholar]
- 28. Singh SN, Poole J, Anderson J, Hellkamp AS, Karasik P, Mark DB, Lee KL, Bardy GH; SCD‐HeFT Investigators . Role of amiodarone or implantable cardioverter/defibrillator in patients with atrial fibrillation and heart failure. Am Heart J. 2006;152:974.e7–974.e11. [DOI] [PubMed] [Google Scholar]
- 29. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NAM, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH; Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wyse DG, Love JC, Yao Q, Carlson MD, Cassidy P, Greene LH, Martins JB, Ocampo C, Raitt MH, Schron E, Stamato NJ, Olarte A. Atrial fibrillation: a risk factor for increased mortality—an AVID registry analysis. J Interv Card Electrophysioly. 2001;5:267–273. [DOI] [PubMed] [Google Scholar]
- 34. Dries D, Exner D, Gersh B, Domanski M, Waclawiw M, Stevenson L. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. J Am Coll Cardiol. 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 35. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 36. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 37. Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748–754. [DOI] [PubMed] [Google Scholar]
- 38. Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453–1458. [DOI] [PubMed] [Google Scholar]
- 39. Powell BD, Saxon LAS, Boehmer JP, Day JD, Gilliam R, Heidenreich PA, Jones PW, Rousseau MJ, Hayes DL. Survival after shock therapy in implantable cardioverter‐defibrillator and cardiac resynchronization therapy‐defibrillator recipients according to rhythm shocked. J Am Coll Cardiol. 2013;62:1674–1679. [DOI] [PubMed] [Google Scholar]
- 40. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Brady GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Streitner F, Herrmann T, Kuschyk J, Lang S, Doesch C, Papavassiliu T, Streitner I, Veltmann C, Haghi D, Borggrefe M. Impact of shocks on mortality in patients with ischemic or dilated cardiomyopathy and defibrillators implanted for primary prevention. PLoS One. 2013;8:e63911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck KH, Gent M, Connolly SJ. Mechanisms underlying the lack of effect of implantable cardioverter‐defibrillator therapy on mortality in high‐risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation. 2010;122:2645–2652. [DOI] [PubMed] [Google Scholar]
- 43. Moss AJ, Greenberg H, Case R, Zareba W, Hall J, Brown M, Daubert JP, McNitt S, Andrews M, Elkin A. Long‐term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. [DOI] [PubMed] [Google Scholar]
- 44. Walcott GP, Killingsworth CR, Ideker RE. Do clinically relevant transthoracic defibrillation energies cause myocardial damage and dysfunction? Resuscitation. 2003;59:59–70. [DOI] [PubMed] [Google Scholar]
- 45. Tokano T, Bach D, Chang J, Davis J, Souza JJ, Zivin A, Knight BP, Goyal R, Man KC, Morady F, Strickberger SA. Effect of ventricular shock strength on cardiac hemodynamics. J Cardiovasc Electrophysiol. 1998;9:791–797. [DOI] [PubMed] [Google Scholar]
- 46. Sham'a RA, Nery P, Sadek M, Yung D, Redpath C, Perrin M, Sarak B, Birnie D. Myocardial injury secondary to ICD shocks: insights from patients with lead fracture. Pacing Clin Electrophysiol. 2013;37:237–241. [DOI] [PubMed] [Google Scholar]
- 47. Tenma T, Yokoshiki H, Mizukami K, Mitsuyama H, Watanabe M, Sasaki R, Maeno M, Matsui Y, Tsutsui H. Predictors and proarrhythmic consequences of inappropriate implantable cardioverter‐defibrillator therapy. Circ J. 2015;79:1920–1927. [DOI] [PubMed] [Google Scholar]
- 48. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. [DOI] [PubMed] [Google Scholar]
- 49. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes M III, Greenberg H, Hall J, Haung DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 50. Kutyifa V, Moss AJ, Schuger C, McNitt S, Polonsky B, Ruwald ACH, Ruwald MH, Daubert JP, Zareba W. Reduction in inappropriate ICD therapy in MADIT‐RIT patients without history of atrial tachyarrhythmia. J Cardiovasc Electrophysiol. 2015;26:879–884. [DOI] [PubMed] [Google Scholar]
- 51. Klein G, Lissel C, Fuchs AC, Gardiwal A, Oswald H, deSousa M, Pichlmaier AM, Lichtinghagen R, Geerlings H, Lippolt P, Niehaus M, Drexler H, Korte T. Predictors of VT/VF‐occurrence in ICD patients: results from the PROFIT‐Study. Europace. 2006;8:618–624. [DOI] [PubMed] [Google Scholar]
- 52. Stein KM, Euler DE, Mehra R, Seidl K, Slotwiner DJ, Mittal S, Markowitz SM, Lerman BB; Jewel AF Worldwide Investigators . Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol. 2002;40:335–340. [DOI] [PubMed] [Google Scholar]
- 53. Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 1992;20:527–532. [DOI] [PubMed] [Google Scholar]
- 54. Lafuente‐Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for Maintaining Sinus Rhythm After Cardioversion of Atrial Fibrillation. Chichester, UK: John Wiley & Sons; 1996:1043–1089. [Google Scholar]
- 55. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 56. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, Ullah W, Unsworth B, Mayet J, Dhinoja M, Earley MJ. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 57. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol. 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot of studies comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S2. Funnel plot for all‐cause mortality.
Figure S3. Forest plot of studies with a priori hypothesis, comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR).
Figure S4. Forest plot of studies with a priori hypothesis, comparing all‐cause mortality in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S5. Forest plot comparing mortality in AF patients with implantable cardioverter defibrillator (ICD) vs goal‐directed medical therapy (GDMT). A fixed‐effects model.
Figure S6. Forest plot comparing appropriate shock therapy in implantable cardioverter‐defibrillator (ICD) patients with atrial fibrillation (AF) and normal sinus rhythm (NSR). A fixed‐effects model.
Figure S7. Funnel plot for appropriate shock therapy.


